Abstract
In 2011, statewide newborn screening programs for critical congenital heart defects began in the United States, and subsequently screening has been implemented widely. In this review, we focus on data reports and collection efforts related to both prenatal diagnosis and newborn screening. Defect-specific, maternal, and geographic factors are associated with variations in prenatal detection, so newborn screening provides a population-wide safety net for early diagnosis. A new web-based repository is collecting information on newborn screening program policies, quality indicators related to screening programs, and specific case-level data on infants with these defects. Birth defects surveillance programs also collect data about critical congenital heart defects, particularly related to diagnostic timing, mortality, and services. Individuals from state programs, federal agencies, and national organizations will be interested in these data to further refine algorithms for screening in normal newborn nurseries, neonatal intensive care settings, and other special populations; and ultimately to evaluate the impact of screening on outcomes.
Keywords: Neonatal screening, Prenatal diagnosis, Heart defects, Congenital, Population surveillance, Public health
Introduction
In this review, we define critical congenital heart defects (CCHD) as structural malformations of the heart that are present at birth and require intervention in the first year of life, and we focus on prenatal and postnatal screening for CCHD in the United States (U.S.). Seven categories of CCHD that usually present in newborns with hypoxemia were considered by the Secretary of Health and Human Service’s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) as the primary targets for pulse oximetry screening.1 These seven types are (1) dextro-transposition of the great arteries; (2) hypoplastic left heart syndrome; (3) pulmonary atresia (with intact ventricular septum); (4) tetralogy of Fallot; (5) total anomalous pulmonary venous return; (6) tricuspid atresia; and (7) truncus arteriosus. The National Birth Defects Prevention Network (NBDPN), an organization of state and population-based birth defects programs partially funded by the Centers for Disease Control and Prevention (CDC), has developed case definitions for these primary targets.2 These definitions include inclusion and exclusion terminology for each category of defects, coding lists to be used by birth defects programs for ascertainment of records from population-based sources, and diagnostic criteria used in reviewing records of prenatally and postnatally diagnosed defects. Other types of CCHD that sometimes present with hypoxemia and are considered “secondary targets” include critical aortic coarctation, atresia/hypoplasia/interruption of the aortic arch, double outlet right ventricle, Ebstein anomaly, severe aortic valve stenosis, severe pulmonic stenosis, and single ventricle complex.3
Public health importance of CCHD and early detection
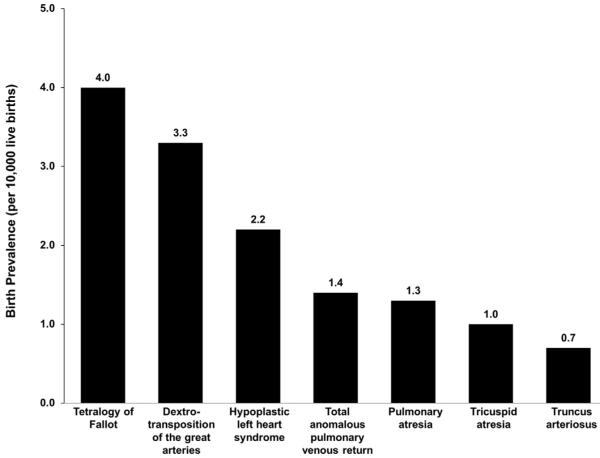
The importance of CCHD in the perinatal context results from the frequency of detection in both prenatal and neonatal settings, the necessity of early detection to prevent complications, and the contribution of these defects to infant mortality. Practicing maternal-fetal medicine specialists and neonatologists are well aware of the impact of malformations in general, and practitioners are involved with many of the approximately 3% of newborns affected with birth defects. Nearly 1% of newborns have congenital heart defects, and approximately one-quarter of those defects are in the CCHD category.4-6 Some newborns with CCHD will have obvious clinical signs in the nursery, but since a subset of affected infants depend on circulation through the ductus arteriosus, closure of the ductus after nursery discharge can be catastrophic and is a major impetus for hospital-based screening in order to avoid unexpected deaths. Congenital heart disease has been reported to be responsible for 30–50% of infant mortality due to birth defects, and from 1999 to 2006 (before the advent of CCHD newborn screening), more than 13,000 infant deaths resulting from congenital heart defects were reported in the U.S.7 These deaths occurred most commonly with diagnoses of hypoplastic left heart syndrome, transposition of the great arteries, or tetralogy of Fallot, in that order of severity, and these three types of CCHD (in reverse order of reported frequency) also have the highest rates among live births in NBDPN surveillance data (Fig.).
