Abstract
Background
This is the first controlled study to explore whether adjunctive immersive virtual reality (VR) can reduce excessive pain of soldiers with combat-related burn injuries during wound debridement.
Methods
Patients were US soldiers burned in combat attacks involving explosive devices in Iraq or Afghanistan. During the same wound care session using a within-subject experimental design, 12 patients received half of their severe burn wound cleaning procedure (∼6 minutes) with standard of care pharmacologies and half while in VR (treatment order randomized). Three 0 to 10 Graphic Rating Scale pain scores for each of the treatment conditions served as the primary variables.
Results
Patients reported significantly less pain when distracted with VR. “Worst pain” (pain intensity) dropped from 6.25 of 10 to 4.50 of 10. “Pain unpleasantness” ratings dropped from “moderate” (6.25 of 10) to “mild” (2.83 of 10). “Time spent thinking about pain” dropped from 76% during no VR to 22% during VR. Patients rated “no VR” as “no fun at all” (<1 of 10) and rated VR as “pretty fun” (7.5 of 10). Follow-up analyses showed VR was especially effective for the six patients who scored 7 of 10 or higher (severe to excruciating) on the “worst pain” (pain intensity) ratings.
Conclusions
These preliminary results provide the first evidence from a controlled study that adjunctive immersive VR reduced pain of patients with combat-related burn injuries during severe burn wound debridement. Pain reduction during VR was greatest in patients with the highest pain during no VR. These patients were the first to use a unique custom robot-like arm mounted VR goggle system.
Keywords: Combat, Analgesia, Burn pain, Wound care, Virtual reality
As the result of frequent use of explosive devices against US troops by enemy insurgents in Iraq and Afghanistan, thousands of US military personnel have suffered severe burn wounds and/or other trauma injuries. Malchow and Black1 cite personal reports that more than 80% of American casualties are transported from Baghdad to Germany with uncontrolled pain. Based on military medical records, Holbrook et al.2 found that 39% of posttraumatic stress disorder (PTSD)-positive and 24% of PTSD-negative injured military personnel received no morphine during resuscitation and early trauma care. Even with use of powerful pharmacologic analgesics, severe-to-excruciating pain often continues during hospitalization due to medical procedures.
US casualties with severe combat-related blast injuries, such as burned hands, amputations, and multiple traumatic injuries, must undergo frequent wound care/rehabilitation sessions as part of their recovery. For patients with severe burns, wound care/debridement typically involves cleaning the wound and scrubbing dead skin away as it sloughs off the wound during healing, to help avoid infection. Debridement typically occurs daily, for weeks or months. Burned skin naturally contracts as it heals. Physical therapy stretches help to counteract contraction, increasing skin elasticity, and enhancing range of motion.3 Although pharmacological agents can usually control pain while patients are resting with little or no movement, most burn patients report severe to excruciating pain during medical procedures such as wound cleaning and physical therapy.4
Although opioids are the cornerstone analgesic for patients with severe burn injuries and other trauma injuries,1,5 side effects of opioid narcotic analgesics limit dose levels and frequency of use.6 Opioid side effects frequently include nausea and constipation, and opioids may cause immunosup-pression.7 Patients often experience gradually reduced analgesic effects with repeated administration of opioids, a phenomenon known as tolerance. In other words, with frequent medications over days, weeks or months, escalating doses of opioid analgesics are needed to achieve the same analgesic effect. And over time, daily use of opioids is frequently accompanied by physical dependence, the need for continued drug use to prevent physical and emotional withdrawal symptoms.8 At high doses, opioid side effects pose a significant challenge to medical providers trying to management acute pain during daily severe burn wound medical procedures.1,6 In an effort to reduce opioid usage, ketamine may be given for its opioid sparing effects.9 Used at low doses, ketamine is a non-barbiturate intravenous anesthetic that is used as part of a multi-modal therapy. Ketamine does not cause respiratory depression, but ketamine is associated with psychoactive effects (e.g., dissociative and psychotic states).10
Pain is often self-reported as a number on a scale. The validity of Graphic Rating Scale (GRS) subjective measure of pain intensity has been demonstrated by their strong associations with other measures of pain intensity (e.g., changes in pain-related brain activity during functional magnetic resonance imaging [fMRI] brain scans, which measures changes in blood flow related to neuronal activity)11,12 and through their ability to detect treatment effects. The word GRS has also demonstrated convergent validity, and strong test-retest reliability.13 Previous studies indicate that Virtual Reality (VR) distraction can affect all of the components of pain measured in the present study, and that the GRS used in this study is sensitive to these effects.14
