Abstract
Type 2 porcine circovirus (PCV2) is associated with postweaning multisystemic wasting syndrome in pigs, whereas the genetically related type 1 PCV (PCV1) is nonpathogenic. In this study, seven monoclonal antibodies (MAbs) against PCV2-ORF2 capsid protein were generated, biologically characterized, and subsequently used to map the antigenic sites of PCV2 capsid protein by using infectious PCV DNA clones containing PCV1/PCV2-ORF2 chimeras. The PCV1/PCV2-ORF2 chimeras were constructed by serial deletions of PCV2-ORF2 and replacement with the corresponding sequences of the PCV1-ORF2. The reactivities of chimeric PCV1/PCV2 clones in transfected PK-15 cells with the seven MAbs were detected by an immunofluorescence assay (IFA). The chimera (r140) with a deletion of 47 amino acids at the N terminus of PCV2-ORF2 reacted strongly to all seven MAbs. Expanding the deletion of PCV2-ORF2 from residues 47 to 57 (r175) abolished the recognition of MAb 3B7, 3C11, 4A10, 6H2, or 8F6 to the chimera. Further deletion of PCV2-ORF2 to 62 residues disrupted the binding of this chimera to all seven MAbs. IFA reactivities with all MAbs were absent when residues 165 to 233 at the C terminus of PCV2-ORF2 was replaced with that of PCV1-ORF2. Extending the sequence of PCV2-ORF2 from residues 165 (r464) to 185 (r526), 200 (r588), or 224 (r652) restored the ability of the three chimeras to react with MAbs 3C11, 6H2, 9H7, and 12G3 but not with 8F6, 3B7, or 4A10. When the four amino acids at the C terminus of r588 were replaced with that of PCV2-ORF2, the resulting chimera (r588F) reacted with all seven MAbs. The results from this study suggest that these seven MAbs recognized at least five different but overlapping conformational epitopes within residues 47 to 63 and 165 to 200 and the last four amino acids at the C terminus of the PCV2 capsid protein.
Porcine circovirus (PCV), classified in the family Circoviridae (17), is a small nonenveloped DNA virus with a circular genome (33). PCV was first isolated as a contaminant of a porcine kidney cell line, PK-15 (33). The PK-15 cell line-derived PCV, designated PCV1, was nonpathogenic in swine (2, 34). Recently, a new disease, named postweaning multisystemic wasting syndrome (PMWS), has emerged in pigs (7, 12). A genetic variant strain of PCV, designated PCV2, was isolated from pigs with PMWS (3, 8, 22). Genetic and pathogenesis studies revealed that the nonpathogenic PCV1 and the PMWS-associated PCV2 belong to two different genotypes (9-11, 20-22).
PMWS is currently considered an important swine disease and potentially has a serious economic impact on the global swine industry. Clinical signs of the disease include progressive weight loss, emaciation, difficult breathing, and jaundice (7, 12). The disease frequently occurs in pigs 5 to 18 weeks old (12). Morbidity is usually low, but case fatality can be more than 50% in epidemic herds (12). The pathogenesis of PCV2-induced PMWS is not well defined, but the disease is believed to be mediated by the host immune response (15). Cases of PMWS/PCV2 in Midwestern swine farms increased sharply from 16 affected herds in 1997 to more than 400 affected herds in 1999 (31). PCV2 is considered the primary causative agent of PMWS (3, 8-10), and incidences of PMWS and PCV2 infections have been reported worldwide (3, 6, 8, 16, 22, 25, 30, 32, 36). PCV2 infection was also found to be associated with porcine dermatitis and nephropathy syndrome (4, 28).
PCV contains a single-stranded, close-circular DNA genome of 1,759 bp for PCV1 and 1,768 bp for PCV2 (11, 20-22). The genomic DNA of both PCV1 and PCV2 consists of two major open reading frames, ORF1 and ORF2, oriented in opposite directions. ORF1 of PCV1 and PCV2 is 936 and 942 bp in length, respectively, and the ORF1 nucleotide sequence identity between these two strains is about 86%. Amino acid sequence (11, 20-21) and transcriptional (5) analyses of PCV2 as well as the demonstrated ability of the ORF1 protein to drive the replication of plasmids with the PCV origin of replication (19) suggested that ORF1 encodes a replication-associated protein. The ORF2 of both PCV1 and PCV2 is 699 nucleotides in length (11, 20-22) and encodes a major capsid protein of approximately 30 kDa (23). ORF2 sequence identity between PCV1 and PCV2 is about 67 and 65% at the nucleotide and amino acid levels, respectively (22). Reactivities between anti-PCV2 swine sera and synthetic peptides revealed at least three immunoreactive regions on the PCV2 capsid protein (18).
