Abstract
Cancer biologists and other healthcare researchers face an increasing challenge in addressing the molecular complexity of disease. Biomarker measurement tools and techniques now contribute to both basic science and translational research. In particular, liquid chromatography-multiple reaction monitoring mass spectrometry (LC-MRM) for multiplexed measurements of protein biomarkers has emerged as a versatile tool for systems biology. Assays can be developed for specific peptides that report on protein expression, mutation, or post-translational modification; discovery proteomics data rapidly translated into multiplexed quantitative approaches. Complementary advances in affinity purification enrich classes of enzymes or peptides representing post-translationally modified or chemically labeled substrates. Here, we illustrate the process for the relative quantification of hundreds of peptides in a single LC-MRM experiment. Desthiobiotinylated peptides produced by activity-based protein profiling (ABPP) using ATP probes and tyrosine-phosphorylated peptides are used as examples. These targeted quantification panels can be applied to further understand the biology of human disease.
Keywords: Activity-Based Protein Profiling, Phosphotyrosine Profiling, Kinase Signaling, Spectral Library, Liquid Chromatography-Multiple Reaction Monitoring Mass Spectrometry (LC-MRM), Cancer
Introduction
Discovery proteomics has myriad uses in biology; one of the most valuable contributions is made through the ability to identify and localize post-translational modifications. However, systems biology now requires quantitative data to elucidate signaling mechanisms and functionally investigate complex biological processes.[1] In cancer, many signaling pathways are controlled by post-translational modifications, like phosphorylation, which mediate protein-protein interactions, enzyme activity levels, and subcellular localization. These molecular cues change cellular phenotype in response to internal and external stimuli. Dynamic kinase activity and changes in protein phosphorylation sites and their levels must be quantified in order to characterize signaling networks and their downstream biological effects. Novel tools are still required to effectively study these changes in model systems and in patients.
One current paradigm for developing the required assays involves translation of discovery proteomics data into quantitative measurements using liquid chromatography-multiple reaction monitoring mass spectrometry (LC-MRM).[2–7] LC-MRM can quantify molecularly diverse biomarkers in complex biological and clinical samples with high degree of portability between labs.[8,9] Protein quantification using tryptic peptides as surrogates has been used to evaluate the expression, mutation, and phosphorylation of individual proteins.[10–17] The capability for multiplexing has also been demonstrated, making LC-MRM an ideal tool for quantitative systems biology.[18,19] Indeed, the technique has already been applied to monitoring the outcomes for individual signaling pathways and multiple components of complex biological processes.[20–24] Large panels of post-translationally modified peptides can also be developed from liquid chromatography tandem mass spectrometry (LC-MS/MS) data, using these previously reported biomarker development strategies. The critical requirement is a viable affinity enrichment technique for the modified peptides.
Two examples illustrate this point: chemically labeled peptides and post-translationally modified. Activity-based protein profiling (ABPP) uses chemical probes as biotinylating reagents to label and enrich classes of target proteins; the use of ATP mimics has provided the ability to enrich kinases for mass spectrometry analysis via desthiobiotinylation of lysines near the ATP-binding pocket and active site (and potentially lysines on substrates as well).[25–28] Because a broad spectrum ATP-utilizing enzymes can be captured, LC-MRM is highly useful for selectively detecting the kinases from cells and tissues, where the background of other proteins can be overwhelming compared to the lower abundance kinases. These experiments provide insight into the levels of kinase ATP uptake and may be used to infer either the expression or the activity of the kinases in a biological sample (depending on whether the ATP binding pocket is always accessible or tightly regulated). Furthermore, kinase inhibitors can block probe binding; consequently, the reduction in labeled peptide ion signal can be used to determine potential targets and effected downstream kinases. ABPP-LC-MRM approaches with isotope-coded probes and extensive development of stable isotope-labeled standard (SIS) peptides have recently been reported.[29,30] As an example of an endogenous post-translational modification, phosphotyrosine-containing (pY) peptides can be enriched by immunoprecipitation, providing the ability to examine signaling networks and the consequences of kinase inhibitor treatment.[21,31–32] The complement of these two techniques provides additional insight and higher confidence in altered signaling pathways by combining kinase activity and substrate phosphorylation into pathway maps generated from the differentially modified peptides and their proteins of origin.
Using these molecularly-specific enrichment techniques and recently developed strategies for LC-MRM assay development, ABPP-LC-MRM and pY-LC-MRM panels containing hundreds of peptides have been prepared. Each of the two experiments requires only a single LC-MRM analysis. Due to the cost of synthesizing and characterizing standards, this method relies on a small pool of commercially available unmodified standard peptides, similar to the labeled reference peptide strategy.[33] Because of the large number of peptide targets and the limited sampling ability of the triple quadrupole mass spectrometer, scheduled methods are required. Data from multiple discovery proteomics representing different tumor types are integrated into a single LC-MRM for each type of post-translationally modified peptide and scheduled using in silico retention time calculations (iRT).[34]
In the methods described here, ABPP-LC-MRM and pY-LC-MRM experiments are developed and used to interrogate the kinome of lung cancer cell lines and tissues. The steps in the workflow for peptide selection and LC-MRM analysis are described and applied to the cell lines and frozen tumor and normal specimens from patients. Ultimately, these LC-MRM platforms will be applied for multiplexed analyses of multiple signaling pathways in fresh or frozen patient tissues to elucidate dominant cancer signaling pathways with the goal of directing personalized therapy strategies. Finally, an example of the promise of parallel reaction monitoring mass spectrometry, PRM, [35,36] in minimal amounts of total cellular protein is shown to illustrate the ability to translate selected targeted measurements into biopsy specimens.
1. In Silico Data Processing and LC-MRM Experiment Development
This part of the method requires three steps: collection of tandem mass spectra into spectral libraries (1.1), selection of peptides and transitions from the existing data (1.2), and mapping discovery data to reversed phase liquid chromatography retention times to enable scheduling for the LC-MRM data acquisition (1.3).
