Abstract
Background
US soldiers injured in Iraq, and civilian burn trauma patients are treated at the US Army Institute of Surgical Research. Burn patients experience extrem pain during wound care, and they typically receive opioid analgesics and anxiolytics for debridement. Virtual Reality (VR) has been applied as an adjunct to opioid analgesics for procedural pain. We describe the first use of ketamine combined with immersive VR to reduce excessive pain during wound care.
Case Report
A 21-year-old male US Army soldier stationed in Iraq, and a 41-year-old civilian male sustained a 13% and 50% total body surface area (TBSA) burn, respectively. Each patient received 40 mg ketamine intraveneous (IV) for wound care. Using a within-subject design, nurses conducted half of a painful segment of wound care treatments with no VR and the other half with immersive VR. Graphic pain rating scores for each of the two treatment conditions served as the dependent variables.
Results
Compared to ketamine + no VR, both patients reported less pain during ketamine + VR for all three pain ratings. Both patients rated wound care during no VR as “no fun at all”, but those same patients rated wound care during virtual reality as either “pretty fun” or “extremely fun”, and rated nausea as either “mild” or “none”.
Conclusions
Results from these first two cases suggest that a moderate dose of ketamine combined with immersive virtual reality distraction may be an effective multimodal analgesic regimen for reducing acute procedural pain during severe burn wound cleanings.
Keywords: Analgesia, Burn Pain, Wound Care, Distraction, Virtual Reality
Introduction
Opioids are the cornerstone analgesics for patients with severe burn and trauma injuries, but the side effects profile of opioids limit their escalation in dose for acute procedural pain [1–3]. Opioid side effects include nausea/vomiting, constipation, sedation, interference with sleep cycles, increased irritability, itching, urinary retention, cognitive impairment, habituation, respiratory depression, and immunosuppression [4,5]. Opioid analgesia and opioid side effects both become more pronounced as the opioid dose is increased. The fact that burn patients typically undergo one or more painful procedures per day, over the course of weeks or months, makes effective pharmacologic treatment of pain for burn patients especially challenging. Most burn patients report severe to excruciating pain during wound care procedures [6–8]. Additionally, excessive pain on the first day can increase expectation of pain the next day, leading to nocebo hyperalgesia, the functional opposite of the placebo effect [9].
Ketamine, a nonbarbiturate intravenous (IV) analgesic, is commonly used at low doses either alone or as part of a multimodal approach for treating procedural pain in a number of patient populations, including physical trauma and severe burn. Ketamine is used to potentiate opioid analgesia and to allow better pain control without excessive opioid side effects [10,11]. In these two case studies, we explored the multimodal combination of ketamine analgesia plus immersive virtual reality (VR), a non-pharmacologic analgesic. The rationale for adding adjunctive immersive VR to the standard treatment dose of ketamine was to capitalize on the combined analgesic action of the two treatment modalities. Ketamine is a pharmacologic analgesic, which works by reducing transmission of neural nociceptive signals via noncompetitive antagonism of the N-methyl-D-aspartic acid (NMDA) receptor as well as interactions with other receptors, including opioid, muscarinic, monoaminergic, and voltage-sensitive calcium channels. Exposing NMDA receptors to ketamine inhibits neuronal signaling and reduces the number of nociceptive signals transmitted from the pain receptors to the brain, thereby decreasing pain.
In contrast to the pharmacologic mechanism for ketamine analgesia, immersive VR is postulated to reduce pain via a non-pharmacologic attentional mechanism [12–16]. Patients gaze into the VR goggles, interact with a computer generated world, and listen to sound effects and soothing music in their noise-canceling headphones. The goggles block patients’ view of their hospital rooms, obstruct patients from watching their wound care, and substitute synthetic computer-generated sight. Noise-canceling headphones block sounds from the hospital room and substitute more calming music and sound effects. The patient interacts with the virtual world by throwing snowballs at virtual objects, making it more immersive and attention-grabbing. According to this logic, pain requires attention and patients have a limited amount of attention available [17,18]. VR draws a patient’s focus, leaving less concentration available to process incoming nociceptive signals. In support of this attention mechanism, analog studies have shown that, on tasks where the primary objective is to monitor number strings, performance drops significantly when participants engage in VR [13].