The objective of this study is to use PCV1/PCV2 chimeric viruses and PCV2 monoclonal antibodies to map the antigenic epitopes of the PCV2 capsid protein. We have previously reported the generation and characterization of an infectious DNA clone of PCV2 (9) and chimeric PCV1/PCV2 infectious DNA clones (10). In this study, we mapped the conformational epitopes of the PCV2 capsid protein by analyses of PCV1/PCV2 ORF2 chimeras in the context of the PCV2 infectious genome using seven PCV2 monoclonal antibodies (MAbs) recognizing conformational epitopes.
MATERIALS AND METHODS
Cells and viruses.
A porcine kidney PK-15 cell line free of PCV1 contamination (kindly provided by Kelly Lager of the National Animal Diseases Center, Ames, Iowa) as well as the PK-15 cells permanently infected by PCV1 (ATCC CCL 33) were maintained at 37°C with 5% CO2 in minimum essential medium (MEM) (Gibco BRL) and 5% heat-inactivated fetal bovine serum (FBS) (Gibco BRL). PCV2 was propagated in PK-15 cells as previously described (23).
Spodoptera frugiperda (Sf9) cells (Invitrogen) were cultured at 27°C in Grace's insect medium (Gibco BRL) supplemented with 10% FBS. Recombinant baculovirus expressing the ORF2 gene of PCV2 (AcMNPV.ORF2) (23) was inoculated onto a 96-well plate at a multiplicity of infection of 2.5. The infected Sf9 cells were used in an immunofluorescence assay (IFA) with anti-PCV2 MAbs and other antibodies. Wild-type baculovirus (AcMNPV.wt), a recombinant baculovirus expressing the ORF4 gene of porcine reproductive and respiratory syndrome virus (AcMNPV.PRRSV) (38), and mock-infected Sf9 cells were prepared similarly as controls.
Preparation of MAbs against PCV2 capsid protein.
To generate MAbs against the PCV2 capsid protein, PCV2 virions from infected PK-15 cells were purified through CsCl gradient and used as the antigen for the immunization of mice. Briefly, CsCl gradient-purified PCV2 virions were diluted with PBS buffer to a final concentration of 1 mg/ml. Thirty micrograms of the antigen mixed with complete Freund's adjuvant were used to immunize each female BALB/c mouse intraperitoneally. A total of four mice were immunized, each for three times at 2-week intervals. The appearance of antibodies to PCV2 in immunized mice was tested on PCV2-infected PK-15 cells by IFA. Three days prior to fusion, the mice with anti-PCV2 antibody response were primed by intravenous injection of 30 μg of the PCV2 antigen. The mice were euthanatized, and their splenocytes were collected and fused with SP2/O myeloma cells as described elsewhere (38). Hybridoma supernatants were screened by IFA for the presence of PCV2-specific antibodies.
Isotyping of MAbs.
The classes and subclasses of the seven MAbs secreted by each hybridoma were determined using the mouse MonoAB ID kit (Zymed Laboratories). A sandwich immunoenzyme assay was performed according to the manufacturer's instructions. Briefly, 2HB polystyrene microtiter plates (Dynex) were coated overnight at 4°C with 50 μl (10 μg/ml) per well of anti-mouse immunoglobulin AGM (IgAGM). The coated plates were blocked for 1 h at 37°C with the blocking reagent supplied by the manufacturer, washed, and subsequently incubated at 37°C with 50 μl per well of undiluted hybridoma supernatant. After washing, 50 μl of Tris-buffered saline (TBS) (50 mM Tris [pH 7.5], 200 mM NaCl), a preimmune rabbit serum, and anti-IgG1, -IgG2a, -IgG2b, -IgG3, -IgA, -IgM, -λ-light chain, and -κ-light chain rabbit sera were each added to a well. TBS and preimmune rabbit serum served as background and negative serum controls. After subsequent incubation and washing, the immune complex was incubated at 37°C for 1 h with 50 μl of anti-rabbit IgG conjugated with alkaline phosphatase (Zymed Laboratories). A positive signal was detected following incubation with the p-nitrophenylphosphate substrate (Kirkegaard & Perry Laboratories, Inc.).