1.1. Spectral Library Construction
LC-MRM is performed on triple quadrupole mass spectrometers and relies on the instrument’s ability to select the intact m/z ratio in the first quadrupole, fragment the intact peptide using collisions with background gas in the second stage of the instrument, and then sequentially mass select a series of structural fragments (typically y ions for tryptic peptides) using the third quadrupole prior to ion detection. Combined with reversed phase liquid chromatography, this experiment provides three degrees of separation to isolate the signal of interest. The critical step in assay development is the selection of appropriate peptides and transitions (pairs of intact m/z and fragment m/z with optimized collision energy values), which uniquely report on the expression, mutation status, chemical labeling, or modification of the protein. General guidance and in silico prediction rules include restrictions on peptide length (7–25 a.a.) as well as avoidance of amino acids that can be artifactually modified (e.g. Cys or Met) and certain motifs (e.g. consensus glycosylation sequences or peptides with adjacent/neighboring tryptic cleavage sites). However, empirical data from discovery proteomics are often the best resource to pick peptides and fragment ions. In cases with synthetic stable isotope-labeled standard (SIS) peptides, the data generation and transition selection can be performed on the QqQ-MS used for LC-MRM by infusion of the peptide standard. However, in cases with hundreds of peptides as described here, costs are prohibitive and standards are not developed for each target molecule. Discovery proteomics data from liquid chromatography-tandem mass spectrometry peptide sequencing experiments can also be relied on, because studies have shown high correlations between fragmentation patterns observed in LC-MS/MS and LC-MRM analyses.[4,37] To rapidly translate discovery proteomics into targeted measurements, a spectral library can be either downloaded or constructed. Spectral libraries are available for unmodified peptides from PeptideAtlas (http://www.peptideatlas.org/speclib/), the National Institute of Standards and Technology (NIST, http://chemdata.nist.gov/dokuwiki/doku.php?id=peptidew:start) and the Global Proteome Machine (GPM, ftp://ftp.thegpm.org/projects/xhunter/libs/) inter al. However for these studies of chemically labeled and post-translationally modified peptides, custom libraries for ABPP and pY peptides (which are available in the Supplemental Materials) were built in-house using previously acquired discovery data. All raw data files from discovery LC-MS/MS on LTQ-Orbitrap or Q-Exactive Plus mass spectrometers were searched against human entries in the UniProt database (downloaded 05/01/2013) using Sequest HT inside Proteome Discoverer 1.4 (Thermo) with Percolator analysis. In our case, the use of vendor software is straightforward, because conversion of data and search result file formats is not necessary. For Thermo instrument platforms, Proteome Discoverer (Thermo) is also capable of building spectral libraries using the Crystal library module. An alternative approach is to use the open source, freely-available Trans-Proteomic Pipeline (TPP, http://tools.proteomecenter.org/wiki/index.php?title=Software:TPP)[38]. In TPP, the raw data files are converted into mzXML format after database searching, the search results are then converted to pepXML format. Peptide identifications can then be validated using PeptideProphet, followed by library construction inside TPP. Each of these library construction methods are compatible with viewing, transition selection, and spectral library generation in Skyline, which is vendor neutral.[39] With the peptide identification probability cut off value set to 0.8, the final ABPP spectral library contains 13,012 ABPP peptides with a subset from kinases, and the pY spectral library contains 1,347 pY peptides. The key to content development in spectral libraries is not the raw number of samples analyzed, but rather in the judicious selection of different cancer types and samples (e.g. cell lines and tissues). While variation is observed among samples from the same type of cancer, greater contribution to the spectral libraries is observed by examining a new type of cancer. Data are included here from multiple myeloma, melanoma, and sarcoma as well as cancers of the lung and thyroid.
1.2. Selection of Target Peptides and Transitions
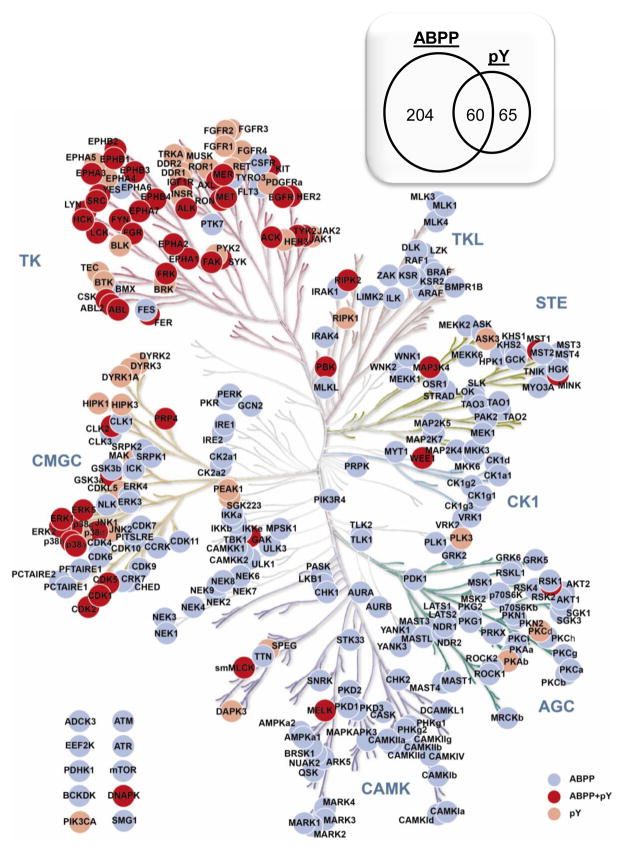
For ABPP, 412 peptides from 264 protein kinases were selected, which represents about 50% of the kinome (Figure 1). For pY, 377 peptides were selected from 126 proteins, including protein kinases, phosphatases, transcription factors and adaptor proteins, inter al. For peptides with multiple charge states observed, intensities from discovery LC-MS/MS are examined and the charge states with higher intensities are chosen based on the assumption that charge state ratios are consistent from sample to sample. Preliminary LC-MRM experiments are used to be certain that a single charge state is representative of total ion signal, and lower intensity charge states (< 20% of total signal) are eliminated from the list of targets. When intensities are similar between different charge states, doubly protonated peptides are preferred. However, the charge state that produces the largest number of high intensity, sequence-specific (or high m/z) fragment ions can also be selected. For each peptide, four fragment ions are monitored, and singly charged y ions are preferable. Generally, fragment ions observed at m/z values higher than parent peptide are chosen for uniqueness (with the exception of the y(n-1)) unless there are not enough predominant fragments observed in that region. In those cases, fragment ions smaller than the parent m/z are also monitored for sensitivity (e.g. b3 ions from peptides starting DIK or DLK, where that lysine is desthiobiotinylated). Overall, 1,628 transitions are monitored in the ABPP-LC-MRM experiment and 1,488 transitions are monitored in pY-LC-MRM (both transition lists are provided in the Supplemental Materials).
Figure 1. ABPP-LC-MRM and pY-LC-MRM Kinome Coverage.
Kinases are color coded for whether LC-MRM is able to detect ABPP peptides (gray), pY peptides (light brown), or both (dark brown). The inset (upper right) shows the total numbers of proteins included unique each assay and the overlap between ABPP and pY datasets. In total, 264 protein kinases are monitored with 412 ABPP peptides using 1,628 total transitions. For phosphotyrosine quantification, 126 proteins (including 60 kinases) are monitored with 377 pY peptides using 1,488 total transitions.
