Abstract
Our standard of care for rodent corneal lesions previously included treatment of the primary lesion, application of topical NSAIDs, and systemic NSAIDs in severe cases. When intensive medical management was unsuccessful, animals were euthanized, leading to premature loss of valuable genetically modified animals and those on long-term studies. We investigated enucleation surgery as a treatment for 15 cases of rodent corneal disease that did not respond to medical management. Enucleation was performed under isoflurane anesthesia and involved removal of the globe, extensive hemostasis, and packing the orbital space with absorbable gelatin sponge. The lid margins were closed by tarsorrhaphy and tissue glue. Analgesia was provided by using buprenorphine preoperatively and carprofen chew tabs postoperatively. To date, we have a 100% success rate with this procedure (n = 20; 15 clinically affected rodents [2 rats, 13 mice], 5 healthy controls), which included a 60-d follow-up period. The single complication involved dehiscence of the tarsorrhaphy site and was repaired by trimming the lid margins to provide fresh tissue for closure. Histologic examination at both 1 and 3 mo after surgery revealed no evidence of infection of the enucleation site. Enucleation in rodents is a straightforward procedure that represents a refinement to our current standard of care for rodents, does not cause significant inflammation of remaining periocular structures, and has reduced the number of animals euthanized prior to study endpoint because of severe ocular lesions.
Corneal ulcerations are common clinical presentations for laboratory rodents. This is likely due to their large corneas, leading to exposure to exogenous irritants such as bedding and ammonia, which can cause contact keratitis.7,8,10 Corneal lesions also can result from inadequate eye lubrication during anesthesia and from complications of retrobulbar (retroorbital) blood collection, such as exophthalmos.1,3,14
At our institution, the current standard of care for rodent ocular lesions includes appropriate treatment of any detectable corneal lesions and the provision of topical NSAID for analgesia (with the addition of systemic NSAID for severe cases) and topical antibiotics to prevent secondary infection in the affected eye. Topical treatment of rodent ocular lesions is challenging because the globe is small, and it is often difficult to administer drops appropriately.5 Because rodent eyes are small, applying even a small drop of topical ocular medication can flood the ocular surface and lead to nasolacrimal overflow and potentially increase systemic absorption.16 In addition, topical ocular medications are diluted and eliminated from the eye quickly, requiring frequent administration.5 Furthermore, most ocular medications are optimally effective when applied at a frequency of no less than 10 to 15 min between doses. When intensive medical management is unsuccessful or animals have severe lesions, they often are euthanized because ocular pain can severely compromise animal wellbeing,16 leading to premature loss of valuable genetically altered animals or those on long-term studies.
In nonrodent animal species, enucleation is the most common orbital surgery performed by ophthalmologists and general practitioners.11 Enucleation involves removing the globe, nictitating membrane, eyelids, and potentially the conjunctiva while leaving the adjacent periorbital glands and muscles in place.5 The most common postenucleation complication is a sunken orbit,6 which is a cosmetic complication of concern to some pet owners but is not a concern in the laboratory animal setting. Other complications in nonrodents include hemorrhage and dehiscence of the surgical site.6 In addition, incomplete removal of associated secretory tissue within the orbit can result in draining fistulas from the orbit, cysts, or chronic production of tear components, leading to the formation of a mucocoele.6
Because enucleation is a common surgical treatment for ocular disease in nonrodents and because ocular disease is a common presentation in laboratory rodents, we used a series of clinical cases to assess the possibility of adapting this surgical technique to rodents. Surgical treatment of severe ocular lesions in rodents represents a refinement to our current standard of care for rodents and could reduce the number of animals that were euthanized prior to study endpoint because of severe ocular lesions. To this end, we developed a surgical enucleation procedure for rodents. Our procedure has had a 100% success rate (defined as survival postoperatively until study endpoint with no ocular complications, such as infection or self-trauma), is easy to perform, and causes at most only mild, localized inflammation of the enucleation site.
