Abstract
Behavioral genetic studies have robustly indicated that parenting behaviors are heritable – that is, individual differences in parenting are at least partially a function of genetic differences between persons. Few studies, however, have sought to identify the specific genetic variants that are associated with individual differences in parenting. Genes that influence the oxytocin system are of particular interest, given the growing body of evidence that points to the role of oxytocin for social behaviors, including parenting. The current study conducted examinations of associations between a variant in the oxytocin receptor gene (OXTR rs53576) and parental warmth, control, and negativity in a sample of 1,000 twin children and their parents (N=500 families) from the Michigan State University Twin Registry to constructively replicate and extend prior work (Bakermans-Kranenburg & van IJzendoorn, 2008; Michalska et al., 2014). Analyses were conducted both at the level of the child and the level of the parent, allowing us to examine both child-driven (via evocative gene-environment correlation) and parent-driven genetic effects on parenting. Mothers’ OXTR genotype predicted her warmth towards her children, even after controlling for child genotype. This association was not found for fathers. These findings add to the growing body of evidence linking oxytocin functioning to parental behavior and also highlight potential etiological differences in parenting across mothers and fathers.
Keywords: parenting, genetic, evocative rGE, oxytocin, OXTR
Parenting practices vary dramatically across individuals (Belsky, 1984, 2011), and do so with long-term consequences for their offspring (Fearon, Bakermans-Kranenburg, van Ijzendoorn, Lapsley, & Roisman, 2012; Groh et al., 2012). Understanding the etiology of individual differences in parenting is thus a critical area of inquiry. Existing research has found that parenting is associated with a wide range of contextual (Kotchick & Forehand, 2002; Wu et al., 2002) and familial characteristics (Bradley, Corwyn, McAdoo, & García Coll, 2001; Hoff, Laursen, Tardif, & Bornstein, 2002). Individual characteristics of the parents and their children are also important, including parental personality (Kochanska, Friesenborg, Lange, & Martel, 2004), child behavior and temperament (Anderson, Lytton, & Romney, 1986; Kerr, Stattin, & Özdemir, 2012), and parent and child genetic makeup (Kendler, 1996; Neiderhiser et al., 2004; Neiderhiser, Reiss, Lichtenstein, Spotts, & Ganiban, 2007). Indeed, a recent meta-analytic examination of twin and adoption studies of parenting (Klahr & Burt, 2014) confirmed the importance of both ‘direct’ genetic effects (whereby the parent’s own genotype influences his or her parenting) and ‘indirect’ genetic effects (whereby the child evokes parental reactions that are consistent with their genotype; often referred to as an evocative genetic-environment correlation; Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983). Despite this, little is known about how specific genetic variants might contribute to the heritability of parenting.
Genes that influence the oxytocinergic systems are of particular interest when examining parenting. Oxytocin plays a key role in parturition and nursing (Soloff, Alexandrova, & Fernstrom, 1979) as well as affiliation and the formation of social bonds (Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010). Oxytocin has been clearly linked to specific maternal behaviors in several species of mammals. These include licking and grooming in rats (Champagne, Diorio, Sharma, & Meaney, 2001; Pedersen, Ascher, Monroe, & Prange, 1982), interest and involvement in sheep (Kendrick, Keverne, & Baldwin, 1987), and time spent grooming and nursing in rhesus macaques (Maestripieri, Hoffman, Anderson, Carter, & Higley, 2009). In humans, plasma oxytocin is associated with maternal bonding (Levine, Zagoory-Sharon, Feldman, & Weller, 2007), affection (Feldman, Weller, Zagoory-Sharon, & Levine, 2007), parental touch and gaze synchrony with infants (Feldman et al., 2012), maternal and paternal social engagement and affect synchrony with infants (Feldman, Gordon, & Zagoory-Sharon, 2011) and paternal positive parenting (Gordon et al., 2010).
Given the robust association between oxytocin and parenting, the oxytocin receptor gene OXTR (3p25) is a likely candidate for genetic influences on parenting. Oxytocin receptors facilitate the binding of oxytocin to the cell membrane and are widespread throughout the central nervous system. OXTR knock-out mice demonstrate gross deficiencies in maternal behavior (Ragnauth et al., 2005; Takayanagi et al., 2005) and it has been suggested that differences in the genetic regulation of oxytocin receptors may be the basis for differences in social behavior both within species and across species (Donaldson & Young, 2008). In humans, recent work has highlighted an association between several OXTR polymorphisms (OXTR rs2254298, OXTR rs1042778, and OXTR rs53576) and observed parental behaviors (Bakermans-Kranenburg & van IJzendoorn, 2008; Feldman et al., 2012; Michalska et al., 2014).
Variant rs53576, a single nucleotide variant, is a silent G to A change in the third intron of OXTR. The functional significance of this change is currently unknown, although at least one study has suggested it may impact the oxytocin receptor’s shape and/or oxytocin’s ability to bind with the receptor (Tost et al., 2010). Prior research has indicated that the A allele is associated with reduced positive affect (Lucht et al., 2009), reduced prosocial behavior (Israel et al., 2009; Kogan et al., 2011), reduced optimism and increased depressive symptoms (Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011), impaired attachment (Costa et al., 2009), and reduced empathy (Rodrigues, Saslow, Garcia, John, & Keltner, 2009). Carriers of the A allele also show a significant decrease in hypothalamus gray matter volume and a significant increase in functional connectivity between the hypothalamus and the amygdala (Tost et al., 2010), regions that have been associated with maternal responses to infant stimuli (Swain, Lorberbaum, Kose, & Strathearn, 2007; Swain, 2011)1.
