Abstract
Turnip yellow mosaic virus (TYMV), a positive-strand RNA virus in the alphavirus-like supergroup, encodes two nonstructural replication proteins (140K and 66K), both of which are required for its RNA genome replication. The 140K protein contains domains indicative of methyltransferase, proteinase, and NTPase/helicase activities, while the 66K protein encompasses the RNA-dependent RNA polymerase domain. Recruitment of the 66K protein to the sites of viral replication, located at the periphery of chloroplasts, is dependent upon the expression of the 140K protein. Using antibodies raised against the 140K and 66K proteins and confocal microscopy, we report the colocalization of the TYMV replication proteins at the periphery of chloroplasts in transfected or infected cells. The replication proteins cofractionated in functional replication complexes or with purified chloroplast envelope membranes prepared from infected plants. Using a two-hybrid system and coimmunoprecipitation experiments, we also provide evidence for a physical interaction of the TYMV replication proteins. In contrast to what has been found for other members of the alphavirus-like supergroup, the interaction domains were mapped to the proteinase domain of the 140K protein and to a large region encompassing the core polymerase domain within the 66K protein. Coexpression and colocalization experiments confirmed that the helicase domain of the 140K protein is unnecessary for the proper recruitment of the 66K protein to the chloroplast envelope, while the proteinase domain appears to be essential for that process. These results support a novel model for the interaction of TYMV replication proteins and suggest that viruses in the alphavirus-like supergroup may have selected different pathways to assemble their replication complexes.
The replication of positive-strand RNA virus genomes is dependent upon the assembly of a replication complex that is an intricate machinery comprising both virus and host components (reviewed in references 1 and 7). Replication complexes are closely associated with intracellular membranes, and many critical interactions among RNA, proteins, and lipids likely take place to allow a successful replication process.
Despite the identification and characterization of many viral replication proteins and the purification of a number of positive-strand RNA virus replication complexes that were extensively used in vitro, there is only a limited understanding of the higher-order interactions among these proteins and the ratios in which they are present in viral replication complexes. Understanding how the various proteins interact in these enzyme complexes is essential for unraveling the mechanism of RNA replication and elucidating the three-dimensional structure and function of RNA virus replication complexes. Furthermore, the fine molecular mapping of the interaction sites may constitute an important step toward the identification of the mechanisms that may eventually regulate these interactions or may constitute a way to identify specific targets for new antiviral compounds.
To gain insight into the assembly of positive-strand RNA virus replication complexes, we have studied the interactions among the replication proteins of Turnip yellow mosaic virus (TYMV), the type member of the tymovirus group. TYMV is a small spherical plant virus that shares viral replication features with other positive-strand RNA viruses in the alphavirus-like supergroup (17) and has proven useful for investigating fundamental aspects of viral multiplication (3, 60). The TYMV genome is composed of a monopartite, positive-sense RNA of 6,318 nucleotides (Fig. 1A) that directs the expression of two extensively overlapping nonstructural proteins with molecular weights of 69,000 (69K) and 206,000 (206K) (40, 61). A third open reading frame (ORF) encodes the 20-kDa coat protein, which is expressed from a subgenomic RNA.
FIG. 1.
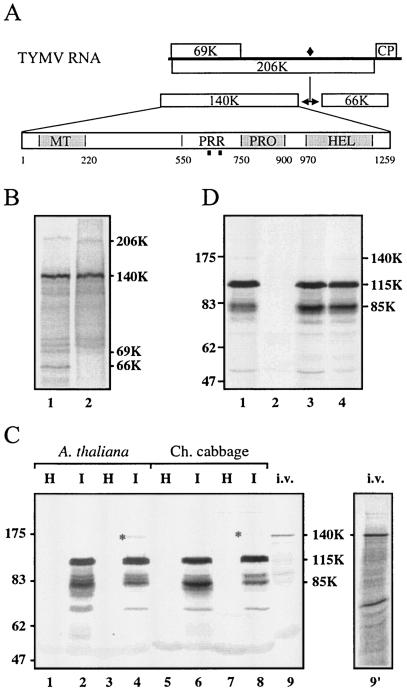
Generation and characterization of anti-140K antibodies. (A) Schematic representation of the genomic organization of TYMV RNA. Open bars denote viral ORFs. The encoded 206K protein is proteolytically processed at a peptide bond signified by a filled diamond. Protein domains within the 140K protein are indicated (MT, methyltransferase; PRR, proline-rich region; PRO, proteinase; HEL, helicase), and the locations of peptides used for immunization are indicated by filled squares. (B) 35S-labeled proteins obtained through in vitro translation of TYMV RNA (lane 1) were immunoprecipitated with anti-140K antiserum (lane 2). The proteins were separated by SDS-PAGE on a 10% polyacrylamide gel and were revealed by autoradiography. The major labeled products synthesized by TYMV RNA (206K, 140K, 69K, and 66K proteins) are indicated on the right. (C) Total proteins from healthy (H) and TYMV-infected (I) Arabidopsis leaves (lanes 1 to 4) and healthy and TYMV-infected Chinese (Ch.) cabbage leaves (lanes 5 to 8) were analyzed by SDS-PAGE on an 8% polyacrylamide gel. The proteins were extracted by a standard method (47) (lanes 1, 2, 5, and 6) or under conditions optimized for the extraction of membrane proteins (23) (lanes 3, 4, 7, and 8). 35S-labeled proteins obtained through in vitro (i.v.) translation of TYMV RNA were loaded in parallel lanes of the same gel (lanes 9 and 9′). The proteins were electroblotted onto a nitrocellulose filter and were revealed both by Western blotting with anti-140K antiserum (lanes 1 to 9) and by autoradiography (lane 9′). The positions of molecular weight markers (Biolabs) are indicated on the left (in thousands). The position of the 140K protein detected in infected plants is indicated by asterisks in lanes 4 and 8. (D) Arabidopsis protoplasts were transfected with TYMV RNA (lane 1), water (lane 2), or expression plasmid pΩ-140K (lane 3) or pΩ-206K (lane 4). The cells were harvested 24 h (lane 1) or 48 h (lanes 2 to 4) posttransfection. Total proteins were subjected to SDS-8% PAGE and immunoblot analysis with anti-140K polyclonal antibodies. The positions of molecular weight markers (Biolabs) are indicated on the left (in thousands).
The 206K protein is the only viral protein required for TYMV RNA replication (61), and it shows considerable amino acid sequence similarities with nonstructural putative replication proteins of several positive-strand RNA viruses (31). The 206K protein possesses a modular organization, and domains indicative of methyltransferase, proteinase, NTPase/helicase, and RNA-dependent RNA polymerase (RdRp) activities have been highlighted in its sequence (4, 18, 28, 40, 49, 50). The methyltransferase and proteinase enzymatic modules are separated by an ∼200-amino-acid-long proline-rich sequence with a predicted nonregular secondary structure (36) that may constitute a hinge between these two domains. The papain-like cysteine proteinase is responsible for the proteolytic cleavage of the 206K protein in vitro (6, 41), leading to the synthesis of a 140-kDa N-terminal protein product (140K) containing the methyltransferase, proteinase, and NTPase/helicase domains and a 66-kDa C-terminal protein product (66K) encompassing the RdRp domain (5, 26) (Fig. 1A). This cleavage was demonstrated to be functional in vivo (47), and both the 140K and the 66K viral proteins appear to be essential for TYMV RNA replication (60).
Besides providing enzymatic functions for RNA replication, the 140K protein also appears to be a key organizer of the assembly of TYMV replication complexes, which are associated with membrane vesicles present at the chloroplast envelope (33, 47). Prod'homme et al. showed previously that the 140K protein localizes to the chloroplast envelope in the absence of any other viral factors, thus revealing its role as a major determinant for the chloroplast localization and retention of viral replication complexes (46). In contrast, the 66K protein, encompassing the RdRp domain, has a cytoplasmic distribution when expressed alone and depends on the 140K protein for recruitment to the sites of replication (46). Targeting of the 66K protein to the replication complexes was therefore proposed to involve protein-protein interactions with the membrane-bound 140K protein.
Recruitment of the viral RdRp to replication complexes via an interaction with a membrane-anchored viral protein was reported previously for several positive-strand RNA viruses (9, 22, 32, 48). In recent years, evidence has accumulated to suggest that interactions of helicase and polymerase domains are essential for the assembly of replication complexes of members of the alphavirus-like supergroup. In Brome mosaic virus (BMV), a direct interaction of the nonconserved N terminus of polymerase protein 2a and the helicase domain of membrane-associated protein 1a has been well documented (29, 43, 44), and similar interactions have been reported for the related Alfalfa mosaic virus (AMV) and Cucumber mosaic virus (30, 57). This type of model has also been presented for virus families belonging to other supergroups of positive-strand RNA viruses, such as the Flaviviridae (25).
In this study, using antibodies raised against the 140K and 66K proteins, we report the colocalization of TYMV replication proteins both in transfected cells and in infected cells as well as their cofractionation in functional replication complexes or with purified chloroplast envelope membranes. Using a two-hybrid system, we also provide evidence for a physical interaction of the TYMV nonstructural replication proteins in yeast cells and the fine mapping of the minimal protein domains required for this interaction. In contrast to what has been found for other members of the alphavirus-like supergroup (29, 43, 57), we have identified the interaction domains as involving the proteinase domain of the 140K protein and a large region encompassing the core polymerase domain within the 66K protein. Coexpression and colocalization experiments confirmed that the helicase domain of the 140K protein is not required for proper recruitment of the 66K protein to the chloroplast envelope. These results support a novel model for the interactions of TYMV replication proteins and suggest that viruses in the alphavirus-like supergroup may have selected different pathways to assemble their replication complexes.