Solving the problem of excessive pain may prove more challenging in military populations than in civilian populations. Both physical and emotional suffering, including PTSD and depression, are particularly problematic in survivors of combat-related injuries caused by explosions. In one recent study,15 patients who had experienced combat-related blasts had more extensive physical injuries (i.e., they were more likely to have amputations) and used higher levels of opioid analgesics. Patients with combat-related blast injuries showed significantly less improvement in pain severity (10% reduction) as a function of treatment/hospitalization than either combat/non-blast (43% reduction) or non-combat (53% reduction) groups. And servicemen with blast-related injuries showed much higher rates of PTSD than those injured via other means.15
Alternatives to pharmacological agents are needed and one such method is immersive VR. Controlled studies with civilians show preliminary evidence that allowing patients to “go into” VR during painful procedures can help to reduce excessive pain nonpharmacologically. Compared with standard of care (i.e., pain medications with no VR) researchers consistently find 30% to 50% reductions in pain ratings when VR is used adjunctively with opioids during civilian severe burn wound care14,16 and physical therapy.17 In addition, analog laboratory studies using fMRI brain scans have shown large reductions in pain-related brain activity associated with VR analgesia.11 Immersive VR has the potential to decrease suffering for US casualties with combat-related burn injuries who must undergo frequent (e.g., daily) painful wound de-bridement and rehabilitative procedures. VR is typically used adjunctively, in addition to any pain medications the patient is already receiving. One case study has recently reported VR analgesia while treating soldiers with combat-related burn injuries,18 but to date no controlled studies on this important topic have been published.
Immersive VR is hypothesized to reduce pain via a non-pharmacologic attentional mechanism.11 Patients look into VR goggles which block patients' view of the hospital room so they cannot see the wound care. The goggles substitute the real world with synthetic computer-generated images from an illusory 3D virtual world of SnowWorld. Noise canceling earphones block sounds from the hospital room, and substitute more calming music and sound effects. The patient interacts with the virtual world, throwing snowballs at objects in the virtual world by clicking a mouse button, this makes it even more interactive and effective.19,20
SnowWorld is a 3D computer graphic system that uses the imagery of an icy canyon with a river flowing through it as a backdrop for snowmen, penguins, woolly mammoth, fish, and snowfall. The object of the system is to distract the patient by allowing the participant to focus on throwing snowballs at objects within the canyon while moving through the canyon. The snowmen freeze with one snowball hit and shatter with two hits. The white and blue colors are soothing and the snowy images are the opposite of the hot burn that resulted in their injuries.
Pain requires attention,21 and patients have a limited amount of attention available. VR draws upon these limited controlled attentional resources, leaving less attention available to process incoming pain signals. Consistent with the involvement of an attentional mechanism, burn ss report spending much less time thinking about their pain during wound care while in SnowWorld.14,16,18 In addition, laboratory pain studies have shown that on a divided attention task, where the primary task is to monitor a string of numbers, performance on the primary task dropped significantly when participants went into VR.22 And, there appears to be a dose-response relationship between the physical properties of the VR system (immersiveness) and the amount VR reduces pain.19,20
In contrast, opioids work by reducing transmission of neural nociceptive signals. Exposing receptors to opioids inhibits neuronal signaling, and reduces the number of noci-ceptive signals transmitted from the pain receptors to the brain.1 More recently, laboratory studies involving brief thermal pain stimuli in healthy volunteers undergoing VR during fMRI found the amount of pain reduction and associated reduction in pain related brain activity were comparable to analgesia from a moderate dose of hydromorphone.12 The largest decreases in pain and pain-related brain activity were observed when VR and opioids were combined.12 This approach capitalizes on the combined analgesic action of the two treatment modalities (pharmacologic vs. VR), each thought to reduce pain via different mechanisms.
