Abstract
Tail biopsy of laboratory mice for genotyping purposes has been studied extensively to develop refinements for this common procedure. Our prior work assessed tail vertebral development in different mouse strains (age, 3 to 42 d) and analyzed behavior and activity in mice (age, 21 to 45 d) biopsied under isoflurane anesthesia. To assess the effects of biopsy on preweanling mice, we here evaluated BALB/cAnNCrl mice (n = 80; age, 18 to 21 d) that received topical vapocoolant (ethyl chloride), topical anesthetic (Cetacaine), or isoflurane anesthesia before undergoing a 5-mm or sham biopsy. Control mice did not receive any anesthetic intervention. Regardless of the anesthetic used, acute observation scores indicative of distress were increased at 10 min after biopsy, and locomotor activity was decreased, in biopsied compared with control mice. Acute observation scores at 10 min after biopsy were higher in mice that received ethyl chloride compared with isoflurane or no anesthesia. Microscopic analysis revealed that inflammatory changes in the distal tail remained elevated until 7 d after biopsy and were higher in tails exposed to ethyl chloride. Our findings indicate that vapocoolant, topical anesthesia, and inhaled isoflurane do not enhance the wellbeing of preweanling mice undergoing tail biopsy. Due to the lack of appreciable benefits and the presence of notable adverse effects, using vapocoolants or Cetacaine for this tail biopsy procedure in laboratory mice is unadvisable and we encourage the removal of these agents from institutional tail biopsy guidelines.
Abbreviations: DT, distal tail; PT, proximal tail
Laboratory mice can be genotyped by analyzing samples derived from multiple sources, including distal tail biopsies,4,11,21,25,31,46,60,61,65,74 blood,4,14,17,28,33,57 buccal36,53,74 or rectal45 swabs, fecal pellets,10 hair,8,57,64 and toe12,49 and ear pinnal biopsies.17,24,25,62 Of all available options, distal tail biopsy remains the most widely used method for obtaining tissue from both preweanling and older mice, likely due to superior aspects of DNA quantification, accuracy, reproducibility and practicality of this source.9,24 The tail biopsy procedure has not been linked to measurable outcomes indicative of prolonged distress or considerable pain, beyond the stress associated with capture and restraint.3,18,29,55 Regardless of genetic background, the tail vertebrae of all mice have calcification and ossification, with mature vertebral endplates, within the distal 5 mm of tail prior to typical weaning age (21 d).30 Therefore, contemporary recommendations are to harvest a minimal amount of distal tail tissue (less than 5 mm) from mice, to limit discomfort from the procedure and maximize DNA yield from highly cellular cartilaginous (nonossified) tissue.24,30,60,61
In our earlier work, we advocated for the application of anesthesia or topical analgesia for biopsies in weanling mice.30 We elected to determine whether isoflurane anesthesia is an appropriate refinement for added comfort during the procedure of tail collection.29 Due to its high safety profile for both the user and patient, isoflurane is one of the most commonly used inhalant anesthetics in the laboratory animal setting; therefore, isoflurane is frequently recommended in guidance regarding tail biopsy procedures. Unexpectedly, we demonstrated that brief (less than 1 min) exposure of isoflurane anesthesia to mice aged 21 to 45 d. resulted in significant locomotor and behavior deficits that persisted until 5 h postinhalation.29 In addition, measurable benefits (for example, diminished anxiety-like responses to tail biopsy and normal activity levels after isoflurane exposure) were not evident when we compared sham and biopsied mice. A growing body of literature has identified additional undesirable outcomes of isoflurane on neonatal and young adult mice. This inhalant has been shown to have neurodegenerative effects and can impact cognitive function, spatial learning, and memory.15,19,37,43,47,67 Continued approaches to management of perceived discomfort after tail biopsy in laboratory mice have provoked controversy regarding the type, timing, and application of anesthesia and analgesia for the procedure. Therefore, for potential pain relief from biopsy, the topical application of different types of local anesthetics (vapocoolants, liquid analgesics, and alcohol-based agents) prior to tail and digit biopsy has been a recent topic of examination.23,38,50,51,58,59
To extend our assessment of refinements to the current practices for tail biopsy in mice, we compared inhaled isoflurane with topical treatments, including the vapocoolant spray ethyl chloride and the topical anesthetic Cetacaine, both of which are recommended specifically by the NIH and other institutions34,39,56,69,70 as anesthetic options for young mice. We determined whether local anesthesia was effective at mitigating the acute behavioral response to tail biopsy in preweanling (age, 18 to 21 d) mice, as well as whether there were any influences on locomotor activity. In addition, we assessed effect of these topical therapies on the tail by performing histologic analyses of biopsied tissues.
Materials and Methods
Mice and testing paradigm.
All mice were cared for in compliance with the Guide for the Care and Use of Laboratory Animals,35 and experiments were approved by the University of Pennsylvania's IACUC. Facilities housing the mice were AAALAC-accredited. Mature male and female mice (BALB/cAnNCrl) were procured from an approved vendor (Charles River Laboratories, Wilmington, MA) at approximately 6 wk of age and maintained on an IACUC-approved protocol. Mice were housed in trios (2 female:1 male) to facilitate breeding. Resulting litters were sexed and grouped into age-specific cohorts (age, 18 to 21 d) for the experimental purpose to emulate when tail biopsies are typically performed around the age of weaning. From these trios, 80 offspring (38 female, 42 male) were tested over a 2-mo period. The 8 experimental treatments tested included: biopsy or sham without anesthesia, biopsy or sham with isoflurane, biopsy or sham with ethyl chloride, and biopsy or sham with Cetacaine. We chose to use BALB/c mice because they are a commonly used strain of laboratory mice and have been included in our previous studies.
Caging and husbandry.
Animals were housed under a 12:12-h light:dark cycle in static microisolation cages (Max 75, Alt Design, Siloam Springs, AR) that were autoclaved with bedding (0.12-in diameter Bed-O-Cob, Animal Specialties and Provisions, Quakertown, PA) prior to use. Mice received water via autoclaved water bottles and were provided ad libitum access to autoclaved chow (LabDiet 5010, Animal Specialties and Provisions). Cotton nest pads (Nestlets, Ancare, Bellmore, NY) and ‘Shepherd shacks’ (Shepherd/Specialty Papers, distributed by Animal Specialties and Provisions) were provided to all breeding and weanling cages.
Sentinel mice at our institution were tested inhouse for 3 quarters of the year and were found to be free from fur mites and pinworms (Syphacia spp. and Aspiculuris spp., by cecal exam). In addition, sentinel mice tested negative for antibodies to pathogens including: mouse hepatitis virus, mouse parvoviruses, rotavirus, Ectromelia virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, and Sendai virus. For 1 quarter of the year, sentinels from the housing facility were tested by an outside contract laboratory and were found to be free from all pathogens contained on a comprehensive assessment panel (HM Assessment Plus Panel, Charles River Laboratories).
Experimental design.
Tail biopsy.