MATERIALS AND METHODS
Plasmid construction.
All DNA manipulations were performed by standard techniques (2, 52). The sequences of PCR-generated DNA fragments were confirmed by DNA sequencing, and the overall structures of all plasmids were confirmed by restriction analysis. Laboratory designations for plasmids are given in parentheses below.
(i) Construction of plasmids for the YTHS.
The vectors used for the yeast two-hybrid system (YTHS) were pLexA (59), encoding the LexA DNA binding domain (BD); pAS2ΔΔ (15), encoding the GAL4 BD; and pGADGH (56), encoding the GAL4 activation domain (AD). SpeI and EcoRI restriction sites were introduced in the multiple cloning site of pAS2ΔΔ to facilitate cloning approaches, leading to vector 2P. DNA fragments corresponding to the 140K and 66K ORFs were amplified by PCR with Pfu DNA polymerase (Promega) and E17 (13) as a template. The fragments were cloned in vector pLexA to create pLex-140K and pLex-66K, respectively. Similar cloning in vector 2P created 2P-140K and 2P-66K, respectively. Plasmids pGad-140K and pGad-66K were described previously (46). Plasmids derived from these constructs and encoding N- and C-terminal truncations of the 140K and 66K proteins fused in frame with the BD or AD of GAL4 were generated. To facilitate designations, the amino acids of the viral protein encoded in the fusion are indicated within parentheses in the plasmid name.
Some constructs were generated by standard cloning techniques with available restriction sites unique to the coding sequences. Alternatively, other constructs were generated by gap repair reconstitution of plasmids in vivo by using the efficient homologous recombination process in yeast cells. For this purpose, DNA fragments were PCR amplified with primers containing 24 to 27 nucleotides identical to the TYMV cDNA sequence and 38 nucleotides homologous to pGADGH or 2P to promote integration in the linearized vector (37). Details of the procedures and sequences of the oligonucleotides used will be made available upon request.
Yeast colonies were screened by PCR analysis (15) with plasmid-specific primers to select those containing a recombinant plasmid vector. The recovery of plasmids from yeast cells was performed by extracting total DNA from yeast cells as described previously (21), followed by transformation of Escherichia coli HB101 and selection of colonies on a medium containing ampicillin.
(ii) Construction of plasmids for expression in plant cells.
Expression vectors pΩ-66K, pΩ-140K, pΩ-EGFP-66K, and pΩ-EGFP-140K were described previously (46). Plasmid pΩ-EGFP-140K(1- 939) (ajJ1) was obtained by replacing the AvrII-SalI fragment of pΩ-EGFP-140K (46) with that of 2P-140K(687- 939). Plasmid pΩ-EGFP-140K(1- 686) (ijAI4) was obtained by replacing the BamHI-SphI fragment of pΩ-EGFP-140K with a PCR-generated DNA fragment containing an introduced stop codon interrupting the 140K ORF at amino acid 686.
Yeast transformation and two-hybrid assays.
All media, buffers, and methods used for yeast cells were adopted from previously described procedures (27) and from the Clontech Yeast Protocols Handbook.
Saccharomyces cerevisiae L40 (MATa ade2 his3 leu2 trp1 LYS::lexA-HIS3 URA3::lexA-lacZ), PJ696 (MATa a1− his3-200 leu2-3,112 trp1-901 ura3-52 gal4Δ gal80Δ CYH2R canR1 LYS2::GAL1 UAS-HIS3 GAL2-ADE2 met2::GAL7 UAS-lacZ), Y187 (MATα ade2-101 met-his3-200 leu2-3,112 trp1-901 ura3-52 gal4Δ gal80Δ URA3::GAL1 UAS-GAL1 TATA-lacZ MEL1), CG1945 [MATa ade2-101 his3-200 leu2-3,112 trp1-901 ura3-52 lys2-801 gal4-542 gal80-538 CYHR2 URA3::(GAL4 17-mer)3-CYC1 TATA-lacZ LYS2::GAL1 UAS-GAL1 TATA-HIS3], and diploid strain Y187×CG1945 were used as reporter strains for two-hybrid experiments. L40 was used in the LexA-based two-hybrid system, while the other strains were used in the GAL4-based two-hybrid system.
Yeast cells were grown on complete YPD medium (1% yeast extract, 2% peptone, and 2% glucose) and were transformed by the lithium acetate method (24) with either supercoiled plasmid DNA or PCR products mixed together with BamHI-linearized vector pGADGH or EcoRI-linearized vector 2P DNA to allow gap repair reconstitution of plasmids in vivo (37). Because plasmids with BD (pLexA or 2P) and AD (pGADGH) carry tryptophan and leucine auxotrophic markers, respectively, transformants were selected on synthetic media lacking tryptophan (−W) and lacking leucine (−L), respectively, and were maintained on these media. Yeast cells containing combinations of vectors were obtained either by cotransformation and plating on medium lacking both leucine and tryptophan (−LW medium) or by mating single transformants of appropriate mating types. To test for interactions, aliquots of 5 μl and serial dilutions (1/5 in phosphate-buffered saline [PBS]) were dropped on an appropriate synthetic medium (−LW medium lacking histidine [−LWH]), and growth was observed after 48 to 72 h at 30°C. In some instances, 2.5 mM 3-amino-1,2,4-triazole was added to the medium to inhibit low levels of self-activation of the HIS3 reporter gene. The interaction of oncoproteins RAS and RAF was used as a positive control (56).
Transformed PJ696 strains were also assayed quantitatively for the production of β-galactosidase with o-nitrophenyl-β-d-galactopyranoside enzymatic assays by previously described procedures (54) modified according to the Clontech Yeast Protocols Handbook. The assays were performed in triplicate with at least seven transformants for each plasmid combination, and β-galactosidase activity units were calculated as described previously (39). The statistical significance of the differences between assay and control values was evaluated by using a Mann-Whitney rank test.
Plant inoculation, replication complex isolation, and activity assay.
Chinese cabbage (Brassica pekinensis cv. Granaat) plants were grown and inoculated with TYMV as described previously (47). At 8 days postinoculation, the young developing leaves from the center of the rosette were collected, and the replication complexes were purified as described previously (11), omitting the DEAE ion-exchange chromatography step. The glycerol gradient was subdivided into 18 fractions that were assayed for in vitro RdRp activity with TYMV RNA as a template. The standard assay was performed by mixing 17.5 μl of enzyme fraction and 7.5 μl of a reaction mixture containing 75 mM Tris-HCl (pH 9), 8 mM MgCl2, 5 mM dithiothreitol, 1 μg of actinomycin D, 2.5 μg of TYMV RNA, 4.16 mM each ATP, GTP, and CTP, 2.5 μCi of [32P]UTP, and 10 U of RNasin (Promega) to give a final volume of 25 μl. The mixture was incubated for 40 min at 30°C, and the incorporation of radioactivity into the acid-insoluble fraction was determined as previously described (11).
Isolation of chloroplast envelope membranes from Chinese cabbage leaves.
TYMV-infected Chinese cabbage plants were grown with the addition of fertilizer and were kept in the dark for 3 days before being harvested in order to minimize the accumulation of starch. At 4 to 6 weeks postinoculation, freshly collected leaves were homogenized in ice-cold 0.45 M mannitol- 20 mM Tricine-NaOH (pH 8.4)-5 mM EDTA- 0.1% bovine serum albumin (BSA) (5 ml/g of fresh weight). Crude chloroplast pellets obtained after centrifugation at 4,000 × g for 5 min were further purified in 0.45 M mannitol- 20 mM morpholinepropanesulfonic acid (MOPS) (pH 7.6)-1 mM MgCl2- 1 mM EDTA (buffer A) on a step gradient formed with 33 and 80% Percoll. After 30 min of centrifugation at 4,000 × g, intact chloroplasts were recovered on the 80% Percoll layer, washed twice by centrifugation in buffer A (10 min at 1,500 × g), and broken in 10 mM MOPS (pH 7.6)-4 mM MgCl2- 1 mM phenylmethylsulfonyl fluoride- 1 mM caproic acid. Chloroplast subfractions were separated by centrifugation at 70,000 × g for 80 min on a step gradient of 0.93 to 0.4 M sucrose in 10 mM MOPS (pH 7.6). The envelope was collected at the interface of the 0.4 and 0.93 M sucrose layers and washed by centrifugation for 1 h at 100,000 × g in 10 mM MOPS (pH 7.6). The top of the saccharose gradient corresponds to the chloroplast stroma, while the pellet contains the thylakoids. Typically, 100 to 200 μg of chloroplast envelope proteins was obtained per kg of TYMV-infected leaves.
Preparation and transfection of protoplasts.
Protoplasts of Arabidopsis thaliana ecotype Columbia were prepared from a cell suspension culture and transfected with 5 μg of viral RNA, plasmid expression vector, or a mixture of them essentially as described previously (46, 47).
Protein extraction methods.
Yeast cells transformed with plasmids encoding hybrid proteins were grown in synthetic liquid medium to an optical density at 600 nm of 0.6. The cells were collected and frozen in liquid nitrogen, and proteins were prepared by the urea-sodium dodecyl sulfate (SDS) extraction method (45) in the presence of a mixture of protease inhibitors (Roche).
Proteins were extracted from plant tissues as described previously (47) or by the phenol extraction procedure (23) to optimize the extraction of membrane proteins.
Antibodies, SDS-polyacrylamide gel electrophoresis (PAGE), and immunoblot analysis.