1.3. LC-MRM Scheduling using Retention Time Alignment
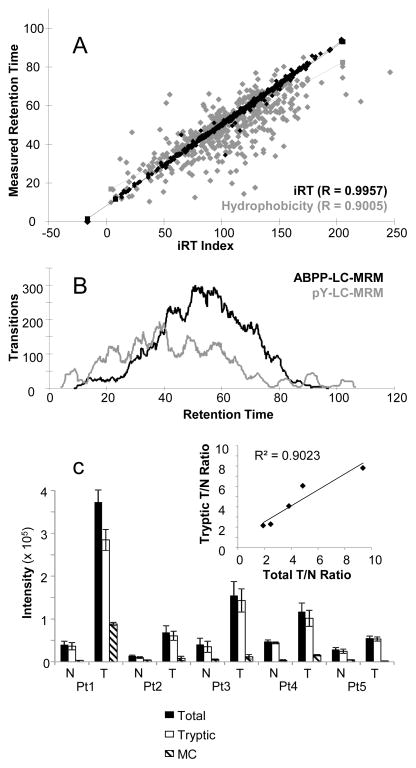
While the instrument software can be programmed with thousands of transitions, triple quadrupole mass spectrometers sequentially scan each one, so the number of measurements per unit time must be limited. Using 10 milliseconds per transition and the goal of having a minimum of 7–10 measurements per fragment ion over a 20–30 second window of peak elution, the number of transitions should not exceed ~300 at a given time in the experiment (allowing overhead time to switch between transitions). In order to monitor thousands of transitions as required for these LC-MRM panels, extended gradients must be used. In addition, scheduled MRM is applied, meaning that the instrument is programmed to monitor each peptide during a specific retention time window based on its elution. For example, a peptide that elutes at 30 minutes would be monitored only from 27 to 33 minutes, if six minute windows were used. Thus, retention time information for each target peptide is essential. However, numerous challenges can be met and overcome when samples were analyzed on different instruments using different columns under varied experimental conditions, etc. In addition, datasets may contain different standard peptides or lacked internal standards, making it challenging and time consuming to align retention times across all of the acquired data. The in silico retention time (iRT) platform [18] creates an index of relative retention time for each peptide in the multiplexed LC-MRM analysis; a tutorial is available online at https://brendanx-uw1.gs.washington.edu/labkey/wiki/home/software/Skyline/page.view?name=tutorial_irt. First, we start by analyzing a set of commercial standards (e.g. the Peptide Retention Time Calibration Mixture or PRTC, Pierce # 88321, Thermo) as backbone template to compare disparate analyses and make the assay portable between instruments. PRTC peptides were analyzed using the desired gradient (see Supplemental Material for experimental details and examples of scheduling), and the index of first eluted peptide is arbitrarily set to 0 and the index of last eluted peptide is set to 100. Then, ABPP or pY test samples with spiked PRTC (5 fmol per injection) are analyzed using the same gradient, and the iRT indices for detectable ABPP or pY peptides are assigned relative to the scale created by the PRTC peptides (1,628 transitions for ABPP are split into 7 unscheduled assays; 1,488 transitions for pY are split into 5 unscheduled assays for the initial scheduling experiments). When enough peptides with iRT indexes are observed from previous datasets, the whole spectral library can be loaded into iRT even though no PRTC peptides were present in the previous samples. The existing empirical data are enough to align the retention times with iRT indices using the other ABPP-labeled or tyrosine-phosphorylated peptides. In the end, a single set of iRT indices is created for each dataset, and a single LC-MRM analysis is scheduled for each panel of peptides. As shown in Figure 2A, the iRT retention time prediction (black) is more precise than hydrophobicity based prediction (gray), which will translate to more accurate retention time prediction and tighter scheduling windows. This advantage shortens development time and reduces the number of preliminary analyses that are required for testing the scheduled method, which in turn reduces the amount of biological sample needed. With 2 hour total analysis time and 1 hour for 2 blanks between samples, throughput for each assay is approximately 8 samples per day.
Figure 2. Selection of Peptides and Transitions.
LC-MS/MS peptide libraries are translated into LC-MRM experiments. First, a map of relative retention time in reversed phase liquid chromatography is created using iRT, which provides more specific values than hydrophobicity calculations (A). Then, the total number of transitions per unit time is plotted to examine the limits of scheduling (B). Finally, the contribution of additional peptide forms (e.g. oxM or missed cleavages) is evaluated to enable elimination of peptides and transitions that contribute little to quantification (C). The inset shows the correlation of the measurement of the fully tryptic sequence plotted against the total ion signal for both peptides.
The selection of the scheduling window must balance the improvement of quantification from higher numbers of measurements per transition with the potential for retention time drifts over the course of the entire instrument. Because of the potential size of the sample sets for clinical projects (n = 60 to 100 typically in duplicate as a minimum), the time window is set to a larger value than would be preferable for shorter term projects. Scheduling for ABPP-LC-MRM and pY LC-MRM both used 5 minute windows (Figure 2B) with the column compartment heated to 40 °C to minimize retention time drift. Part of the goal of using wider acquisition windows is to accommodate even rare events with high impact, like column replacement. However, scheduling is checked in every batch of clinical specimens (n = 10) using cell line standards at the beginning and end of the analysis sequence.
2. Multiplexed LC-MRM Analysis of Chemically Labeled and Post-Translationally Modified Peptides
LC-MRM platforms must be extensively tested prior to analysis of patient samples. For that purpose, cell line model systems for the tumor type of interest are analyzed first to see if the discovery data can be both recapitulated and refined (2.1). Then, modulation with drugs that have known effect can be performed to further test the system (2.1). Finally, experiments are designed and implemented with clinical samples (2.2).