Case Series
We performed enucleation surgery on 15 clinically affected animals (2 rats and 13 mice) from various investigator colonies in the vivarium of the University of Michigan. These rodents were of various ages, stocks, strains, and genetic alterations. We chose these animals as surgical candidates either because they had corneal lesions that did not respond to topical treatment or because their lesions were too severe to warrant topical therapy. All lesions were unilateral and varied from severe corneal ulceration to overt rupture of the globe. Causes of corneal disease in these animals varied and included unknown etiology, complication from retroorbital blood collection, and inadequate lubrication during prolonged inhalation anesthesia. On physical examination at presentation, all rodents appeared otherwise healthy prior to enucleation surgery. In addition, we performed right unilateral enucleation on 5 clinically normal rodents for histologic studies. All animals were monitored after surgery for 60 d and then were euthanized for histologic evaluation.
Materials and Methods
Experimental animals.
Mice and rats in this study had ad libitum access to food (Laboratory Rodent Diet 5001, PMI LabDiet, St Louis, MO) and water. The animal housing room was maintained on a 12:12-h light:dark cycle with constant temperature (72 ± 2 °F [22.2 ± 1.1 °C]). Mice and rats were acclimated for at least 7 d prior to experimental use. All procedures were approved by the University of Michigan's Animal Care and Use Committee. Mice were SPF for viruses including mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus. Rats were SPF for sialodacryoadenitis virus, Kilham rat virus, rat parvovirus, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, and mouse adenovirus.
Surgical procedure.
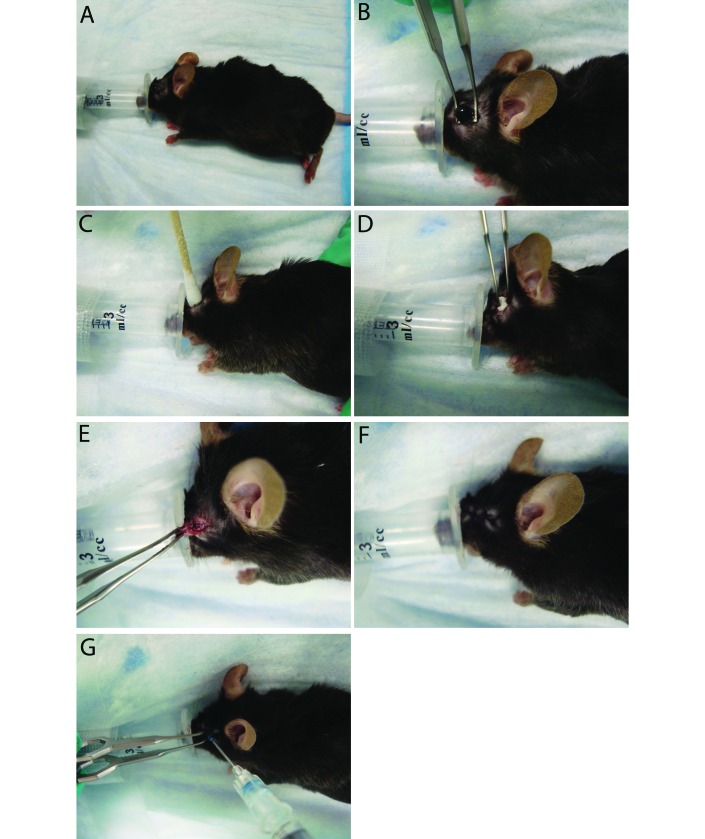
Anesthesia was induced by administering isoflurane (4%) in an induction box, with subsequent transfer to a modified nosecone, which was created by removing the top from a 3-mL syringe (Figure 1 A), thus providing adequate access to the surgical area and maintaining the rodents on a surgical plane of anesthesia. After the induction of anesthesia and prior to surgery, rodents received buprenorphine (0.1 mg/kg) and the healthy eye was lubricated to prevent ulceration. Once a surgical plane of anesthesia was reached, the periorbital area was shaved and cleaned by using alternating chlorhexidine and saline scrubs.
Figure 1.
Enucleation procedure. (A) An anesthetic nose cone was created by removing the top of a 3-mL syringe. This replaced the standard nose cone, allowing for adequate access to the eye while maintaining a surgical plane of anesthesia. Each mouse received a preoperative dose of buprenorphine at induction. (B) Once a surgical plane of anesthesia was obtained, the periorbital region was shaved. The globe was proptosed and isolated with Iris forceps. The globe was removed (without ligation of vessels), and the orbit was flushed with sterile saline. (C) A sterile swab was used to provide hemostasis until active bleeding had ceased. (D) The orbit was packed tightly with absorbable gelatin sponge. (E) The eyelid margins were trimmed to provide fresh tissue for closure. (F) The site was closed using a simple interrupted pattern of 4-0 polydioxanone suture. (G) A drop of skin glue was added to the site to prevent dehiscence due to self-trauma.