The TYMV 66K protein-specific rabbit polyclonal antiserum was described previously (47). The polyclonal antiserum against the TYMV 140K protein was obtained by injecting rabbits with a mixture of two synthetic peptides (amino acids 621 to 635 and amino acids 667 to 681) conjugated to keyhole limpet hemocyanin. Peptide synthesis, coupling, and immunization were performed by Eurogentec according to standard protocols.
SDS-PAGE and immunoblot analysis were performed essentially as described previously (47) with a 2,000-fold dilution of the anti-140K antiserum. To eliminate any nonspecific reactions, the anti-66K and anti-140K antisera were preadsorbed by overnight incubation at 4°C with nitrocellulose membranes to which proteins from healthy plants had been transferred. In some instances, the nitrocellulose membranes were probed successively with the anti-66K and anti-140K antisera by using a Blot Restore membrane rejuvenation kit (Chemicon) between the immunodetection steps; nitroblue tetrazolium (NBT)-5-bromo-4-chloro-3-indolylphosphate (BCIP) and Fast red (Roche) were sequentially used as substrates of the alkaline phosphatase-conjugated secondary antibodies to allow dual-color detection of the viral proteins.
Anti-Toc75 antiserum was kindly provided by J. Soll (20) and was diluted 100-fold in Tris-buffered saline- 5% milk. Detection was performed by using peroxidase-conjugated affinity-purified goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch) and enhanced chemiluminescence reagent (Amersham).
In vitro translation and immunoprecipitation.
In vitro translation reactions of viral RNA were carried out with micrococcal nuclease-treated reticulocyte lysates and separation by SDS-PAGE as previously described (13).
For immunoprecipitation, the translation mixture (10 μl) was mixed with 5 μl of extraction buffer (150 mM Tris-HCl [pH 6.8], 8% SDS, 2 M β-mercaptoethanol, 30% glycerol) and boiled for 5 min. The sample then was diluted to 300 μl with NET buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 0.05% NP-40). After the addition of antiserum (0.5 μl) and overnight incubation at 4°C, the antigen-antibody complexes were precipitated with Pansorbin (Calbiochem). After successive washes with NET buffer and buffer B (20 mM Tris-HCl [pH 7.5], 0.05% NP-40), the immunoprecipitates were removed from the Pansorbin by boiling in extraction buffer, separated by SDS-PAGE as previously described (13), and visualized by autoradiography.
For coimmunoprecipitation, the active glycerol gradient fraction (20 μl of fraction 13) was diluted to 500 μl with buffer D (50 mM Tris-HCl [pH 7.5], 50 mM KCl, 0.5 mM EDTA, 20% glycerol, 0.5% Lubrol W) and incubated overnight at 4°C in the presence of antiserum (either 4 μl of anti-66K antiserum or its corresponding preimmune serum or 2 μl of anti-140K antiserum or its corresponding preimmune serum). The antigen-antibody complexes were precipitated with Pansorbin, washed twice with buffer D, eluted from the Pansorbin by boiling in extraction buffer, separated by SDS-7% PAGE, and analyzed by immunoblotting as described above.
Immunofluorescence and confocal microscopy.
TYMV-infected or plasmid-transfected protoplasts were harvested for immunofluorescence staining or observation of enhanced green fluorescent protein (EGFP) fluorescence at 24 to 48 h posttransfection. They were processed essentially as described previously (46, 47), except that the anti-140K antiserum was diluted 500-fold in PBS- 1% BSA and goat anti-rabbit immunoglobulin G conjugated to Alexa-fluor 594 (Molecular Probes) (diluted 1,000-fold in PBS-1% BSA) was used as the secondary antibody.
Epifluorescence microscopy and image acquisitions were performed by using a Leica DMR epifluorescence microscope equipped with a charge-coupled device camera. Standard filters for fluorescein isothiocyanate and rhodamine were used to monitor and record EGFP fluorescence and Alexa-fluor 594 fluorescence, respectively. Chloroplasts in fixed cells were identified by 4′,6′-diamidino-2-phenylindole (DAPI) staining of the chloroplast DNA as described previously (47).
Confocal images were collected by using a Leica TCS SP2-AOBS laser scanning confocal microscope equipped with a ×63 objective (Leica numerical aperture 1.32). Images with EGFP fluorescence were acquired by using a 488-nm argon laser line and were collected at between 500 and 560 nm. The Alexa-fluor 594 fluorochrome was excited with a 543-nm helium-neon laser line, and fluorescence was collected at between 600 and 700 nm. Images were acquired in the sequential mode and were digitally superimposed. Color level and contrast adjustment were processed by using Photoshop (Adobe Systems).
RESULTS
Generation and characterization of anti-140K antibodies.
To produce a polyclonal antibody raised against the TYMV 140K protein, rabbits were immunized with two synthetic peptides derived from the amino acid sequence of the 140K protein. These peptides are localized in the central region of the 140K protein, within an ∼200-amino-acid-long proline-rich region with a predicted nonregular secondary structure (36) that we expected to be accessible in the folded protein (Fig. 1A).
The reactivity of the anti-140K antiserum was first investigated by performing immunoprecipitation of 35S-labeled in vitro translation products of TYMV RNA. As shown in Fig. 1B, lane 1, translation of TYMV RNA typically gives rise to the synthesis of the 69K protein, the 206K polyprotein, and its self-processed products, the 140K and 66K proteins, as well as several minor bands in the range of 75 to 140 kDa that are most likely incomplete in vitro translation products of the 206K ORF (13, 41). The anti-140K antiserum precipitated both the 140K protein and its 206K precursor (Fig. 1B, lane 2), as well as most of the minor bands mentioned above, but did not precipitate the 69K and 66K proteins; these results demonstrate its specificity.
The anti-140K antiserum then was tested further by immunoblot analysis. Because preliminary experiments revealed that the anti-140K antiserum cross-reacted with some host proteins (data not shown), the antiserum was first preadsorbed against healthy plant protein extracts to minimize this background signal. The resulting preadsorbed antiserum then was tested in immunoblot analysis for its capacity to detect virus-specific proteins in TYMV-infected A. thaliana and Chinese cabbage protein extracts. A major immunoreactive protein with an apparent molecular mass of approximately 115 kDa (115K) was detected in infected Arabidopsis (Fig. 1C, lane 2) and Chinese cabbage (lane 6) plants. Some bands of approximately 85 kDa (85K) were also specifically detected in the protein extracts from infected plants, as were fainter bands of lower molecular masses that were likely to correspond to degradation products, since their proportions were highly variable from preparation to preparation.
No products of higher molecular masses could be detected by standard protein extraction protocols (47). However, when conditions optimized for the extraction of membrane proteins were used (23), an additional faint protein band with a higher molecular mass could be immunodetected in the samples from infected plants (Fig. 1C, lanes 4 and 8). This minor band was specifically recognized by the anti-140K antiserum, and its electrophoretic mobility appeared to be similar to that of the 140K protein obtained through in vitro translation of TYMV RNA (Fig. 1C, lanes 9 and 9′). Therefore, we assume that this minor species actually corresponds to the 140K protein, i.e., the N-terminal cleavage product of the 206K protein. This observation provides the first evidence for the occurrence of the 140K protein in planta.
Transfection of Arabidopsis protoplasts with TYMV RNA (Fig. 1D, lane 1) also led to the immunodetection of the 140K, 115K, and 85K proteins. A similar protein profile was observed upon transfection of expression vector pΩ-140K, which encodes the TYMV 140K protein (46) (Fig. 1D, lane 3). This observation demonstrates that the 115K and 85K proteins are derived solely from the 140K ORF. Therefore, in the following text, we refer to these two protein products as 140K-derived proteins. When expression vector pΩ-206K, which encodes the 206K protein precursor (46), was transfected in protoplasts (Fig. 1D, lane 4), the unprocessed form of the 206K protein could not be detected by the anti-140K antiserum; these results confirm the previous report of the efficient processing of the 206K protein into its 66K and 140K-derived protein products in plant cells (46, 47).
Colocalization of 66K and 140K-derived proteins within Arabidopsis cells.
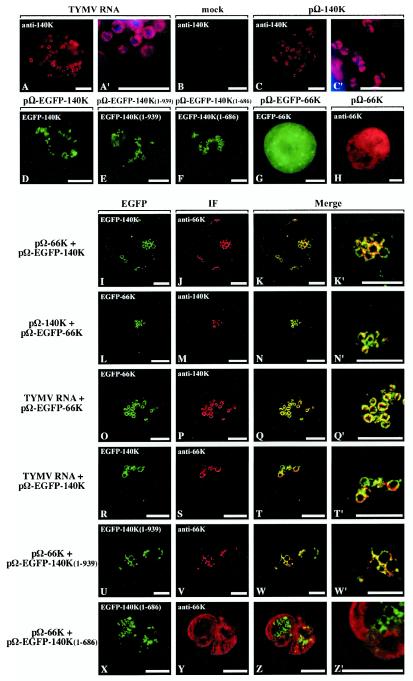
In order to analyze the intracellular sites of accumulation of the TYMV 140K protein, indirect immunofluorescence microscopy was carried out on mock- or TYMV-infected protoplasts at 24 h posttransfection. After fixation, the protoplasts were probed with anti-140K antibodies and secondary antibodies coupled to Alexa-fluor 594. Figure 2 shows representative images of such cells. TYMV-infected cells displayed bright fluorescent staining of the 140K protein (pseudo-colored red) in the shape of rings or groups of rings scattered throughout the cytoplasm (Fig. 2A). These rings correspond to the periphery of the chloroplasts, as confirmed by counterstaining of chloroplast DNA with DAPI (Fig. 2A′). Negligible fluorescence was detected in mock-infected cells (Fig. 2B). A similar distribution of the 140K protein was observed when Arabidopsis protoplasts were transfected with expression vector pΩ-140K (Fig. 2C and 2C′). The results of these immunofluorescence experiments are consistent with previous results obtained upon the expression of an EGFP-140K fusion protein in plant cells (46) (Fig. 2D). They demonstrate that the 140K protein is predominantly associated with the periphery of chloroplasts both in the course of a viral infection or when expressed alone, and they support the idea that the 140K protein is a major determinant for the chloroplast localization and retention of TYMV replication complexes.