2.1. ABPP-LC-MRM of Cancer Cell Lines and Modulation of ATP Uptake with Inhibitors
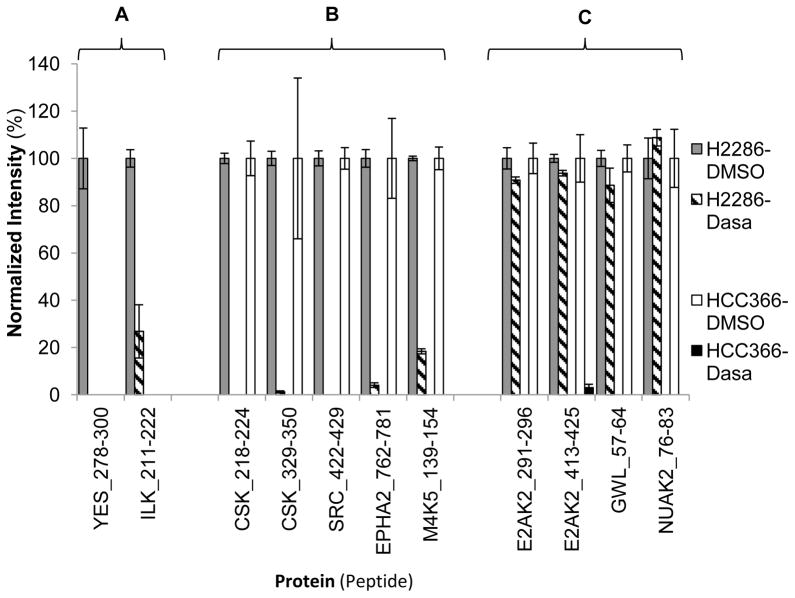
Samples are prepared according to the manufacturer’s instructions with minor modifications; experimental details are available in the Supplemental Material. Using ABPP discovery data, kinase inhibitors are selected for each cell line based on high ATP uptake by their expected target (e.g. Dasatinib in HCC366 and H2286 cells). In order to examine how promiscuous kinase inhibitors displace the ATP probes across the detectable kinome, lysates from each cell line are incubated either with the matched inhibitor at 10 μM concentration or with DMSO as a control for 10 minutes before ATP probe labeling. Then, ABPP-LC-MRM is applied to measure the changes in kinase ATP uptake pre- and post-treatment. Observation of the drugs’ impact on ATP uptake across the kinome can ultimately enable more complex strategies to select appropriate therapies for patients. As Figure 3 shows, the changes in ATP uptake after inhibitor treatment are context-dependent; each cell line shows different responses to inhibitor treatment. Dasatinib, a highly promiscuous SRC family kinase inhibitor, can completely block the ATP uptake by some kinases in HCC366 cell lysates. However, some of the ABPP peptides for those same kinases were unchanged during treatment in H2286 cell lysates. These treatment scenarios provide additional proof of function for the LC-MRM panels, which increases confidence in their performance for precious clinical samples.
Figure 3. Drug Interference Experiments with ABPP-LC-MRM.
Lysates from HCC366 and H2286 are treated with DMSO or Dasatinib (Dasa) prior to ABPP labeling; peptide ion signals are normalized to the DMSO control average values. Three groups are observed, including labeled peptides unique to one cell line (A), observed and inhibited in both cell lines (B), and observed and inhibited only in 1 cell line, but not in the other (C).
2.2. Experimental Designs for ABPP-LC-MRM of Clinical Samples
ABPP-LC-MRM is then applied to profile protein kinases in frozen lung tumors and adjacent control tissues using ~1 mg of total protein, as described above in section 2.1, to examine the differences in kinase ATP uptake and indicate potential oncogenic signaling mechanisms that can be targeted to develop therapeutic strategies. The PRTC standard mixture (5 fmol per injection) is spiked into each sample to monitor instrument performance and sample-to-sample variability. Because these samples were obtained as tumor and matched normal tissue from the same lobe of the lung, a randomized, blocked study design is used. For each batch of 10 clinical samples (5 patients’ tumor/normal pairs), 2 lung cancer cell lines of matched histologic type were also processed to monitor the ABPP labeling variability and overall experimental outcomes. For squamous cell carcinoma, HCC366 and H2286 cells were chosen, because they provided the most comprehensive coverage of detectable protein kinases of any 2 of the 8 SCC cell lines profiled by ABPP-LC-MRM.
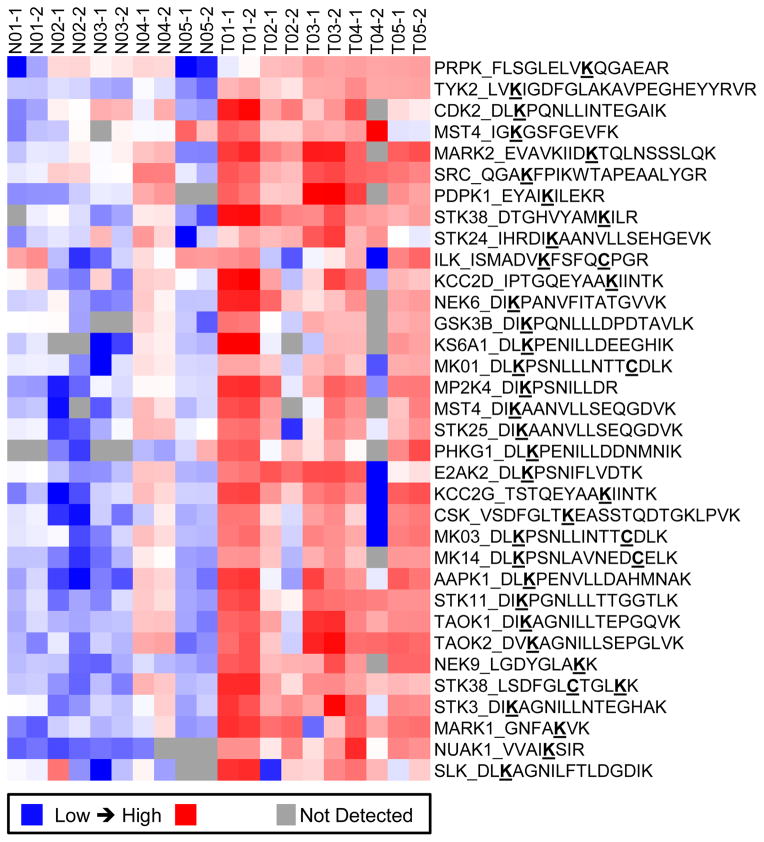
ABPP-LC-MRM is able to detect significant differences in kinase ATP uptake for tumors and matched normal frozen tissue samples (Figure 4), which shows peptides detected in at least 80% of samples and 2-fold higher in every tumor compared to its corresponding control tissue from the same patient. Heat maps are produced using Cluster 3.0 (bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) and Java Treeview (jtreeview.sourceforge.net). These data provide not only a list of kinases elevated in tumor tissues, but also unique protein kinase profiles for certain patients. Comparisons can be made using the peak area/intensity data or the tumor-to-normal expression ratios (T/N). In experiments with high variability or those conducted over extended periods of time, the comparison of T/N values may be more effective, due to the blocked experimental design. Equipped with the knowledge from cell line drug treatment results, kinase profiling may ultimately be able to support clinical trial design by matching therapeutics to individual patients using their kinase profiles.