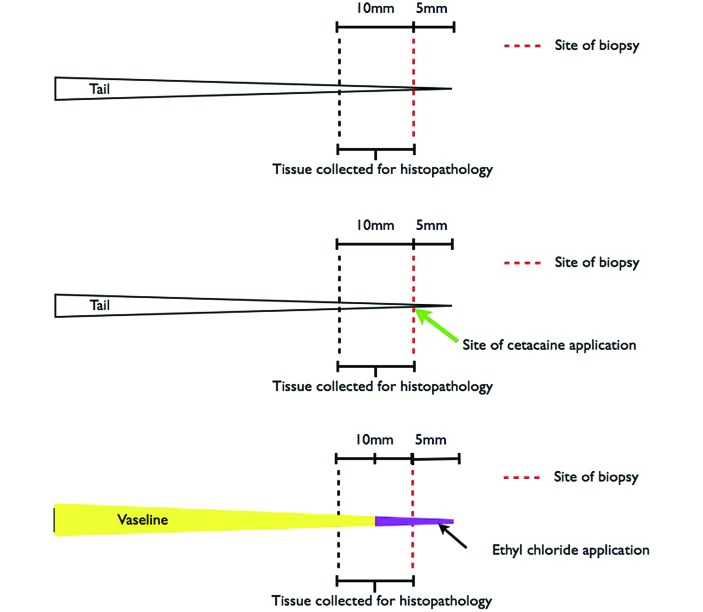
All experimental mice were randomized into groups (8 experimental groups, each with 10 animals) from age-specific litters and uniquely coded by using marker ink (Fine Point Permanent Marker, Sharpie, Downers Grove, IL) at the tail base. Operators restrained mice manually by using one hand and placed them on a plastic block with permanent grooves denoting 5-mm increments or, for mice that received ethyl chloride or Cetacaine, placed them on a clean disposable (C-fold) paper towel next to a marked ruler (in 1-cm increments). Individual paper towels were discarded between mice. The tail was held in position next to the measured line indicating 5 mm (Figure 1). Scissors (Roboz Surigcal Instrument, Gaithersburg, MD), which had been placed in a glass-bead sterilizer (Germinator 500, Stoelting, Wood Dale, IL) for at least 15 s between mice and then allowed to cool for at least 30 s, were used to make a transverse biopsy cut 5 mm proximal to the tip of the tail. To promote hemostasis after biopsy, manual pressure was applied by using a disposable gauze sponge (Curity Gauze Sponge, Kendell–Coviden, Hampshire, MA) for 30 s. Mice that received a sham biopsy were restrained in the same manner, but instead of a biopsy cut, brief pressure was applied by gloved hand, using the thumbnail at the site 5 mm proximal to the tail tip. Mice were then returned to a clean standard shoebox-style cage with a microfilter top and wire lid for acute behavioral assessments. The plastic block and ruler were both wiped with alcohol between mice. All biopsy procedures and acute observations were performed in a dedicated procedure room that was equipped with a fume hood and that was located remotely from the animal housing area.
Figure 1.
Diagram of tail biopsy procedure. The distal 5 mm of tail was transected in all mice, and then the next most distal 10 mm of tail was collected for histopathology. Cetacaine was applied directly to the site of transection. For ethyl chloride application, Vasoline was applied to along the length of the tail, sparing the most distal 1 cm in these mice.
Isoflurane.
Individual mice were exposed to isoflurane delivered by vaporizer (SurgiVet, Smith Medical, Dublin, OH) into an induction chamber (Braintree Scientific, Braintree, MA) at 5% with 0.75 L/min O2. Mice underwent a 5-mm biopsy or sham biopsy as described earlier. Experimental mice, from the 2 groups receiving inhalant anesthesia, were placed in the induction box and exposed to isoflurane for less than 1 min to render them unconscious, as determined by a loss of righting reflex and nonresponsiveness to toe pinch. Unconscious animals were manually restrained on a plastic block, as previously described.29 The tail was held in position next to the measured 5-mm grooves and the tail wiped briefly with alcohol before the biopsy cut or sham biopsy was performed. Mice regained consciousness within 45-s after removal from the induction chamber.
Ethyl chloride.
Individual mice were restrained on a C-fold paper towel as described earlier. The tail was positioned next to a ruler and coated along its length with a layer of Vaseline Petroleum Jelly (Unilever, Englewood Cliffs, NJ) for protection; the distal 1 cm was left uncoated. The distal 1 cm of tail was then wiped with alcohol. Ethyl Chloride Fine Jet Stream Spray (Gebauer, Cleveland, OH) was held approximately 3 inches (approximately 8 cm) away from the tail and sprayed for 3 to 5 s or until the exposed tail area began to exhibit a blanched appearance due to freezing and vasoconstriction. The biopsy or sham procedure was then performed immediately. Before mice were returned to cages, their tails were wiped with a gauze sponge to remove excess Vaseline.
Cetacaine.
Individual mice were restrained on a C-fold paper towel as described above. The tail was held in position next to a ruler and wiped briefly with alcohol before Cetacaine Topical Anesthetic liquid (14.0% benzocaine, 2.0% butamben, and 2.0% tetracaine hydrochloride; Cetylite Industries, Pennsauken, NJ) was sprayed directly at the planned biopsy site (5 mm proximal to distal tail tip) for 1 s. The mouse then was gently restrained for 1 min to allow to achieve the potential maximum anesthetic effect (as described by the manufacturer's instructions) before biopsy or sham procedure was performed, as described earlier.
Acute observations.
Two observers (FCH and GB) were randomly assigned to conduct experiments; therefore, on a given experimental day, mice were scored by direct observation by a single observer. Observers trained together by using a preprinted scoring sheet to avoid the introduction of subjective bias. Blinding of observers to biopsy was not possible for the described testing paradigm; however, they were blinded to anesthetic exposure and the specific ages of mice through coding of animals (as described earlier).
Observations of mouse behavior began at the moment of biopsy; a numerical point was recorded when mice made an audible response or moved the tail from the restrained position at the time of tissue transection or application of manual pressure. Mice were then observed immediately and continuously after biopsy for a 10-min period and then again at 60 min after biopsy (for a second 10-min period) to ascertain the ‘acute observation’ responses, as described previously.29 In summary, each time a mouse was observed to be grooming, licking at the tail, or was subdued compared with cagemates (e.g., not exploring the cage environment), a numerical point was recorded, and all points were tallied (at the end of each 10-min period) to generate a cumulative score, hereafter referred to as the acute observation score. After completion of the first 10-min observation time point, mice were housed within cages but not handled until completion of the acute observation recordings.
Locomotor activity.
After acute observations, mice were relocated to an adjacent behavioral testing room for locomotor testing, which provides a measure of the physical health and coordination of the animals. Mice were assessed individually in clean autoclaved standard mouse cages, with a single layer of corncob bedding, inserted into an adjustable-height frame (30 × 24 × 8 cm) to permit infrared motion detection by 2 levels of sensors, as described previously.29 The sensors were arranged in an 8-beam array strip with approximately 1.5-cm spacing. This arrangement allowed for detection of both horizontal and vertical movements within the cage. Mice were placed randomly into available boxes, and locomotion assessments were conducted in a procedure room without observers present. Mice were recorded for 120 min (30 min light, 90 min dark), and beam break data were collected directly by using commercial software (MED Associates, St Albans City, VT). The variable ‘activity’ was quantified as the number of single horizontal beam breaks, ‘rears’ refers the number of vertical beam breaks, and ‘crosses’ refers to a continuous horizontal motion across one side of the cage.