FIG.2.
Colocalization of 66K and 140K-derived proteins within Arabidopsis cells. Arabidopsis protoplasts were transfected with viral RNA (A and A′); with water (B); with expression plasmid pΩ-140K (C and C′), pΩ-EGFP-140K (D), pΩ-EGFP-140K(1-939) (E), pΩ-EGFP-140K(1- 686) (F), pΩ-EGFP-66K (G), or pΩ-66K (H); with plasmid pΩ-66K together with pΩ-EGFP-140K (I to K′); with plasmid pΩ-140K together with pΩ-EGFP-66K (L to N′); with plasmid pΩ-EGFP-66K together with TYMV RNA (O to Q′); with plasmid pΩ-EGFP-140K together with TYMV RNA (R to T′); with plasmid pΩ-EGFP-140K(1-939) together with pΩ-66K (U to W′); or with plasmid pΩ-EGFP-140K(1- 686) together with pΩ-66K (X to Z′). The transfected protoplasts were collected at 24 h posttransfection (A to C′ and H to Z′) or 47 h posttransfection (D to G). The cells either were processed for indirect immunofluorescence labeling with anti-140K antiserum (A to C′ and L to Q′) or anti-66K antiserum (H to K′ and R to Z′) followed by secondary antibodies coupled to Alexa-fluor 594 or were observed for GFP fluorescence (D to G). Single protoplasts were observed either by epifluorescence microscopy (A to H) or by CLSM with sequential acquisition of EGFP fluorescence (green) (I, L, O, R, U, and X) and Alexa-fluor 594 fluorescence (red) (J, M, P, S, V, and Y). The merged images in panels K, N, Q, T, W, and Z represent digital superimpositions of red and green signals in which areas of fluorescence colocalization appear yellow. Scale bars, 10 μm. Panels A′, C′, K′, N′, Q′, T′, W′, and Z′ correspond to enlargements of panels A, C, K, N, Q, T, W, and Z, respectively. In order to visualize the location of chloroplasts, DAPI staining (blue) of chloroplast DNA was acquired and superimposed onto the fluorescence signal of the viral protein (red) in panels A′ and C′.
Because it was shown previously that the 66K protein expressed alone has a cytoplasmic distribution (46) (Fig. 2G and H) and that its recruitment to chloroplast envelope replication sites is dependent upon the simultaneous expression of the 140K protein (46), the possible colocalization of TYMV 66K and 140K-derived proteins in plant cells was analyzed by confocal laser scanning microscopy (CLSM). Indirect immunofluorescence studies were combined with the use of EGFP fusion proteins to allow the simultaneous detection of the two viral proteins.
As a first step, we performed cotransfection of Arabidopsis protoplasts with expression vectors pΩ-66K and pΩ-EGFP-140K. At 24 h posttransfection, the transfected cells were fixed, and indirect immunofluorescence microscopy was carried out with anti-66K antibodies and secondary antibodies conjugated to Alexa-fluor 594, while the autofluorescence of the GFP moiety allowed the detection of the 140K protein. Figure 2I to K′ show representative fluorescence micrographs of such a transfected cell. Analysis of the cells by CLSM revealed a clear colocalization of the two viral proteins (Fig. 2K and K′). Similar results were obtained when the reciprocal combination of expression vectors (pΩ-EGFP-66K and pΩ-140K) was transfected in Arabidopsis protoplasts and when immunodetection was performed with anti-140K antibodies (Fig. 2L to N′).
To determine whether the TYMV 140K and 66K proteins also colocalize during the infection process, Arabidopsis protoplasts were cotransfected with TYMV viral RNA and expression vector pΩ-EGFP-66K. It was shown previously that the EGFP-66K protein had a subcellular distribution that was indistinguishable from that of the nonfusion protein in coinfection experiments involving wild-type viral RNA (46). Indirect immunofluorescence was carried out with anti-140K antibodies, and localization of the proteins was observed by CLSM of infected cells. As shown by representative fluorescence micrographs of such cells, the 140K and EGFP-66K proteins clearly colocalized during viral infection (Fig. 2O to Q′). Similarly, cotransfection of TYMV viral RNA and expression vector pΩ-EGFP-140K and CLSM observation of infected cells after immunodetection of the 66K protein revealed that the 66K and EGFP-140K proteins colocalized during viral infection (Fig. 2R to T′).
Altogether, these results demonstrate that the TYMV 140K and 66K proteins colocalize at the periphery of chloroplasts, either when expressed simultaneously from expression vectors or during the course of the viral infection process.
Cofractionation of 66K and 140K-derived proteins in TYMV replication complexes.
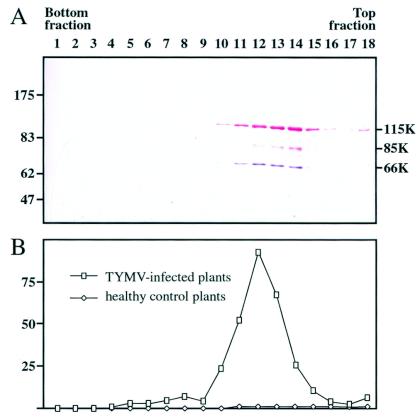
To investigate whether the 66K and 140K-derived proteins constituted a functional viral RdRp, replication complexes were prepared from TYMV-infected Chinese cabbage plants and from healthy control plants essentially as previously described (11). They were solubilized from membrane structures by using Lubrol W and centrifuged in a glycerol gradient. The gradient was subdivided into 18 fractions, each of which was analyzed for polymerase activity by an in vitro assay and for the presence of the 66K and 140K-derived proteins by Western blotting. Over 80% of the total RdRp activity was present in fractions 10 to 14, with a peak of 30% in fraction 12 (Fig. 3B). As a control, an identical procedure performed with healthy Chinese cabbage leaves revealed no significant RdRp activity. The sedimentation pattern of the 66K protein (Fig. 3A) closely corresponded to the distribution of the RdRp activity. The 140K-derived proteins were present in all fractions that showed RdRp activity, but some of the proteins were also found in fractions of the gradient that showed little or no RdRp activity (Fig. 3A).
FIG. 3.
Cofractionation of 66K and 140K-derived proteins in TYMV replication complexes. Replication complexes prepared from TYMV-infected Chinese cabbage plants were solubilized from membranes and centrifuged in a glycerol gradient that was subdivided into 18 fractions. (A) Samples of proteins from each fraction were analyzed by SDS-8% PAGE. The gel was electroblotted onto a nitrocellulose filter, and the proteins were sequentially revealed by Western blotting with anti-66K and anti-140K antisera and NBT-BCIP (purple) and Fast red (red) substrates, respectively. The positions of molecular weight markers (Biolabs) are indicated on the left (in thousands). (B) The in vitro RdRp activity of each fraction was determined with TYMV RNA as a template. The activity is expressed as 104 cpm of [32P]UMP incorporated. Fractions obtained from healthy control plants were analyzed in parallel.
These data demonstrate that the fractions displaying RdRp activity contain both 66K and 140K-derived proteins and support the notion that the 66K and 140K-derived proteins are all subunits of the TYMV replication complex.
Cofractionation of 66K and 140K-derived proteins in chloroplast envelope fractions from TYMV-infected plants.
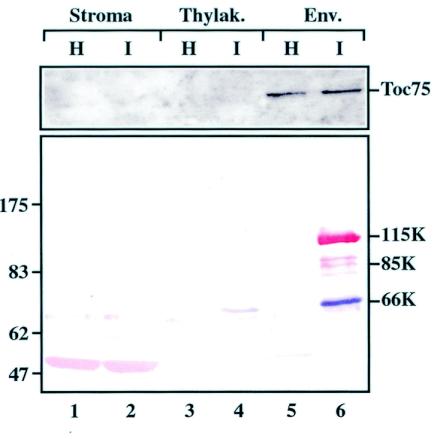
As a complementary approach, we also investigated whether the TYMV 66K and 140K-derived proteins were present in chloroplast envelope fractions prepared from TYMV-infected Chinese cabbage leaves. For this purpose, intact chloroplasts were first purified by centrifugation through a Percoll gradient. Following purification, the chloroplasts were lysed by osmotic shock, and envelope membranes, stroma, and thylakoids were fractionated over a sucrose density gradient and collected separately. Published procedures (12) were adapted to take into account the modified density of the chloroplast envelope as a consequence of TYMV infection. As a control, chloroplast purification and fractionation were performed identically with healthy Chinese cabbage leaves. Each fraction then was analyzed for the presence of the 66K and 140K-derived proteins by Western blotting. Antibodies raised against Toc75, a component of the chloroplast protein import apparatus, were used as specific markers of the envelope fractions (20).
As shown in Fig. 4, lane 6, the 66K protein and the two major proteins derived from the 140K protein, the 115K and 85K proteins, were detected in chloroplast envelope fractions from infected leaves. These data support the notion that the 66K and 140K-derived proteins are all closely associated with chloroplast envelope membranes in infected plants.
FIG. 4.