More importantly, however, three prior studies have examined associations between OXTR rs53576 genotype and maternal behavior. Bakermans-Kranenburg and van IJzendoorn (2008) examined the association in a sample of 159 Caucasian mothers who were observed with their 2 year old children. They found that mothers carrying the A allele demonstrated less sensitive parenting (Bakermans-Kranenburg & van IJzendoorn, 2008). A subsequent study similarly found that the absence of the A allele (i.e., the G/G genotype) was associated with increased preference for infant faces following intranasal administration of oxytocin in a sample of 57 adults (Marsh et al., 2012). Lastly, Michalska et al. (2014) examined the associations between rs53576 genotype, observed parenting (assessed when children were aged 4–6 years), and maternal neural activation while viewing photographs of their own and other children (assessed 15 years later) in a sample of 40 mothers (mothers were selected for follow-up genotyping and brain imaging if they demonstrated extreme levels of either positive and/or negative parenting at Wave 1). In contrast to Bakermans-Kranenburg and van IJzendoorn (2008) and prior studies linking the A allele to a social-empathy disadvantage (as reviewed above), Michalska and colleagues found higher levels of positive parenting in carriers of the A allele, along with greater activation in regions associated with positive parenting (bilateral orbitofrontal cortex and left anterior cingulate cortex) while viewing photos of their own (vs. other children).
The Current Study
The current study sought to constructively replicate and extend prior research on the association between OXTR rs53576 genotype and parenting, and in this way, further inform our understanding of the biological bases of parenting. We specifically sought to provide not only confirmation of this association, but also to evaluate whether this effect is apparent in parents of school-age children and in both mothers and fathers. There are several important features of this study that we would like to highlight here. First, although still underpowered by current molecular genetic study standards, our sample represents the largest molecular genetic examination of human parenting to date (for any genetic variant; Current N = 500 families; prior studies of rs53576 N ≤ 159 mothers). Secondly, our sample includes both mothers and fathers, while the prior studies only examined mothers. Next, because our assessment included collection of DNA from parents and children, we were able to examine the possibility of both child-driven and parent-driven genetic effects (all existing studies focused on one without controlling for the other). This represents an important advance over prior work, since genetic influences on parenting appear to be both direct (via the parent’s own genotype) and indirect (via the child’s genotype and evocative gene-environment correlation; Klahr & Burt, 2014).
Methods
Participants
Participants were drawn from the population-based Michigan State University Twin Registry (MSUTR), which includes several independent twin projects (Burt & Klump, 2013; Klump & Burt, 2006). The current study included a population-based sample of 500 twin families (N=1,000 twins, 50.2% monozygotic pairs, 494 mothers, and 430 fathers) who were assessed as part of the Twin Study of Behavioral and Emotional Development in Children (TBED-C) within the MSUTR. Assessments took place either in our laboratory at Michigan State University or in participant’s homes (in the event that families were unable to travel to the university). Children provided informed assent, while parents provided informed consent for themselves and their children. The twins were 47% female and ranged in age from 6 to 10 years (M = 8.23, SD = 1.44; although a few twins had turned 11 by the time they participated). 86.4% of twins were Caucasian, 5.4% were African American, 1.2% were Asian, 1% were Hispanic/Latino, 0.4% were Native American, 0.2% were Pacific Islanders, and 5.4% were “other” (including mixed race/ethnicity). To be eligible for participation in the TBED-C, neither twin could have a cognitive or physical disability (as assessed via parental screen) that would preclude completion of the assessment. Recruitment details and participation rates have been provided at length in prior work (see details in Burt & Klump, 2013).
Measures
We examined three core parenting dimensions here: warmth, negativity, and control. Each of these domains of parenting is heritable (Klahr & Burt, 2014) and has been clearly associated with important child outcomes, including child antisocial behavior, anxiety, and substance use (Feinberg, Button, Neiderhiser, Reiss, & Hetherington, 2007; Lynch et al., 2006; McLeod, Wood, & Weisz, 2007). Observer-ratings of each of the three dimensions of parenting were obtained using 8 minute video-taped interactions of each parent-child dyad (i.e., mother-twin 1, mother-twin 2, father-twin 1, and father-twin 2). The on-campus interactions took place in laboratory offices that were set-up to resemble living rooms, with cameras inconspicuously installed in the ceiling. For those assessments occurring in participants’ homes, interactions took place in a family living space with a video camera placed on a tripod in the room. Each parent-child dyad was asked to complete a mildly frustrating task (i.e., use an Etch-a Sketch to draw specific pictures, but parent and child may only use one dial each, thereby requiring cooperation). The task was originally designed for use in child twin families, and has been found to be a reliable and valid tool for assessing the parent-child relationship with school-age children (Deater-Deckard, Pylas, & Petrill, 1997). The videotaped interactions for each parent-child dyad (n=964 mother-child dyads and 817 father-child dyads; some videos were not codeable; e.g., there was no sound, the file was corrupted, etc. In addition, some fathers participated via the mail) were then coded using two independent coding systems, each of which is described below.