Figure 4. ABPP-LC-MRM of Tumor and Normal Tissues from 5 Lung Cancer Patients.
The heat map shows ABPP-labeled peptides significantly and consistently upregulated in tumors (T) as compared with normal lung (N); each peptide plotted here is detected in at least 80% of the samples and observed at 2-fold higher levels in each tumor specimen compared to its matched normal. The sample numbers indicate the patient of origin and run order. The bold and underlined residues are modified: K indicates desthiobiotinylated lysine and C indicated carboxamidomethyl cysteine.
3. Data Analysis and Evaluation
As in many proteomics experiments, the acquisition of the data can be more straightforward than the analysis and evaluation. Extensive quality control metrics are needed and should be more stringent in experiments that lack SIS peptides (3.1). A critical step in the LC-MRM platform development is iterative evaluation of peptides and transitions to optimize the experiment and eliminate measurements that never detect the peptide target (3.2).
3.1. Quality Control
Before each batch of samples, PRTC standards were analyzed without scheduling using the same gradient. The retention time results were used to adjust the scheduling windows by plotting the linear regression between the two sets of retention times of PRTC standards. The patient sample analysis order was randomized while keeping the same patient tumor/normal pair together. Because we are interested primarily in increases in ATP uptake and phosphorylation, which are typically higher in tumors, the normal samples typically precede the tumor specimens in run order. Clinical samples (n = 10) are bracketed by the two cell line analyses: the same cell line is analyzed first in each run order and the other is analyzed last for consistent batch-to-batch evaluation. Scheduling is evaluated using the cell line sample and recalculated using the iRT software, as needed.
When reviewing the data, the signal intensity, peak shape, peak width, and retention times of the PRTC standards were evaluated. In addition, ABPP labeling and tissue cellularity are monitored using a desthiobiotinylated enolase α peptide (TIAPALVSKK). Data can be plotted either per sample or by run order (e.g. time of acquisition) to detect sample-to-sample variability or batch effects. Upon review of each batch and the overall dataset, outliers can be selected based on poor signal of the PRTC standards or the enolase peptide. Because tissues can vary in content and cellularity, the signal for enolase may vary by one order of magnitude across a batch. Decreases of the enolase ABPP-peptide signal greater than that clearly indicate either a poor quality sample or an analysis that needs to be repeated. In tissue batch 1 (which produced the data shown in Figure 4), the median variability between replicates for the enolase peptide was 4%. The use of these peptides for normalization purposes could also be explored for samples that have low signal intensity; however, without SIS peptides, the definition of the linear response range with external calibration curves would be necessary to avoid errors due either to thresholding or saturation effects.
Because the data are acquired for desthiobiotinylated peptides without matched standards, the confidence in the data must be bolstered by observing consistent trends. As an example, the spectral library and iRT index provide reference transition ratios and the rank order of retention times. These data can be used to show that the behavior of the individual peptides and the behavior of the overall population are consistent with both the LC-MS/MS discovery data and the other LC-MRM analyses.
3.2. Method Refinement
The ABPP peptide list from protein kinases is iteratively updated based on additional discovery data from different cancer types and specimens. With the analysis of a more diverse sample set from different disease types and the application of instruments with improved sensitivity probing deeper into the proteome, the target list is expected to increase. Method refinement by prioritization or elimination of some target peptides is necessary. After reviewing the existing data, we retained only the detectable peptides for kinases, and those that were not detected in this set of tissues were discarded. This strategy is only appropriate for further application to the same sample type and should be rigorously tested for each tumor or tissue type. For peptides with different missed cleavages and potential modifications (e.g. methionine oxidation), the intensity of each peptide form must be monitored separately. In 5 patients’ samples, the detection of the peptide with 1 missed cleavage was a minor part of the total signal, which had a consistent ratio across samples. Therefore, the differences between normal and tumor tissues could be clearly elucidated without monitoring that minor contributor (Figure 2C). However, the precision and accuracy may decrease by removing these measurements.
An alternative strategy to prioritize peptides is based on either on biological knowledge or clinical relevance; the LC-MRM panel can be restricted to ABPP peptides containing or near known, annotated ATP binding or kinase activity sites. In addition, kinase profiling can tailored to focused assays targeting specific biological questions or signaling pathways. For clinical interest, the LC-MRM platform can be tuned to monitor only protein kinases that have pharmacological inhibitors relevant to the cancer type under investigation. By targeting a shorter list of peptides, we can increase the assay specificity and quality by including more transitions for each peptide and/or increasing the number of measurements for each peak. The robustness of the assay can also be increased by applying a wider retention time scheduling windows or no scheduling at all. Furthermore, a shorter gradient can be considered to increase throughput. Finally, the restriction to a small number of targets would make it cost effective to synthesize SIS desthiobiotinylated peptides for each assay (Note: We have synthesized biotinylated peptides, rather than SIS peptides, but had problems with oxidation of the sulfur moiety during storage).
4. Alternative Strategy for Mass Spectrometry Analysis
When developing targeted LC-MRM assay for modified (or mutant) peptides, the choice of the peptide is strictly limited by the biochemical characteristics and the primary sequence of the protein. Therefore, the option to avoid certain amino acid residues or motifs by choosing other peptides more amenable to mass spectrometry is lost. In addition, alternative strategies must be considered when the tryptic peptides are too long for LC-MRM analysis; the distribution of fragment ions for peptides with 20 or more amino acids almost always produces transitions with poor sensitivity due to the high degree of signal splitting (in both intact peptide charge states and the fragment ion spectra). To address this issue, recently developed LC-MS/MS instruments can be used; a comparison between LC-MRM and these approaches is shown in Table 1. The ability to trap the peptide ions for analysis and enrich them using longer accumulation times can increase sensitivity. However, the trade-off is currently in the limited number of peptides that can be queried in the same experiment. Quantitation using targeted MS/MS or parallel reaction monitoring (PRM) takes advantage of instruments with high resolution and accurate mass measurement capability.[35,36] In PRM, all fragments of a target peptide are monitored in high resolution instruments with orbital ion trap, Fourier Transform, or time-of-flight mass analyzers. Higher mass resolution offers additional specificity, potentially reducing interference and noise contributions. In addition, simultaneous fragment monitoring simplifies assay development when compared to LC-MRM, because prediction of transition performance is no longer necessary. Transitions can be selected a priori or reviewed and chosen after evaluation of the full dataset. Furthermore, database searches can still be performed to increase confidence in peptide assignments. Finally, the isolation window could be set wide enough to accommodate both endogenous and SIS peptides, eliminating the need for separate sets of transitions.
Table 1.