Fig.
Pooled birth prevalence among live births of primary critical congenital heart defects (CCHD) screening targets, by CCHD type, among population-based birth defects surveillance systems that use active case finding methodologies, United States, 2005–2009. The exact years and number of programs included (range: 11–16) vary by defect. Pooled prevalence was calculated by dividing the total number of cases across programs by total number of combined live births. (Adapted from Mai et al.2)
In spite of the relatively common occurrence of CCHD among children with birth defects and the adverse consequences they have for cardiac physiology, newborns are often unsuspected of having CCHD before transitional events from fetal circulation occur. Cardiac defects might not be suspected if there is no multiple malformation syndrome, no prenatal cardiac diagnoses by screening ultrasound and/or fetal echocardiography (discussed below), or in the absence of any family history that would alert parents and providers about the need to investigate for CCHD. Among infants with any congenital heart defect, conventional cytogenetic abnormalities such as trisomies have only been reported in approximately one-tenth of those affected.8,9 In isolated CCHD, physical examination might not detect cyanosis or other clinical signs before transitions from fetal circulation are completed, which can occur after nursery discharge.
Implementing CCHD newborn screening in the United States
Statewide newborn screening for CCHD has developed rapidly after a series of events in recent years. Pilot screening programs for subclinical cyanosis related to CCHD had been reported from normal newborn nurseries in the U.S. for more than 10 years.10 The centerpiece of these early programs was pulse oximetry, already with a well-established presence in hospitals for noninvasive hypoxemia screening. As pilot data accumulated internationally, in 2009 the American Heart Association (AHA) and the American Academy of Pediatrics (AAP) published a scientific statement reviewing pulse oximetry screening studies with recommendations for larger and more diverse studies.3 This statement and other published data such as a prospective study from Sweden led to considerations for population-based screening implementation.11 After further evidence reviews in 2010–2011, the U.S. Secretary of Health and Human Services adopted the SACHDNC’s endorsement of CCHD screening for the Recommended Uniform Screening Panel (RUSP) for newborns. Subsequently, an expert panel published an algorithm for pulse oximetry screening that was endorsed by the AAP, AHA, and the American College of Cardiology (ACC).1,12,13
U.S. public health departments began implementing statewide CCHD newborn screening programs in 2011.14,15 The earliest statewide programs were legislatively mandated, while other programs initiated over the 3 years subsequent to the RUSP addition came about through changes in state screening rules and regulations, with or without legislation or recommendations from state newborn screening advisory boards. Examples of these developments were summarized in a 2013 Issue Brief published online by the Association of Maternal and Child Health Programs: http://www.amchp.org/programsandtopics/CHILD-HEALTH/projects/newborn-screen ing/Documents/AMCHP_Screening_for_CCHD_Issue_Brief_FI NAL-Oct2013.pdf. Adding to the clinical evidence that led to the RUSP decision, results of a subsequent study indicated that CCHD screening appeared to be cost-effective, using modeled data and survey results from one of the early-adopting states.16 With the exception of just a few states, screening is either in the process of statewide implementation or has already been adopted statewide in U.S. birth hospitals; readers can find specific state status updates at http://www.aap.org/en-us/advocacy-and-policy/state-advocacy/Documents/Newborn%20Screening%20for%20Critical%20Congenital%20Heart%20Disease.pdf or links to state-specific websites for local mandates and guidance at http://www.babysfirsttest.org/newborn-screening/conditions/critical-congenital-heart-disease-cchd. Even without mandates or regulations including CCHD screening on state panels, newborn screening programs in most of the states without a current screening requirement report that pulse oximetry screening is occurring (in a few such states, all hospitals are reportedly screening) (Hudson et al., submitted). In these states without requirements, the influence of the endorsements by national organizations such as AAP might be leading to screening practices that are based on “standards for care delivery.”17
Data collection by state health departments is sometimes also legislatively mandated, and with or without mandates many are attempting to collect information from birth hospitals (Hudson et al., submitted). Results from early statewide data collection efforts now have been published.14,15 As predicted, these studies demonstrated detection of otherwise unsuspected CCHD through pulse oximetry screening, which must be understood in the context of prenatal diagnosis and pre-screening clinical detection rates.