Joystick Coding Method
Highly trained research assistants who were blind to all other participant data viewed the interactions and coded parental warmth (e.g., eye contact, interest, praise, physical affection) and parental directiveness or control (e.g., giving directions, taking control of the etch-a-sketch) using the joystick method of video coding (Sadler, Ethier, Gunn, Duong, & Woody, 2009). In this method, a computer joystick apparatus (the Microsoft Sidewinder Force Feedback 2) is utilized to provide momentary ratings of an individual’s interpersonal behavior throughout the course of an interaction. Observers were trained to use movement along the horizontal axis to indicate changes in warmth-related behaviors (from -1,000 to 1,000), and movement along the vertical axis to indicate changes in control-related behaviors (from -1,000 to 1,000). The software program was set to write the joystick position within the Cartesian plane (i.e., the x and y coordinates) to a text file twice per second. Trained observers were able to view the video-taped interactions and the joystick monitor on the screen simultaneously, in order to continuously watch the interaction and move the joystick position accordingly. Each video was observed twice: once to code the parent and once to code the child. In order to minimize error, each interaction was coded by 3–4 observers whose ratings were then averaged. Within each family, different teams coded the dyads of twin 1 and 2, respectively. See Burt, Klahr, Neale, & Klump (2013) and Klahr, Thomas, Hopwood, Klump, & Burt (2013) for more information on the joystick coding system.
For the current study, we made use of overall scores for warmth and control (averaged across the 8 minutes of the interaction). Reliability was calculated within teams of coders on the overall warmth and control scores for all completed videos (interrater alphas averaged .89 for control and .71 for warmth). To ensure consistency in coding across teams, both teams watched and rated the same set of reliability videos each month (Warmth: α = 0.90, Control: α = 0.73). Maternal ratings were available for 92.9% of twins and paternal ratings were available for 75.0% of twins.
PARCHISY Coding Method
An independent team of highly trained research assistants (also blind to all participant data) separately coded the same parent-child interaction videos using the global Parent Child Interaction System (PARCHISY; Deater-Deckard, et al., 1997). In this coding scheme, each video was watched three times: once to code the behavior of the parent, once to code the behavior of the child, and once to code dyadic behaviors between parent and child. Each observer received approximately 85 hours of training and was required to pass observation examinations before coding videotapes. Different coders rated each of the four parent-child dyads within a family, eliminating the possibility of shared method variance. See Burt & Klump, 2014 for more information on the use of the PARCHISY coding system in these data.
For the current study, we focused on global measures of positive and negative parental behavior across the entire interaction: positive content (e.g., use of praise, explanation, open-ended questions), positive affect with child (e.g., smiling, laughing), negative content (e.g., use of criticism, physical control of the dials, and physical control of the child’s hand or body), and negative affect with child (e.g., rejection, frowning, cold/harsh tone). Observer reliability was assessed by randomly assigning 10% of all videos to be rated by a second observer, and then comparing the primary and secondary ratings using intraclass correlations. Intraclass correlations between coders were as follows: r = .88 for positive content, r = .95 for positive affect, r = .97 for negative content, and r = .96 for negative affect. Maternal ratings were available for 93.4% of twins and paternal ratings were available for 74.9% of twins.
Creating parenting composites
Because prior behavioral genetic studies of parenting have focused on the three parenting dimensions of warmth, control, and negativity (Klahr & Burt, 2014), a confirmatory factor analysis was conducted for warmth and negativity, including positive and negative content, positive and negative affect, and warmth (control was not included, as we have only a single indicator of control, and CFA factors must have at least two indicators). The resulting model demonstrated an excellent fit with the observed data (X2 (4) = 5.74, p = 0.22; RMSEA = 0.015). In order to confirm that control should be separated from warmth and negativity, the six observer-rated measures of parenting (positive content, negative content, positive affect, negative affect, warmth, and control) were submitted to a principle-axis exploratory factor analysis followed by a promax rotation. A scree plot suggested a break between the three- and four-factor solutions. The three factors accounted for 35.27%, 17.45%, and 14.64% of the variance in the six measures, respectively. The factors corresponded to warmth (warmth, positive control, and positive affect), negativity (negative control, negative affect), and control (control).
Based on these factor analytic results, composites were created for warmth and negativity by averaging across standardized scores (i.e., joystick-rated warmth, PARCHISY-rated positive content, and PARCHISY-rated positive affect for the warmth composite; PARCHISY-rated negative content and PARCHISY-rated negative affect for the negativity composite). Control was represented using control scores from the joystick coding method only.
Genotyping
Noninvasive saliva samples were collected from all participants via Oragene saliva collection kits (Ottawa, Ontario, Canada). All assays were performed with predesigned and validated TaqMan based assays from Life Technologies following the manufacturer’s recommendations. All reactions were cycled in a PE9700 Thermocycler and read on an Applied Biosystems 7900ht Sequence Detection System. Analysis was performed using the SDS 2.4 software (Life Technologies). The OXTR genotype was coded according to the number of A alleles (G/G = 0; A/G = 1; A/A = 2).
Statistical Analyses
We evaluated OXTR rs53576 associations with parenting via a series of multi-level regressions in which OXTR genotype was regressed onto each of the three aspects of parenting (e.g., regression 1: child OXTR genotype predicting warmth; regression 2: parent OXTR genotype predicting warmth, etc.). Separate analyses were conducted for the three parenting phenotypes (warmth, control, and negativity). Child age, child gender, and participant ethnicity were entered as covariates into all regressions. Regressions were also completed separately by parent gender. A final regression examined parent and child genotypes simultaneously. Analyses were conducted using hierarchical linear modeling (HLM) in SPSS 20.0 (IBMCorp., 2011) to account for the nonindependence of observations within families while maximizing statistical power. In order to adjust for the effects of multiple testing (warmth, control, and negativity regressed onto OXTR genotype, separately for parents and children), we used a Bonferroni correction to establish our significance cut-point (.05 / 6 = .008).