Cofractionation of 66K and 140K-derived proteins in chloroplast envelope fractions from TYMV-infected plants. Chloroplasts from TYMV-infected Chinese cabbage plants (I) and healthy control plants (H) were purified, lysed, and subjected to fractionation on a sucrose gradient. Equivalent amounts (7 μg) of proteins of the stroma (lanes 1 and 2), of the thylakoids (Thylak.) (lanes 3 and 4), and of the envelope membrane fractions (Env.) (lanes 5 and 6) were analyzed by SDS-8% PAGE. The gel was electroblotted onto a nitrocellulose filter, and the proteins were sequentially revealed by Western blotting with anti-66K and anti-140K antisera and NBT-BCIP (purple) and Fast red (red) substrates, respectively. Western blotting with anti-Toc75 antiserum and enhanced chemiluminescence detection were performed with 10 μg of the same samples as a specific marker of the envelope fractions. The positions of molecular weight markers (Biolabs) are indicated on the left (in thousands).
Interaction of 66K and 140K proteins in the YTHS.
To investigate the physical interaction of the TYMV 140K and 66K proteins, we made use of the YTHS. This system is a genetic approach that aims to detect protein-protein interactions in vivo in yeast cells by taking advantage of the modular nature of some eukaryotic transcription factors, such as GAL4 (14). This system has been shown to be suitable for mapping interactions among the replication proteins of a number of viruses (10, 22, 25, 35, 43, 57).
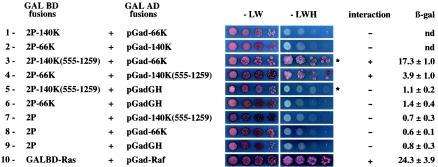
In order to assay the interaction of the TYMV 140K and 66K proteins in the YTHS, plasmid constructs 2P-66K, 2P-140K, pGad-66K, and pGad-140K, encoding the 66K and 140K viral proteins fused in frame to the C terminus of the GAL4 BD or the GAL4 AD, were obtained and used to transform yeast strains. After verification that none of these constructs was able to self-activate the HIS3 and lacZ selectable marker genes, yeast strains were transformed with a combination of plasmids (2P-140K plus pGad-66K) and plated on nonselective (−LW) or selective (−LWH) medium. As shown in Fig. 5, row 1, no interaction was detected for full-length 140K and 66K proteins. Similarly, reciprocal assays with the combination of plasmids (2P-66K plus pGad-140K) did not give rise to any reporter activity (Fig. 5, row 2).
FIG. 5.
Interactions of TYMV 66K and 140K proteins in the YTHS. TYMV proteins (or protein segments) fused to the GAL4 BD or AD were used to transform yeast cells. Aliquots of transformed yeast cells and serial fivefold dilutions were dropped on both −LW and −LWH media. For some plasmid combinations (indicated by asterisks), 2.5 mM 3-amino-1,2,4-triazole was added to the medium to prevent self-activation of the HIS3 reporter gene. No growth (−) or growth (+) of yeast colonies on interaction-selective medium (−LWH) after 48 to 72 h of incubation at 30°C is indicated for each combination. Typical interactions are shown along with negative and positive controls (rows 9 and 10, respectively). For quantitative β-galactosidase assays for interactions in the YTHS, liquid cultures were grown under interaction-nonselective conditions at 30°C. β-Galactosidase assays were performed in triplicate sets with at least seven independent cultures for each strain, and the means (in Miller units) and standard deviations are indicated; nd, not determined.
In order to determine whether the above lack of interaction was the result of poor expression of the proteins in yeast cells, Western blot analyses were performed with yeast protein extracts. They revealed the proper expression of the fusion proteins in transformed yeast cells (data not shown). We also ruled out the possibility that the interaction of the viral proteins would be temperature sensitive and inhibited at 30°C, since cotransformed yeast colonies also failed to grow on selective (−LWH) medium after 14 days at 22°C (data not shown). As an alternative, we tested a YTHS which makes use of the bacterial LexA BD, because in some instances, this system is more sensitive than the GAL4-based system (38). The 66K and 140K proteins were fused in frame to the C terminus of the LexA BD, generating pLex-66K and pLex-140K, respectively. However, upon transformation of appropriate yeast cells with plasmid combinations (pLex-66K plus pGad-140K and pLex-140K plus pGad-66K), the results were also negative (data not shown).
There are many examples in the literature of false-negative results in the two-hybrid system, especially when large proteins are involved. Possible explanations include misfolding of the fusion protein, steric hindrance of the interaction site by cellular factors, and/or incorrect localization through exclusion of the proteins from the nucleus. In an attempt to circumvent these problems, plasmids 2P-140K(555-1259) and pGad-140K(555-1259) were constructed; in these plasmids, the DNA sequences corresponding to amino acids 1 to 554 of the 140K protein were deleted. As shown in Fig. 5, rows 3 and 4, growth on selective (−LWH) medium of yeast colonies transformed with a combination of plasmids [2P-140K(555-1259) plus pGad-66K] and with the reciprocal combination [2P-66K plus pGad-140K(555-1259)] was clearly visible within 2 to 3 days at 30°C. The strength of the interaction of the two viral proteins was also assessed by a liquid β-galactosidase assay (Fig. 5). Yeast cells transformed with pairs of plasmids encoding the TYMV replication proteins showed levels of β-galactosidase activity that were significantly higher (3- to 15-fold) than than those in control yeast cells lacking either one or both of the two viral sequences.
These results demonstrate that the TYMV 66K protein is able to physically interact with the C-terminal region of the 140K protein.
Coimmunoprecipitation of 66K and 140K-derived proteins from purified RdRp.
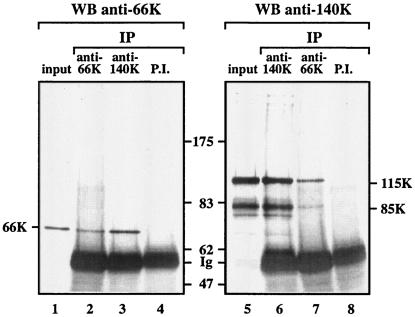
Confirmation of the physical interaction of the TYMV replication proteins was assessed by performing coimmunoprecipitation experiments. For this purpose, viral replication complexes purified by glycerol gradient centrifugation (fraction 13 of the gradient shown in Fig. 3) were subjected to immunoprecipitation under nondenaturing conditions with either the anti-140K antiserum or the anti-66K antiserum or their corresponding preimmune sera. The immunoprecipitates were analyzed by Western blotting with the anti-66K antiserum or the anti-140K antiserum. The anti-66K antiserum was able to immunoprecipitate both the 66K protein (Fig. 6, lane 2) and the 140K-derived proteins (lane 7). Reciprocally, the anti-140K antiserum immunoprecipitated both the 140K-derived proteins (Fig. 6, lane 6) and the 66K protein (lane 3). These results therefore demonstrate that the 66K and 140K-derived proteins interact physically with each other in planta and corroborate the interaction observed in the YTHS.
FIG. 6.
Coimmunoprecipitation of 66K and 140K-derived proteins in TYMV replication complexes. TYMV replication complexes purified by glycerol gradient centrifugation (Fig. 3, fraction 13) were subjected to immunoprecipitation (IP) with anti-66K antiserum (lanes 2 and 7), the corresponding preimmune (P.I.) serum (lane 8), anti-140K antiserum (lanes 3 and 6), or the corresponding preimmune serum (lane 4). The immunoprecipitates and an aliquot of the replicase fraction corresponding to 40% of the input sample (lanes 1 and 5) were subjected to SDS-7% PAGE and immunoblot analysis with anti-66K (lanes 1 to 4) or anti-140K (lanes 5 to 8) polyclonal antibodies. The positions of molecular weight markers (Biolabs) (in thousands) and immunoglobulin (Ig) heavy chains are indicated.
Further mapping of minimal protein domains required for the interaction.
In order to delineate further the protein domains required for the interaction of the 140K and 66K proteins in yeast cells, plasmids that direct the synthesis of various truncated forms of both proteins were constructed. Those plasmids were obtained either by standard cloning techniques with available restriction sites or by gap repair reconstitution of plasmids in vivo by use of the efficient homologous recombination process in yeast cells and PCR-amplified DNA fragments.
Initially, truncated versions of the viral proteins were cloned both in vector 2P and in vector pGADGH. However, a number of 2P-derived plasmids appeared to have intrinsic transcriptional activation activity (data not shown) and were thus useless for interaction analysis. To circumvent this problem, the truncated 140K and 66K protein fragments were expressed as AD fusions only, and their interaction capability was challenged against that of the BD fusions encoded by vectors 2P-66K and 2P-140K(555-1259), respectively. Interaction assays were performed by plating aliquots of transformed yeast cells on both nonselective (−LW) and selective (−LWH) media, and growth was observed after 48 to 72 h at 30°C. The results obtained are summarized in Table 1.
TABLE 1.
