Introduction
Progress in the biomedical sciences has accelerated enormously over the last two decades. Technology to enable discovery of ligands to perturb the function of biological targets has also advanced such that many complementary approaches exist for creating small molecule tools to interrogate biological processes and potentially serve as drug leads.1 However, while pharmaceutical industry investment in R&D has grown exponentially, approval of new medicines has remained constant2 and pricing pressures and litigation have further eroded profitability.3 Frequent mergers, reorganizations and reductions in scientific staff in the for-profit drug discovery sector have made it increasingly difficult for a research project or strategy to bear fruit before it is abandoned for nontechnical reasons.4 As a result, there is a growing trend for large pharmaceutical companies to externalize the early phases of drug discovery via either active partnerships or opportunistic in-licensing of novel compounds.
In this context, there is a clear societal need for enhanced innovation and productivity in drug discovery in order for advances in biomedical research to result in new medicines. Academia is a critical area where innovation in drug discovery can flourish. Indeed, two recent reviews provided strong support for the role of academics and biotechnology companies (often spawned from academia) in producing 56% of the new small molecule and biologic drugs judged by the FDA to warrant priority review in the period spanning 1998-2007.5, 6 While over the years individual investigators in academia have been successful at small molecule drug discovery, the conditions outlined above have led to more formal academic drug discovery (ADD) efforts during the last decade. This trend has been noted and a number of scientists engaged in this endeavor have provided commentary on the emerging field.7-11 However, the analyses to date have relied almost exclusively on expert opinion and very rarely upon data.
In the current article we report the results of a scientific survey of academic and non-profit drug discovery entities in the United States and our analysis of the current state of this enterprise and prospects for the future. We intentionally excluded work on large molecules such as monoclonal antibodies or siRNA because our intent was to capture the emerging trend of systematic focus on small molecule drug discovery. To accomplish this we utilized survey methodology currently employed in organizational and general social science research, as described in the Supplementary Materials. Seventy-eight units were identified as small molecule drug discovery centers sited within universities or non-profit research organizations and our response rate was 71% which compares very favorably to average response rates in web surveys.