To prevent the formation of orbital cysts or infection or severe inflammation of accessory ocular structures, nucleation surgery in nonrodent species often involves the removal of epithelially lined structures, including the third eyelid, the gland of the third eyelid, the palpebral conjunctiva, and the lacrimal caruncle (located at the medial canthus).6 However, due to the small globe and associated glandular structures in rodents, this technique was infeasible in our animals. Instead, we used iris forceps to proptose and isolate the globe (Figure 1 B). The globe was removed and the orbit flushed with sterile saline; vessels were too small to ligate even with 5-0 suture. In addition, ligation of an individual vessel likely would be insufficient, given that mice have an orbital venous sinus (a confluence of several vessels).18 Instead, sterile swabs were used to provide hemostasis until active bleeding ceased (Figure 1 C). The orbit was tightly packed with absorbable gelatin sponge to facilitate continued hemostasis and to minimize dead space (Figure 1 D). The eyelid margins were trimmed to provide fresh tissue for closure (Figure 1 E), and the site was closed with 4-0 polydioxanone suture in a simple interrupted pattern (Figure 1F). A drop of skin glue was added to the surgical site to prevent dehiscence due to self-trauma (Figure 1 G).
Rodents recovered postoperatively in a clean cage and were housed individually for 7 to 10 d. Each cage contained 1/4 of a carprofen chew tab (2-mg tablet, Bio-serv, Flemington, NJ), shredded paper strips, and a cotton nesting square; none of the animals required additional postoperative analgesia, according to their clinical appearance, activity level, and body condition.
Results
Clinical outcomes.
Surgical success was defined as survival postoperatively until study endpoint with no ocular complications (infection, self-mutilation). Animals were monitored daily after surgery for 7 d and then monthly for 2 mo or until their study endpoint (whichever came first). After surgery, animals ambulated normally, had normal activity levels, ate and drank normally, and maintained body condition. No ocular discharge occurred postoperatively in any animal. During the first enucleation surgery that we performed, the eyelids were not trimmed sufficiently prior to tarsorraphy, resulting in dehiscence in that animal. The dehiscence was repaired, and this complication was eliminated in future surgeries by taking care to trim the lid margins completely. After repair of the dehiscence, this animal survived until its study endpoint.
All animals groomed the surgical site almost immediately after recovery; however, the use of both tarsorrhaphy with trimmed lid margins and closure with tissue glue prevented future dehiscence of the site despite grooming. All 20 mice and rats that underwent surgery survived to the study endpoint (2 mo postoperatively, n = 18) or to their planned endpoint (1 or 3 wk postoperatively, n = 2).
Histologic outcomes.