When VR is used in combination with opioids, researchers consistently find 30–50% reductions in pain ratings during severe burn wound care, physical therapy, and treatment of soldiers with combat-related burn injuries [19–24]. In addition, analog laboratory studies using functional magnetic resonance imaging brain scans have shown large reductions in pain-related brain activity associated with VR analgesia [16]. Burn patients often receive moderate doses of opioids during daily burn wound care. When comparing the reduction of pain-related brain activity of moderate doses of opioid analgesics with brief laboratory VR treatments, the amount of reduction in pain-related brain activity was similar. Furthermore, the largest drops in pain scores and pain-related brain activity were observed when VR and opioids were combined [25].
VR also appears to show a non-pharmacologic dose-response relationship in which more immersive VR systems (presumed to be more attention-grabbing) reduce pain more effectively than less immersive VR systems [15,26]. For example, in a between-groups, double-blind analog pain study manipulating only helmet quality, more immersive wide field of view VR goggles led to clinically meaningful reductions in pain in two out of three participants, whereas less immersive, narrower field of view VR goggles led to clinically meaningful reductions in pain in only one out of three participants [26]. SnowWorld VR analgesia (University of Washington, Seattle, WA) (http://www.vrpain.com) was specifically designed for burn patients being treated with traditional opioid pain medications. Studies using SnowWorld report little or no side effects from either highly immersive or less immersive VR. We predicted that the multimodal analgesia approach of combining ketamine (pharmacologic) and immersive VR (non-pharmacologic) analgesia would reduce pain more effectively than ketamine alone, without increases in VR side effects.
Subjects
Patient 1 was a 21-year-old male US Army soldier who was injured while stationed in Iraq. The patient suffered severe electrical burns covering 13% of his body while taking a shower. He sustained electrical burns to his right thigh (10 cm × 10 cm exit wound on the anterior right thigh), groin, right chest and flank, right medial upper and lower arm, dorsum of his left hand, and circumferential burn to the left ring finger. He was taken to Landstuhl Regional Medical Center in Landstuhl, Germany and admitted to the ICU for evaluation. The patient was transferred to Brooke Army Medical Center at Fort Sam Houston, Texas and admitted to the US Army Institute of Surgical Research (USAISR) Burn Center for initial acute burn care. In keeping with the standard wound care treatment for severe burns, the patient received frequent dressing changes to maximize wound healing and limit wound infection.
Patient 2 was a 41-year-old civilian male injured in a building fire in Houston, Texas caused by Hurricane Ike. The patient’s initial fluid resuscitation was begun at a civilian hospital in Houston, Texas before evacuation by air ambulance to Brooke Army Medical Center for initial acute burn care at the USAISR Burn Center. During the fire, the patient sustained burns covering 50% of his body, including partial and full thickness burns involving the face, neck, back, and bilateral upper and lower extremities. This patient also had moderate inhalation injury.
Methods
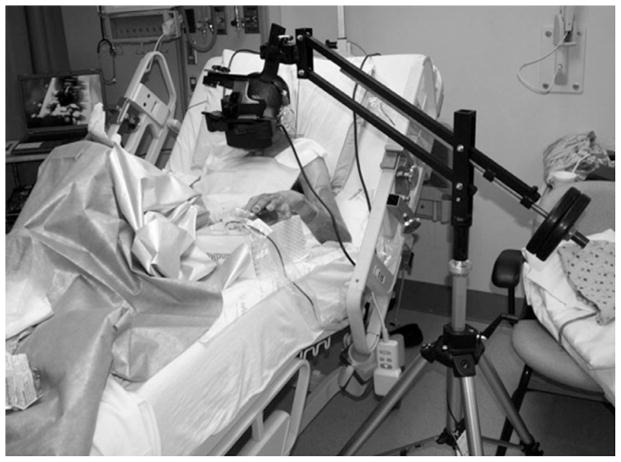
One 10-minute segment of burn wound treatment was divided into two equivalent 5-minute wound care segments. Premedication with 40 mg ketamine IV, given approximately 20 minutes prior to wound care, served as the pharmacologic analgesic for this wound care session. The patients received no VR distraction (standard pre-medication only) during one of the 5-minute wound care sessions. During the second 5-minute wound care session, the patients interacted with the virtual world via the immersive VR goggles, shown in Figure 1.