Prenatal and postnatal estimates of CCHD detection before 2011
Prenatal diagnosis of CCHD depends upon the recognition of structural heart defects via ultrasound or fetal echocardiography, typically during the second trimester. Guidelines put forth by the American Institute of Ultrasound in Medicine suggest that a basic cardiac examination should include a four-chamber view of the heart and, when technically feasible, views of the outflow tracts.18,19 Similar guidelines were issued by the American College of Obstetricians and Gynecologists.20 Further examination via fetal echocardiography is warranted if the ultrasound is abnormal, but also under circumstances such as a family history of congenital heart disease, presence of maternal diabetes, or use of in vitro fertilization.21
Some CCHD are more amenable to visualization through these methods than others. Using 1997–2007 data from the Utah Birth Defect Network, Pinto et al.22 found that the defects most likely to be detected prenatally included those with abnormal four-chamber views, while defects exhibiting abnormal outflow tracts were much less likely to be detected prenatally. In a study of members of a large health maintenance organization (HMO) in California from 2005 to 2010, Levy et al.23 showed that women who received care from HMO clinics that had instituted a policy to examine outflow tracts during prenatal ultrasound had much higher prenatal diagnosis rates (59%) compared to HMO clinics that had not instituted such a policy (28%).
Given these challenges, it is not surprising that many studies have noted variation in the prenatal detection rates of CCHD (Table).22-26 Among the primary CCHD screening targets, hypoplastic left heart syndrome, for example, has been shown to be detected prenatally quite frequently, with estimates ranging from 53% to 88%.24,26 Other primary screening targets, such as total anomalous pulmonary venous return, are much less likely to be detected prenatally, with a few studies reporting no prenatally detected cases, though these studies often have a very small number of cases overall. Of the secondary screening targets, coarctation of the aorta is infrequently diagnosed before birth, with studies estimating that only 11–37% of cases have a prenatal diagnosis.24,26 Other secondary targets, such as double outlet right ventricle, seem to be diagnosed prenatally more frequently.
Table.
Select studies of prenatal critical congenital heart defects (CCHD) diagnosis and late CCHD detection, United States, 1997–2009.
| Prenatal diagnosis (%) |
Late detection (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Location, study years | Utah, 1997–200722 | Multiple sites, 1998–200524 | Northern California 2004–200525 |
Massachusetts, 2004–200926 |
Southern California, 2005–201023 |
Multiple sites, 1998–200931 |
Florida, 1998–200733 | Massachusetts, 2004–200926 |
| Definition of prenatal diagnosis/late detection |
Abnormal fetal ultrasound |
Maternal report of abnormal fetal ultrasound/ echocardiogram |
Parental report of prenatal diagnosis (+terminations) |
Documented in maternal and/or infant medical record |
Abnormal fetal ultrasound/ echocardiogram |
Fetal echocardiogram >3 days of birth |
Diagnosis after birth/transfer hospital discharge |
Diagnosis after birth/ transfer hospital discharge |
| Primary CCHD screening targets | ||||||||
| Dextro- transposition of the great arteries |
13a | 17 | 17 (19)b | 60 | 71c | 14 | 10 | 0 |
| Hypoplastic left heart syndrome |
70 | 53 | 56 (61) | 88 | 100d | 13 | 12 | 0 |
| Pulmonary atresia | 26 | 44 | 43 (50)e | 77 | – | 8 | 23 | 0 |
| Total anomalous pulmonary venous return |
6 | 1 | 0 (0) | 25 | 0 | 41 | 40 | 23 |
| Tetralogy of Fallot | 26f | 18 | 18 (31) | 58 | 69 | 28 | 25 | 11 |
| Tricuspid atresia | 52 | – | 25 (25)g | 89 | 100 | 12 | 16 | 0 |
| Truncus arteriosus | 24 | – | 50 (50) | 54 | – | 31 | 32 | 8 |
| Secondary CCHD screening targets | ||||||||
| Coarctation of the aorta |
19 | 11 | – | 37 | 33 | 62 | 37h | 30 |
| Double-outlet right ventricle |
89i | – | 18 (25) | 83 | 100d | 31 | 29 | 2 |
| Ebstein anomaly | 43 | 22 | – | 80 | – | 21 | 13 | 0 |
| Single ventricle complex |
100j | 56 (64) | 92 | 100d | 22 | 25 | 0 | |
d-Transposition of the great arteries (d-TGA) with intact ventricular septum.
d- and l-Transposition of the great arteries.
Transposition of the great arteries.
Includes single ventricle, double outlet right ventricle, and hypoplastic left heart syndrome.
Pulmonary stenosis or atresia with intact ventricular septum.