Results
Descriptive Statistics and Correlations
The majority of participants were either homozygous for the G allele (G/G genotype: 43.9% of mothers, 46.8% of fathers, and 43.9% of children) or heterozygous (A/G genotype: 44.0% of mothers, 42.4% of fathers, and 42.4% of children). 9.8% of mothers, 9.4% of fathers, and 10.0% of children carried two minor ‘A’ alleles (i.e., A/A genotype)2. These genotype frequencies are consistent with expected frequencies in the population (48% G/G, 41% A/G, 10% A/A; “SNP rs53576,” 2014).
Descriptive parenting data is presented in Table 1, separately for mothers and fathers. Comparisons across mothers and fathers revealed significant differences across all measures of parenting, with the exception of observed negative affect. Mothers exhibited higher positive content (d = .09, p =.05), positive affect (d = .43, p <.01), and warmth (d = .09, p =.01) than fathers. Fathers exhibited higher negative content (d = .09, p =.05) and higher levels of control (d = .37, p <.01).
Table 1.
Mean levels of parenting, separately for mothers and fathers
| Mothers | Fathers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | N | M | SD | Min | Max | N | |
| Positive content | 2.86* | 0.50 | 1.00 | 5.00 | 932.00 | 2.81* | 0.59 | 1.00 | 5.00 | 749.00 |
| Positive affect | 2.95** | 0.50 | 1.00 | 6.00 | 932.00 | 2.70** | 0.65 | 1.00 | 5.00 | 749.00 |
| Warmth | 213.18** | 193.55 | −601.22 | 687.53 | 929.00 | 196.41** | 168.07 | −471.06 | 770.38 | 751.00 |
|
| ||||||||||
| Control | 307.76** | 283.94 | −647.38 | 942.09 | 929.00 | 408.16** | 257.98 | −715.40 | 820.49 | 751.00 |
|
| ||||||||||
| Negative content | 1.71* | 0.77 | 1.00 | 6.00 | 932.00 | 1.78* | 0.79 | 1.00 | 4.00 | 749.00 |
| Negative affect | 1.35 | 0.65 | 1.00 | 5.00 | 932.00 | 1.33 | 0.63 | 1.00 | 5.00 | 749.00 |
Note. SD = standard deviation; Min = minimum; Max = maximum; N = number of children. Warmth and negativity composites were created using standardized variables. Possible minimum and maximum scores for positive content, positive affect, negative content, and negative content = 1 (min) and 7 (max). Possible minimum and maximum scores for warmth and control = −1,000 (min) and 1,000 (max).
Means are significantly different across mothers and fathers, p < .05.
Means are significantly different across mothers and fathers, p < .01.
Phenotypic correlations among the three parenting composites along with twin age and ethnicity are presented in Table 2. As seen there, warmth and negativity were modestly and negatively associated in both mothers and fathers. Control was negatively associated with warmth and positively associated with negativity. Importantly, however, all of these associations were small-to-moderate in magnitude (no single correlation exceeded .33). Twin age was not associated with warmth or negativity, but was negatively associated with control (such that parents exhibited more control with younger children, as expected). Ethnicity was associated with warmth and negativity, such that minority parents exhibited lower levels of warmth and higher levels of negativity.
Table 2.
Phenotypic correlations for parenting composites with child age and ethnicity
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Mothers | ||||
| 1. Warmth | ||||
| 2. Control | −0.25 | |||
| 3. Negativity | −0.33 | 0.26 | ||
| 4. Twin age | 0.03 | −0.23 | −0.02 | |
| 5. Ethnicity | −0.12 | 0.04 | 0.09 | 0.02 |
|
| ||||
| Fathers | ||||
| 1. Warmth | ||||
| 2. Control | −0.22 | |||
| 3. Negativity | −0.30 | 0.21 | ||
| 4. Twin age | −0.01 | −0.20 | −0.02 | |
| 5. Ethnicity | −0.18 | 0.08 | 0.12 | 0.02 |
Note. Bolded correlations are significant at p <.01. Correlations in italics are significant at p <.05. Ethnicity was coded as 0 = white/Caucasian and 1 = non-white/Caucasian
HLM regressions
We next performed a series of HLM regressions, regressing parenting onto OXTR genotype along with child gender, age, and ethnicity. To adjust for non-independence, families (rather than individual family members) were the unit of analysis3. We first examined evocative genetic effects of child genotype on parenting, examining whether child genotype predicted the parenting he or she received. We then examined the impact of parent genotype on parenting. All regressions were computed separately for mothers and for fathers.
HLM results are presented in Table 3. As seen there, child OXTR genotype did not predict the parenting received by the child. Paternal OXTR genotype also did not predict father’s parenting. By contrast, maternal OXTR genotype was significantly associated with maternal warmth (β = −0.28, SE = 0.10, p = .004). As seen in Figure 1, this effect was largely driven by the AA genotype, such that mothers with the AA genotype were significantly less warm with their children than were mother with the GG or AG genotypes (p < .01 and p = .01, respectively, for each pair-wise comparison). In order to confirm the robustness of these findings, mother OXTR genotype was entered into an HLM regression along with child OXTR genotype (retaining child age, gender, and ethnicity as covariates). The effect of maternal OXTR genotype on warmth persisted even when controlling for child OXTR genotype (Child OXTR: β = −0.05, SE = 0.05, p = .32; Mother OXTR: β = −0.27, SE = 0.10, p = .008), providing further confirmation of the parent-driven nature of this effect. In short, maternal OXTR appears to uniquely predict her warmth with her children4.
Table 3.