Histopathologic analysis.
Experimental groups of mice were divided into 3 subcategories for histopathologic analysis. A 1-cm section of tail (starting from the 5 mm proximal to the distal tail-tip mark in sham animals or at the biopsied tip in sampled animals) was collected from each mouse. To standardize microscopic examinations of tail, this 1-cm section was halved into proximal tail (PT, 5 mm) and distal tail (DT, 5 mm) sections (Figure 1). Of the 10 mice per group, tail samples from 4 mice from each group were collected at the end of the first day of testing (day 0). Tails from the remaining mice from each group were harvested at 24 h later (3 mice) and 7 d later (3 mice). Mice were euthanized in a CO2 chamber before collection of tail tissue for histopathology. Each tail was loaded into an individual pathology cassette and placed immediately in a 10% formaldehyde solution prior to submission to the Laboratory of Pathology and Toxicology (Matthew J Ryan Veterinary Hospital, University of Pennsylvania) for decalcification, processing, and embedding. Tails were decalcified in 15% formic acid for as long as 2 d. Sections of the tail tissue were taken on a longitudinal plane from the middle of the tail, placed on a slide, and stained with hematoxylin and eosin. The tail sections were then examined by a board-certified veterinary pathologist who was blinded to the experimental groups. The tails were scored on an inflammation scale of 0 to 3 (0, minimal to no inflammation; 1, mild; 2, moderate; and 3, severe) for both the PT and DT sections individually. The entire tail section was assessed and the percentage of the section infiltrated and the density of inflammatory cells were used to determine the inflammatory score.
Statistical analysis.
The data were summarized by using means and standard errors for continuous outcome measures and by using proportions for categorical outcome measures. For the analysis of acute observation and locomotor activity data, ANOVA was used to test the interactions among 4 treatment groups and between 2 biopsy groups. When interaction was not statistically significant (2-sided, P > 0.05), the ANOVA only considered main effect of treatment and biopsy. When the overall difference was statistically significant (2-sided P ≤ 0.05) among 4 treatment groups, the posthoc pairwise comparisons were made by using a 2-sided P value of less than 0.05 as statistically significant. P values without adjustment and with adjustment for multiple comparisons, using the approach of controlling false-discovery rate, were calculated; where appropriate, multiple comparison (adjusted) P values are included.7 When the data were not normally distributed, the Kruskal–Wallis test was used for comparison among the 4 treatment groups, and the Wilcoxon rank-sum test was used for comparison between the 2 biopsy groups. For the analysis of histologic data, the mean inflammation scores for PT and DT on different experimental harvest days were calculated for each treatment group and compared by using ANOVA, considering the main effect of treatment, biopsy, and day and the 2-way interactions among them. When none of the 2-way interactions was statistically significant, the main effect of treatment, biopsy, and day were statistically tested without interaction terms by using ANOVA. When none of the 2-way interactions was statistically significant (such as biopsy and treatment group for DT), the main effect of treatment and day were tested separately for each biopsy group by using ANOVA. All data analyses were performed by using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Acute observations.
Overall, during the biopsy itself, no mouse pulled its tail away from the platform or vocalized when the tail was transected (biopsy) or when manual pressure was applied (sham). Mice that vocalized only at the time of initial manual restraint were not scored. When data for treatment groups were combined, mice that received a 5-mm biopsy had significantly (P ≤ 0.05) higher acute observation scores at 10 min compared with those mice who received a sham biopsy (Figure 2). Mice that received ethyl chloride, either with a 5-mm biopsy or sham biopsy, had significantly (P = 0.04) increased acute observation scores at 10 min, compared with control mice and mice that received isoflurane. Similar to mice that received ethyl chloride, biopsied mice that received Cetacaine had increases in acute behavioral responses compared with sham animals. Cetacaine was the only anesthetic to increase responses when sham and biopsied mice were compared (P < 0.02). There were no significant differences in acute observation score related to biopsy or anesthetic treatment at 60 min after biopsy.
Figure 2.
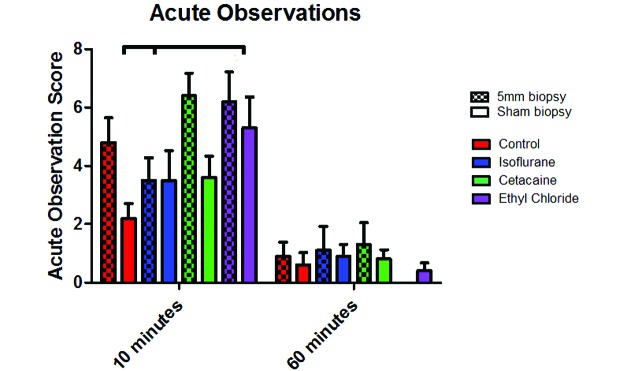
Acute observations. Anesthesia-associated changes in acute behavioral responses after tail biopsy of mice. For all cohorts of mice, acute observation scores were recorded for the first 10 min after biopsy and for a second 10-min interval beginning at 60 min after biopsy. Mice that received a 5-mm biopsy had significantly (P ≤ 0.05) increased acute observation scores at 10 min. Neither isoflurane, Cetacaine, nor ethyl chloride significantly diminished acute responses. The horizontal bar denotes that all mice that received ethyl chloride (both biopsy and sham) had significantly increased acute observation scores at 10 min compared with control mice and mice that received isoflurane anesthesia (P = 0.01 for both). There were no significant differences in acute observation score related to biopsy or anesthetic treatment at 60 min after biopsy.
Locomotor activity.