Research Portfolios, Capabilities and Innovative Posture
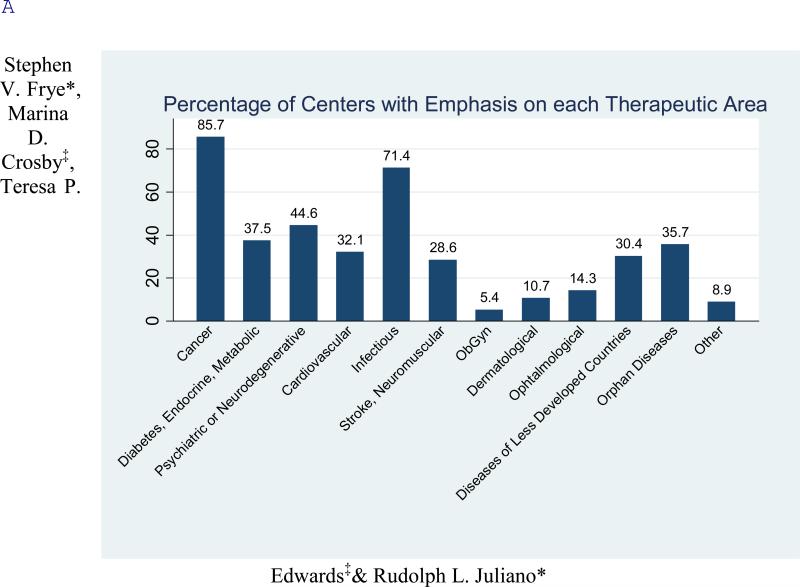
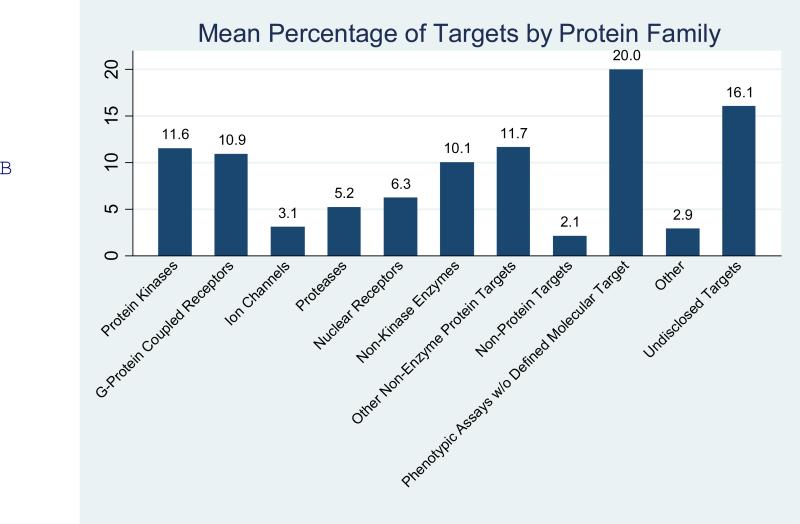
A broad range of therapeutic areas were included among the interests of the various ADD centers (Figure 1A). Not surprisingly cancer and infectious disease were the most common, with 86% and 71% respectively of centers reporting these areas in their project portfolios. In an interesting contrast to the historic direction of commercial drug research, diseases of less developed countries (30%) and orphan diseases (36%) were major concerns of many centers. Other important areas of emphasis were cardiovascular, CNS and metabolic diseases. Only one center reported activity in airway diseases such as asthma and COPD although there is an increasing burden of this type of disease in the US. A broad array of potential drug targets is being addressed by the centers (Figure 1B). This includes many classes of known ‘druggable’ targets such as protein kinases, G-protein coupled receptors, and nuclear receptors.12 However, it also includes considerable activity involving less traditional targets such as non-enzyme proteins (12%) and phenotypic assays lacking a defined target (20%).
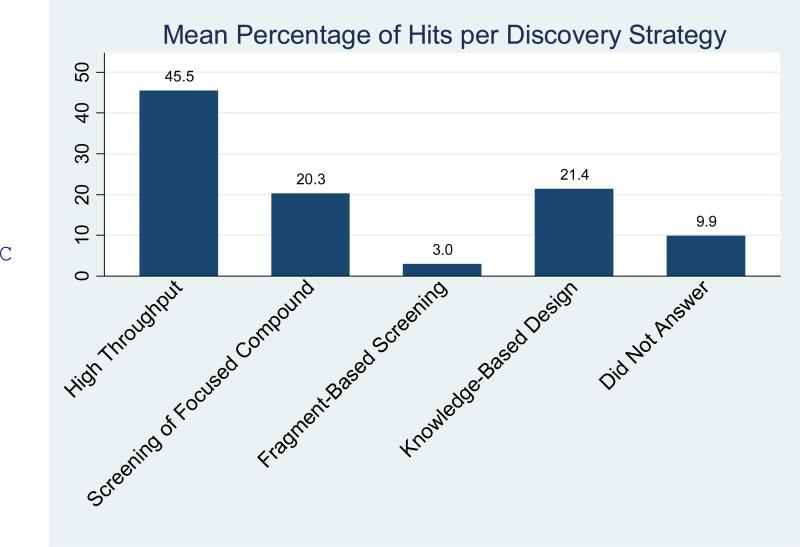
Figure 1.
Therapeutic (A) and target-class focus (B) (see supplemental information, survey questions 14 and 15). The undisclosed targets result from a small number of centers providing no information. (C) Sources of hits by discovery strategy. The figure indicates tractable hits that originated from each strategy averaged over all respondents (see supplemental information, survey question 19).
The research capabilities resident within the centers seemed to emphasize the early phases of drug discovery (Supplementary Figure S1). Thus most of the centers reported capabilities for in vitro or cell based primary assay development (93%), target identification (77%), and cellular biology and secondary functional assay development (79%). With 72% of centers reporting capabilities in hit to lead medicinal chemistry, the integration of chemistry into academic centers has also progressed significantly. By contrast, only about one half of the centers reported capabilities for in vivo efficacy testing (51%) or dmpk studies (42%). Somewhat surprisingly, given the recent emphasis on this aspect, only 65% of centers reported high throughput screening capabilities for libraries of one hundred thousand compounds or more. Nonetheless, high throughput screening accounted for 45% of the generation of tractable hits with focused library screening (20%) and knowledge-based design (21%) being the other large contributors to hit generation (Figure 1C).