Figure 1.
US Army soldier receiving immersive virtual reality (VR) to reduce his pain during severe burn wound care. The unique robot-like arm mounted VR goggles designed by Hoffman and built by Jeff Magula at the University of Washington, Seattle, holds the VR goggles near the patient’s eyes weightlessly, reducing the amount of surface contact (if any) needed with the patient. Photo and copyright Hunter Hoffman.
During the two brief pauses in the wound care procedure (once after each 5-minute wound care period), the patients completed three subjective pain ratings using graphic rating scales (GRSs) labelled 0–10 with respect to the preceding 5 minutes of wound care. Each patient was asked to “Please indicate how you felt during the past five minute session by rating your response on the following scales.” Each question was accompanied by a pictorial example of the labelled GRS such as the “pain unpleasantness.”
The pain ratings were obtained using the following questions and scales: “How much time did you spend thinking about your pain during the past five minutes? I thought about my pain during Virtual Reality (0 = none of the time, 1–4 = some of the time, 5 = half of the time, 6–9 = most of the time, and 10 = all of the time),” “Rate your worst pain during the past five minutes during the Virtual Reality (0 = no pain at all, 1–4 = mild pain, 5–6 = moderate pain, 7–9 = severe pain, and 10 = worst pain),” and “How unpleasant was your pain during the past 5 minutes during the Virtual Reality? (GRS as shown in Figure 2 and 0 = not unpleasant at all, 1–4 = mildly unpleasant, 5–6 = moderately unpleasant, 7–9 = severely unpleasant, and 10 = excruciatingly unpleasant).”
Figure 2.
SnowWorld. A screenshot of what patients see in the goggles during immersive virtual reality pain distraction. World designed/developed by Hoffman and Patterson, University of Washington, Seattle, and software created by world builders at Firsthand. Image captured by Firsthand Technologies, copyright Hunter Hoffman.
The patients were also asked “How much fun did you have during Virtual Reality? (0 = no fun at all, 1–4 = mildly fun, 5–6 = moderately fun, 7–9 = pretty fun, 10 = extremely fun),” “To what extent (if at all) did you feel nausea for any reason during Virtual Reality? (10-cm line with numeric and verbal descriptors: 0 = no nausea at all, 1–4 = mild nausea, 5–6 = moderate nausea, 7–9 = severe nausea, and 10 = vomit),” and “While experiencing the virtual world, to what extent did you feel like you went inside the computer-generated world? (10-cm line with numeric and verbal descriptors: 0 = I did not feel like I went inside at all, 1–4 = mild sense of going inside, 5–6 = moderate sense of going inside, 7–9 = strong sense of going inside, 10 = I went completely inside the virtual world).” After the wound care session with no VR, each patient was asked the same questions but “during Virtual Reality” was replaced by “without Virtual Reality.” After wound care with no VR, patients were not asked the question about presence.
Such GRSs have been shown to be valid through their strong associations with other measures of pain intensity as well as through their ability to detect treatment effects [27,28]. The specific measures used in the current study were designed to assess the cognitive component of pain (amount of time spent thinking about pain), the affective component of pain (unpleasantness), and the sensory component of pain (worst pain). Affective and sensory pain are two separately measurable and sometimes differentially influenced components of the pain experience [29]. Gracely et al. have shown ratio scale measures such as the labelled GRSs used in this study to be highly reliable. In addition, a GRS rating of “fun” during wound care was taken [19,30].
Both patients utilized the VR system consisting of a Voodoo Envy laptop with NVIDIA GForce Go 7900 GTX (512 MB) video card, Intel Core 2 Duo (T7400) CPU @ 2.16 GHz, 2 GB RAM @ 994 MHz (Hewlett-Packard, Palo Alto, CA). While in high-tech VR, each subject followed a predetermined path, “gliding” through an icy three-dimensional (3-D) virtual canyon (Figure 2). Patients used robot-like articulated arm mounted goggles, which do not require wearing a helmet and minimize contact with the patients’ face/head (Figure 1).