Western blot.
Cell lysates containing proteins from PCV2-infected cells, recombinant and wild-type baculovirus-infected insect cells, and mock-infected cells were prepared as described previously (23). The lysates were electrophoresed through a 15% mini-slab polyacrylamide gel (Bio-Rad). Subsequently, the proteins were transferred onto nitrocellulose membranes (Bio-Rad) using a mini-Trans Blot Transfer cell (Bio-Rad) in a transfer buffer (25 mM Tris, 192 mM glycine 20% [vol/vol] methanol). After washing, the membranes were then allowed to react overnight at 4°C with 1:20-diluted hybridoma supernatants in TBS buffer containing 0.1% Tween 20 using a ProteanII multiscreen apparatus (Bio-Rad). The membranes were washed prior to incubation with an optimal concentration of peroxidase-labeled anti-mouse IgAGM (Kirkegaard & Perry Laboratories, Inc.). Color development in the membrane was achieved using substrate 3,3′-5, 5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Inc.).
Purification of MAbs.
Hybridoma supernatants were precipitated with saturated ammonium sulfate solution and desalted prior to affinity chromatography purification. All hybridomas with IgG classes were separated using the ImmunoPure Plus protein A purification kit (Pierce). Clone 3B7 with IgA class was purified using the ImmunoPure (L) immunoglobulin purification kit (Pierce). Briefly, the ammonium sulfate-treated MAbs were mixed with the binding buffer and applied onto each of the protein A columns for IgG-class MAbs or a protein L column for MAb 3B7. Subsequently, the unbound proteins were washed out with the binding buffer. MAbs were eluted with an elution buffer, and the eluants were monitored for the presence of MAbs by measuring the optical density at an absorbance of 280 nm. The eluted MAbs were further desalted by size exclusion chromatography.
ELISA.
To establish an enzyme-linked immunosorbent assay (ELISA) for MAbs, each well of the high-binding polystyrene microtiter plates (Dynex) was coated with 100 μl of 0.01 M PBS buffer containing approximately 2.5 μg of the purified PCV2 virion. The affinity-purified MAbs were biotinylated with an immunoprobe biotinylation kit (Sigma) according to the manufacturer's instruction. The labeled MAbs were passed through a Sephadex G25 M column (Amersham) to remove salts and uncoupled biotin. Serial 10-fold dilutions of each biotinylated MAb in 5% milk diluents (Kirkegaard & Perry Laboratories, Inc.) were incubated with the coated PCV2 antigen. A MAb of an irrelevant virus, anti-canine coronavirus, was included as a background control. The peroxidase-conjugated Extravidin (Sigma) was incubated with the biotinylated MAb. All incubations were performed at 37°C for 1 h, and three washes with 0.1 M PBS buffer were performed between each incubation step. The 2,2′-azino-di-3-ethylbenzthiazoline-6-sulfonate substrate (Kirkegaard & Perry Laboratories, Inc.) was used for color development at 37°C for 30 min.
Virus neutralization (VN) assay.
A focus reduction assay was used to assess the VN activity of the MAbs. Briefly, PCV2 virus stock was adjusted to a concentration of 105 50% tissue culture infective doses/ml, and 50 μl of the virus stock was added into each well of the 96-well plates. An equal volume of undiluted hybridoma supernatant from each primary clone was mixed with the virus using a mini-orbital shaker (Bellco Biotechnology). The hybridoma supernatant containing an irrelevant MAb (anti-porcine reproductive and respiratory syndrome virus [PRRSV] MAb) and 1:100-diluted anti-PCV2 swine convalescent-phase serum were included as negative and positive controls, respectively. Three replications were performed for each MAb and control. Prior to the inoculation of PK-15 cells, the virus-antibody mixtures were incubated at 37°C for 1 h. IFA was performed as previously described, using anti-PCV2 rabbit hyperimmune serum and fluorescein isothiocyanate-labeled anti-rabbit IgG (Kirkegaard & Perry Laboratories, Inc.) to determine the neutralizing activity of the MAbs.