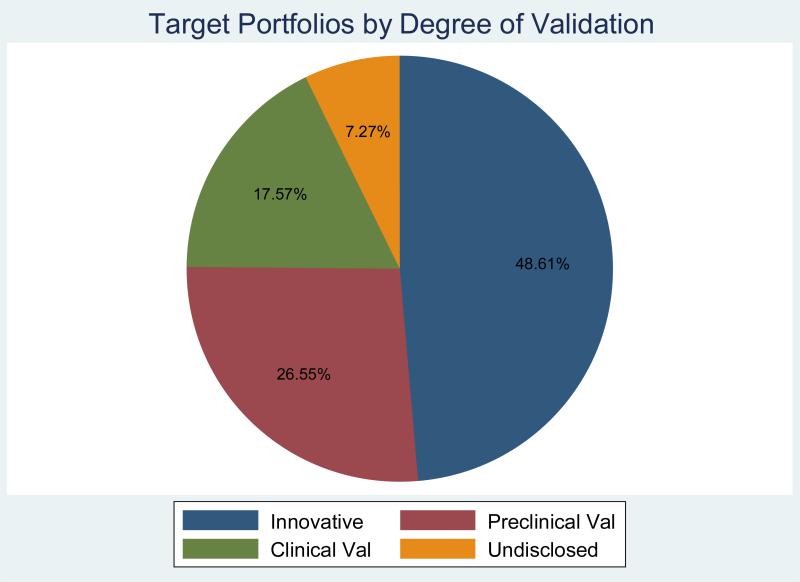
There was much emphasis on innovation in the choice of targets. Thus centers reported that on average 49% of their targets were based on unique discoveries within their institutions with little literature validation. Another large cohort involved targets with significant preclinical scientific validation in the literature but no clinical validation (27%). Only 18% of targets chosen were associated with clinical evidence of validity (Figure 2A). Thus the academic centers are clearly choosing to pursue long-term, higher risk strategies rather than focus on short-term movement of precedented candidates into the clinic consistent with the role of academia in driving innovation in drug discovery.5, 6
Figure 2A.
Degree of validation of target portfolios. Responders were asked to give the approximate percentage of their center's targets in three categories: innovative targets based on unique discoveries or expertise within your institution with little validation evidence in the literature (“Innovative” above); targets with significant pre-clinical evidence for validity in the literature but lacking clinical validation (“Preclinical Val” above); targets with existing clinical evidence of validity (“Clinical Val” above); or they could choose not to answer (“Undisclosed” above) (see supplemental information, survey question 16).
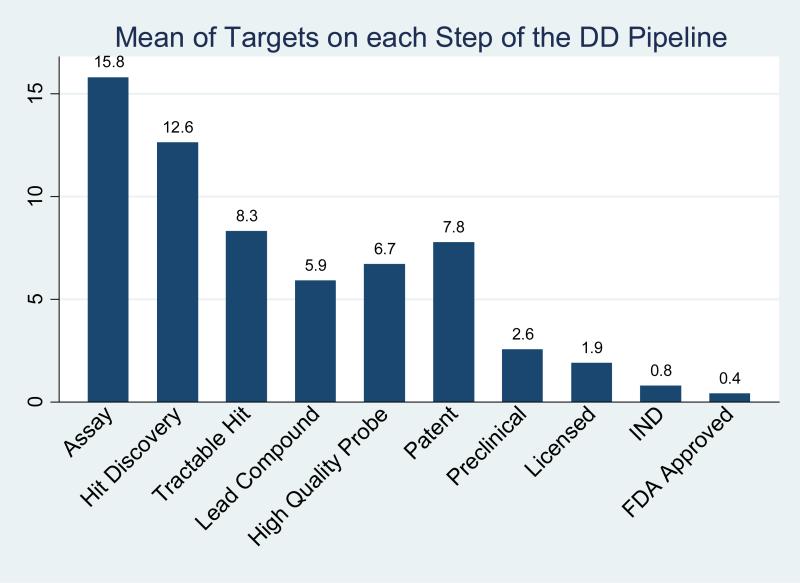
We have sought to evaluate the ADD ‘pipeline’ by aggregating several survey questions concerning the number of biological targets that have progressed to various stages of drug development (Figure 2B). Not surprisingly many targets were still at the assay development stage that precedes generation of compounds, or were in the stage of hit discovery. The number of targets progressing to more advanced stages such as identification of lead compounds or high quality chemical probes, compounds in preclinical development, agents licensed to industry, or drugs at the IND or FDA approval stages, largely followed a monotonic decline, with the notable exception of the number of targets covered by patents. The reasons for this deviation from the trend are unclear; possibly centers are patenting targets or assays at an early stage of development before lead compounds have been identified. Given that the typical drug discovery project, if successful, has a lifetime somewhat longer than most of the centers have been in existence (see below), this analysis by stage represents a snap-shot and not a steady-state analysis from which reliable attrition/progression data could be derived.