Summary of two-hybrid interactions tested
| Vector encoding GAL BD fusion | Vector encoding GAL AD fusion | Interactiona |
|---|---|---|
| 2P-66K | pGadGH | − |
| pGad-140K(1-1259) | − | |
| pGad-140K(1-1044) | − | |
| pGad-140K(1-877) | − | |
| pGad-140K(1-555) | − | |
| pGad-140K(555-1259) | + | |
| pGad-140K(877-1259) | − | |
| pGad-140K(1044-1259) | − | |
| pGad-140K(555-1044) | + | |
| pGad-140K(622-1044) | + | |
| pGad-140K(687-1044) | + | |
| pGad-140K(748-1044) | ± | |
| pGad-140K(812-1044) | − | |
| pGad-140K(877-1044) | − | |
| pGad-140K(553-1044) | + | |
| pGad-140K(553-983) | + | |
| pGad-140K(553-968) | + | |
| pGad-140K(553-958) | + | |
| pGad-140K(553-949) | + | |
| pGad-140K(553-939) | + | |
| pGad-140K(553-930) | − | |
| pGad-140K(553-877) | − | |
| pGad-140K(687-983) | + | |
| pGad-140K(687-939) | + | |
| pGad-140K(731-939) | ± | |
| pGad-140K(748-939) | ± | |
| 2P-140K(555-1259) | pGadGH | − |
| pGad-66K(1-585) | + | |
| pGad-66K(1-511) | + | |
| pGad-66K(1-395) | + | |
| pGad-66K(1-382) | + | |
| pGad-66K(1-369) | + | |
| pGad-66K(1-356) | − | |
| pGad-66K(1-237) | − | |
| pGad-66K(1-202) | − | |
| pGad-66K(1-172) | − | |
| pGad-66K(1-78) | − | |
| pGad-66K(80-585) | − | |
| pGad-66K(172-585) | − | |
| pGad-66K(203-585) | − | |
| pGad-66K(237-585) | − | |
| pGad-66K(395-585) | − | |
| pGad-66K(17-395) | ± | |
| pGad-66K(33-395) | ± | |
| pGad-66K(49-395) | ± | |
| pGad-66K(65-395) | ± | |
| pGad-66K(81-395) | − | |
| pGad-66K(1-369) | + | |
| pGad-66K(49-369) | ± | |
| pGad-66K(65-369) | ± | |
| pGad-66K(81-369) | − | |
| 2P-140K(687-939) | pGadGH | − |
| pGad-66K(1-369) | + |
Interactions were scored as follows: −, no growth of colonies on −LWH selective medium after 48 to 72 h at 30°C; ±, growth of petite white colonies; +, growth of regularly sized pink colonies.
Although the 705 C-terminal amino acids of the 140K protein were found to interact with the 66K protein (Fig. 5), further truncation of the 140K protein to its 383 or 216 C-terminal amino acids [encoded by pGad-140K(877-1259) and pGad-140K(1044-1259), respectively] abolished the interaction (Table 1). Construct pGad-140K(555-1044), however, was still capable of inducing reporter gene activity in the presence of 2P-66K. Further deletions at the N terminus revealed that constructs with deletions up to amino acid 687 of the 140K protein retained the ability to interact with the 66K protein in vivo, while further N-terminal truncations caused a decline in the interaction or abolished the interaction. Similarly, C-terminal nested deletions in the 140K protein revealed that constructs with deletions up to amino acid 939 showed wild-type levels of interaction with the 66K protein, while extending the deletion end point to amino acid 930 abolished the interaction.
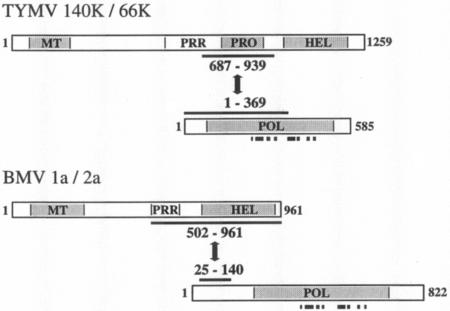
Thus, amino acids 687 to 939 of the 140K protein were identified as being the minimal protein domain able to interact with the 66K protein. Interestingly, this region of the 140K protein corresponds to the previously characterized papain-like proteinase domain (4, 50) (Fig. 1A).
Reciprocally, a series of plasmids encoding truncated 66K polypeptides were constructed and assayed in the YTHS for their ability to interact with the 140K-AD fusion. As shown in Table 1, C-terminal truncation of the 66K protein in pGad-66K(1- 369) led to a wild-type interaction, while the removal of an additional 13 amino acids in pGad-66K(1- 356) abolished it. Nested N-terminal deletions extending from the start codon of the 66K protein to amino acid 81 caused a progressive decline in the ability to interact with the 140K protein. These data therefore demonstrate that amino acids 1 to 369 of the 66K protein correspond to the minimal protein domain able to interact with the 140K protein. About two-thirds of the conserved polymerase domain is thus required for an interaction with the C-terminal region of the 140K protein.
To investigate whether these minimal binding domains were sufficient to interact with themselves, constructs pGad-66K(1-369) and 2P-140K(687- 939) were obtained and assayed in the YTHS. As shown in Table 1, when these truncated 140K and 66K protein fragments were expressed in yeast cells, colonies were able to grow on a selective synthetic medium, demonstrating that no additional protein sequences are required for the interaction of the viral proteins.
Altogether, these results demonstrate that the physical interaction of the TYMV replication proteins involves the proteinase domain of the 140K protein and a region of the 66K protein encompassing two-thirds of the conserved polymerase domain (Fig. 7).
FIG. 7.
Comparison of the protein domains involved in interactions between replication proteins of different members of the alphavirus-like supergroup. Schematic representation of the protein fragments involved in the interactions of the TYMV 140K and 66K and the BMV 1a and 2a replication proteins. Protein domains are indicated (MT, methyltransferase; PRR, proline-rich region; PRO, proteinase; HEL, helicase; POL, polymerase). The limits of the interaction domains characterized with the two-hybrid system (43; this study) are indicated. The locations of the eight conserved sequence motifs in RdRp of positive-strand RNA viruses (31) are indicated by filled squares.
The helicase domain of the 140K protein is unnecessary for promoting the relocalization of RdRp to the chloroplast envelope in plant cells.
Since the results obtained in the above-described two-hybrid experiments were strikingly different from those described previously for other members of the alphavirus-like supergroup (29, 43, 57) (Fig. 7), experiments were carried out to ascertain whether the requirements for the interaction of TYMV protein domains were similar in plant cells and yeast cells. Of particular interest was the role of the helicase domain, which has been implicated in the assembly of the replication complexes of other members of the alphavirus-like supergroup but which appeared to be dispensable in the above-described YTHS experiments implicating TYMV replication proteins.
To probe the possible involvement of the helicase and/or proteinase domains in the 140K protein-mediated recruitment of the 66K protein to the chloroplast envelope, expression vectors pΩ-EGFP-140K(1-939) and pΩ-EGFP-140K(1-686) were constructed. They encode EGFP-fused C-terminally truncated versions of the 140K protein lacking the helicase domain and lacking both the helicase and the proteinase domains, respectively. When expressed in Arabidopsis protoplasts, the corresponding proteins retained a distribution at the periphery of chloroplasts similar to that of the EGFP-140K protein, as visualized by fluorescence microscopy of the GFP moiety (Fig. 2D to F). Arabidopsis protoplasts then were cotransfected with expression vector pΩ-EGFP-140K(1-939) together with construct pΩ-66K. The localization of the EGFP fusion was determined by fluorescence microscopy of the GFP moiety, while the distribution of the 66K protein was visualized by indirect immunofluorescence with anti-66K antibodies and Alexa-fluor 594 conjugates. As shown by representative confocal laser scanning micrographs of such cells at 24 h posttransfection, both the EGFP-140K(1-939) protein and the 66K protein were targeted to the chloroplast envelope and were clearly colocalized (Fig. 2U to W′). In contrast, when Arabidopsis protoplasts were cotransfected with expression vector pΩ-EGFP-140K(1-686) together with construct pΩ-66K, the 66K protein displayed a cytoplasmic distribution (Fig. 2X to Z′), demonstrating that the EGFP-140K(1- 686) protein is unable to promote the relocalization of the 66K protein to the chloroplast envelope.
These results therefore demonstrate that the helicase domain of the TYMV 140K protein is dispensable for the proper recruitment of the 66K protein to the chloroplast envelope, while the proteinase domain appears to be essential for that process.
DISCUSSION
Proteolytic processing of the TYMV 206K protein in vivo.
TYMV encodes a 206K polyprotein which is the only viral protein required for RNA replication (61). The 206K protein is proteolytically processed in vitro and in vivo to release the C-terminal 66K protein encompassing the RdRp domain (6, 41, 47). However, the mode of TYMV genome expression in vivo is not completely understood. In particular, highly purified TYMV replicase fractions were shown earlier to contain a 115K protein of viral origin that is likely to arise from the processing of the 206K protein (8, 42), but investigation on the nature of this polypeptide has not been pursued further.
In this study, we raised specific antibodies against synthetic peptides located within the central proline-rich region of the 140K protein (Fig. 1A). Probing of protein blots of TYMV-infected plant and protoplast samples with the anti-140K antiserum (Fig. 1C and D) resulted in the detection of two major products, 115K and 85K proteins. A minor protein of 140 kDa could also be detected, and based on its electrophoretic mobility and antibody recognition, we assume that this product corresponds to the 140K protein, i.e., the N-terminal cleavage product of the 206K protein (6, 41). The detection of the 140K protein was improved when conditions optimized for the extraction of membrane proteins were used (Fig. 1C), a finding that is consistent with previous (46) and current results demonstrating that the 140K protein is targeted to chloroplast envelope membranes in plant cells (Fig. 2A to D). This observation provides the first evidence for the occurrence of the 140K protein in planta.
We do not currently know whether the occurrence of these multiple bands is due to alternative proteolytic cleavage(s) of the 140K protein by the viral proteinase domain, to a trans-mediated cleavage by a host enzyme, or to unspecific proteolytic degradation occurring during extraction or analysis. It should be noted that the proteinase domain of alphaviruses is responsible for multiple sequential cis and trans cleavages (reviewed in reference 55) that play a pivotal role in the temporal regulation of minus- and plus-strand RNA synthesis (34). This raises the question as to whether a similar processing scheme occurs in TYMV as well.