Each patient scanned the virtual environment and aimed at virtual objects via a computer mouse. The patients projected virtual snowballs at virtual snowmen, igloos, and penguins using the mouse trigger button (see http://www.vrpain.com). In addition to the VR environment scenes and sound effects, auditory background music by recording artist Paul Simon (http://www.paulsimon.com) was played to enhance the immersion VR effects. Participants viewed the VR world through a pair of Rockwell Collins SR-80 VR goggles (Rockwell Collins Optronics, Carlsbad, CA) with a custom-made neoprene blinder on the top and sides of the viewer. These VR goggles afforded an approximately 80° diagonal field of view for each of the rectangular eyepieces with 100% overlap between the right and left eye images. The goggles were held in place near the patient’s eyes by a custom made robot-like arm goggle holding system.
Results
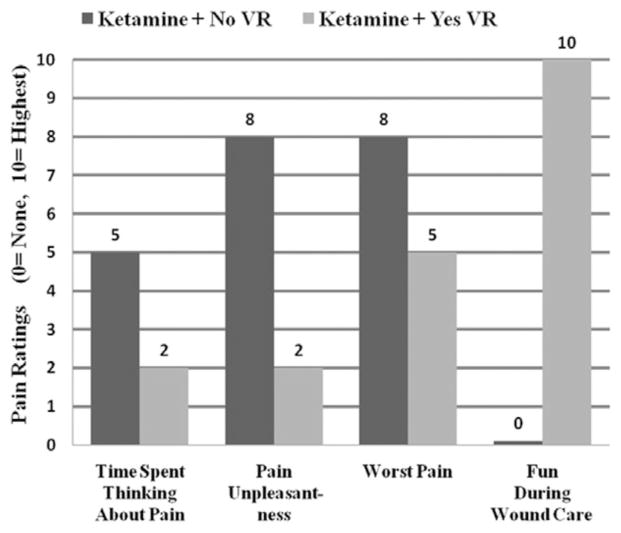
As shown in Figure 3, patient 1 reported less pain on all three pain measures when distracted with VR. “Time spent thinking about pain” ratings dropped from 5/10 (half of the time) to 2/10 (some of the time), “pain unpleasantness” ratings dropped from 8/10 (severe) to 2/10 (mild), and “worst pain” ratings dropped from 8/10 (severe pain) to 5/10 (moderate pain). In addition, wound care was “no fun at all” (0/10) during ketamine + no VR but was “extremely fun” (10/10) during ketamine + VR. The patient reported a “moderate sense of going inside the computer-generated world” during ketamine + VR (presence in VR = 5/10) and rated nausea during VR as zero.
Figure 3.
Patient 1 pain ratings. Compared with ketamine + no virtual reality (VR) (shown in dark gray), patient 1 reported large reductions in pain unpleasantness during ketamine + immersive VR (shown in light gray) during severe burn wound care of burn injury.
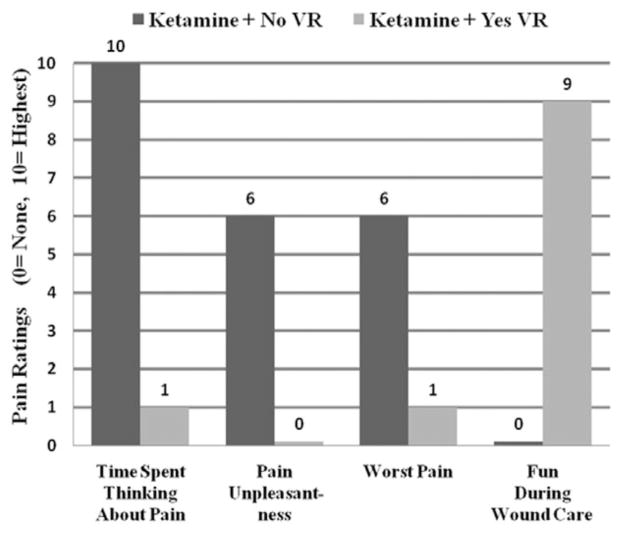
As shown in Figure 4, patient 2 reported a reduction in all three pain measures during ketamine + VR as compared with ketamine alone (no VR). The “Time spent thinking about pain” ratings dropped from 10/10 (all of the time) to 1/10 (some of the time), “pain unpleasantness” ratings dropped from “moderate” (6/10) to “none” (0/10), and “worst pain” ratings dropped from “moderate” (6/10) to “mild pain” (1/10). Wound care was “no fun at all” (0/10) during ketamine + no VR but was “pretty fun” (9/10) during ketamine + VR. The patient reported a “strong sense of going inside the computer-generated world” (presence in VR = 9/10) and rated nausea for any reason during VR as mild (1/10).