The present study explored for the first time (1) whether adjunctive VR can reduce pain in military patients with combat-related blast severe burn injuries, (2) the use a robotlike arm mounted (helmet-less) VR system designed to reduce barriers to using VR with combat-related burn patients (e.g., face and head injuries, discomfort), and (3) whether soldiers reporting the highest pain levels during wound care still benefited from adjunctive VR. It measured whether VR could reduce pain in an unusually challenging patient population: military patients with severe pain intensity and whether patients reporting the highest pain levels benefited as much as patients experiencing more moderate procedural pain levels.
Materials and Methods
This study was approved by the Brook Army Medical Center Institutional Review Board. Patients met inclusion criteria if pain was documented as excessively painful during the previous days wound care session, were 18 years or older with thermal injuries and had the ability to operate a computer mouse or keyboard. The exclusionary criteria used were as follows: (1) history of susceptibility to motion sickness, (2) presence of open wounds to the hands that could not be covered with a dressing when operating the mouse/control button, and (3) patients who reported a feeling of anxiety or discomfort while viewing the SnowWorld software on a desktop computer without using VR goggles.
After patients met eligibility requirements, an informed consent from the patient was obtained by physicians or research nursing staff. Once informed consent was obtained, patients received VR first or second. Treatment order was randomized using a random number generator (e.g., www.random.org) such that each participant was approximately equally likely to get VR first and No VR second or No VR first and yes VR second. Individual medication regimens were determined by the treating physician and were independent of study protocol. Pre-medication (e.g., fast acting opioids and/or ketamine) ∼20 minutes before wound care served as the pharmacologic analgesic for this wound care session. Subjects received the same standard analgesic medications during VR and No VR (both conditions occurred within minutes of each other during one wound care session on the same day). Patients received approximately 12 minutes of wound care during this study (mean duration of the study per patient = 11.32 minutes), identified from previous days' procedures as being excessively painful. The 12-minute segment was divided into two equivalent wound care segments (∼6 minutes per segment, mean treatment segment duration = 5.66 minutes, range = 3–11 minutes). A patient interacting with the virtual world via the immersive VR goggles and mouse is shown in Figure 1, A and B. Because all patients received wound care with and without VR study participants and providers administering interventions and assessing outcomes were not blinded.
Figure 1.
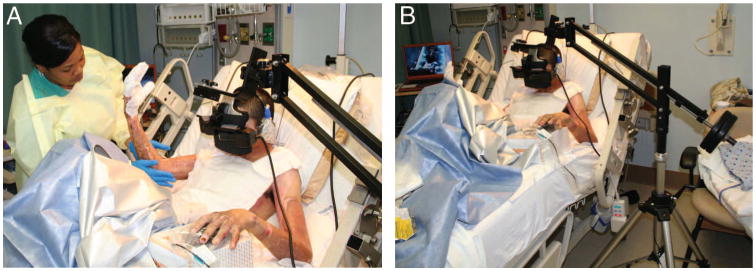
(A and B) US Army soldier receiving immersive VR to reduce his pain during severe burn wound care. The unique robot-like arm mounted VR goggle holder designed by Hoffman and Magula at the University of Washington, Seattle holds the VR goggles near the patient's eyes weightlessly, reducing the amount of surface contact (if any) needed with the patient. Photos and copyrights Hunter Hoffman, U.W. see also http://www.youtube.com/watch?v=jNIqyyypojg.
The VR system consisted of a Voodoo Envy laptop with NVIDIA GForce Go 7900 GTX (512 MB) video card; Intel Core 2 Duo (T7400) CPU at 2.16 GHz, 2 GB RAM at 994 MHz (HP, Palo Alto, CA). Although in immersive VR, each subject followed a pre-determined path, “gliding” through an icy 3D virtual canyon (Fig. 2). Each patient “looked” around the virtual environment of an icy canyon with an icy river and aimed snowballs at snowmen, penguins, wooly mammoths, and jumping fish via a mouse. Patients pushed a mouse trigger button to throw virtual snowballs at virtual snowmen, igloos, and penguins. Each patient saw the sky when they looked up, saw a canyon wall when they looked to the left or right, a flowing river when they looked down, and heard sound effects (e.g., a splash when a snowball hit the river) mixed with background music by recording artist Paul Simon (www.paulsimon.com). Participants looked into a pair of Rockwell Collins SR-80A VR goggles (Rockwell Collins, Cedar Rapids, IA) with a custom made neoprene blinder on top and sides, which blocked the patient's view of the real world. The SR-80A VR goggles afforded ∼80° diagonal field of view for each of the rectangular eyepieces with 100% overlap between the right and left eye images. The goggles were held in place near the patient's eyes by a custom made robot-like arm goggle holding system (Fig. 1, A and B).18
Figure 2.

