Abstract
The N-methyl-D-aspartate (NMDA) receptor has been implicated in a variety of systems that undergo plastic changes in the central nervous system. We used electron microscopic immunocytochemistry with an antibody directed against an alternatively spliced exon near the C terminus of NMDAR1, the essential functional subunit of the NMDA receptor, to study the distribution of the NMDA receptor in the spinal cord and CA1 region of the hippocampus, two regions where NMDA-mediated long-term plasticity has been demonstrated. In CA1, we found that the NMDA receptor is exclusively expressed on postsynaptic structures. By contrast, in the spinal cord we found that in about one-third of labeled synapses, the receptor is located in the presynaptic terminal, immediately adjacent to the vesicle release site at the active zone. Using combined postembedding immunocytochemistry, we also showed that > 70% of the NMDA receptor immunoreactive terminals are glutamate positive, which suggests that the presynaptic NMDA receptor is an autoreceptor. Nerve ligation studies demonstrated that the receptor is transported in dorsal roots and sciatic nerve to the spinal cord and periphery, respectively. These data indicate that an NMDA autoreceptor is located in terminals of primary afferent fibers, where it could facilitate the transmission of inputs to the spinal cord by increasing the release of neurotransmitter from the primary afferent terminal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. G., Evans R. H. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986 Feb;87(2):345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bustos G., Abarca J., Forray M. I., Gysling K., Bradberry C. W., Roth R. H. Regulation of excitatory amino acid release by N-methyl-D-aspartate receptors in rat striatum: in vivo microdialysis studies. Brain Res. 1992 Jul 10;585(1-2):105–115. doi: 10.1016/0006-8993(92)91195-k. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Methods for antagonizing glutamate neurotoxicity. Cerebrovasc Brain Metab Rev. 1990 Summer;2(2):105–147. [PubMed] [Google Scholar]
- Cincotta M., Beart P. M., Summers R. J., Lodge D. Bidirectional transport of NMDA receptor and ionophore in the vagus nerve. Eur J Pharmacol. 1989 Jan 24;160(1):167–171. doi: 10.1016/0014-2999(89)90668-7. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990 Feb;11(2):77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lodge D. Evidence for involvement of N-methylaspartate receptors in 'wind-up' of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987 Oct 27;424(2):402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- De Biasi S., Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay-Goyet P., Satoh H., Lundberg J. M. Relative involvement of substance P and CGRP mechanisms in antidromic vasodilation in the rat skin. Acta Physiol Scand. 1992 Dec;146(4):537–538. doi: 10.1111/j.1748-1716.1992.tb09460.x. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987 Aug;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dubner R., Ruda M. A. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992 Mar;15(3):96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Durand G. M., Bennett M. V., Zukin R. S. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison C. J., Dougherty P. M., Carlton S. M. Quantitative analysis of substance P and calcitonin gene-related peptide immunohistochemical staining in the dorsal horn of neuropathic MK-801-treated rats. Brain Res. 1993 Apr 2;607(1-2):205–214. doi: 10.1016/0006-8993(93)91508-p. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Young A. B., Penney J. B. Quantitative autoradiographic distribution of L-[3H]glutamate-binding sites in rat central nervous system. J Neurosci. 1984 Aug;4(8):2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Henley J. M., Jenkins R., Hunt S. P. Localisation of glutamate receptor binding sites and mRNAs to the dorsal horn of the rat spinal cord. Neuropharmacology. 1993 Jan;32(1):37–41. doi: 10.1016/0028-3908(93)90127-o. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Huettner J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990 Sep;5(3):255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Kolhekar R., Meller S. T., Gebhart G. F. Characterization of the role of spinal N-methyl-D-aspartate receptors in thermal nociception in the rat. Neuroscience. 1993 Nov;57(2):385–395. doi: 10.1016/0306-4522(93)90070-v. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith I. J., Minson J. B. Complete penetration of antibodies into vibratome sections after glutaraldehyde fixation and ethanol treatment: light and electron microscopy for neuropeptides. J Histochem Cytochem. 1992 Nov;40(11):1741–1749. doi: 10.1177/40.11.1431060. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Weight F. F. Glutamate induces a depolarization of adult rat dorsal root ganglion neurons that is mediated predominantly by NMDA receptors. Neurosci Lett. 1988 Dec 5;94(3):314–320. doi: 10.1016/0304-3940(88)90037-7. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Mao J., Price D. D., Mayer D. J., Lu J., Hayes R. L. Intrathecal MK-801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res. 1992 Apr 3;576(2):254–262. doi: 10.1016/0006-8993(92)90688-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Mosinger J. L., Price M. T., Bai H. Y., Xiao H., Wozniak D. F., Olney J. W. Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in the in vivo adult mammalian retina. Exp Neurol. 1991 Jul;113(1):10–17. doi: 10.1016/0014-4886(91)90140-8. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Malenka R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992 Nov;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
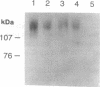
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randić M., Jiang M. C., Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993 Dec;13(12):5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Pignatelli D., Coimbra A. Synaptic architecture of glomeruli in superficial dorsal horn of rat spinal cord, as shown in serial reconstructions. J Neurocytol. 1985 Apr;14(2):203–220. doi: 10.1007/BF01258448. [DOI] [PubMed] [Google Scholar]
- Sheng M., Cummings J., Roldan L. A., Jan Y. N., Jan L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994 Mar 10;368(6467):144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Ohishi H., Nakanishi S., Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett. 1992 Sep 14;144(1-2):229–232. doi: 10.1016/0304-3940(92)90756-w. [DOI] [PubMed] [Google Scholar]
- Siegel S. J., Brose N., Janssen W. G., Gasic G. P., Jahn R., Heinemann S. F., Morrison J. H. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. K., Prusky G. T., O'Leary D. D., Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova T., Stinnakre J., Mallet J. Characterization of a presynaptic glutamate receptor. Science. 1993 Oct 15;262(5132):430–433. doi: 10.1126/science.8105537. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Moriyoshi K., Ishii T., Masu M., Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992 Jun 30;185(3):826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Tingley W. G., Roche K. W., Thompson A. K., Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993 Jul 1;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Tölle T. R., Berthele A., Zieglgänsberger W., Seeburg P. H., Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993 Dec;13(12):5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J., Thompson S. W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991 Mar;44(3):293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]