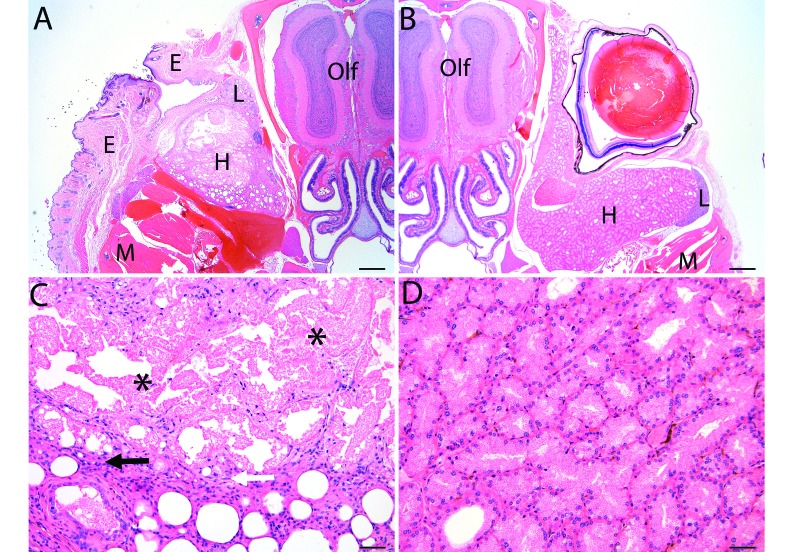
Because infection and severe inflammation have the potential to interfere with various types of research studies, we performed right unilateral enucleation in 5 clinically normal mice to histologically evaluate the orbital space for evidence of infection or severe chronic inflammation. Clinically normal mice were used for this arm of the study so that we could investigate inflammation associated with the enucleation procedure itself and not underlying disease. We examined the enucleation site (and contralateral control eye) at 4 wk (n = 2 mice) and 10 wk (n = 3 mice) postoperatively to look for signs of cyst formation, active infection, and chronic inflammation of the associated ocular structures. Histologic evaluation revealed mild histologic changes without evidence of cyst formation or active infection in all of the animals evaluated. There was no evidence of the absorbable sponge that had been placed during the procedure, and granulation tissue was present in the eyelid at the site of tarsorrhaphy (Figure 2 A and B). The remaining Harderian gland demonstrated localized necrosis accompanied by dilation or atrophy of remaining acini. Occasionally mild granulomatous inflammation was present within and immediately adjacent to the area of necrosis but was not purulent and did not extend into the surrounding soft tissue (Figure 2 C and D). Findings at both time points were the same.
Figure 2.
Histologic evaluation of (A) the enucleation site at 10 wk postoperatively compared with (B) contralateral control. Necrosis and mild granulation tissue occurred within the eyelid at the site of enucleation, but no evidence of infection, such as suppurative inflammation or bacteria, was present. Original , 20×; scale bar, 500 μm. Higher magnification of the enucleation site revealed necrosis (asterisks) with acinar dilation or atrophy in the Harderian gland, which was occasionally accompanied by mild granulomatous inflammation (arrows) that did not extend into the surrounding soft tissue. E, eyelid; H, Harderian gland; L, lacrimal gland; M, muscle; Olf, olfactory lobe of brain. Scale bar, 50 μm.
Discussion
We describe a series of cases of rodent ocular disease that were treated successfully by enucleation, which was performed in less than 5 min under inhalation anesthesia. After surgery, rodents did not show signs of distress, were able to navigate their surroundings, and ate and drank normally. There were no incidences of postoperative hemorrhage, which is the most common complication of enucleation in nonrodents,11 and the procedure had a 100% success rate. In addition, no gross infection occurred, and histology revealed only mild granulomatous inflammation confined to the Harderian glands. However, some animals groomed the enucleation site after surgery, perhaps indicating irritation or pain locally at the site. Therefore, the addition of a local anesthetic to the analgesic protocol may be beneficial. Furthermore, we suggest using dilute povidone–iodine instead of chlorhexidine, given that chlorhexidine might damage the corneal epithelium of the healthy eye.12
Rodent corneal disease is a common laboratory animal problem that likely is due to a combination of factors, including husbandry issues (exposure to irritants such as corncob bedding and ammonia), trauma (self-induced or by a cagemate), and procedure-related injury (ulceration due to inadequate lubrication during anesthesia and incorrect technique during retroorbital blood collection). For example, rodents can develop exposure keratopathy and corneal ulceration occur after prolonged anesthesia, particularly with ketamine–xylazine combinations, despite sufficient lubrication.4,13,17 In addition to husbandry and procedure-related causes for corneal disease in rodents, corneal lesions can occur secondary to systemic disease, including coronavirus infection and mycoplasmosis.17 Furthermore, spontaneous corneal lesions have been reported in rodents.2,15 The incidence of these spontaneous inflammatory corneal lesions varies by stock and strain as well as by microbiologic environment.2,15 An additional consideration regarding ocular disease in rodents is the increasing use of genetically altered mice in biomedical research. Although transgenic rodents are created to study specific genes or organ systems, they often have unexpected phenotypes that can cause health problems for the animals. For example, genetic manipulation to generate transgenic mice that express a mutant form of the GTPase Rab27a unexpectedly yielded mice with corneal disease that in some cases led to phthisical eyes.9
One limitation of this study is that all enucleations were unilateral. However, ocular abnormalities resulting from genetic alterations could be bilateral. During the time frame of our case series, no animals presented with bilateral corneal or ocular disease that could have been addressed via bilateral enucleation. We cannot directly comment on the outcome of performing the procedure bilaterally. However, the clinical presentation of our animals postoperatively and the fact that rodent stocks and strains (SJL, CBA, C3H) that carry the retinal degeneration (rd) mutation16 (and thus could be blind) do well in the research setting suggest that enucleation could be used successfully as a ‘rescue’ strategy for a valuable animal with bilateral corneal disease. If a particular line of genetically altered animals consistently presents with bilateral ocular disease prior to reaching their research endpoint, the animal welfare implications of bilateral enucleation should be assessed on a case-by-case basis. In addition, bilateral enucleation may prompt scientific concerns, given that blind animals may have alterations of their other senses, learning, and memory.