Hierarchical linear modeling results for evocative genetic and direct genetic effects, separately for mothers and fathers
| Mother’s Parenting | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Warmth | Control | Negativity | |||||||
| β | SE | p | β | SE | p | β | SE | p | |
|
|
|||||||||
| Child OXTR | −0.09 | 0.05 | 0.08 | 0.10 | 0.07 | 0.19 | 0.18 | 0.10 | 0.07 |
| Mother OXTR | −0.28 | 0.10 | 0.004* | 0.11 | 0.14 | 0.43 | 0.19 | 0.12 | 0.10 |
|
Father’s Parenting | |||||||||
| Child OXTR | −0.08 | 0.06 | 0.23 | 0.20 | 0.13 | 0.14 | 0.04 | 0.12 | 0.70 |
| Father OXTR | −0.02 | 0.11 | 0.83 | 0.07 | 0.15 | 0.65 | 0.17 | 0.12 | 0.16 |
Note. SE = standard error. All models included child gender, age, and ethnicity entered as covariates. OXTR was coded as G/G = 0, A/G = 1, and A/A = 2.
significant at p < .0008 (Bonferroni-corrected).
Figure 1.
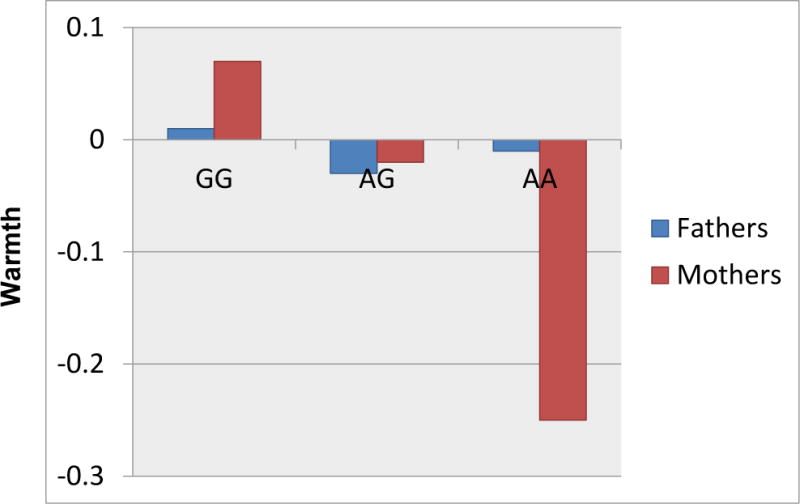
Parent OXTR Genotype and Warmth
Note. Warmth scores are standard scores.
Discussion
The current study investigated the association between child and parent OXTR genotype (OXTR rs53576) and observed parental warmth, control, and negativity in both mothers and fathers in a population-based sample of 500 families with twin children between the ages of 6 and 10, in an attempt to replicate and extend prior research (Bakermans-Kranenburg & van IJzendoorn, 2008; Michalska et al., 2014). Although only preliminary given the sample size, our results revealed a significant association between maternal OXTR rs53576 genotype and warmth, even when controlling for the effects of child age, gender, and ethnicity, as well as child-driven evocative effects. This finding not only serves as a constructive replication of previous research examining the relationship between variants within OXTR and parenting (Bakermans-Kranenburg & van IJzendoorn, 2008; Feldman et al., 2012; Michalska et al., 2014), but also adds to the growing body of research supporting a link between oxytocin functioning and parenting (Feldman et al., 2012; Weisman, Zagoory-Sharon, & Feldman, 2012).
There are several limitations to the current study, however. As indicated above, the first of these relates to the small sample size. In particular, although this is the largest study to examine a molecular genetic association with parenting to date, the current sample size is still very small by molecular genetic standards. We therefore consider these results to be only preliminary, and emphasize that replication of the current results is needed in order to draw firmer conclusions about OXTR rs53576 and its associations with parenting. Next, these results only apply to families with elementary school-aged children. Indeed, recent meta-analytic work suggests that the genetic and environmental etiology of parenting shifts across development (Klahr & Burt, 2014). It is thus unclear whether maternal OXTR genotype is associated with warmth during other developmental periods (although some evidence suggests that this association is also present during early childhood; Bakermans-Kranenburg & van IJzendoorn, 2008).
A third possible limitation relates to our use of observer ratings of parenting, which capture only a very small slice of parental behavior. Despite this, we would argue that the use of observer-ratings represents a strength of the current study overall, as it avoids the pitfalls associated with child and parent reports of parenting (e.g., recency effects, social desirability biases; De Los Reyes & Kazdin, 2005; Morsbach & Prinz, 2006; Paulhus & Vazire, 2007). Another possible limitation relates to our approach to addressing ethnic heterogeneity. We elected to include all participants in order to increase the applicability of our findings. An alternative approach, however, is to limit ethnic heterogeneity within a sample. We re-ran our analyses using the latter approach, examining only Caucasian families (the largest ethnic group in our sample). Our results were entirely unchanged. However, because of population differences in minor allele frequencies for rs53576 (“SNP rs53576,” 2014)5, additional research examining associations between rs53576 genotype and parenting within other racial and ethnic groups is needed. In addition, we relied upon self-reported ethnicity for our analyses. Future work should make use of ancestry markers to more accurately control for possible confounding by population stratification.
Finally, and most importantly, the current study relied upon a candidate gene approach, which has come under pointed criticism. Non-replication is frequent (Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001) and the focus on a single candidate gene does not capture the genetic complexity of most human behaviors. The variant examined herein represents one of many hundreds or even thousands of variants that are likely to be associated with parenting. In particular, additional variants within the OXTR gene (e.g., rs2254298 and rs1042778) and other candidate genes should be considered. Future research should examine parenting using genome-wide association study (GWAS) techniques for a more comprehensive approach to identifying variants, and should also consider the role of epigenetic effects. Ultimately, however, research across multiple levels of analysis is necessary for understanding the biological bases of parenting (e.g., animal research, genetics, neuroscience).