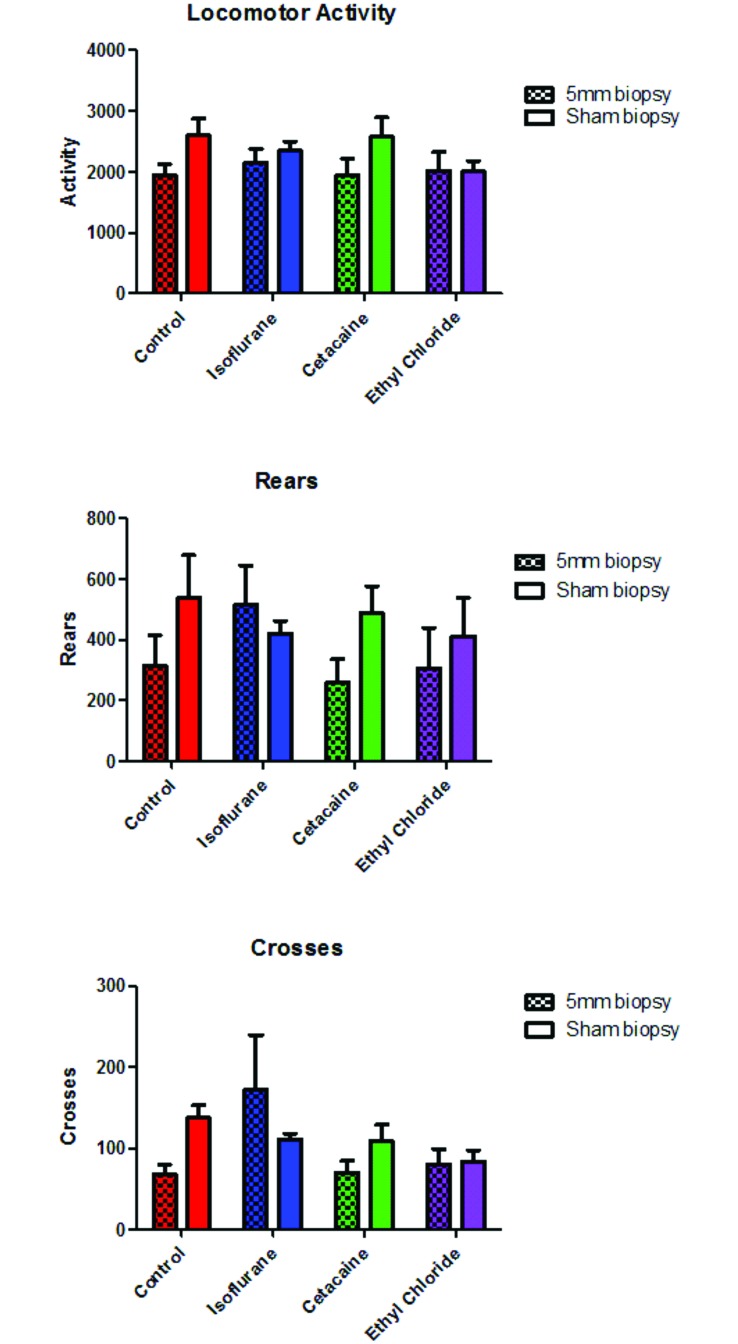
Biopsied mice exhibited significantly (P < 0.05) decreased activity, crosses, and rears compared with those who received a sham biopsy, regardless of whether they had received local or general anesthesia. Mice that received either ethyl chloride or Cetacaine exhibited fewer rears and crosses, compared with control and isoflurane groups, although this finding was not statistically significant (Figure 3).
Figure 3.
Locomotor activity. Activity was measured over 120 min (30 min light, 90 min dark). Whereas biopsy decreased activity across all groups, anesthetic treatment had no significant effect. Of interest, mice that received a topical treatment (ethyl chloride or Cetacaine) tended to have decreased locomotor activity, with fewer rears and crosses, than did the untreated and isoflurane-anesthetized groups. When data between treatment groups were combined, activity was significantly (P < 0.05) decreased in mice that received a 5-mm biopsy compared with a sham biopsy, regardless of whether they had received topical or general anesthesia.
Histopathology.
For assessment of tissue changes, the identified inflammatory cells consisted of predominantly neutrophils with fewer lymphocytes and plasma cells and were located in the dermis and subcutis. Cells were distributed multifocally to diffusely throughout the section, and the overall density was scored.
With additional time after the biopsy procedure (days 0, 1, and 7), the inflammation score for the PT section decreased (P ≤ 0.01) in both biopsied and sham mice. Inflammation scores significantly (P ≤ 0.02) decreased between days 0 and 1 and days 0 and 7 for both sham and biopsied mice. Mice that received a 5-mm biopsy had greater inflammation scores in the PT section compared with mice that received a sham biopsy (P = 0.01). Anesthetic treatment had no significant effect in the PT section.
For the DT section, when data from all mice that received a 5-mm biopsy were combined (Table 1), inflammation scores decreased from day 0 to day 1 and increased from day 1 to day 7 (P < 0.0.1 and P = 0.02, respectively). For mice that received a sham biopsy, the DT inflammation score decreased significantly with time (from day 0 to day 7, P = 0.04). Regardless of the type of anesthetic used, mice that received a 5-mm biopsy had increased inflammation scores for the DT section over all time points when compared with mice that had received a sham biopsy (P < 0.01). In sham-biopsied mice, the DT inflammation score was affected by the type of anesthetic used. Sham-biopsied mice that received isoflurane had significantly (P < 0.008) lower inflammation scores than did mice that received Cetacaine, ethyl chloride, or no anesthesia. Mice that received a sham biopsy sprayed with ethyl chloride had a significantly (P < 0.01) increased score in the DT portion compared with mice that received Cetacaine, isoflurane, or no anesthesia. In addition, mild focal hemorrhage was noted on day 1 after sham biopsy only in tails treated with ethyl chloride. Representative tail sections with corresponding treatments and inflammation scores are highlighted in Figure 4.
Table 1.
Histologic inflammation scores (mean [SE]) of the distal tail across treatment groups
| Day 0 | Day 1 | Day 7 | ||
| Control | ||||
| 5-mm biopsy | 3.00 (0) | 2.67 (0.25) | 3.00 (0) | |
| Sham | 1.75 (0.25) | 1.00 (0) | 0.33 (0.33) | |
| Isoflurane | ||||
| 5-mm biopsy | 3.00 (0) | 2.00 (0.58) | 3.00 (0) | |
| Sham | 0.50 (0.29) | 0 (0) | 0.33 (0.33) | |
| Cetacaine | ||||
| 5-mm biopsy | 3.00 (0) | 2.00 (0.58) | 2.67 (0.33) | |
| Sham | 1.50 (0.29) | 1.33 (0.67) | 0.67 (0.33) | |
| Ethyl chloride | ||||
| 5-mm biopsy | 3.00 (0) | 3.00 (0) | 3 (0) | |
| Sham | 2.25 (0.25) | 2.00 (0) | 2.33 (0.33) | |
Figure 4.