Rodents may be predisposed to corneal lesions in a laboratory setting for any number of reasons, including the size of the cornea, exposure to environmental irritants, complications from anesthesia and blood collection, trauma, and so forth. This predisposition makes enucleation a valuable treatment option for rodents in biomedical research. An additional consideration for this procedure is in situations where severe ocular lesions are an unexpected result of genetic manipulation. The ability to enucleate painful, diseased eyes (rather than euthanize animals) could reduce the incidence of premature euthanasia among animals carrying valuable genetic alterations. The procedure described herein represents a refinement to clinical rodent medicine that can be used to reduce the loss of valuable animals that present with ocular disease.
Acknowledgments
We thank the histology personnel of the ULAM In Vivo Animal Core (IVAC) for preparation of histological sections.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Bellhorn RW, Korte GE, Abrutyn D. 1988. Spontaneous corneal degeneration in the rat. Lab Anim Sci 38:46–50. [PubMed] [Google Scholar]
- 3.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods 2001. A good-practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 4.Kufoy EA, Pakalnis VA, Parks CD, Wells A, Yang CH, Fox A. 1989. Keratoconjunctivitis sicca with associated secondary uveitis elicited in rats after systemic xylazine–ketamine anesthesia. Exp Eye Res 49:861–871. [DOI] [PubMed] [Google Scholar]
- 5.Maggs DJ, Miller PE, Ron O, Slatter DH. 2008. Slatter's fundamentals of veterinary ophthalmology, 5th ed. Philadelphia (PA): Elsevier Saunders. [Google Scholar]
- 6.Martin CL. 2010. Ophthalmic disease in veterinary medicine. London (UK): Manson Publishing. [Google Scholar]
- 7.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits, 3rd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 8.Pettan-Brewer C, Treuting PM. 2011. Practical pathology of aging mice. Pathobiol Aging Age Relat Dis. 1 doi:10.3402/pba.v1i0.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramalho JS, Gregory-Evans K, Huxley C, Seabra MC. 2004. Mouse genetic corneal disease resulting from transgenic insertional mutagenesis. Br J Ophthalmol 88:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith R. 2002. Systematic evaluation of the mouse eye: anatomy, pathology, and biomethods (research methods for mutant mice). Boca Raton (FA): CRC Press LLC. [Google Scholar]
- 11.Spiess BM. 2007. Canine Orbit: Diseases and Surgery, p 539–558. In: Gelatt KN. Essentials of Veterinary Ophthalmology, 4th ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 12.Tabor E, Bostwick DC, Evans CC. 1989. Corneal damage due to eye contact with chlorhexidine gluconate. JAMA 261:557–558. [DOI] [PubMed] [Google Scholar]
- 13.Turner PV, Albassam MA. 2005. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med 55:175–182. [PubMed] [Google Scholar]
- 14.van Herck H, Baumans V, Brandt CJ, Hesp AP, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. 1998. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim 32:377–386. [DOI] [PubMed] [Google Scholar]
- 15.Van Winkle TJ, Balk MW. 1986. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci 36:248–255. [PubMed] [Google Scholar]
- 16.Williams D. 2007. Rabbit and rodent ophthalmology. European J Companion Anim Pract 17:242–252. [Google Scholar]
- 17.Williams DL. 2002. Ocular disease in rats: a review. Vet Ophthalmol 5:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. 2011. Retroorbital injections in mice. Lab Anim (NY) 40:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]