Comparison of LC-MRM and LC-PRM Strategies.
| Comparator | LC-MRM | LC-PRM |
|---|---|---|
| Mass Analyzer | Triple Quadrupole | Quadrupole-Orbital Ion Trap (QExactive) Quadrupole-Time-of-Flight |
| MS2 Resolution | Low (~5,000) | High (≥17,500) |
| MS2 Scan Type | Filtering Pre-Defined Transitions | Full Scan MS2 |
| Specificity | Co-Elution of 3-6 Fragments | Co-Elution of Fragments Selected a posteriori |
| Maximum # of Peptides (Max per Unit Time) | 100s can be Scheduled (~100, Dependent on # Transitions) | 100s can be Scheduled (3-10 Dependent on Ion Accumulation Time) |
| Maximum # of Transitions (Max per Unit Time) | 1000s Scheduled in Total, but < 300 at any Time (10 ms/Transition) | Not Applicable (Transition Selection Is Retrospective) |
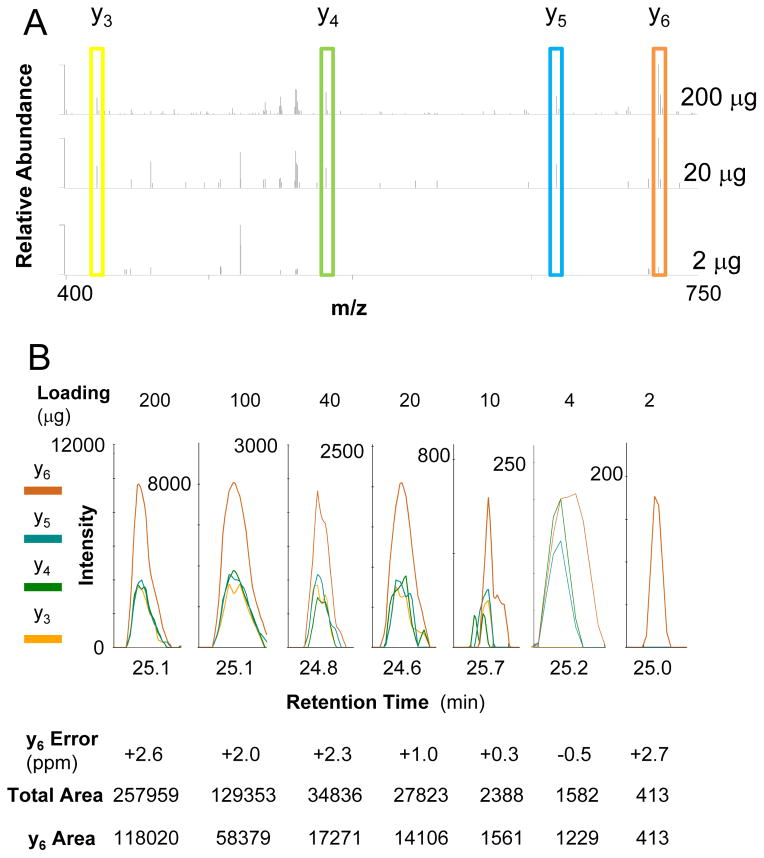
In an example PRM evaluation experiment, a dilution series consisting of 100 μg, 40 μg, 20 μg, 10 μg, 4 μg and 2 μg aliquots of total protein were labeled with ABPP probes and processed for mass analysis using a hybrid quadrupole-orbital ion trap mass spectrometer (Q Exactive Plus, Thermo) with MS resolution set to 70,000, AGC target 10E6 with maximum injection time 100 ms as well as MS2 resolution set to 17,500, AGC target 5E5 and maximum injection time 500 ms. As shown in Figure 5 for the peptide, KGEQQNR, from PHKG2_HUMAN, this strategy significantly improves the data quality (the mass accuracy for MS and MS/MS < 5 ppm with little interference or noise). Even at the lowest concentration, 1 fragment from the target peptide can still be detected. Because of the long MS/MS accumulation time, PRM may not be able to monitor all peptides during the entire chromatographic separation; only 1–7 should be scheduled at any given time depending on the accumulation time, as compared with ~80 peptides scheduled at a given time in LC-MRM. Therefore, PRM and similar methods are most suited analysis of a short list of target peptides with retention time scheduling. The most effective use of this strategy would be to focus on the peptides most relevant to FDA-approved treatment regimens (e.g. EGFR, BRAF, inter al.).
Figure 5. Example Data for ABPP-LC-PRM.
Quantification of the ABPP-labeled peptide, KGEQQNR, from PHKG2_HUMAN, shows the potential to use this method for small amounts of sample, because the peptide could still be detected and quantified using only 2 micrograms of total protein loading. Raw MS/MS data are shown for different amounts of total protein processed for ABPP-LC-PRM (A), and the data from the calibration curve are tabulated (B).
5. Data Integration for ABPP-LC-MRM and pY-LC-MRM
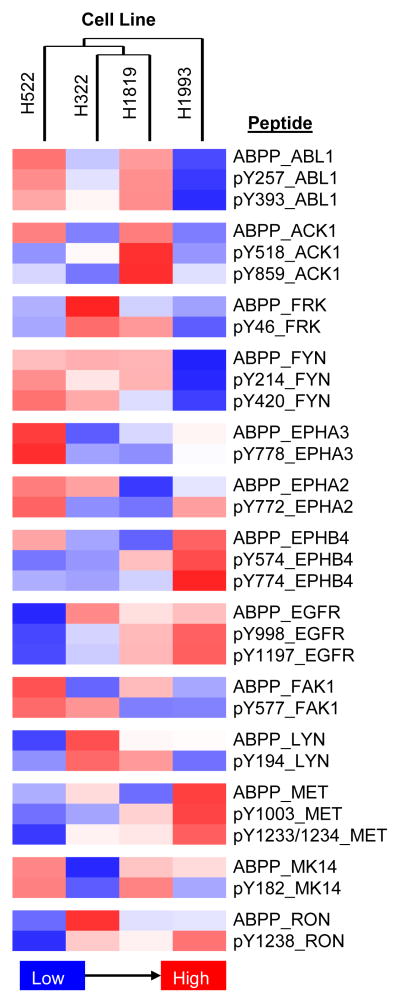
Acquisition of both dataset for cell lines and patient specimens can give unique insight into the signaling mechanisms supporting cancer cell survival and progression. Data can be mapped onto pre-defined or canonical pathways using different software tools. The Pathway Map Creator in GeneGO (Metacore) can be used to display the relationships between proteins of interest; similar approaches can be applied using Cytoscape or Ingenuity Pathway Analysis. However, each of these strategies focuses on relationships defined at the protein level; the peptide-based data from these LC-MRM experiments links to specific lysine desthiobiotinylation and tyrosine phosphorylation sites. To complement the protein-based informatics strategies, heat maps are prepared for the integrated dataset (Figure 6) to examine correlation between ABPP and pY relative quantification. Each site of labeling or modification needs to be explored via literature review to understand the underlying biology. With that information, a more detailed picture of the relevant cancer signaling comes into focus.