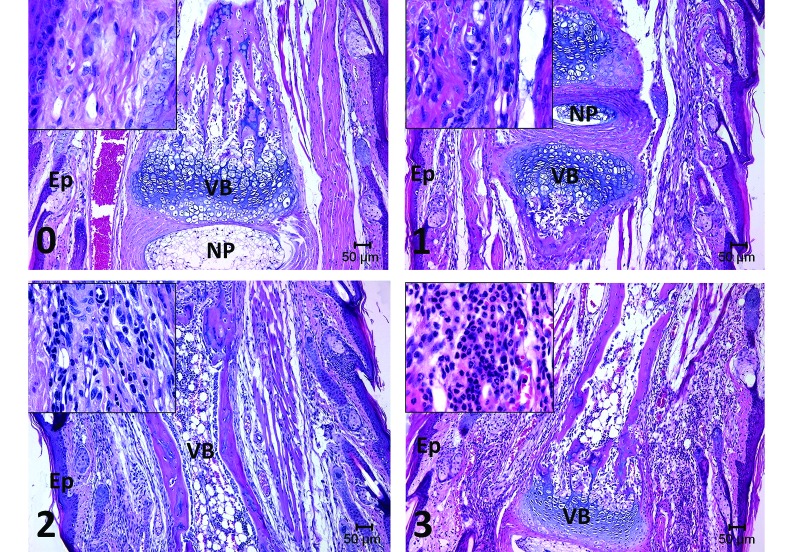
Distal tail histology. Representative images of distal tail histology demonstrate inflammation scores of 0 (none or minimal; example: day 7, isoflurane-treated mouse with sham biopsy), 1 (mild; example: day 7, Cetacaine-treated mouse with sham biopsy); 2(moderate; example: day 7, mouse treated with ethyl chloride and sham biopsy), and 3 (severe; example: day 7, untreated control mouse with biopsy). Overall, inflammation scores were higher (P = 0.01) in biopsied mice compared with sham mice for as long as 7 d after biopsy. Inflammation scores for the distal portion of the tail were significantly (P < 0.01) higher in mice that received ethyl chloride compared with mice that received Cetacaine, isoflurane, or no anesthesia. Ep, epidermis; NP, nucleus pulposa; VB, vertebral body; magnification, 10× (inset, 40×).
Discussion
The influence of tissue biopsy on pain responses and objective alterations in the behaviors of mice has been a primary objective of research for many years;29,30,38,50,59 therefore, we undertook the current study to help further elucidate the efficacy of various topical analgesics on the procedure. The procedure of tail biopsy for laboratory mice elicits an immediate response, with significantly more acute behavioral responses than are seen for mice that undergo a sham biopsy.29 Our previous work demonstrated that although isoflurane is effective for diminishing responses to tail biopsy in older mice (age, 41 to 45 d), it results in prolonged behavioral changes of decreased activity and increased anxiety-like responses when used in younger mice of weaning age.29 Therefore, in our continued effort toward establishing refinements in tail-biopsy procedures for laboratory mice, the current study evaluated various topical treatments—specifically a vapocoolant and a topical anesthetic—on behavioral responses, locomotor activity, and the integrity of tail tissues.
Our interest in assessing vapocoolants, as compared with systemic anesthesia, was derived from anecdotal reports and NIH recommendations56 that these ‘skin-refrigerant’ agents would be effective in providing some measure of comfort after biopsy. Vapocoolants typically are delivered by aerosol application, and the cooling effect is thought to be the direct result of the immediate evaporation of the alcohol-based ingredients.16,22,32 The intended mechanism of action is for the rapid onset of cold to numb the skin temporarily by decreasing nerve conduction, thus interrupting the stimuli to the brain that typically would process the sensation of pain.16,44 These agents are often used in human and sports medicine to assist with pain control associated with injections, minor surgical procedures, sprains, bruising and muscle injury; therefore their use in certain animal species has been extrapolated to have potential efficacy.22,32,44,54,71,72
Although ethyl chloride has long been used as a vapocoolant for pediatric patients and others receiving injections or undergoing catheter placement, it has been shown to be less effective than are other local anesthetics.16,63,66 In addition, inappropriate use of ethyl chloride has the potential to cause tissue damage and frostbite;40 therefore, the labeled use advocates for protection of surrounding skin with petroleum jelly (Vaseline). In our study, despite the application of Vaseline along the majority of the mice's tails, they had increased acute responses (likely due to the detected temperature change of the tail). In addition, mice that received ethyl chloride had the greatest inflammatory infiltrate in DT sections at all time points, compared with all other treatment groups. Furthermore, mice that had applications of ethyl chloride were more likely to lick and groom the tail within the first 10 min after application.
A previous study assessed outbred preweanling Non-Swiss Albino (NSA) mice at 17 d of age after application of ethyl chloride spray (Med-Vet International, Mettawa, IL), buprenorphine (0.05 mg/kg SC), or 30-s immersion in 0.75% bupivicaine to determine whether these agents provided appropriate analgesia for tail biopsy.38 The group found that local anesthetics were ineffective in alleviating pain associated with tail biopsy and further stated that topical ethyl chloride spray resulted in an increase in tail grooming behavior for as long as 60 min when compared with other treatment groups. Importantly, this same group verified that submersion of tails into ice-cold ethanol, currently endorsed by various institutional guidelines,34,39,56,69,70 is ineffective for providing topical analgesia.38
One group sought to evaluate effects of a vapocoolant (95% 1,1,1,3,3-pentafluoropropane and 5% 1,1,1,2-tetrafluoroethane, Spray and Stretch, Gebauer) for toe-clipping procedures in C57Bl/6J mice. This group applied vapocoolant to the digits of young pups (7 to 17 d of age) and determined that the immediate change in tissue temperature appeared to be distressful and often fused digits together. The authors also noted that treating animals elicited vocalizations, limb withdrawal, prolonged tissue swelling, and increased blood loss after toe clipping, likely related to subsequent hypothermia-induced vasodilation. These authors ultimately did not recommend the use of a vapocoolant, given that the adverse effects on the animals markedly outweighed any potential benefits.59
Our findings are consistent with the 2 studies we have just described, in that the use of vapocoolant—in particular, ethyl chloride spray—is ineffective for providing cryoanalgesia and may have serious drawbacks regarding its routine use. Our assertions are in contrast to a published study that supported cryoanalgesia for tail biopsy of laboratory mice (25 to 28 d of age).50 The report stated that the use of ‘ethylene chloride’ spray (noted as Fluor-Ethyl Spray, Gebauer) was preferable to ethyl chloride spray because ethylene chloride is nontoxic, nonflammable, and ecologically friendly.50 According to our communication with the manufacturer, Fluor-Ethyl spray, which has been discontinued, contained ethyl chloride and fluoromethane. In addition, another study referenced to support the use of ethylene chloride,40 instead used the product PainEase (Gebauer)—that is, 1,1,1,3,3-pentafluropropane and 1,1,1,2-tetrafluroethane, the same constituents of the product used by authors who recommended against the use of a vapocoolant, given its pronounced adverse effects.58 The compound ethylene chloride (1,2-dichloroethane) itself does not appear to have been tested, rendering the recommendations potentially ambiguous about the efficacy of cryoanalgesia for older mice.
To our knowledge, the efficacy of Cetacaine as a local anesthetic for tail biopsy in laboratory mice has not been studied previously, although its use is recommended by certain institutions.69 Cetacaine is a fast-acting, long-lasting topical anesthetic that combines benzocaine (fast-onset, short-acting) with butamben (intermediate-onset, intermediate-acting) and tetracaine (slow-onset and long-acting) to provide a potentially broader therapeutic range of topical effect. Cetacaine is specifically labeled for use on mucous membranes; therefore, it was not unexpected to find that this agent appeared to have minimal to no anesthetic effect when applied to intact murine tail tissue. Related to Cetacaine use in our study, other colleagues found that pretreatment of mouse tails by submersion for 2 min into a solution of lidocaine, bupivacaine, or their combination did not seem to permit sufficient penetration into tail tissue to ensure adequate localized analgesia.38 Another study similarly found no significant differences in hot-plate withdrawal times between untreated mice and those treated with lidocaine, bupivicaine, or their combinations when delivered by tail submersion in DMSO.23 Reports of methemoglobinemia due to oropharyngeal use of topical analgesics in both humans and various species of laboratory animals, including mice, have been published.5,13,20,41 A very common behavior after tail biopsy or application of topical anesthetic was for mice to lick the tip of the tail, leading to the likely ingestion of Cetacaine. Due to the extensively studied concerns of methemoglobinemia when ingested or applied topically to the mucosa of the oropharynx, the use of Cetacaine may pose an additional safety hazard to laboratory mice after application.