Despite these limitations, the current study has several important (if preliminary) implications. Previous research has highlighted the possible role of OXTR rs53576 for socially relevant phenotypes including prosocial behavior (Israel et al., 2009; Kogan et al., 2011), empathy (Rodrigues, Saslow, Garcia, John, & Keltner, 2009), and attachment (Costa et al., 2009). The current results confirm and extend this previous work, suggesting that the association between the A/A genotype of OXTR and social behavior extends to low maternal warmth. That said, it is important to note that a recent meta-analysis found that effect sizes for rs53576 did not differ from zero in any outcome domain (biology, personality, social behavior, psychopathology, and autism) nor in all domains combined (N = 52 effect sizes, 17,557 participants; Bakermans-Kranenburg & van Ijzendoorn, 2014). Although the original study of parenting and rs53576 (i.e., Bakermans-Kranenburg & van IJzendoorn, 2008) was included in the meta-analysis, there were not enough existing studies to conduct a meta-analysis focused solely on rs53576 and parental behavior. Thus, it is possible that rs53576 may be particularly important for maternal warmth and less so for other social phenotypes. Recent genetic neuroimaging work provides some insight into links between rs53576, brain function, and parenting (Michalska et al., 2014; Tost et al., 2010), but more research is needed.
In addition to highlighting the role of OXTR, our results point to a possible distinction between mothers and fathers in the role of OXTR for individual differences in parenting. Previous research has demonstrated that mothers and fathers differ in their parenting practices (Collins & Russell, 1991; Cowan, Cowan, & Kerig, 1993; Craig, 2006) and that the etiology of parenting differs across mothers and fathers (e.g., evocative genetic effects exert a greater influence on maternal parenting while fathers are more influenced by the shared environment; Klahr & Burt, 2014). The current results add to this growing body of research by suggesting that different variants may comprise genetic effects on parenting in mothers vs. fathers, at least to some extent. This is not to say that oxytocin is unimportant for parenting in fathers. Indeed, there is evidence supporting the role of oxytocin in fathering (Feldman et al., 2011, 2007; Gordon et al., 2010; Naber, Poslawsky, van Ijzendoorn, van Engeland, & Bakermans-Kranenburg, 2012; Weisman et al., 2012). However, the etiology of oxytocin functioning and its association with parenting may differ for mothers and fathers (e.g., paternal oxytocin might be more influenced by exposure to infant/child cues (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010) and possible epigenetic changes, while maternal oxytocin functioning might be more genetically “hard-wired”). The current findings further highlight the known phenotypic and etiological distinctions between mothering and fathering, and point to the need for additional research.
It is also worth noting that we did not find evidence for an evocative genetic effect of child rs53576 genotype on parenting. This finding does not suggest that evocative genetic effects are unimportant, however, as behavioral genetic research suggests that evocative genetic effects appear to account for a moderate proportion of the variance in parenting (Klahr & Burt, 2014). Instead, our results suggest that other genetic variants (and not rs53576) are likely to comprise evocative genetic effects. The behavioral genetic literature can provide some insight into which genes may be most important. For example, twin research has found that the association between child aggression and maternal negativity appears to be partly driven by evocative genetic effects (Klahr, Klump, & Burt, 2014; Marceau et al., 2013). Therefore, research seeking to identify evocative genetic effects on parenting might begin by identifying genes that are associated with childhood aggression and then testing for the possibility of evocative effects on parenting.
In sum, there is growing evidence for a link between oxytocin functioning and parenting. This association has multiple translational implications for clinical practice. In particular, the OXTR rs53576 genotype, along with peripheral measures of oxytocin functioning, may serve as markers of biological risk for poor parenting in mothers (although a great deal of additional research is needed to establish the validity and clinical utility of biological risk markers for poor parenting). Such markers, along with other risk indicators, could conceivably be used to target psychosocial parenting interventions to the most at-risk families. Given the foundational role of maternal warmth for healthy developmental outcomes in children (Fearon et al., 2012; Groh et al., 2012), the early identification of at-risk mothers (i.e., before problems in parenting precipitate deleterious child outcomes) may well pay dividends down the line. Oxytocin manipulations are also a promising avenue of research (Manning, Hoffman, Anderson, Carter, & Higley, 2008). For example, two recent double-blind studies found that intranasal administration of oxytocin was associated with increased sensitivity in fathers of toddlers with autism spectrum disorders and in fathers of typically developing toddlers (Naber et al., 2012) and increased social reciprocity in fathers toward their infants (Weisman et al., 2012). Future work should further elucidate the role of oxytocin in parenting behavior in order to inform clinical assessment and intervention practices for the benefit of both parents and children.
Acknowledgments
This project was supported by R01-MH081813 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, or the National Institutes of Health. The authors thank all participants for making this work possible.