A screenshot of what patients see in the VR goggles during immersive VR pain distraction. This 2006 version of SnowWorld was designed by Hoffman, developed by Hoffman and Patterson, University of Washington, Seattle, WA, and created for the UW/Harborview Burn Center by worldbuilders at Firsthand. Image by Firsthand Inc., Seattle, WA, copyright Hunter Hoffman, UW, www.vrpain.com.
Although for ease of exposition “6 minutes” is used throughout the manuscript, the exact amount of treatment time for each individual varied depending on their injuries, and was accurately substituted into the questions by the researchers. The following questions and rating scales were used to assess the patient's response to VR analgesia:
How much “time” did you spend thinking about your pain during the past 6 minutes? I thought about my pain during VR 0 = none of the time, 1 to 4 = some of the time, 5 = half of the time, 6 to 9 = most of the time, and 10 = all of the time.
Rate your “worst pain” during the past 6 minutes during the VR (a similar 10-cm line with numeric and word descriptors beneath it: 0 = no pain at all, 1 to 4 = mild pain, 5 to 6 = moderate pain, 7 to 9 = severe pain, and 10 = worst pain).
How “unpleasant” was your pain during the past 6 minutes during the VR? 0 = not unpleasant at all, 1 to 4 = mildly unpleasant, 5 to 6 = moderately unpleasant, 7 to 9 = severely unpleasant, and 10 = excruciatingly unpleasant (see Hoffman et al,14 for a graphic example).
How much “fun” did you have during VR? (10-cm line with numeric and verbal descriptors: 0 = no fun at all, 1 to 4 = mildly fun, 5 to 6 = moderately fun, 7 to 9 = pretty fun, and 10 = extremely fun).
To what extent (if at all) did you feel “nausea” for any reason during VR? (10-cm line with numeric and verbal descriptors: 0 = no nausea at all, 1 to 4 = mild nausea, 5 to 6 = moderate nausea, 7 to 9 = severe nausea, and 10 = vomit).
While experiencing the virtual world, to what extent did you feel like you “went inside” the computer-generated world? (10-cm line with numeric and verbal descriptors: 0 = I did not feel like I went inside at all, 1 to 4 = mild sense of going inside, 5 to 6 = moderate sense of going inside, 7 to 9 = strong sense of going inside, and 10 = I went completely inside the virtual world).
During two brief pauses in the wound care procedure (once after each 6 minute wound care period), each patient completed three subjective pain ratings using GRS labeled 0 to 10 with respect to the preceding 6 minutes of wound care. “Please indicate how you felt during the past 6-minute session by rating your response on the following scales.” Each question was accompanied by a pictorial example of the labeled GRS.
After the wound care session with no VR, each patient was asked the same questions but “during VR” was replaced by “without VR.” After wound care with no VR, patients were not asked the question about presence.
Statistical Analysis
A paired t test was used to test for significance after it was determined that the data were normally distributed. All tests for significance were two-tailed, with level of α = 0.05.
Results
Twelve male patients aged 20 years to 27 years (mean age, 22 years) with a mean TBSA burn of 20.68% (range, 4–57.5%) were transferred from their point of injury in Iraq or Afghanistan to Brooke Army Medical Center at Fort Sam, Houston, TX, and admitted to the United States Army Institute of Surgical Research Burn Center for initial inpatient acute burn care. All were injured in separate incidents/attacks involving explosive devices (e.g., roadside bombs) and all participated in this study while inpatients on the 4th floor burn unit (i.e., not in the ICU and not yet discharged from the hospital).
The specific measures used in the current study were designed to assess the cognitive component of pain (amount of time spent thinking about pain), the affective component of pain (unpleasantness), and the sensory component of pain (worst pain). Gracely et al.23 have shown ratio scale measures, including the labeled GRS used in this study, to be highly reliable. Mean pain ratings were lower during VR than in the control condition (no distraction) for all three pain measures, and the differences were all statistically significant (Fig. 3). Patients (n = 12) reported less pain when distracted with VR. A rating of time spent thinking about pain was included as a measure of a cognitive component of pain, a domain that is understudied in pain outcome studies. “Time spent thinking about pain” dropped from “most of the time” 7.58 (76% of the time) to “some of the time” 2.17 (22%), “pain unpleasantness” ratings dropped from “moderate” (6.25) to “mild” (2.83), and worst pain dropped from “moderate” (6.25) to mild pain (4.50).