Work by our group and others have reported on the histology of naive mouse tail tissues for vertebral development and presence of nervous tissue.3,30 However, microscopic assessment of tail tissues, secondary to biopsy and after therapeutic treatments, has not previously been conducted. Inflammation as an entity is presumed to be an evolutionary adaptive response for restoring homeostasis.52 Our study involved exogenous inducers of inflammation, particularly direct tissue transection and the application of skin irritants. Various degrees of inflammation were found in most of the tail samples analyzed. Sham-biopsied animals did have evidence of neutrophilic infiltration in tail tissues, potentially secondary to the manual pressure applied to the tail at the faux biopsy site; however, this response was not statistically significant compared with that in biopsied sections. For those mice that were biopsied, inflammation would have been an expected adaptive tissue response to injury that persisted through the 7-d period that we assessed. Inflammation varied between solely neutrophilic and combined neutrophilic and lymphoplasmacytic; however, the type of inflammatory infiltrate was not dependent on treatment group or day assessed after biopsy. Mice directly sprayed with ethyl chloride had significantly more inflammation in tail tissues than did all other groups, likely due to a cellular response to the acute tissue damage caused by this vapocoolant.
Mice exposed to brief isoflurane anesthesia had the least amount of inflammatory infiltration to the tail as compared with all other treatment groups, including controls. This finding suggests that isoflurane may contribute antiinflammatory effects to the subcutaneous tissues and skin, as has been described in other organ systems. For example, pre- and posttreatment with isoflurane and other volatile anesthetics has been shown to reduce brain edema and improve neurobehavioral function in mice with traumatic brain injury or induced subarachnoid hemorrhage;2,42,48 decrease renal and myocardial ischemic-reperfusion injury in mice;68,73 and attenuate sepsis-induced lung inflammation.6 The mechanism by which this antiinflammatory effect is exerted is still unknown, but isoflurane has been shown to decrease several proinflammatory cytokines involved with neutrophil recruitment.1,26,27
Contrary to our previous assertion that anesthesia or topical analgesia should be given to preweanling mice for tail biopsy,30 the topical treatments tested herein did not ameliorate acute behavioral responses within 10 min after biopsy as compared with those of sham mice that did not receive anesthesia. In fact, both ethyl chloride and Cetacaine resulted in increased acute responses and decreased locomotor activity in mice, compared with isoflurane-anesthetized and untreated control mice, regardless of biopsy.
Given our findings and in accordance with the work of others, we do not support the application of the vapocoolant, ethyl chloride, or the topical anesthetic Cetacaine for tail biopsy of laboratory mice, regardless of age. In addition to the aversive responses to ethyl chloride, this topical vapocoolant caused the greatest degree of inflammatory changes and focal hemorrhage within mouse tail tissues. Ultimately, neither inhaled isoflurane nor topical anesthetics appreciably enhanced the wellbeing of preweanling mice undergoing tail biopsy for genotyping purposes.
Acknowledgments
We thank Dr Gui-shuang Ying for statistical assistance and the laboratory of Dr Julie Blendy for access to experimental equipment. We thank the Office of the Vice Provost at the University of Pennsylvania for funding support. In addition, we are grateful to the ULAR staff at the TRL facility for the excellent oversight and care of our animals.
References
- 1.Al-Mousawi AM, Kulp GA, Branski LK, Kraft R, Mecott GA, Williams FN, Herndon DN, Jeschke MG. 2010. Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock 34:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH. 2012. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med 40:1908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45. [DOI] [PubMed] [Google Scholar]
- 4.Attal J, Cajero-Juarez M, Houdebine LM. 1995. A simple method of DNA extraction from whole tissues and blood using glass powder for detection of transgenic animals by PCR. Transgenic Res 4:149–150. [DOI] [PubMed] [Google Scholar]
- 5.Basra SK, Vives MJ, Reilly MC, Reiter MF, Kushins LG. 2006. Methemoglobinemia after fiberoptic intubation in a patient with an unstable cervical fracture: a case report. J Spinal Disord Tech 19:302–304. [DOI] [PubMed] [Google Scholar]
- 6.Bedirli N, Demirtas CY, Akkaya T, Salman B, Alper M, Bedirli A, Pasaoglu H. 2012. Volatile anesthetic preconditioning attenuated sepsis-induced lung inflammation. J Surg Res 178:e17–e23. [DOI] [PubMed] [Google Scholar]
- 7.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. 2001. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125:279–284. [DOI] [PubMed] [Google Scholar]
- 8.Boivin GP, Otaño-Rivera V, Boakye A, Grobe N, Di Fulvio M. 2013. Genotyping mice DNA isolated using cross-linked iminodiacetate sytrene divinylbenzene copolymer beads. Abstract presented at the American Association for Laboratory Animal Science National Meeting, Baltimore Maryland, October 2013. J Am Assoc Lab Anim Sci 52:682. [Google Scholar]
- 9.Bonaparte (Convenor) D, Cinelli P, Douni E, Hérault Y, Maas M, Pakarinen P, Poutanen M, Lafuente MS, Scavizzi F. 2013. FELASA guidelines for the refinement of methods for genotyping genetically modified rodents: a report of the Federation of European Laboratory Animal Science Associations Working Group. Lab Anim 47:134–145. [DOI] [PubMed] [Google Scholar]
- 10.Broome RL, Feng L, Zhou Q, Smith A, Hahn N, Matsui SM, Omary MB. 1999. Noninvasive transgenic mouse genotyping using stool analysis. FEBS Lett 462:159–160. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart CA, Norris MD, Haber M. 2002. A simple method for the isolation of genomic DNA from mouse tail free of real-time PCR inhibitors. J Biochem Biophys Methods 52:145–149. [DOI] [PubMed] [Google Scholar]
- 12.Busler DE, Li SW. 1996. Rapid screening of transgenic type II and type XI collagen knockout mice with 3-primer PCR. Biotechniques 21:1002–1004. [DOI] [PubMed] [Google Scholar]