The 115K protein is of particular interest because it has been known for many years to be a major component of the purified TYMV replicase (8, 42). Interestingly, the protein profile immunodetected with the anti-140K antiserum was identical when Arabidopsis protoplasts were either infected by TYMV viral RNA or transfected by an expression vector carrying the 140K ORF (Fig. 1D). This observation demonstrates that the 115K and 85K proteins are derived solely from the 140K ORF and rule out the possibility that the 115K protein corresponds to a fusion of the helicase and the RdRp protein domains, as previously suggested (11). Whether the 115K protein lacks the N-terminal methyltransferase domain or the C-terminal helicase domain remains to be determined by use of other domain-specific antisera.
Because the 115K and 85K polypeptides copurify along with the replicase activity on glycerol gradients (Fig. 3), cofractionate with the chloroplast envelope membranes that host the replication complexes (Fig. 4), and coimmunoprecipitate with the 66K protein (Fig. 6), they may represent specific cleavage products involved as subunits of the replicase. However, until the processing strategy and the identity of the mature protein(s) are clarified, we will refer to these two protein products as 140K-derived proteins.
Colocalization, cofractionation, and physical interaction of TYMV replication proteins.
Tymovirus infection is accompanied by the induction of characteristic membranous vesicles at the periphery of chloroplast envelopes, which have been implicated in virus replication (16, 33, 47). The 140K protein localizes to the chloroplast envelope when expressed alone (46) (Fig. 2C and C′), demonstrating its key role in the targeting of the replication complexes to these particular subcellular membranes. Unlike the 140K protein, the 66K polymerase appears not to be directed toward replication complexes in the form of a membrane-binding protein, since it is cytoplasmic when produced alone from an expression vector (46) (Fig. 2G and H). By genetic complementation analysis, the 66K protein produced independently was shown to be active, since it can trans-complement a TYMV mutant genome that lacks a functional 66K coding sequence (46). In such a situation, the 66K protein is relocated from the cytoplasm to the chloroplast envelope, and this relocation process appears to be solely dependent upon the simultaneous expression of the 140K protein (46). Therefore, we hypothesized that the trans-active 66K protein may be recruited to replication complexes through protein-protein interactions with the membrane-bound 140K protein.
The observed subcellular colocalization of the TYMV replication proteins at the periphery of chloroplasts, as revealed by immunofluorescence experiments and expression of GFP fusions (Fig. 2), is in good agreement with this hypothesis. In addition, chloroplast envelope membrane fractions purified from TYMV-infected plants were shown to contain both the 66K protein and the 140K-derived proteins (Fig. 4). Those proteins also copurified in enzymatically active RdRp fractions from TYMV-infected plants (Fig. 3), supporting the notion that the 66K and 140K-derived proteins are components of the active replication complex.
Given the previous observations that the TYMV replication proteins may have to interact to bring the different replication proteins together for functional reasons, we searched for physical interactions of the viral proteins. Using both the two-hybrid system (Fig. 5) and coimmunoprecipitation of purified replication complex fractions (Fig. 6), we showed that the TYMV replication proteins interact physically with each other. Through deletion analysis, we mapped the regions required for this interaction (Table 1). The fine molecular mapping of the interaction sites in the YTHS may ultimately lead to a test that can be used to screen for molecules or peptides that prevent the protein-protein interaction and thus inhibit viral replication (51, 58). Alternatively, it can constitute an important step toward the identification of mechanisms that may regulate these interactions. In that respect, it was recently shown that the phosphorylation of the cucumber mosaic virus 2a protein RdRp by a host kinase inhibited its interaction with the 1a protein in vitro (30). Interestingly, the peptides that were previously reported to be phosphorylated in the recombinant 66K protein (19) are located within the interaction domain with the 140K protein. It is therefore possible that the 140K-66K interaction is regulated by conformational changes within the 66K protein, triggered by phosphorylation events. Because it is likely that the TYMV RNA replication machinery may be more complex or dynamic than a simple 140K-66K complex, further issues to be investigated also include the stoichiometry of the proteins in the replication complexes of TYMV.
Comparison of the assembly of replication complexes of positive-strand RNA viruses.
It is interesting to note that the interaction of the TYMV replication proteins differs from protein-protein interactions of some other alphavirus-like viruses. In a number of them (alphaviruses, rubiviruses, and several tripartite RNA plant viruses), the viral protein encompassing the polymerase domain contains a less conserved N-terminal segment in front of the core RdRp domain. In the BMV 2a protein, this discrete N-terminal segment was shown to mediate the interaction with the cognate helicase-like 1a protein (29, 43, 44) (Fig. 7). The corresponding segment in the 66K protein is much smaller (∼80 amino acids versus 220 and 280 amino acids in the BMV 2a and AMV P2 proteins, respectively) and was not sufficient to promote binding to the 140K protein (Table 1). Instead, a large region of the 66K protein was required; this region includes four of the eight conserved sequence motifs that are characteristic of the viral polymerases (31) (Fig. 7). This finding suggests that the interaction function of the 66K protein may depend on multiple regions within the protein or that it may involve a stringent structural requirement throughout the entire protein.
It is even more remarkable that the interaction domain mapped within the TYMV 140K protein did not correspond to the helicase domain (Table 1 and Fig. 7), as previously reported for other members of the alphavirus-like supergroup (29, 43, 44, 57). This result was further corroborated by coexpression and localization experiments of the 66K protein with C-terminally truncated forms of EGFP-140K protein in plant cells (Fig. 2U to Z′); these experiments demonstrated that the helicase domain was unnecessary for the recruitment of the 66K protein to the chloroplast envelope, while the proteinase domain appeared to be essential for that process. These findings ruled out the possibility that significant deviations from the protein-protein interactions detected in the YTHS may have occurred in the context of plant cells.
The results presented in this study therefore support a novel model for the assembly of TYMV replication complexes based on the interaction of the polymerase and proteinase domains and suggest that different members of the alphavirus-like supergroup may have selected different pathways to assemble their replication complexes. Interestingly, an interaction of viral polymerase and proteinase domains has been reported for other positive-strand RNA viruses; in the case of potyviruses, which are members of the picornavirus-like supergroup, the recruitment of the NIb polymerase to the replication initiation complexes was shown to occur through protein-protein interactions with the proteinase domain of the membrane-bound 6K/NIa precursor (10, 35, 53). It has been proposed that such an interaction (functionally homologous to the involvement of 3Cpro-3Dpol in poliovirus replication) may represent a highly conserved core feature of the RNA replication apparatus of viruses within the picornavirus supergroup (10). The finding of a similar interaction in the phylogenetically distinct TYMV is striking and raises some interesting questions about the evolution of the assembly of replication complexes within positive-strand RNA viruses.
Based on the comparative analysis of amino acid sequences of replication proteins of positive-strand RNA viruses, phylogenetic subdivisions and hypothetical evolutionary scenarios have been proposed. Koonin and Dolja (31) suggested that the “common ancestor” of the alphavirus-like viruses possessed a proteinase domain that would have been lost in the evolutionary process leading to the Tricornaviridae family (whose members include BMV and AMV). One can therefore speculate that the polymerase-proteinase interaction corresponds to the “ancestor” type of interaction leading to the assembly of replication complexes. It might have evolved to a polymerase-helicase interaction upon the loss of the proteinase domain during the evolution of some viral families. It will be interesting in the future to determine whether members of the alphavirus-like supergroup possessing a proteinase domain, for instance, alphaviruses and rubiviruses, also rely on an interaction of the polymerase and proteinase domains for the assembly of their replication complexes.
Acknowledgments
A.J. and F.H. were recipients of a fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT). This work was supported in part by grants from CNRS (Programme Jeunes Equipes) and MENRT (Action Concertée Incitative Jeunes Chercheurs). I.J. also acknowledges the support of a Human Frontier Science Program short-term fellowship (SF-399/95) for a training period at Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
We are grateful to M. Fromont-Racine (Institut Pasteur, Paris, France) for kindly providing to us plasmid pAS2ΔΔ and several yeast strains used in this study and to J. Soll (Ludwig-Maximilians-Universität, Munich, Germany) for the kind gift of anti-Toc75 antibodies. We acknowledge C. Chamot for help with confocal microscopy experiments, O. Delmas and E. Meunier for constructing some of the plasmids used in this study, D. Prod'homme and S. Pflieger for help with the Arabidopsis cell culture, C. Albrieux for excellent technical assistance, and S. Planchais for comments on the manuscript. Special thanks are due to C.J. and F.-A. for constant encouragement.