Figure 2B.
Aggregated depiction of the drug discovery pipeline by stage for all centers. Note: the mean number of instances in each drug discovery stage was calculated with the mid points of the ranges reported (see supplemental information, survey questions 18, 20 & 21).
Questions regarding comparisons between academic and industrial drug discovery evoked intense and informative responses. As depicted in Figure S2 there was clear agreement among the respondents concerning the relative strengths and weaknesses of the two discovery environments. Academia was perceived to be much stronger than industry in disease biology expertise and in innovation, and was considered to be better aligned with societal goals. However, industry was perceived to be much stronger in assay development and screening, and particularly in medicinal chemistry. In terms of stability and organizational commitment to goals academia and industry were ranked about equally. While these overall trends are not surprising, the highly skewed nature of the responses implies important unmet issues for both academic and industrial drug discovery and strongly supports the value of collaborations to bring these complimentary skills together.
Mission, Staffing and Financing
With a few exceptions, ADD centers are very new entities in the context of historic timelines for drug development (Figure S3). Thirty-three of the sixty four responding centers were established during the 2003-08 period. Thus for many centers there has not been sufficient time for internally generated hits or leads to progress very far down the path toward clinical deployment, as discussed above. The rationales given for establishing centers were quite varied. Interestingly, self-description of center missions indicated that traditional academic goals such as publication (ranked first out of eight items, 1st/8) and training of graduate students (4th/8) were deemed more important than goals with a commercial orientation such as developing institutional revenue streams (6th/8) or creation of new companies and economic development (7th/8) (Figure S4). However the generation of intellectual property (3rd/8), a goal that clearly has commercial implications, was also highly ranked. In terms of perception of mission success, the respondents overwhelmingly (53%) indicated that their centers had exceeded initial institutional expectations rather than falling below expectation (11%), with the remainder indicating that expectations had been met.
In terms of placement of drug discovery centers in the academic organizational hierarchy, the most frequent category was that of being a sub-center of a larger academic center (32%), with the next most frequent association simply being a center within a university (19%). Only one center reported being part of a multi-university consortium, while a significant number (15%) were associated with independent, non-profit research institutes rather than universities. (Figure S5).
In terms of staffing, center leadership was equally divided between individuals having a nonindustrial background (49%) and individuals with a substantial background in industry (51%). In contrast, only 29% of centers reported having more than 50% of their total staff with a background in industry while 53% of centers had 25% or less staff with industrial experience. A result that was somewhat surprising to the authors was the large size of the staff at some of the centers with 23% reporting the employment of 21 or more Ph.D. scientists. Similarly, 37% of the centers reported quite a large number (7+) of tenure-track faculty whose primary research focus is drug discovery (Figures S6-8). Although historical data are not readily available, it seems likely that this reflects a major change in orientation for tenure track faculty at many institutions. In summary, our information suggests that current drug discovery centers are largely staffed by individuals with an academic background, including substantial involvement of tenure track faculty, but that leadership by an individual with industrial experience is frequently favored.
By far the largest source of financial support reported for ADD centers was federal grants or contracts, accounting on average for 41% of total funding. Contributions from the center's home university or other academic unit comprised the next largest source at 21%. Lesser contributions were derived from user fees, revenue from intellectual property, or support from for-profit organizations, suggesting that most centers have not moved very far into the commercial arena (Figure S9A). Consistent with this, only 14% of centers reported a long–term relationship with a for-profit organization. However, there were exceptions to these patterns with some centers deriving 100% support from user/client fees and some centers obtaining up to 50% support from commercial organizations. There was tremendous variation in the total operating expenses reported by the centers with the lowest being $25,000 and several centers reporting >$10,000,000. The distribution was skewed toward moderate levels of total funding with 57% of respondents reporting $2,000,000 or less (Figure S9B). Centers were quite optimistic about future funding prospects with 64% expecting moderate to substantial increases while only 18% expected moderate to substantial decreases (Figure S10).