Figure 4.
Patient 2 pain ratings. Patient 2 reported large reductions in pain during virtual reality (VR) (shown in light gray) compared with no VR (shown in dark gray) during burn wound care of a severe burn injury resulting from a house fire during Hurricane Ike.
Both patients and their wound care nurses noted that they would prefer VR be available for subsequent dressing changes as they found it to be helpful as an adjunctive modality for pain control.
Discussion
The present case comparison explores the use of ketamine combined with immersive VR as a multimodal analgesia regimen. Results from the present report provide preliminary evidence that ketamine combined with immersive VR is effective for reducing the cognitive, emotional, and sensory components of moderate to severe acute procedural pain in burn patients. In addition to reducing pain, patients reported that wound care was either “pretty fun” or “extremely fun” and rated their illusion of going inside the computer generated world as either moderate or strong. In these two patients, ketamine plus immersive VR provided superior pain relief compared to ketamine alone during burn wound care.
Conventional efforts to control procedural pain via opioid analgesics alone can be problematic. Increased opioid doses are often required and may also increase physical dependence, ileus, respiratory depression, and opioid-induced hyperalgesia [1,31]. Ketamine, a non-barbituate and non-opioid analgesic, is commonly used at low doses, either alone or as part of a multimodal approach to treating procedural pain in a number of patient populations, including physical trauma and severe burn. Ketamine is used to potentiate opioid analgesics and to allow better pain control without excessive opioid side effects [10,11]. Adding non-pharmacologic adjunct such as immersive virtual reality to the multimodal pain regime has potential to reduce pain more than opioids or ketamine alone (or opioids + ketamine combined), with little or no increase in side effects from the VR.
The substantial limitations of case studies are well known. Case studies are scientifically inconclusive by nature [32]. Although case studies are a good way to introduce descriptions of innovative techniques, evidence for effectiveness requires larger clinical studies, for example, randomized controlled clinical studies where patients and ideally researchers are kept blind to some experimental manipulations, to help control for placebo effect and demand characteristics. Future clinical research involving a randomized controlled study might explore whether ketamine combined with a more highly immersive VR system (e.g., using wide field of view VR goggles) is more effective than ketamine combined with a less immersive VR system (e.g., using lower field of view VR goggles) [33]. Experimentally manipulating the immersiveness of the VR system will lead to a better understanding of the relation between VR and pain, helping us improve nonpharmacologic analgesia. Since patients will not know the immersiveness of the VR system is being manipulated, findings could greatly reduce the viability of a demand characteristics/placebo effect explanation for VR pain control. To determine the clinical implications of VR analgesia, research will be needed to assess whether VR remains effective for pain management with repeated use and more clinically relevant treatment durations. Further research may determine whether VR distraction generalizes to reducing acute pain during other painful medical procedures.
Excessive acute pain during medical procedures for burn injuries remains a widespread medical problem in the veteran, military, and civilian health care systems. Additional research on how to reduce excessive acute procedural pain is needed.
Acknowledgments
This study was funded by US Army Institute of Surgical Research. Hunter Hoffman’s time was funded with help from the following NIH grants to Drs. Patterson and Sharar at the UW: NIH 1R01AR054115-01A1, R01GM042725-17A1, the Scan Design Foundation by Inger and Jens Bruun, and private donations from Ross Chambers. Thanks to Admiral Mullens, General Chiarelli, COL Sutherland, COL Friedl, Dr. Belard, Luke Knittig, and to musician Paul Simon for their encouragement.