REFERENCES
- 1.Ahlquist, P., A. O. Noueiry, W. M. Lee, D. B. Kushner, and B. T. Dye. 2003. Host factors in positive-strand RNA virus genome replication. J. Virol. 77:8181-8186. [DOI] [PMC free article] [PubMed]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Barends, S., H. H. Bink, S. H. van den Worm, C. W. Pleij, and B. Kraal. 2003. Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell 112:123-129. [DOI] [PubMed] [Google Scholar]
- 4.Bransom, K. L., and T. W. Dreher. 1994. Identification of the essential cysteine and histidine residues of the turnip yellow mosaic virus protease. Virology 198:148-154. [DOI] [PubMed] [Google Scholar]
- 5.Bransom, K. L., S. E. Wallace, and T. W. Dreher. 1996. Identification of the cleavage site recognized by the turnip yellow mosaic virus protease. Virology 217:404-406. [DOI] [PubMed] [Google Scholar]
- 6.Bransom, K. L., J. J. Weiland, and T. W. Dreher. 1991. Proteolytic maturation of the 206-kDa nonstructural protein encoded by turnip yellow mosaic virus RNA. Virology 184:351-358. [DOI] [PubMed] [Google Scholar]
- 7.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candresse, T., C. Mouchès, and J. M. Bové. 1986. Characterization of the virus encoded subunit of turnip yellow mosaic virus RNA replicase. Virology 152:322-330. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daros, J. A., M. C. Schaad, and J. C. Carrington. 1999. Functional analysis of the interaction between VPg-proteinase (NIa) and RNA polymerase (NIb) of tobacco etch potyvirus, using conditional and suppressor mutants. J. Virol. 73:8732-8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deiman, B. A., K. Séron, E. M. Jaspars, and C. W. Pleij. 1997. Efficient transcription of the tRNA-like structure of turnip yellow mosaic virus by a template-dependent and specific viral RNA polymerase obtained by a new procedure. J. Virol. Methods 64:181-195. [DOI] [PubMed] [Google Scholar]
- 12.Douce, R., and J. Joyard. 1982. Purification of the chloroplast envelope, p. 239-256. In M. Edelman, R. B. Hallick, and N. H. Chua (ed.), Methods in chloroplast molecular biology. Elsevier Biomedical, Amsterdam, The Netherlands.
- 13.Drugeon, G., and I. Jupin. 2002. Stability in vitro of the 69K movement protein of turnip yellow mosaic virus is regulated by the ubiquitin-mediated proteasome pathway. J. Gen. Virol. 83:3187-3197. [DOI] [PubMed] [Google Scholar]
- 14.Fields, S., and R. Sternglanz. 1994. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 10:286-292. [DOI] [PubMed] [Google Scholar]
- 15.Fromont-Racine, M., J. C. Rain, and P. Legrain. 1997. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat. Genet. 16:277-282. [DOI] [PubMed] [Google Scholar]
- 16.Garnier, M., T. Candresse, and J. M. Bové. 1986. Immunocytochemical localization of TYMV-coded structural and nonstructural proteins by the protein A-gold technique. Virology 151:100-109. [DOI] [PubMed] [Google Scholar]
- 17.Goldbach, R., and J. Wellink. 1988. Evolution of plus-strand RNA viruses. Intervirology 29:260-267. [DOI] [PubMed] [Google Scholar]
- 18.Gorbalenya, A. E., E. V. Koonin, A. P. Donchenko, and V. M. Blinov. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 17:4713-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Héricourt, F., S. Blanc, V. Redeker, and I. Jupin. 2000. Evidence for phosphorylation and ubiquitinylation of the turnip yellow mosaic virus RNA-dependent RNA polymerase domain expressed in a baculovirus-insect cell system. Biochem. J. 349:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinnah, S. C., K. Hill, R. Wagner, T. Schilcher, and J. Soll. 1997. Reconstitution of a chloroplast protein import channel. EMBO J. 16:7351-7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 22.Hope, D. A., S. E. Diamond, and K. Kirkegaard. 1997. Genetic dissection of interaction between poliovirus 3D polymerase and viral protein 3AB. J. Virol. 71:9490-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurkman, M. A., and C. K. Tanaka. 1986. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 81:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson, M., A. J. Brooks, D. A. Jans, and S. G. Vasudevan. 2001. A small region of the dengue virus-encoded RNA-dependent RNA polymerase, NS5, confers interaction with both the nuclear transport receptor importin-beta and the viral helicase, NS3. J. Gen. Virol. 82:735-745. [DOI] [PubMed] [Google Scholar]
- 26.Kadaré, G., M. Rozanov, and A. L. Haenni. 1995. Expression of the turnip yellow mosaic virus proteinase in Escherichia coli and determination of the cleavage site within the 206 kDa protein. J. Gen. Virol. 76:2853-2857. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Kamer, G., and P. Argos. 1984. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 12:7269-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao, C. C., and P. Ahlquist. 1992. Identification of the domains required for direct interaction of the helicase-like and polymerase-like RNA replication proteins of brome mosaic virus. J. Virol. 66:7293-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, S. H., P. Palukaitis, and Y. I. Park. 2002. Phosphorylation of cucumber mosaic virus RNA polymerase 2a protein inhibits formation of replicase complex. EMBO J. 21:2292-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonin, E. V., and V. V. Dolja. 1993. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28:375-430. [DOI] [PubMed] [Google Scholar]
- 32.Kujala, P., A. Ikaheimonen, N. Ehsani, H. Vihinen, P. Auvinen, and L. Kaariainen. 2001. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 75:3873-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laflèche, D., C. Bové, G. Dupont, C. Mouchès, T. Astier, M. Garnier, and J. M. Bové. 1972. Site of viral replication in the cells of higher plants: TYMV—RNA synthesis on the chloroplast outer membrane system, p. 43-71. In Proceedings of the 8th FEBS Meeting: RNA viruses/ribosomes. Elsevier/North Holland Publishing Co., Amsterdam, The Netherlands.
- 34.Lemm, J. A., T. Rumenapf, E. G. Strauss, J. H. Strauss, and C. M. Rice. 1994. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 13:2925-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, X. H., P. Valdez, R. E. Olvera, and J. C. Carrington. 1997. Functions of the tobacco etch virus RNA polymerase (NIb): subcellular transport and protein-protein interaction with VPg/proteinase (NIa). J. Virol. 71:1598-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, J., and B. Rost. 2003. NORSp: predictions of long regions without regular secondary structure. Nucleic Acids Res. 31:3833-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manivasakam, P., S. C. Weber, J. McElver, and R. H. Schiestl. 1995. Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res. 23:2799-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus, G. A., N. Silverman, S. L. Berger, J. Horiuchi, and L. Guarente. 1994. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 13:4807-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Morch, M. D., J. C. Boyer, and A. L. Haenni. 1988. Overlapping open reading frames revealed by complete nucleotide sequencing of turnip yellow mosaic virus genomic RNA. Nucleic Acids Res. 16:6157-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morch, M. D., G. Drugeon, P. Szafranski, and A. L. Haenni. 1989. Proteolytic origin of the 150-kilodalton protein encoded by turnip yellow mosaic virus genomic RNA. J. Virol. 63:5153-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouchès, C., T. Candresse, and J. M. Bové. 1984. Turnip yellow mosaic virus RNA-replicase contains hosts and virus-encoded subunits. Virology 134:78-90. [DOI] [PubMed] [Google Scholar]
- 43.O'Reilly, E. K., J. D. Paul, and C. C. Kao. 1997. Analysis of the interaction of viral RNA replication proteins by using the yeast two-hybrid assay. J. Virol. 71:7526-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Reilly, E. K., N. Tang, P. Ahlquist, and C. C. Kao. 1995. Biochemical and genetic analyses of the interaction between the helicase-like and polymerase-like proteins of the brome mosaic virus. Virology 214:59-71. [DOI] [PubMed] [Google Scholar]
- 45.Printen, J. A., and G. F. J. Sprague. 1994. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics 138:609-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prod'homme, D., A. Jakubiec, V. Tournier, G. Drugeon, and I. Jupin. 2003. Targeting of the turnip yellow mosaic virus 66K replication protein to the chloroplast envelope is mediated by the 140K protein. J. Virol. 77:9124-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prod'homme, D., S. Le Panse, G. Drugeon, and I. Jupin. 2001. Detection and subcellular localization of the turnip yellow mosaic virus 66K replication protein in infected cells. Virology 281:88-101. [DOI] [PubMed] [Google Scholar]
- 48.Restrepo-Hartwig, M. A., and J. C. Carrington. 1994. The tobacco etch potyvirus 6-kilodalton protein is membrane associated and involved in viral replication. J. Virol. 68:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozanov, M., E. Koonin, and A. Gorbalenya. 1992. Conservation of the putative methyltransferase domain: a hallmark of the ′Sindbis-like' supergroup of positive-strand RNA viruses. J. Gen. Virol. 73:2129-2134. [DOI] [PubMed] [Google Scholar]
- 50.Rozanov, M. N., G. Drugeon, and A.-L. Haenni. 1995. Papain-like proteinase of turnip yellow mosaic virus: a prototype of a new viral proteinase group. Arch. Virol. 140:4545-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudolph, C., P. H. Schreier, and J. F. Uhrig. 2003. Peptide-mediated broad-spectrum plant resistance to tospoviruses. Proc. Natl. Acad. Sci. USA 100:4429-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider, S., M. Buchert, and C. M. Hovens. 1996. An in vitro assay of beta-galactosidase from yeast. BioTechniques 20:960-962. [DOI] [PubMed] [Google Scholar]
- 55.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Aelst, L., M. Barr, S. Marcus, A. Polverino, and M. Wigler. 1993. Complex formation between RAS and RAF and other protein kinases. Proc. Natl. Acad. Sci. USA 90:6213-6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Der Heijden, M. W., J. E. Carette, P. J. Reinhoud, A. Haegi, and J. F. Bol. 2001. Alfalfa mosaic virus replicase proteins P1 and P2 interact and colocalize at the vacuolar membrane. J. Virol. 75:1879-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal, M., and H. Endoh. 1999. Prospects for drug screening using the reverse two-hybrid system. Trends Biotechnol. 17:374-381. [DOI] [PubMed] [Google Scholar]
- 59.Vojtek, A. B., S. M. Hollenberg, and J. A. Cooper. 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74:205-214. [DOI] [PubMed] [Google Scholar]
- 60.Weiland, J. J., and T. W. Dreher. 1993. Cis-preferential replication of the turnip yellow mosaic virus RNA genome. Proc. Natl. Acad. Sci. USA 90:6095-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiland, J. J., and T. W. Dreher. 1989. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 17:4675-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]