Tetralogy of Fallot with pulmonary stenosis.
Tricuspid valve anomalies.
Coarctation/hypoplasia of the aortic arch.
Estimate for double outlet right ventricle (DORV) not otherwise specified; for DORV with normal arteries: 71%; for DORV with transposition of the great arteries: 58%.
Single ventricle, not otherwise specified.
Maternal factors associated with increased rates of prenatal detection have included carrying multiple gestations, increased maternal age, maternal diabetes, and family history of CCHD, while non-Hispanic white maternal race/ethnicity and increased prepregnancy body-mass index have been associated with decreased rates of prenatal diagnosis.24,27 Infant/fetal characteristics associated with increased prenatal diagnosis have included the presence of other birth defects or chromosomal syndromes, as well as increasing complexity of CCHD. Improvements in ultrasonography and changes in guidelines to include both the four-chamber view of the heart and outflow tracts may be responsible for some increase in overall prenatal detection of congenital heart defects seen since the mid-2000s.18,20,26
Broad geographic as well as hospital-level variation in prenatal detection has likewise been noted.24,28,29 In a study of live born infants with “a single morphologic right ventricle with systemic outflow obstruction” seen at 15 centers participating in the Pediatric Cardiac Network from 2005 to 2009, Atz et al.28 noted that prenatal diagnosis rates ranged by center from 59% to 85% and that centers that saw a higher volume of patients were more likely to have higher prenatal diagnosis rates. In a study of factors associated with maternal report of prenatal diagnosis of any congenital heart defect (i.e., not restricted to CCHD), Ailes et al.24 found maternal residence to be one of the strongest predictors of maternal report of prenatal diagnosis. Even among select CCHD types in these maternal reports, such as hypoplastic left heart syndrome, there was a four-fold difference between the geographic location with the lowest prevalence of maternal report of prenatal diagnosis (21%) and highest (81%). Geographic variation in access to high-quality imaging technology and skilled ultrasound technicians might play a role in the geographic variation observed in prenatal detection. The Utah study by Pinto et al.,22 for instance, also noted that access to high-risk clinics increased the likelihood of having a prenatal diagnosis by 10-fold.
As mentioned above, the decision to add CCHD to the RUSP was motivated by the realization that prenatal detection was incomplete and so-called “late” postnatal detection of CCHD led to potentially preventable deaths and morbidity. In studies of pre-2011 data, however, variation exists in how “late” detection has been operationalized, with authors alternatively defining late diagnosis as diagnosis after birth hospital discharge, after 3 days of birth, or even at death.30-32 Late detection of a number of the primary CCHD screening targets is relatively low, with an estimated 0–16% of cases of hypoplastic left heart syndrome, dextro-transposition of the great arteries, and tricuspid atresia reported to be late detected (Table).26,31 Among secondary screening targets, cases of coarctation of the aorta were estimated in three studies to be late detected much more frequently, with study estimates ranging from 30% to 62% of cases.26,31,33 In these three studies, factors associated with the late detection were less often related to maternal characteristics and more so associated with CCHD type, nursery level of care, and presence/absence of extracardiac defects.
Data collection in the screening era
Going forward, there is a widespread interest in collecting national data that will examine the performance of pulse oximetry screening programs and the impact of these programs on the overall timing of CCHD detection. In the U.S., NBDPN programs continue to compile CCHD surveillance data. Many birth defects surveillance programs unfortunately do not have the capacity or authority to collect CCHD newborn screening results, or to do so in a timely fashion, which has led to a need for national collection of data specific to CCHD newborn screening program identification and quality improvement. These data are collected through a recently funded Health Resources and Services Administration initiative: the Newborn Screening Technical assistance and Evaluation Program (NewSTEPs).
NewSTEPs functions as a partnership between the Association of Public Health Laboratories and the Colorado School of Public Health and operates with the mission of supporting the highest quality for newborn screening systems by providing relevant, accurate tools and resources, and facilitating collaboration between newborn screening partners for both dried blood spot and point-of-care newborn screening. This initiative builds on the previously funded HRSA efforts, extending data collection to CCHD screening activities as well.34 Previous efforts for data collection in point-of-care newborn screening through early hearing detection and intervention have been funded by CDC.35 Aggregate data collected by CDC has provided the evidence of improved screening and identification of infants with early hearing loss. These efforts have demonstrated that central data collection and surveillance by newborn screening programs can provide much needed data for local quality improvement, and point to the need for consistent definitions for newborn screening.