Appendix I: Genotypes for children, mothers, and fathers, separately by reported race/ethnicity
| Children | Mothers | Fathers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Race/Ethnicity | GG | AG | AA | GG | AG | AA | GG | AG | AA |
| Caucasian | 376 (45.2%) |
372 (44.8%) |
83 (10%) |
182 (44.3%) |
190 (46.2%) |
39 (9.5%) |
160 (47.6%) |
142 (42.3%) |
32 (9.5%) |
| African-American | 32 (62.7%) |
16 (31.4%) |
3 (5.9%) |
13 (54.2%) |
8 (33.3%) |
3 (12.5%) |
8 (47%) |
9 (53%) |
0 (0.0%) |
| Hispanic/Latino | 6 (60%) |
4 (40%) |
0 (0.0%) |
2 (50%) |
2 (50%) |
0 (0.0%) |
4 (57.1%) |
2 (28.6%) |
1 (14.3%) |
| Asian | 1 (8.3%) |
5 (41.7%) |
6 (50%) |
1 (25%) |
1 (25%) |
2 (50%) |
2 (50%) |
2 (50%) |
0 (0.0%) |
| Native American | 2 (50%) |
0 (0.0%) |
2 (50%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
| Pacific Islander | 2 (100%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
2 (66.7%) |
1 (33.3%) |
| Other/Mixed Race | 20 (37.7%) |
27 (50.9%) |
6 (11.3%) |
6 (46.2%) |
7 (53.8%) |
0 (0.0%) |
8 (47.1%) |
7 (41.2%) |
2 (11.8%) |
Note. Race/ethnicity is based on self-reports for parents and parent-reports for children. GG, AG, and AA = OXTR rs53576 genotype.
Footnotes
Notably, a recent meta-analysis found no significant association between rs53576 and any of the domains they considered (biology, personality, social behavior, psychopathology, and autism). Parenting was included in the social behavior domain but could not be considered independently because too few studies had been conducted (Bakermans-Kranenburg & van Ijzendoorn, 2014).
2.7% of mothers, 1.4% of fathers, and 3.7% of children had missing genotypic data due to failure to provide a saliva sample, insufficient quantity of sample, and/or the laboratory’s inability to conclusively determine genotype based on the sample.
Child genotype was used to predict parenting, separately for each twin. Parent genotype was also used to predict the parenting they provided, separately for each twin (i.e., parenting was not averaged across the twins). To adjust for the non-independence of parenting observations within families, analyses were conducted in HLM.
In order to confirm that our results did not differ across biological and non-biological parents, we re-ran our analyses excluding step-parents (n = 1 biological grandmother, 1 biological grandfather, and 10 step-fathers). Conclusions were entirely unchanged. Conclusions were also unchanged when excluding families who participated in home visits rather than in-lab assessments. Finally, we added zygosity as a covariate to ensure that results did not differ across parents of MZ vs. DZ twins. The results were unchanged.
See Appendix for allele frequencies, separately by race/ethnicity, within the current sample.
Works Cited
- Anderson KE, Lytton H, Romney DM. Mothers’ interactions with normal and conduct-disordered boys: Who affects whom? Developmental Psychology. 1986;22(5):604. doi: 10.1037/0012-1649.22.5.604. [DOI] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics. 2014;24(2):45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Belsky J. The determinants of parenting: a process model. Child Development. 1984;55(1):83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6705636. [DOI] [PubMed] [Google Scholar]
- Belsky J. The Determinants of Parenting in GxE Perspective: A Case of Differential Susceptibility? Biosocial Foundations of Family Processes. 2011:61–68. doi: 10.1007/978-1-4419-7361-0_4. [DOI] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, García Coll C. The home environments of children in the United States part I: Variations by age, ethnicity, and poverty status. Child Development. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klahr AM, Neale MC, Klump KL. Maternal warmth and directiveness jointly moderate the etiology of childhood conduct problems. Journal of Child Psychology and Psychiatry. 2013;54(10):1030–7. doi: 10.1111/jcpp.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan state university twin registry (MSUTR): An update. Twin Research and Human Genetics. 2013;16(1):344. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL. Parent-child conflict as an etiological moderator of childhood conduct problems: an example of a “bioecological” gene-environment interaction. Psychological Medicine. 2014;44:1065–1076. doi: 10.1017/S0033291713001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences. 2001;98(22):12736. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, Russell G. Mother-child and father-child relationships in middle childhood and adolescence: A developmental analysis. Developmental Review. 1991;11(2):99–136. doi: 10.1016/0273-2297(91)90004-8. [DOI] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Galderisi S. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34(10):1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Cowan PA, Cowan CP, Kerig PK. Mothers, fathers, sons, and daughters: Gender differences in family formation and parenting style. In: Cowan PA, Field D, Hansen DA, Skolnick A, Swanson GE, editors. Family self and society: Toward a new agenda for family research. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1993. pp. 165–195. [Google Scholar]
- Craig L. Does Father Care Mean Fathers Share?: A Comparison of How Mothers and Fathers in Intact Families Spend Time with Children. Gender & Society;Gender & Society. 2006;20(2):259–281. doi: 10.1177/0891243205285212. [DOI] [Google Scholar]
- De Los Reyes A, Kazdin AE. Informant Discrepancies in the Assessment of Childhood Psychopathology: A Critical Review, Theoretical Framework, and Recommendations for Further Study. Psychological Bulletin. 2005;131(4):483–509. doi: 10.1037/0033-2909.131.4.483. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: a meta-analytic study. Child Development. 2012;81(2):435–56. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Feinberg ME, Button TMM, Neiderhiser JM, Reiss D, Hetherington EM. Parenting and Adolescent Antisocial Behavior and Depression: Evidence of Genotype x Parenting Environment Interaction. Arch Gen Psychiatry. 2007;64(4):457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–41. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011;14(4):752–61. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18(11):965–70. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72(3):175–81. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh AM, Roisman GI, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Fearon RP, Lapsley AM. The significance of insecure and disorganized attachment for children’s internalizing symptoms: A meta-analytic study. Child Development. 2012;81(2):591–610. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Hoff E, Laursen B, Tardif T, Bornstein MH. Socioeconomic status and parenting. Handbook of Parenting: Vol. 2: Biology and Ecology of Parenting. (2) 2002:231–252. [Google Scholar]