Discussion
As this is the first systematic survey of the ADD sector of which we are aware, there is limited objective research with which to compare our results. However, some clear themes emerged from the data that broadly fit with the expectations set by previous commentary.7-11 Thus while creation of intellectual property (IP) is acknowledged as an important part of their mission, the majority of centers are focused on fulfilling the academic objectives of their institutions while creating new medicines – very few centers see creation of revenue from their IP as an end in itself. The innovative posture of academic centers is demonstrated by the relative lack of clinical validation data on ‘targets’ under pursuit, the focus on neglected and orphan diseases, and the fact that approximately 30% of the portfolios are based on novel protein targets and phenotypic assays. As discussed in recent reviews5, 6, ADD efforts over the last decade have provided many of the innovative new drugs – especially in the area of biologic agents. With the expansion of academic interests in small molecule drug discovery, perhaps the coming decade will see a similar impact on innovation in this domain as well.
While there is significant promise, there are also major obstacles to maximizing the impact of these centers on health care. Of respondents who answered our open-ended question about obstacles (Figure S11) 68% identified some aspect of funding (amount, stability, etc.) as an obstacle, while 25% see either a lack of expertise in medicinal chemistry, lack of understanding of drug discovery in academia, or a poor fit between academic individual PI science and the team efforts required for drug discovery as obstacles. The latter issue was raised in several text responses as regards barriers to career development for drug discovery scientists in academia. The availability of career paths for high caliber scientists who are motivated primarily by team achievements and creation of new drugs is perhaps a missing element in some institutions where the tenure-track is the only respected avenue for advancement.
While concerns over funding are not surprising, the expense of lead optimization and preclinical IND enabling studies are so daunting in the face of flat NIH budgets and extreme competition for grant funding that the ‘valley of death’ between lead compounds and viable clinical candidates seems very perilous indeed. Unless public and private funders create mechanisms to progress projects through this phase, much of value may be lost. Unfortunately, the venture capital investments that drove the last decade of innovation6 have largely retreated from preclinical opportunities. Creative models for public/private partnerships to share the costs, risks and rewards are clearly needed to both sustain the current efforts in these centers, combine complimentary skills, and address the key challenge of translation from a lead compound to a potential drug in clinical studies.13
In summary ADD centers were identified and surveyed to gauge the current state of this activity in the United States. We are hopeful that our efforts will serve as a baseline data set for future analysis and a useful data-based assessment of the current status of ADD. Extension of such a study to similar centers in Europe and the rest of the world would be of great interest as some very significant efforts are underway8 and the funding environment and relationship with industrial drug discovery varies significantly as compared to the United States.
Supplementary Material
References
- 1.Schreiber SL. Small molecules: the missing link in the central dogma. Nat Chem Biol. 2005;1:64–66. doi: 10.1038/nchembio0705-64. [DOI] [PubMed] [Google Scholar]
- 2.Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–68. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- 3.Paul SM, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 4.Cuatrecasas P. Drug discovery in jeopardy. J Clin Invest. 2006;116:2837–42. doi: 10.1172/JCI29999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens AJ, et al. The Role of Public-Sector Research in the Discovery of Drugs and Vaccines. New England Journal of Medicine. 2011;364:535–541. doi: 10.1056/NEJMsa1008268. [DOI] [PubMed] [Google Scholar]
- 6.Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov. 2010;9:867–882. doi: 10.1038/nrd3251. [DOI] [PubMed] [Google Scholar]
- 7.Ohlmeyer M, Zhou M-M. Integration of Small-Molecule Discovery in Academic Biomedical Research. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77:350–357. doi: 10.1002/msj.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyatt PG. The emerging academic drug-discovery sector. Future Medicinal Chemistry. 2009;1:1013–1017. doi: 10.4155/fmc.09.78. [DOI] [PubMed] [Google Scholar]
- 9.Tralau-Stewart CJ, Wyatt CA, Kleyn DE, Ayad A. Drug discovery: new models for industry-academic partnerships. Drug Discovery Today. 2009;14:95–101. doi: 10.1016/j.drudis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Frearson JA, Collie IT. HTS and hit finding in academia - from chemical genomics to drug discovery. Drug Discovery Today. 2009;14:1150–1158. doi: 10.1016/j.drudis.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple M, Taylor D, Kettleborough C, Bryans J, Solari R. Academiaindustry partnerships in drug discovery. Expert Opinion on Drug Discovery. 2006;1:1–6. doi: 10.1517/17460441.1.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–30. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 13.Edwards AM, Bountra C, Kerr DJ, Willson TM. Open access chemical and clinical probes to support drug discovery. Nat Chem Biol. 2009;5:436–440. doi: 10.1038/nchembio0709-436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.