Quality improvement and monitoring in CCHD newborn screening requires high-quality data with consistent definitions across newborn screening programs, such as those set forth for NBDPN programs.2 NewSTEPs provides a web-based repository to collect data on newborn screening program policies, quality indicators related to newborn screening programs, and specific case-level information on infants diagnosed with CCHD. Data collected within the NewSTEPs data repository will be summarized and reports will be compiled internally and reviewed by a data review committee comprised of newborn screening program experts. Reports will be distributed and shared at the state, local, and national level, and aggregate reports will be available publically on the NewSTEPs website.
As mentioned above, the policy, or rules and regulations for CCHD newborn screening data collection at the state level vary widely across the nation. Some programs collect every pulse oximetry value for each screening, others collect aggregate data from birthing hospitals, and yet others practice no public health data collection, even though standards for such data collection have been recommended.36 In the absence of centralized state data collection, public health newborn screening programs are unable to evaluate the performance of the screen in a standardized and comparative manner. NewSTEPs has assembled the policies from all state newborn screening programs for both CCHD screening and data collection. Annual review of these policies by NewSTEPs staff in collaboration with the data review committee will be performed to assure accurate representation and will provide a robust assessment of the CCHD policies in the U.S. Harnessing these collective policy differences can enable programs to identify model practices across the nation and to advocate for improved data collection systems with the benefit of shared information. Policy practices will be publically available via the NewSTEPs website to support state, regional, and national policies.
In collaboration with newborn screening stakeholders, NewSTEPs has finalized a core set of quality indicators that encompass key areas of newborn screening activities, which can be found at http://www.newsteps.org. These indicators are consistent with national collection efforts required within the Newborn Screening Saves Lives Act (http://www.con gress.gov/bill/113th-congress/house-bill/1281) and build off of previously developed indicators.37 State newborn screening programs have secure access to the NewSTEPs repository to enter quality indicators on an annual basis. Metrics are tracked between newborn screening programs as well as longitudinally within each program, with reports issued at the state, regional, and national levels. Quality indicators specific to CCHD newborn screening include: percentage of eligible infants screened for CCHD, number of infants who screened positive for CCHD, number of infants with CCHD identified by newborn screening, number of newborns with CCHD who were missed on the newborn screen, and the timing of screening activities (screen, follow-up, and diagnosis). These quality indicators related to CCHD will provide the first national summary data on CCHD newborn screening. Data will be available on all quality indicators in 2015 and will be disseminated publically via the NewSTEPs website and through state, regional, and national newborn screening stakeholders.
In addition to aggregate newborn screening quality indicators, the NewSTEPs data repository collects case-level data related to the newborns identified with CCHD, including the timing of the pulse oximetry testing, follow-up testing, and confirmation of diagnosis. Assessment of the certainty of the diagnosis using standardized public health surveillance case definitions will allow consistent comparisons across programs and over time. State level policies on local data collection vary, and some states are not currently authorized to collect point-of-care testing data for CCHD newborn screening. NewSTEPs will report aggregate data from states that have individual newborn data via the NewSTEPs website and through state, local, and national newborn screening stakeholders.
The activities of NewSTEPs have been reviewed by the Colorado Multiple Institutional Review Board and the Health and Human Services Office of Human Research Protection and have been determined to be non-human subject research. NewSTEPs offers strict privacy to newborn screening programs, with many layers of access, enabling programs to determine the extent of quality- and case-specific information they wish to share on a non-aggregate level. Data dissemination and sharing will be done via national, regional, and local mechanisms, and oversight of all data sharing will be accomplished through the data review committee. All states are participating in the NewSTEPs data repository, and all sensitive data from states (quality indicators and case definitions) are collected from states through a memorandum of understanding. The NewSTEPs repository remains dynamic and evolving, with routine feedback incorporated from the newborn screening community.