Figure 3.
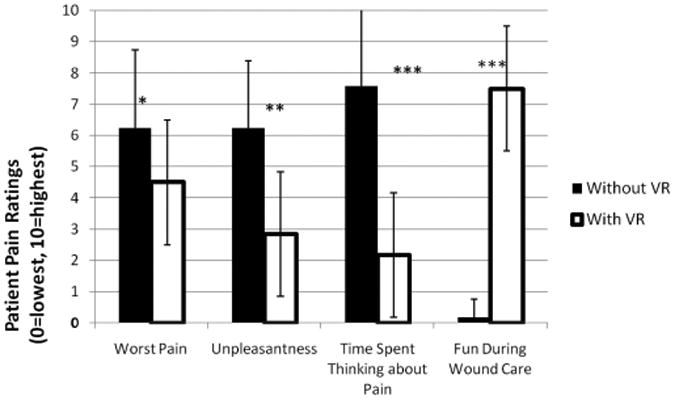
Compared with pharmacologies + No VR (shown in solid), patients (n = 12) reported large reductions pain during pharmacologies + immersive VR (shown as white bars) during severe burn wound care of burn injury. Standard deviations are show in the error bars. (*p < 0.05), (**p < 0.01), (***p < 0.001).
Although the rationale for assessing amount of fun experienced during wound debridement may not be readily apparent, preliminary data suggest that VR distraction can be associated with increased levels of fun even during painful laboratory stimuli during civilian burn physical therapy and wound debridement.14 Exploratory GRS ratings of “fun” during VR versus fun during no VR were measured. No VR was “no fun at all” (less than 1) but VR was “pretty fun” (7.50). GSR ratings of immersiveness of the experience were also collected. Patients reported a moderate sense of “going inside the computer-generated world” during VR (presence in VR = 5.33) and rated nausea during VR as zero.
To determine whether VR was more effective for mild and moderate pain patients or for patients who experienced severe pain during procedures, the patients were grouped into two groups based on the severity of their pain during the No VR portion of the wound care session. The six combat-related (explosion) burn patients with the highest pain intensity (i.e., “worst pain”) ratings during No VR reported a significant 66% reduction in pain unpleasantness during VR, a 70% reduction in time spent thinking about pain during VR and a 100% increase in fun during VR (Table 1, p < 0.001). They also reported a 32% drop in worst pain during VR, which is clinically meaningful in magnitude and statistically significant with n = 6 patients (Table 1, p < 0.05). However, the six patients with only mild to moderate pain during No VR did not show a difference in either the worst pain or the unpleasantness of the treatment. However, these patients reported a significant reduction in time spent thinking about pain during VR (Table 1, p < 0.05), and a significant increase in amount of fun during VR (Table 1, p < 0.001).
Table 1. Patients With Worst Pain ≥7 and <7 (n = 6).
| Worst Pain ≥7 | Worst Pain <7 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control Condition | VR Condition | p | Control Condition | VR Condition | p | |
| Worst pain | 8.33 (1.03) | 5.67 (2.50) | 0.043 | 4.17 (1.47) | 3.33 (2.42) | NS |
| Unpleasant | 7.83 (1.33) | 2.67 (3.01) | 0.004 | 4.67 (1.51) | 3.00 (2.61) | NS |
| Time thinking | 8.83 (1.94) | 2.67 (2.80)) | 0.007 | 6.33 (3.38) | 1.67 (1.03) | 0.03 |
| Fun | 0 (0.0) | 8.00 (2.45) | <0.001 | 0.33 (.82) | 7.00 (1.90) | <0.001 |
Values are expressed as mean (SD). For all statistical comparisons reported in this study, the paired t test was used with the alpha = 0.05, two-tailed.