- 13.Byrne MF, Mitchell RM, Gerke H, Goller S, Stiffler HL, Golioto M, Branch MS, Jowell PS, Baillie J. 2004. The need for caution with topical anesthesia during endoscopic procedures, as liberal use may result in methemoglobinemia. J Clin Gastroenterol 38:225–229. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DB, Hess EJ. 1997. Rapid genotyping of mutant mice using dried blood spots for polymerase chain reaction (PCR) analysis. Brain Res Brain Res Protoc 1:117–123. [DOI] [PubMed] [Google Scholar]
- 15.Campbell LL, Tyson JA, Stackpole EE, Hokenson KE, Sherrill H, McKeon JE, Kim SA, Edmands SD, Suarez C, Hall AC. 2011. Assessment of general anaesthetic cytotoxicity in murine cortical neurones in dissociated culture. Toxicology 283:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Çelik G, Özbek O, Yilmaz M, Duman I, Özbek S, Apiliogullari S. 2011. Vapocoolant spray vs lidocaine–prilocaine cream for reducing the pain of venipuncture in hemodialysis patients: a randomized, placebo-controlled, crossover study. Int J Med Sci 8:623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SZ, Evans GA. 1990. A simple screening method for trangenic mice using the polymerase chain reaction. Biotechniques 8:32–33. [PubMed] [Google Scholar]
- 18.Cinelli P, Rettich A, Seifert B, Bürki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184. [DOI] [PubMed] [Google Scholar]
- 19.Dallasen RM, Bowman JD, Xu Y. 2011. Isoflurane does not cause neuroapoptosis but reduces astroglial processes in young adult mice. Med Gas Res 1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis JA, Greenfield RE, Brewer TG. 1993. Benzocaine-induced methemoglobinemia attributed to topical application of the anesthetic in several laboratory animal species. Am J Vet Res 54:1322–1326. [PubMed] [Google Scholar]
- 21.Drews R, Drohan WN, Lubon H. 1994. Transgene detection in mouse tail digests. Biotechniques 17:866–867. [PubMed] [Google Scholar]
- 22.Fjordbakk CT, Haga HA. 2011. Effect of topical vapocoolant spray on response to arthrocentesis and intravenous catheterization in unsedated horses. Am J Vet Res 72:746–750. [DOI] [PubMed] [Google Scholar]
- 23.Freeman Z. 2010. P151 Practical application of local analgesia for tail amputation in laboratory mice. Abstracts of scientific presentations presented at the 2010 American Association Laboratory Animal Science Naional Meeting, Atlanta, Georgia. J Am Assoc Lab Anim Sci 49:728–729. [Google Scholar]
- 24.Garzel LM, Hankenson FC, Combs J, Hankenson KD. 2010. Use of quantitative polymerase chain reaction analysis to compare quantity and stability of isolated murine DNA. Lab Anim (NY) 39:283–289. [DOI] [PubMed] [Google Scholar]
- 25.Gaw A, Mancini FP, Ishibashi S. 1995. Rapid genotyping of low-density lipoprotein receptor knockout mice using a polymerase chain reaction technique. Lab Anim 29:447–449. [DOI] [PubMed] [Google Scholar]
- 26.Giraud O, Molliex S, Rolland C, Leçon-Malas V, Desmonts JM, Aubier M, Dehoux M. 2003. Halogenated anesthetics reduce interleukin-1β–induced cytokine secretion by rat alveolar type II cells in primary culture. Anesthesiology 98:74–81. [DOI] [PubMed] [Google Scholar]
- 27.Giraud O, Seince PF, Rolland C, Leçon-Malas V, Desmonts JM, Aubier M, Dehoux M. 2000. Halothane reduces the early lipopolysaccharide-induced lung inflammation in mechanically ventilated rats. Am J Respir Crit Care Med 162:2278–2286. [DOI] [PubMed] [Google Scholar]
- 28.Gregory CA, Myal Y, Shiu RP. 1995. Rapid genotyping of transgenic mice using dried blood spots from Guthrie cards for PCR analysis. Biotechniques 18:758–760. [PubMed] [Google Scholar]
- 29.Hankenson FC, Braden-Weiss GC, Blendy JA. 2011. Behavioral and activity assessment of laboratory mice (Mus musculus) after tail biopsy under isoflurane anesthesia. J Am Assoc Lab Anim Sci 50:686–694. [PMC free article] [PubMed] [Google Scholar]
- 30.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. 2008. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertebral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 47:10–18. [PMC free article] [PubMed] [Google Scholar]
- 31.Henneberger C, Grantyn R, Rothe T. 2000. Rapid genotyping of newborn gene mutant mice. J Neurosci Methods 100:123–126. [DOI] [PubMed] [Google Scholar]
- 32.Hijazi R, Taylor D, Richardson J. 2009. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised, double-blind, placebo-controlled trial. Br Med J 338:b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofstetter JR, Zhang A, Mayeda AR, Guscar T, Nurnberger JI, Jr, Lahiri DK. 1997. Genomic DNA from mice: a comparison of recovery methods and tissue sources. Biochem Mol Med 62:197–202. [DOI] [PubMed] [Google Scholar]
- 34.Indiana University Office of Research Compliance. [Internet] 2014. Guidelines for mouse genotyping tissue harvesting. [Cited 30 July 2014]. Available at: http://researchadmin.iu.edu/Policies/IACUC/IUB/BIACUC_Genotyping_Guidelines_02_26_14.pdf
- 35.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): The National Academies Press. [Google Scholar]
- 36.Irwin MH, Moffatt RJ, Pinkert CA. 1996. Identification of transgenic mice by PCR analysis of saliva. Nat Biotechnol 14:1146–1148. [DOI] [PubMed] [Google Scholar]
- 37.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. 2011. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology 114:578–587. [DOI] [PubMed] [Google Scholar]
- 38.Jones CP, Carver S, Kendall LV. 2012. Evaluation of common anesthetic and analgesic techniques for tail biopsy in mice. J Am Assoc Lab Anim Sci 51:808–814. [PMC free article] [PubMed] [Google Scholar]
- 39.John Hopkins University Animal Care and Use Committee. [Internet] 2014. Tail Biopsy of Mice. [Cited 30 July 2014]. Available at: http://web.jhu.edu/animalcare/policies/Tail%20Biopsy%20of%20Mice.pdf
- 40.Kelly JS. 2008. Painless vascular cannulation: ethyl chloride, vapocoolants, and cryoanalgesics. J R Coll Physicians Edinb 38:232–233. [Google Scholar]
- 41.Khan R, Kuppaswamy BS. 2013. Cetacaine induced methemoglobinemia: overview of analysis and treatment strategies. W V Med J 109:24–26. [PubMed] [Google Scholar]
- 42.Khatibi NH, Ma Q, Rolland W, Ostrowski R, Fathali N, Martin R, Applegate R, Stier G, Tang J, Zhang JH. 2011. Isoflurane posttreatment reduces brain injury after an intracerebral hemorrhagic stroke in mice. Anesth Analg 113:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong F, Xu L, He D, Zhang X, Lu H. 2011. Effects of gestational isoflurane exposure on postnatal memory and learning in rats. Eur J Pharmacol 670:168–174. [DOI] [PubMed] [Google Scholar]