NBDPN surveillance programs remain uniquely positioned to contribute population-based CCHD data. More than 40 states collect data that can be used to estimate rates of individual types of CCHD (Fig.), and surveys indicate that most also have the capabilities to examine mortality (including time trends) and links to support services, which will be important in the evaluation of the impact of early identification.2,5,38 These surveys also indicate that some programs are also capable of evaluating interventions and morbidities associated with CCHD. Some programs should be (and have been) able to distinguish between detection along a continuum of key time periods: prenatal diagnoses, clinical detection in the pre-screening period shortly after birth, and newborn screening-related diagnoses.15 Another important role of statewide surveillance programs will be to ascertain missed cases, either those children who were not diagnosed prenatally or somehow avoided screening in a newborn nursery, or true false negatives: failures of the screening algorithm itself or its application and interpretation.39,40 Birth defects programs already systematically ascertain CCHD cases identified clinically or through newborn screening, through a combination of active and passive methods.2 New Jersey has provided a model for ascertaining pulse oximetry results with the use of a diagnostic code for failed screens, combined with linkage to reported CCHD cases to identify missed screens.15
Ultimately, the goal of early identification of CCHD will be not only to decrease mortality and other short-term adverse outcomes, but also to have long-term improvements in quality of life and morbidities for affected individuals. As noted above, birth defects surveillance systems have varying capabilities to measure some of these endpoints. A new public health priority is to evaluate long-term outcomes among older children and even adults with congenital heart defects, and pilot projects have been funded to link databases and do longitudinal surveillance and research.41,42 These projects, which are slated to be expanded in the future, can potentially examine covariates such as the timing of diagnosis along the continuum mentioned above; locations of birth and surgical interventions; and types and timings of all interventions.
Discussion/comment
National implementation of CCHD newborn screening has progressed rapidly since 2011, and while outcome trend data are still forthcoming, this public health program has promising potential to decrease infant mortality from these relatively common birth defects. Prenatal detection has become a significant contributor to early identification of CCHD. As long as disparities in use of prenatal ultrasound exist, due to factors such as location of maternal residence and access to high-risk clinics, CCHD newborn screening will provide an important safety net for early diagnosis across all populations.43
As with any new public health program, newborn screening data collection, including long-term follow-up, will be essential to monitor the progress of implementation and will need to be ongoing for continuous quality improvement.40,44 Unlike traditional screening for disorders detectable in newborn blood spots, which are collected primarily in hospitals but analyzed in central laboratories, there are particular challenges for point-of-care newborn screening programs.17 These challenges include practical issues such as training and use of standardized algorithms in a variety of settings with multiple health care providers, as well as data collection issues that ideally need to occur both at the clinical level as well as involving public health.36
As detailed above, CCHD data surveillance is ongoing and expanding and the NewSTEPs data collection program has also begun. With the results of initial CCHD screening experiences, one particular research need would be to evaluate and optimize the algorithms used for pulse oximetry screening. To do so, efforts have begun to analyze case-level pulse oximetry data, and additional projects will require special data collection protocols beyond the projects outline above, ideally also incorporating false negative information with the collaboration of birth defects surveillance programs.40,45 Of particular interest to neonatologists, there are also data needs for screening protocols in neonatal intensive care units (NICUs). Many infants in NICUs have already been identified with CCHD through prenatal diagnosis, and others are monitored intensively with pulse oximetry. Since NICU oximetry is not necessarily done systematically, incorporating comparisons of pre- and post-ductal oxygen saturation levels or making special provisions for infants treated with oxygen, some neonatologists have proposed specific protocols for screening NICU infants.46 Screening can be similarly problematic in nurseries that are located at moderate or high altitudes where baseline saturation levels in normal newborns are understandably lower, so investigators in such settings have proposed modified algorithms and started collecting data with these protocols.47 Clearly, evidence-based recommendations for special settings will require more CCHD data.
Summary.
Nearly 1% of newborn infants have congenital heart defects, and approximately one-quarter of those defects are in the CCHD category. Infant deaths have occurred most commonly with hypoplastic left heart syndrome, transposition of the great arteries, and tetralogy of Fallot. Pulse oximetry screening of newborns to prevent unexpected deaths has been well-studied.
Based on evidence reviews, in 2011 CCHD screening was added to the U.S. Recommended Uniform Screening Panel for newborns. With a few state exceptions, screening is either in the process of statewide implementation or has already been adopted statewide in birth hospitals.
There have been increases in overall prenatal detection rates of congenital heart defects seen since the mid-2000s; defect-specific, maternal, and geographic factors are associated with variations in prenatal detection, so CCHD newborn screening provides a population-wide safety net for early diagnosis.
Birth defects surveillance programs are collecting data to examine current rates of CCHD detection through prenatal diagnoses, clinical detection in the pre-screening period shortly after birth, and newborn screening-related diagnoses, as well as long-term follow-up data to examine outcomes in older children and adults.
A recently funded program (NewSTEPs) has provided a web-based repository to collect data on newborn screening program policies, quality indicators related to newborn screening programs, and specific case-level information on infants with CCHD.