Discussion
The present study is the first controlled study to explore whether adjunctive immersive VR can reduce excessive pain during wound debridement of soldiers with combat-related burn injuries. All previous controlled studies on VR analgesia have involved civilians, and this is also the first controlled study to use a robot-like VR goggle holder. Results from subjective pain ratings collected in the present study provide preliminary evidence that adjunctive immersive VR can reduce the cognitive, the emotional, and the sensory components of pain of soldiers with combat-related burn injuries during wound debridement.
McCaul and Malott24 proposed that “stimulus intensity is an important determinant of whether and when a distraction will occur. In other words, as a painful stimulus reaches some intense level, it will begin to attract attention and impede the effectiveness of the distraction.” Recent researchers have further argued that distraction will probably fail if the pain is perceived as very threatening, for instance in high pain catastrophizers who have shown difficulty disengaging attention from pain information.25 Thus, in theory, both high pain intensity, and/or powerful affective characteristics of the pain could limit the efficacy of pain distraction techniques.
Contrary to such predictions that distraction would be less effective when attempting to treat more intense pain, results of the present study showed that VR analgesia was unusually effective in the six soldiers reporting severe to excruciating pain with pharmacologies alone, during no VR. There is thus growing evidence14 that VR analgesia is especially effective for patients who need it the most: patients experiencing severe to excruciating pain intensity during burn wound debridement.
Supplementary to reducing pain, patients reported that VR was “pretty fun” and rated their illusion of going inside the computer-generated world as moderate. In addition to being the first controlled study of VR analgesia on a military patient population, the present study is also the first controlled study where patients used robot-like arm mounted goggles, an immersive VR experience that does not require patients to wear a helmet. The goggle holder increases patient comfort, increases the number of patients who can use VR (e.g., patients with bandaged face and head wounds as well as those who otherwise found the helmet too uncomfortable) and reduces or eliminates contact between the patient and the VR equipment.
Individual burn patients typically show large day to day variations in how much pain they experience, due to a wide number of nuisance variables not under the researchers control (e.g., drug dose, amount of sleep the night before, gentleness of the nurse on that day). For this reason, a within-subject design was used in the current study, so this nuisance “noise” variance was the same in both treatment conditions, and thus cancelled out, allowing a much more statistically powerful research design than a between groups design. However, the advantages come at a cost.
The present study has limitations. Although care was taken to standardize the treatment protocol, the nurses performing the wound care were aware of the treatment condition and could (in theory) have inadvertently treated patients more gently in VR. A double-blind (between-groups) replication of the present study, although challenging to perform, would be ideal. Encouragingly, previous reports of VR analgesia in experimental pain settings have shown similar magnitude reductions in pain during VR, using single and double blind between-groups designs.22 Another limitation of the present study is the short treatment duration (∼6 minutes in VR), and small number of treatments (one VR treatment per patient). Larger studies (involving dozens of soldiers) with longer treatment durations (e.g., 20 minutes per patient) on multiple days (e.g., 10 or 20 days per patient) are needed to determine whether VR is viable in clinical practice as a non-pharmacologic adjunct, and to explore whether there are any health benefits from better control of acute procedural pain, above and beyond the short-term reduction of pain. Future research is needed to explore whether better control of procedural pain via adjunctive VR improves physiologic and/or psychologic outcome. Additionally, this study did not consider whether VR reduced the amount of pharmacological agents needed to control pain during the procedure. Future studies are needed to evaluate whether there are drug sparing aspects of VR. Whether VR reduces pain in high pain catastrophizers was not addressed in the present study and is an interesting topic for future research.
The current results suggest adjunctive VR analgesia might prove valuable for military patient populations. Because excessive acute pain during medical procedures for civilian and combat-related injuries remains a widespread medical problem (not limited to burn patients), further research on this topic is justified.
Acknowledgments
The authors thank Judy Martin, RN, and Nancy C. Molter, RN, MN, PhD (USAISR), for their assistance implementing this technology in the Burn Center, and Admiral Mullen, General Chiarelli, Col. Sutherland, Col. Friedl (TATRC), Jean-Louis Belard (TATRC), Luke Knit-tig, Combat Casualty Care, Ross and Gloria Chambers (Seattle), singer/songwriter Paul Simon (New York) for their encouragement. SnowWorld software is made available free to eligible medical centers/researchers via the corresponding author.