- 44.Kostopoulos D, Rizopoulos K. 2008. Effect of topical aerosol skin refrigerant (spray and stretch technique) on passive and active stretching. J Bodyw Mov Ther 12:96–104. [DOI] [PubMed] [Google Scholar]
- 45.Lahm H, Hoeflich A, Rieger N, Wanke R, Wolf E. 1998. Identification of transgenic mice by direct PCR analysis of lysates of epithelial cells obtained from the inner surface of the rectum. Transgenic Res 7:131–134. [DOI] [PubMed] [Google Scholar]
- 46.Lin CS, Magnuson T, Samols D. 1989. A rapid procedure to identify newborn transgenic mice. DNA 8:297–299. [DOI] [PubMed] [Google Scholar]
- 47.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. 2009. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108:90–104. [DOI] [PubMed] [Google Scholar]
- 48.Luh C, Gierth K, Timaru-Kast R, Engelhard K, Werner C, Thal SC. 2011. Influence of a brief episode of anesthesia during the induction of experimental brain trauma on secondary brain damage and inflammation. PLoS ONE 6:e19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malumbres M, Mangues R, Ferrer N, Lu S, Pellicer A. 1997. Isolation of high-molecular–weight DNA for reliable genotyping of transgenic mice. Biotechniques 22:1114–1119. [DOI] [PubMed] [Google Scholar]
- 50.Matthias N, Robinson MA, Crook R, Lockworth CR, Goodwin BS., Jr 2013. Local cryoanalgesia is effective for tail-tip biopsy in mice. J Am Assoc Lab Anim Sci 52:171–175. [PMC free article] [PubMed] [Google Scholar]
- 51.Matthias N, Robinson MA, Goodwin BS. 2010. Local cryoanalgesia: an effective method for tail-tip biopsy in mice. Abstracts of scientific presentations presented at the 2010 American Association Laboratory Animal Science National Meeting, Atlanta, Georgia. J Am Assoc Lab Anim Sci 49:657–658. [Google Scholar]
- 52.Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454:428–435. [DOI] [PubMed] [Google Scholar]
- 53.Meldgaard M, Bollen PJ, Finsen B. 2004. Noninvasive method for sampling and extraction of mouse DNA for PCR. Lab Anim 38:413–417. [DOI] [PubMed] [Google Scholar]
- 54.Moon YE, Kim SH, Choi WH. 2013. Comparison of the effects of vapocoolant spray and topical anesthetic cream on pain during needle electromyography in the medial gastrocnemius. Arch Phys Med Rehabil 94:919–924. [DOI] [PubMed] [Google Scholar]
- 55.Morales ME, Gereau RW. 2009. The effects of tail biopsy for genotyping on behavioral responses to nociceptive stimuli. PLoS ONE 4:e6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Institutes of Health Office of Animal Care and Use. [Internet] 2014. Guidelines for genotyping of mice and rats. [Cited 30 July 2014]. Available at: http://oacu.od.nih.gov/ARAC/documents/Rodent_Genotyping.pdf.
- 57.Ohhara M, Kurosu Y, Esumi M. 1994. Direct PCR of whole blood and hair shafts by microwave treatment. Biotechniques 17:726–728. [PubMed] [Google Scholar]
- 58.Paluch L, Lieggi C, Dumont M, Monette S, Riedel ER, Lipman NS. 2012. Assessment of the effects of vapocoolant anesthesia, reflex development, and behavioral responses following toe clipping of neonatal and preweanling mice. Abstracts of scientific papers presented at the 2012 American Association Laboratory Animal Science National Meeting. J Am Assoc Lab Anim Sci 51:639. [Google Scholar]
- 59.Paluch LR, Lieggi CC, Dumont M, Monette S, Riedel ER, Lipman NS. 2014. Developmental and behavioral effects of toe clipping on neonatal and preweanling mice with and without vapocoolant anesthesia. J Am Assoc Lab Anim Sci 53:132–140. [PMC free article] [PubMed] [Google Scholar]
- 60.Petkov PM, Cassell MA, Sargent EE, Donnelly CJ, Robinson P, Crew V, Asquith S, Haar RV, Wiles MV. 2004. Development of a SNP genotyping panel for genetic monitoring of the laboratory mouse. Genomics 83:902–911. [DOI] [PubMed] [Google Scholar]
- 61.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. 2004. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 14:1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren S, Li M, Cai H, Hudgins S, Furth PA. 2001. A simplified method to prepare PCR template DNA for screening of transgenic and knockout mice. Contemp Top Lab Anim Sci 40:27–30. [PubMed] [Google Scholar]
- 63.Robinson PA, Carr S, Pearson S, Frampton C. 2007. Lignocaine is a better analgesic than either ethyl chloride or nitrous oxide for peripheral intravenous cannulation. Emerg Med Australas 19:427–432. [DOI] [PubMed] [Google Scholar]
- 64.Schmitteckert EM, Prokop CM, Hedrich HJ. 1999. DNA detection in hair of transgenic mice—a simple technique minimizing the distress on the animals. Lab Anim 33:385–389. [DOI] [PubMed] [Google Scholar]
- 65.Sørensen DB, Stub C, Jensen HE, Ritskes-Hoitinga M, Hjorth P, Ottesen JL, Hansen AK. 2007. The impact of tail-tip amputation and ink tattoo on C57BL/6JBomTac mice. Lab Anim 41:19–29. [DOI] [PubMed] [Google Scholar]
- 66.Soueid A, Richard B. 2007. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatr Emerg Care 23:380–383. [DOI] [PubMed] [Google Scholar]
- 67.Su D, Zhao Y, Wang B, Li W, Xiao J, Chen J, Wang X. 2011. Repeated but not single isoflurane exposure improved the spatial memory of young adult mice. Acta Anaesthesiol Scand 55:468–473. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka K, Ludwig LM, Krolikowski JG, Alcindor D, Pratt PF, Kersten JR, Pagel PS, Warltier DC. 2004. Isoflurane produces delayed preconditioning against myocardial ischemia and reperfusion injury: role of cyclooxygenase 2. Anesthesiology 100:525–531. [DOI] [PubMed] [Google Scholar]
- 69.University of Arizona Office for the Responsible Conduct of Research. [Internet] 2014. Biopsy of rodent tails. Institutional Animal Care and Use Committee Guidance 203. [Cited 30 July 2014]. Available at: http://orcr.arizona.edu/sites/orcr.arizona.edu/files/203%20Rodent%20Tail%20Biopsy.pdf.
- 70.University of Minnesota Research Animal Resources. [Internet] 2014. Rodent (<50 grams) tail biopsy guidelines. [Cited 30 July 2014]. Available at: http://www.ahc.umn.edu/rar/tailsnip.html
- 71.Waibel KH, Katial RK. 2005. Effect of topical vapocoolant spray on skin test wheal, flare, and pain responses. Ann Allergy Asthma Immunol 95:149–153. [DOI] [PubMed] [Google Scholar]
- 72.Waterhouse MR, Liu DR, Wang VJ. 2013. Cryotherapeutic topical analgesics for pediatric intravenous catheter placement: ice versus vapocoolant spray. Pediatr Emerg Care 29:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. 2012. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann K, Schwarz HP, Turecek PL. 2000. Deoxyribonucleic acid preparation in polymerase chain reaction genotyping of transgenic mice. Comp Med 50:314–316. [PubMed] [Google Scholar]