Current research related to CCHD newborn screening focuses on evaluating and refining pulse oximetry algorithms and developing protocols for special populations (e.g., in NICUs and at moderate-to-high altitudes).
Acknowledgments
We are grateful to Dr. Cara Mai and her co-authors from the National Birth Defects Prevention Network for the use of the data that they have compiled.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 2.Mai CT, Riehle-Colarusso T, O’Halloran A, et al. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2005-2009: featuring critical congenital heart defects targeted for pulse oximetry screening. Birth Defects Res A Clin Mol Teratol. 2012;94(12):970–983. doi: 10.1002/bdra.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahle WT, Newburger JW, Matherne GP, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 5.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153(6):807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman RJ, Rasmussen SA, Botto LD, et al. The contribution of chromosomal abnormalities to congenital heart defects: a population-based study. Pediatr Cardiol. 2011;32(8):1147–1157. doi: 10.1007/s00246-011-0034-5. [DOI] [PubMed] [Google Scholar]
- 9.Pierpont ME, Basson CT, Benson DW, Jr, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 10.Koppel RI, Druschel CM, Carter T, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111(3):451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 11.de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. Br Med J. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahle WT, Martin GR, Beekman RH, 3rd, Morrow WR. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 13.Mahle WT, Sable CA, Matherne PG, Gaynor JW, Gewitz MH, American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young Key concepts in the evaluation of screening approaches for heart disease in children and adolescents: a science advisory from the American Heart Association. Circulation. 2012;125(22):2796–2801. doi: 10.1161/CIR.0b013e3182579f25. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Rapid implementation of pulse oximetry newborn screening to detect critical congenital heart defects – New Jersey, 2011. Morb Mortal Wkly Rep. 2013;62(15):292–294. [PMC free article] [PubMed] [Google Scholar]
- 15.Garg LF, Van Naarden Braun K, Knapp MM, et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2):e314–e323. doi: 10.1542/peds.2013-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132(3):e595–e603. doi: 10.1542/peds.2013-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemper AR, Kus CA, Ostrander RJ, et al. A framework for key considerations regarding point-of-care screening of newborns. Genet Med. 2012;14(12):951–954. doi: 10.1038/gim.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Institute of Ultrasound in Medicine AIUM practice guideline for the performance of an antepartum obstetric ultrasound examination. J Ultrasound Med. 2003;22(18):1116–1125. doi: 10.7863/jum.2003.22.10.1116. [DOI] [PubMed] [Google Scholar]
- 19.American Institute of Ultrasound in Medicine AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2010;29(1):157–166. doi: 10.7863/jum.2010.29.1.157. [DOI] [PubMed] [Google Scholar]
- 20.Abuhamad AZ, ACOG Committee on Practice Bulletins-Obstetrics Ultrasonography in pregnancy. Obstet Gynecol. 2008;112(4):951–961. doi: 10.1097/AOG.0b013e31818b1fba. ACOG Practice Bulletin Number 98. [DOI] [PubMed] [Google Scholar]
- 21.Fetal Echocardiography Task Force, American Institute of Ultrasound in Medicine Clinical Standards Committee, American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med. 2011;30(1):127–136. doi: 10.7863/jum.2011.30.1.127. [DOI] [PubMed] [Google Scholar]
- 22.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: a population based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 23.Levy DJ, Pretorius DH, Rothman A, et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol. 2013;34(3):670–679. doi: 10.1007/s00246-012-0526-y. [DOI] [PubMed] [Google Scholar]
- 24.Ailes EC, Gilboa SM, Riehle-Colarusso T, et al. Prenatal diagnosis of nonsyndromic congenital heart defects. Prenat Diagn. 2013;34(3):214–222. doi: 10.1002/pd.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Liberman RF, Getz KD, Lin AE, et al. Delayed diagnosis of critical congenital heart defects: trends and associated factors. Pediatrics. 2014;134(2):e373–e381. doi: 10.1542/peds.2013-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036–1040. doi: 10.1016/j.amjcard.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atz AM, Travison TG, Williams IA, et al. Prenatal diagnosis and risk factors for preoperative death in neonates with single right ventricle and systemic outflow obstruction: screening data from the Pediatric Heart Network Single Ventricle Reconstruction Trial. J Thorac Cardiovasc Surg. 2010;140(6):1245–1250. doi: 10.1016/j.jtcvs.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quartermain MD, Pasquali S, Hill K, et al. National variation in prenatal diagnosis of congenital heart disease by state and lesion type: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery (STS-CHS) database. Paper presented at: American College of Cardiology.2014. [Google Scholar]