Supported by U.S. Army Institute of Surgical Research. Hoffman's time was funded with help from the Gustavus and Louise Pfieffer Research Foundation, the Scan Design Foundation by Inger and Jens Bruun, and the following NIH grants to Drs. Patterson and Sharar at the UW: NIH HD40954-01, 1R01AR054115-01A1, R01GM042725-17A1.
Footnotes
The opinions and assertions contained in this article are solely the authors' private ones and are not to be construed as official or reflecting the views of the United States Army or the Department of Defense.
References
- 1.Malchow RJ, Black IH. The evolution of pain management in the critically ill trauma patient: emerging concepts from the global war on terrorism. Crit Care Med. 2008;36(7 suppl):S346–S357. doi: 10.1097/CCM.0b013e31817e2fc9. [DOI] [PubMed] [Google Scholar]
- 2.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 3.Ward RS. Physical rehabilitation. In: Carrougher GJ, editor. Burn Care and Therapy. St. Louis, MO: Mosby; pp. 1998pp. 293–320. [Google Scholar]
- 4.Melzack R. The tragedy of needless pain. Sci Am. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 5.Patterson DR. Non-opioid-based approaches to burn pain. J Burn Care Rehabil. 1995;16:372–376. doi: 10.1097/00004630-199505001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cherny N, Ripamonti C, Pereira J, et al. Expert Working Group of the European Association of Palliative Care Network. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–2554. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 7.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 8.Berger AC, Whistler JL. How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol. 2010;67:559–569. doi: 10.1002/ana.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazan S, Buda I, Nesher N, Paz J, Weinbroum AA. Low-dose ketamine via intravenous patient-controlled analgesia device after various trans-thoracic procedures improves analgesia and patient and family satisfaction. Pain Manag Nurs. 2010;11:169–176. doi: 10.1016/j.pmn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Hirota K, Lambert DG. Ketamine: its mechanism(s) of action and unusual clinical uses. Br J Anaesth. 1996;77:441–444. doi: 10.1093/bja/77.4.441. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HG, Richards TL, Coda B, et al. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15:1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman HG, Richards TL, Van Oostrom T, et al. The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth Analg. 2007;105:1776–1783. doi: 10.1213/01.ane.0000270205.45146.db. [DOI] [PubMed] [Google Scholar]
- 13.Tesler MD, Savedra MC, Holzemer, Wilkie DJ, Ward JA, Paul SM. The word-graphic rating scale as a measure of children's and adolescents' pain intensity. Res Nurs Health. 1991;14:361–371. doi: 10.1002/nur.4770140507. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24:299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 15.Clark ME, Walker RL, Gironda RJ, Scholten JD. Comparison of pain and emotional symptoms in soldiers with polytrauma: unique aspects of blast exposure. Pain Med. 2009;10:447–455. doi: 10.1111/j.1526-4637.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 16.van Twillert B, Bremer M, Faber AW. Computer-generated virtual reality to control pain and anxiety in pediatric and adult burn patients during wound dressing changes. J Burn Care Res. 2007;28:694–702. doi: 10.1097/BCR.0B013E318148C96F. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain. 2000;16:244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Maani C, Hoffman HG, DeSocio PA, et al. Pain control during wound care for combat-related burn injuries using custom articulated arm mounted virtual reality goggles. J Cyber Ther Rehabil. 2008;1:193–198. [Google Scholar]
- 19.Wender R, Hoffman HG, Hunner HH, Seibel EJ, Patterson DR, Sharar SR. Interactivity influences the magnitude of virtual reality analgesia. J Cyber Ther Rehabil. 2009;2:27–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlquist LM, McKenna KD, Jones KK, Dillinger L, Weiss KE, Ackerman CS. Active and passive distraction using a head-mounted display helmet: effects on cold pressor pain in children. Health Psychol. 2007;26:794–780. doi: 10.1037/0278-6133.26.6.794. [DOI] [PubMed] [Google Scholar]
- 21.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HG, Garcia-Palacios A, Kapa VA, et al. Immersive virtual reality for reducing experimental ischemic pain. Int J Hum Comput Interact. 2003;15:469–486. [Google Scholar]
- 23.Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 24.McCaul KD, Malott JM. Distraction and coping with pain. Psychol Bull. 1984;95:516–533. [PubMed] [Google Scholar]
- 25.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998;75:187–198. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]