Figure 6. Heat Map of Selected ABPP and pY Relative Quantification in Four Lung Cancer Cell Lines.
Relative quantification is provided from lowest levels (blue) to highest levels (red); all peptides are detected in all samples. Correlation of the individual sites requires knowledge of the underlying biology. In many cases, the ATP uptake and the tyrosine phosphorylation correlate well (e.g. ABL1, MET). However, the pY sites may not be from autophosphorylation and thus would not be expected to correlate with ATP uptake; additional mechanisms of regulation may also play a role if the kinase activity and phosphorylation do not agree.
Conclusions
LC-MRM continues to develop as a promising tool for quantitative evaluation of proteins and their molecular switching events, which can be used to examine biological system. This biomarker technology also provides a clear path to clinical utility as long as the measurements can be translated from model systems to patient samples. Furthermore, additional sensitivity and quality of measurements can be obtained through LC-PRM experiments, which are promising to translate to biopsy specimens due to the ability to get only microgram quantities of total protein. These panels can be applied to elucidate tumor biology in situ and may ultimately contribute to the development of personalized therapeutic strategies.
Supplementary Material
Highlights.
Spectral Libraries for ABPP-Labeled and Phosphotyrosine Peptides
Portable multiplexed quantitative methods for ABPP and tyrosine phosphorylation.
ABPP- and pY-LC-MRM used for steady state and pharmacodynamic measurements in cells and tissues.
Acknowledgments
Funding was received from the American Lung Association Lung Cancer Discovery Award (LCD-257857 to JK) and the National Cancer Institute (R21-CA169980 to JK, R01-CA178456 to JW, and R21-CA169979 to EH). The Lung Cancer Center of Excellence has been supported by NCI SPORE (P50 CA119997); contributions to this work were made possible through Project 2 and the Career Development Program. This work was partially supported by a Young Investigator Award from the American Head and Neck Society/American Academy of Otolaryngology/Head and Neck Surgery (to PMW). The Moffitt Proteomics Facility is supported by the US Army Medical Research and Materiel Command under Award No. W81XWH-08-2-0101 for a National Functional Genomics Center, the National Cancer Institute Cancer Center Support Grant (P30-CA076292), and the Moffitt Foundation. The triple quadrupole mass spectrometer was purchased with a shared instrument grant from the Bankhead-Coley Cancer Research program of the Florida Department of Health (09BE-04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics. Mol Syst Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, Piening BD, Feng LC, Kasarda E, Gurley KE, Eng JK, Chodosh LA, Kemp CJ, McIntosh MW, Paulovich AG. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J Proteome Res. 2007;6:3962–3975. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- 3.Yocum AK, Gratsch TE, Leff N, Strahler JR, Hunter CL, Walker AK, Michailidis G, Omenn GS, O’Shea KS, Andrews PC. Coupled global and targeted proteomics of human embryonic stem cells during induced differentiation. Mol Cell Proteomics. 2008;7:750–67. doi: 10.1074/mcp.M700399-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash A, Tomazela DM, Frewen B, Maclean B, Merrihew G, Peterman S, Maccoss MJ. Expediting the development of targeted SRM assays: using data from shotgun proteomics to automate method development. J Proteome Res. 2009;8:2733–2739. doi: 10.1021/pr801028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh GM, Lin S, Evans DM, Khosrovi-Eghbal A, Beavis RC, Kast J. Implementation of a data repository-driven approach for targeted proteomics experiments by multiple reaction monitoring. J Proteomics. 2009;72:838–52. doi: 10.1016/j.jprot.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson NL, Anderson NG, Pearson TW, Borchers CH, Paulovich AG, Patterson SD, Gillette M, Aebersold R, Carr SA. A human proteome detection and quantitation project. Mol Cell Proteomics. 2009;8:883–886. doi: 10.1074/mcp.R800015-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Shi T, Brown JN, He J, Gao Y, Fillmore TL, Shukla AK, Moore RJ, Camp DG, II, Rodland KD, Qian W, Liu T, Smith RD. Expediting SRM assay development for large-scale targeted proteomics experiments. J Proteome Res. 2014;13:4479–4487. doi: 10.1021/pr500500d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, Hall SC, Allen S, Blackman RK, Borchers CH, Buck C, Cardasis HL, Cusack MP, Dodder NG, Gibson BW, Held JM, Hiltke T, Jackson A, Johansen EB, Kinsinger CR, Li J, Mesri M, Neubert TA, Niles RK, Pulsipher TC, Ransohoff D, Rodriguez H, Rudnick PA, Smith D, Tabb DL, Tegeler TJ, Variyath AM, Vega-Montoto LJ, Wahlander A, Waldemarson S, Wang M, Whiteaker JR, Zhao L, Anderson NL, Fisher SJ, Liebler DC, Paulovich AG, Regnier FE, Tempst P, Carr SA. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash A, Rezai T, Krastins B, Sarracino D, Athanas M, Russo P, Ross MM, Zhang H, Tian Y, Kulasingam V, Drabovich AP, Smith C, Batruch I, Liotta L, Petricoin E, Diamandis EP, Chan DW, Lopez MF. Platform for establishing interlaboratory reproducibility of selected reaction monitoring-based mass spectrometry peptide assays. J Proteome Res. 2010;9:6678–6688. doi: 10.1021/pr100821m. [DOI] [PubMed] [Google Scholar]