- 30.Kuehl KS, Loffredo CA, Ferencz C. Failure to diagnose congenital heart disease in infancy. Pediatrics. 1999;103:743–747. doi: 10.1542/peds.103.4.743. 4 Pt 1. [DOI] [PubMed] [Google Scholar]
- 31.Peterson C, Ailes E, Riehle-Colarusso T, et al. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370. doi: 10.1001/jamapediatrics.2013.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson C, Dawson A, Grosse SD, et al. Hospitalizations, costs, and mortality among infants with critical congenital heart disease: how important is timely detection? Birth Defects Res A Clin Mol Teratol. 2013;97(10):664–672. doi: 10.1002/bdra.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawson AL, Cassell CH, Riehle-Colarusso T, et al. Factors associated with late detection of critical congenital heart disease in newborns. Pediatrics. 2013;132(3):e604–e611. doi: 10.1542/peds.2013-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Therrell BL, Jr, Lloyd-Puryear MA, Camp KM, Mann MY. Inborn errors of metabolism identified via newborn screening: ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol Genet Metab. 2014;113(1–2):14–26. doi: 10.1016/j.ymgme.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Identifying infants with hearing loss - United States, 1999-2007. MMWR Morb Mortal Wkly Rep. 2010;59(8):220–223. [PubMed] [Google Scholar]
- 36.Martin GR, Beekman RH, 3rd, Mikula EB, et al. Implementing recommended screening for critical congenital heart disease. Pediatrics. 2013;132(1):e185–e192. doi: 10.1542/peds.2012-3926. [DOI] [PubMed] [Google Scholar]
- 37.Therrell BL, Hannon WH. National evaluation of US newborn screening system components. Ment Retard Dev Disabil Res Rev. 2006;12(4):236–245. doi: 10.1002/mrdd.20124. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Newborn screening for critical congenital heart disease: potential roles of birth defects surveillance programs – United States, 2010-2011. MMWR Morb Mortal Wkly Rep. 2012;61(42):849–853. [PubMed] [Google Scholar]
- 39.Kochilas LK, Lohr JL, Bruhn E, et al. Implementation of critical congenital heart disease screening in Minnesota. Pediatrics. 2013;132(3):e587–e594. doi: 10.1542/peds.2013-0803. [DOI] [PubMed] [Google Scholar]
- 40.Olney RS, Botto LD. Newborn screening for critical congenital heart disease: essential public health roles for birth defects monitoring programs. Birth Defects Res A Clin Mol Teratol. 2012;94(12):965–969. doi: 10.1002/bdra.23103. [DOI] [PubMed] [Google Scholar]
- 41.2014 http://dx.doi.org/10.1111/chd.12235.
- 42.Oster ME, Riehle-Colarusso T, Simeone RM, et al. Public health science agenda for congenital heart defects: report from a Centers for Disease Control and Prevention experts meeting. J Am Heart Assoc. 2013;2(5):e000256. doi: 10.1161/JAHA.113.000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brosco JP, Grosse SD, Ross LF. Universal state newborn screening programs can reduce health disparities. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.2465. http://dx.doi.org/10.1001/jamapediatrics.2014.2465. [DOI] [PMC free article] [PubMed]
- 44.Kemper AR, Boyle CA, Aceves J, et al. Long-term follow-up after diagnosis resulting from newborn screening: statement of the US Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Genet Med. 2008;10(4):259–261. doi: 10.1097/GIM.0b013e31816b64f9. [DOI] [PubMed] [Google Scholar]
- 45.Kochilas LK, Menk JS, Saarinen A, Gaviglio A, Lohr JL. A comparison of retesting rates using alternative testing algorithms in the pilot implementation of critical congenital heart disease screening in Minnesota. Pediatr Cardiol. 2014 doi: 10.1007/s00246-014-1048-6. http://dx.doi.org/10.1007/s00246-014-1048-6. [DOI] [PubMed]
- 46.Suresh GK. Pulse oximetry screening for critical congenital heart disease in neonatal intensive care units. J Perinatol. 2013;33(8):586–588. doi: 10.1038/jp.2012.161. [DOI] [PubMed] [Google Scholar]
- 47.Wright J, Kohn M, Niermeyer S, Rausch CM. Feasibility of critical congenital heart disease newborn screening at moderate altitude. Pediatrics. 2014;133(3):e561–e569. doi: 10.1542/peds.2013-3284. [DOI] [PubMed] [Google Scholar]