- Ioannidis JPA, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29(3):306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, Knafo A. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4(5):e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Parenting: A genetic-epidemiologic perspective. The American Journal of Psychiatry. 1996;153(1):11–20. doi: 10.1017/S0033291797004704. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46(1):56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kerr M, Stattin H, Özdemir M. Perceived parenting style and adolescent adjustment: Revisiting directions of effects and the role of parental knowledge. Developmental Psychology. 2012;48(6):1540–1553. doi: 10.1037/a0027720. [DOI] [PubMed] [Google Scholar]
- Klahr AM, Burt SA. Elucidating the etiology of individual differences in parenting: A meta-analysis of behavioral genetic research. Psychological Bulletin. 2014;140:544–586. doi: 10.1037/a0034205. [DOI] [PubMed] [Google Scholar]
- Klahr AM, Klump KL, Burt SA. The etiology of the association between child antisocial behavior and maternal negativity varies across aggressive and non-aggressive rule-breaking forms of antisocial behavior. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr AM, Thomas KM, Hopwood CJ, Klump KL, Burt SA. Evocative gene-enviornment correlation in the mother-child relationship: A twin study of interpersonal processes. Development and Psychopathology. 2013;25(1):105–18. doi: 10.1017/S0954579412000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies. 2006;9(6):971–7. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Friesenborg AE, Lange La, Martel MM. Parents’ personality and infants’ temperament as contributors to their emerging relationship. Journal of Personality and Social Psychology; Journal of Personality and Social Psychology. 2004;86(5):744. doi: 10.1037/0022-3514.86.5.744. [DOI] [PubMed] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rodrigues Saturn S. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(48):19189–92. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchick BA, Forehand R. Putting parenting in perspective: A discussion of the contextual factors that shape parenting practices. Journal of Child and Family Studies. 2002;11(3):255–269. doi: 10.1023/A:1016863921662. [DOI] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28(6):1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Kroemer H. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(5):860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lynch SK, Turkheimer E, D’Onofrio BM, Mendle J, Emery RE, Slutske WS, Martin NG. A genetically informed study of the association between harsh punishment and offspring behavioral problems. Journal of Family Psychology. 2006;20(2):190–198. doi: 10.1037/0893-3200.20.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiology & Behavior. 2009;96(4–5):613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Hoffman CL, Anderson GM, Carter CS, Higley JD. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Progress in Brain Research. 2008:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Marceau K, Horwitz BN, Narusyte J, Ganiban JM, Spotts EL, Reiss D, Neiderhiser JM. Gene-environment correlation underlying the association between parental negativity and adolescent externalizing problems. Child Development. 2013;84(6):2031–46. doi: 10.1111/cdev.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJR. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224(4):469–76. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Wood JJ, Weisz JR. Examining the association between parenting and childhood anxiety: A meta-analysis. Clinical Psychology Review. 2007;27(2):155–172. doi: 10.1016/j.cpr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, Lahey BB. Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Frontiers in Behavioral Neuroscience. 2014;8:21. doi: 10.3389/fnbeh.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsbach S, Prinz R. Understanding and Improving the Validity of Self-Report of Parenting. Clinical Child and Family Psychology Review. 2006;9(1):1–21. doi: 10.1007/s10567-006-0001-5. [DOI] [PubMed] [Google Scholar]
- Naber FBA, Poslawsky IE, van Ijzendoorn MH, van Engeland H, Bakermans-Kranenburg MJ. Brief report: oxytocin enhances paternal sensitivity to a child with autism: a double-blind within-subject experiment with intranasally administered oxytocin. Journal of Autism and Developmental Disorders. 2012;43(1):224–9. doi: 10.1007/s10803-012-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Lichtenstein P, Spotts EL, Ganiban J. Father-adolescent relationships and the role of genotype-environment correlation. Journal of Family Psychology. 2007;21(4):560–571. doi: 10.1037/0893-3200.21.4.560. [DOI] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Pedersen NL, Lichtenstein P, Spotts EL, Hansson K, Elthammer O. Genetic and Environmental Influences on Mothering of Adolescents: A Comparison of Two Samples. Developmental Psychology. 2004;40(3):335–351. doi: 10.1037/0012-1649.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulhus DL, Vazire S. The self-report method. In: Robins RW, Fraley RC, Krueger RF, editors. Handbook of research methods in personality psychology. New York, NY, US: Guilford Press; 2007. pp. 224–239. [Google Scholar]
- Pedersen C, Ascher J, Monroe Y, Prange A. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84(2):309–322. doi: 10.1037/0033-2909.84.2.309. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain and Behavior. 2005;4(4):229–39. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15118–22. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. Retrieved from http://ukpmc.ac.uk/abstract/MED/6683622. [DOI] [PubMed] [Google Scholar]
- SNP rs53576. 2014 Retrieved April 14, 2014, from https://opensnp.org/snps/rs53576.
- Soloff M, Alexandrova M, Fernstrom M. Oxytocin receptors: triggers for parturition and lactation? Science. 1979;204(4399):1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- Swain JE. The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(5):1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48(3–4):262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences. 2010;107(31):13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin Administration to Parent Enhances Infant Physiological and Behavioral Readiness for Social Engagement. Biological Psychiatry. 2012;72(12):982–989. doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Wu P, Robinson CC, Yang C, Hart CH, Olsen SF, Porter CL, Wu X. Similarities and differences in mothers’ parenting of preschoolers in China and the United States. International Journal of Behavioral Development. 2002;26(6):481–491. doi: 10.1080/01650250143000436. [DOI] [Google Scholar]