- 10.Gerber SA, John R, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci USA. 2003;100(12):6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkpatrick DS, Gerber SA, Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods. 2005;35:265–273. doi: 10.1016/j.ymeth.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Chaerkady R, Wu J, Hwang HJ, Papadopoulos N, Kopelovich L, Maitra A, Matthaei H, Eshleman JR, Hruban RH, Kinzler KW, Pandey A, Vogelstein B. Mutant proteins as cancer-specific biomarkers. Proc Natl Acad Sci U S A. 2011;108:2444–2449. doi: 10.1073/pnas.1019203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halvey PJ, Ferrone CR, Liebler DC. GeLC-MRM quantitation of mutant KRAS oncoprotein in complex biological samples. J Proteome Res. 2012;11:3908–3913. doi: 10.1021/pr300161j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttenhain R, Soste M, Selevsek N, Rost H, Sethi A, Carapito C, Farrah T, Deutsch EW, Kusebauch U, Moritz RL, Nimeus-Malmstrom E, Rinner O, Aebersold R. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci Transl Med. 2012;4:142ra94. doi: 10.1126/scitranslmed.3003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Fang B, Liu RZ, Lin H, Kinose F, Bai Y, Oguz U, Remily-Wood ER, Li J, Altiok S, Eschrich S, Koomen J, Haura EB. Mass spectrometry mapping of epidermal growth factor receptor phosphorylation related to oncogenic mutations and tyrosine kinase inhibitor sensitivity. J Proteome Res. 2011;10:305–319. doi: 10.1021/pr1006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccimaro E, Hanks SK, Yu KH, Blair IA. Absolute quantification of phosphorylation on the kinase activation loop of cellular focal adhesion kinase by stable isotope dilution liquid chromatography/mass spectrometry. Anal Chem. 2009;81:3304–3313. doi: 10.1021/ac900204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin LL, Tong J, Prakash A, Peterman SM, St-Germain JR, Taylor P, Trudel S, Moran MF. Measurement of protein phosphorylation stoichiometry by selected reaction monitoring mass spectrometry. J Proteome Res. 2010;9:2752–2761. doi: 10.1021/pr100024a. [DOI] [PubMed] [Google Scholar]
- 18.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, Multiplexed Assays for Low Abundance Proteins in Plasma by Targeted Mass Spectrometry and Stable Isotope Dilution. Mol Cell Proteomics. 2007;6:2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percy AJ, Simon R, Chambers AG, Borchers CH. Enhanced sensitivity and multiplexing with 2D LC/MRM-MS and labeled standards for deeper and more comprehensive protein quantitation. J Proteomics. 2014;106:113–124. doi: 10.1016/j.jprot.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Brasier AR. Applications of selected reaction monitoring (SRM)-mass spectrometry (MS) for quantitative measurement of signaling pathways. Methods. 2013;61:313–322. doi: 10.1016/j.ymeth.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc Natl Acad Sci U S A. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Gruidl M, Remily-Wood E, Liu RZ, Eschrich S, Lloyd M, Nasir A, Bui MM, Huang E, Shibata D, Yeatman T, Koomen JM. Quantification of β-Catenin Signaling Components in Colon Cancer Cell Lines, Tissue Sections, and Microdissected Tumor Cells using Reaction Monitoring Mass Spectrometry. J Proteome Res. 2010;9:4215–4227. doi: 10.1021/pr1005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebecca VW, Wood E, Fedorenko IV, Paraiso KH, Haarberg HE, Chen Y, Xiang Y, Sarnaik A, Gibney GT, Sondak VK, Koomen JM, Smalley KS. Evaluating Melanoma Drug Response and Therapeutic Escape with Quantitative Proteomics. Mol Cell Proteomics. 2014;13:1844–1854. doi: 10.1074/mcp.M113.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Y, Remily-Wood ER, Oliveira V, Yarde D, He L, Cheng JQ, Mathews L, Boucher K, Cubitt C, Perez L, Gauthier TJ, Eschrich SA, Shain KH, Dalton WS, Hazlehurst L, Koomen JM. Monitoring a nuclear factor-kB signature of drug resistance in multiple myeloma. Mol Cell Proteomics. 2011;10:1–16. doi: 10.1074/mcp.M110.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barglow KT, Cravatt BF. Activity-based protein profiling for the functional annotation of enzymes. Nat Methods. 2007;4:822–827. doi: 10.1038/nmeth1092. [DOI] [PubMed] [Google Scholar]
- 26.Patricelli MP, Szardenings AK, Liyanage M, Nomanbhoy TK, Wu M, Weissig H, Aban A, Chun D, Tanner S, Kozarich JW. Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry. 2007;46:350–358. doi: 10.1021/bi062142x. [DOI] [PubMed] [Google Scholar]
- 27.Adachi J, Kishida M, Watanabe S, Hashimoto Y, Fukamizu K, Tomonaga T. Proteome-Wide Discovery of Unknown ATP-Binding Proteins and Kinase Inhibitor Target Proteins Using an ATP Probe. J Proteome Res. 2014 Sep 30; doi: 10.1021/pr500845u. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.McAllister FE, Niepel M, Haas W, Huttlin E, Sorger PK, Gygi SP. Mass Spectrometry Based Method to Increase Throughput for Kinome Analyses Using ATP Probes. Anal Chem. 2013;85:4666–4674. doi: 10.1021/ac303478g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y, Guo L, Wang Y. A targeted quantitative proteomics strategy for global kinome profiling of cancer cells and tissues. Mol Cell Proteomics. 2014;13:1065–1075. doi: 10.1074/mcp.M113.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worboys JD, Sinclair J, Yuan Y, Jørgensen C. Systematic evaluation of quantotypic peptides for targeted analysis of the human kinome. Nat Methods. 2014;11:1041–1044. doi: 10.1038/nmeth.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J, Bennett KL, Gao J, Song L, Eschrich S, Superti-Furga G, Koomen J, Haura EB. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol. 2010;6:291–299. doi: 10.1038/nchembio.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Liu Q, Zimmerman LJ, Ham AJ, Slebos RJ, Rahman J, Kikuchi T, Massion PP, Carbone DP, Billheimer D, Liebler DC. Methods for peptide and protein quantitation by liquid chromatography-multiple reaction monitoring mass spectrometry. Mol Cell Proteomics. 2011;10:M110.006593. doi: 10.1074/mcp.M110.006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escher C, Reiter L, MacLean B, Ossola R, Herzog F, Chilton J, MacCoss MJ, Rinner O. Using iRT, a normalized retention time for more targeted measurement of peptides. Proteomics. 2012;12:1111–1121. doi: 10.1002/pmic.201100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallien S, Bourmaud A, Kim SY, Domon B. Technical considerations for large-scale parallel reaction monitoring analysis. J Proteomics. 2014;100:147–159. doi: 10.1016/j.jprot.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics. 2012;11:1475–1488. doi: 10.1074/mcp.O112.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherwood CA, Eastham A, Lee LW, Risler J, Vitek O, Martin DB. Correlation between y-type ions observed in ion trap and triple quadrupole mass spectrometers. J Proteome Res. 2009;8:4243–4251. doi: 10.1021/pr900298b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrioli PG. Trans-proteomic pipeline: a pipeline for proteomic analysis. Methods Mol Biol. 2010;604:213–238. doi: 10.1007/978-1-60761-444-9_15. [DOI] [PubMed] [Google Scholar]
- 39.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.