Footnotes
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Malchow RJ, Black IH. The evolution of pain management in the critically ill trauma patient: Emerging concepts from the global war on terrorism. Crit Care Med. 2008;36(suppl 7):S346–57. doi: 10.1097/CCM.0b013e31817e2fc9. [DOI] [PubMed] [Google Scholar]
- 2.Patterson DR. Practical applications of psychological techniques in controlling burn pain. J Burn Care Rehabil. 1992;13:13–8. doi: 10.1097/00004630-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Patterson DR. Non-opioid-based approaches to burn pain. J Burn Care Rehabil. 1995;16:372–6. doi: 10.1097/00004630-199505001-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 5.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: A review. Am J Ther. 2004;11:354–65. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 6.Carrougher GJ, Ptacek JT, Sharar SR, et al. Comparison of patient satisfaction and self-reports of pain in adult burn-injured patients. J Burn Care Rehabil. 2003;24:1–8. doi: 10.1097/00004630-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Choiniere M, Melzack R, Rondeau J, Girard N, Paquin MJ. The pain of burns: Characteristics and correlates. J Trauma. 1989;29:1531–9. doi: 10.1097/00005373-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Melzack R. The tragedy of needless pain. Sci Am. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 9.Colloca L, Benedetti F. Nocebo hyperalgesia: How anxiety is turned into pain. Curr Opin Anaesthesiol. 2007;20:435–9. doi: 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- 10.Tucker A, Kim YI, Nadeson R, Goodchild CS. Investigation of the potentiation of the analgesic effects of fentanyl by ketamine in humans: A double-blinded, randomised, placebo controlled, crossover study of experimental pain. BMC Anesthesiol. 2005;5:2. doi: 10.1186/1471-2253-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuerong Y, Yuguang H, Xia J, Hailan W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth Analg. 2008;107(6):2032–7. doi: 10.1213/ane.0b013e3181888061. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, Furness TA., 3rd Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain. 2000;85:305–9. doi: 10.1016/s0304-3959(99)00275-4. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HG, Garcia-Palacios A, Kapa VA, Beecher J, Sharar SR. Immersive virtual reality for reducing experimental ischemic pain. Int J Hum Comput Interact. 2003;15:469–86. [Google Scholar]
- 14.Patterson DR, Hoffman HG, Palacios AG, Jensen MJ. Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. J Abnorm Psychol. 2006;115:834–41. doi: 10.1037/0021-843X.115.4.834. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman HG, Sharar SR, Coda B, et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004;111:162–8. doi: 10.1016/j.pain.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman HG, Richards TL, Coda B, et al. Modulation of thermal pain-related brain activity with virtual reality: Evidence from fMRI. Neuroreport. 2004;15:1245–8. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 17.Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–66. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- 19.Hoffman HG, Patterson DR, Seibel E, et al. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24:299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: A controlled study. Clin J Pain. 2000;16:244–50. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain. 2001;17:229–35. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sharar SR, Carrougher GJ, Nakamura D, et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: Preliminary results from 3 ongoing studies. Arch Phys Med Rehabil. 2007;88:S43–9. doi: 10.1016/j.apmr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman HG, Patterson DR, Soltani M, et al. Virtual reality pain control during physical therapy range of motion exercises for a patient with multiple blunt force trauma injuries. Cyberpsychol Behav. 2009;12:47–9. doi: 10.1089/cpb.2008.0056. [DOI] [PubMed] [Google Scholar]
- 24.Maani C, Hoffman HG, DeSocio PA, et al. Pain control during wound care for combat-related burn injuries using custom articulated arm mounted virtual reality goggles. J Cyber Ther Rehabil. 2008;1:193–8. [Google Scholar]
- 25.Hoffman HG, Richards TL, Van Oostrom T, et al. The analgesic effects of opioids and immersive virtual reality distraction: Evidence from subjective and functional brain imaging assessments. Anesth Analg. 2007;105:1776–83. doi: 10.1213/01.ane.0000270205.45146.db. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman HG, Seibel EJ, Richards TL, et al. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain. 2006;7:843–50. doi: 10.1016/j.jpain.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. 2. New York: Guilford Press; 2001. pp. 15–34. [Google Scholar]
- 29.Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman HG, Patterson DR, Magula J, et al. Water-friendly virtual reality pain control during wound care. J Clin Psychol. 2004;60:189–95. doi: 10.1002/jclp.10244. [DOI] [PubMed] [Google Scholar]
- 31.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman HG. Virtual-reality therapy. Sci Am. 2004;291:58–65. doi: 10.1038/scientificamerican0804-58. [DOI] [PubMed] [Google Scholar]
- 33.Campbell DT, Stanley JC. Experimental and quasi-experimental designs for research. Boston: Houghton Mifflin Company; 1963. p. 84. [Google Scholar]