The positive foci in each field were counted using a fluorescence microscope at a magnification of ×200. For each sample, a total of 15 fields were counted: 5 fields per well and 3 wells per sample. The five fields per well were randomly selected (one field at the far left, one at the far right, one at the top, one at the bottom, and one at the center of the well). Therefore, the positive foci for each sample were reported as average numbers of foci per field. The percentage of foci reduction was calculated from the average numbers of foci per field in the negative control (anti-PRRSV MAb) and in each anti-PCV2 MAb: % foci reduction = [(average numbers of foci in negative control − average numbers of foci in anti-PCV2 MAb)/average numbers of foci in negative control] × 100. A MAb is considered as having neutralizing activity if it can reduce the average numbers of positive foci by more than 80% compared to the negative control.
Constructions of PCV2 DNA clone and PCV1/PCV2 chimeras.
Plasmid p31/31 contains the entire genomic sequence of PCV2 (strain ISU 31). To produce p31/31, the ORF2 of PCV2 (strain ISU 31) was amplified with a forward primer (5′-GAGCAAGCTTTTAGGGTTTAAGTGGGGGGTC-3′) and a reverse primer (5′-GAGTCTCGAGATGACGTATCCAAGGAGGCG-3′) containing XhoI and HindIII sites, respectively. The remaining PCV2 genomic sequence, designated PCV2-ORF1 fragment (containing ORF1 and noncoding regions), was engineered to contain BamHI and XhoI sites at the 5′ end, and a HindIII site at the 3′ end with primers 5′-GACAGGATCCCTCGAGAGCTGAAAACGAAAGAAGTGCG-3′ and 5′-GACAAAGCTTATGAATAAAAACAATTACG-3′, respectively. The PCV2-ORF2 and the PCV2-ORF1 fragments were ligated into plasmid pKSII(+) (Stratagene) at the XhoI/HindIII and HindIII/BamHI sites to produce PCV2 DNA clone p31/31 (Fig. 1).
FIG. 1.
Construction of PCV2 clone p31/31 (PCV2 strain ISU31). The ORF2 gene and the ORF1 fragment (ORF1 plus the remaining genomic sequence of PCV2) were amplified by PCR and cloned into plasmid pKSII+ (hatched line) as shown. B, X, and H are the BamHI, XhoI, and HindIII restriction sites used for cloning. The full-length PCV2 genome is excised with XhoI and recircularized with T4 DNA ligase to form a double-stranded circular DNA molecule for in vitro transfection.
Plasmid p31/15 contains a chimeric PCV1 and PCV2 genome, which consisted of the PCV2-ORF1 fragment and the PCV1-ORF2 sequence. The PCV1-ORF2 sequence was amplified with primers 5′-GAGCAAGCTTTTATTTATTTAGAGGGTCTTTTAG-3′ and 5′-GAGTCTCGAGATGACGTGGCCAAGGAGGCG-3′. The construction of clone p31/15 was done essentially as described for clone p31/31, except that PCV1-ORF2 was inserted in the plasmid instead of PCV2-ORF2.
A series of chimeric PCV1/PCV2 ORF2 cassettes containing sequential deletions of PCV2-ORF2 fused with the remaining ORF2 sequence of PCV1 was also constructed for epitope mapping (Fig. 2). Chimeric PCV1/PCV2 ORF2 cassettes were constructed by PCR using an elongase enzyme (Gibco BRL) for high-fidelity amplification. PCR was performed with pairs of internal primers, each containing an overlapping sequence of approximately 10 to 20 nucleotides at the recombining junction. Primers at the 3′ and 5′ ends of the ORF2 gene were complementary to the template sequences. Plasmids containing various chimeras were constructed essentially as described for plasmid p31/31, except that the recombinant PCV1/PCV2 ORF2 cassettes were used for cloning instead of the PCV2-ORF2. The resulting plasmid constructs were verified by sequencing and sequence analyses.
FIG. 2.
Schematic diagram of PCV1/PCV2 ORF2 chimeras and their reactivities with various polyclonal and monoclonal antibodies. PCV clones p31/31 (PCV2) and p31/15 contained the complete 233-amino-acid sequences of PCV2-ORF2 (empty block) and PCV1-ORF2 (hatched block), respectively. The PCV1/PCV2 ORF2 chimeric cassettes contain serial deletions of the PCV2-ORF2 sequence joined with the remaining sequence of PCV1-ORF2 at a similar position. (A) The first set of chimeras consists of the N-terminal sequence of PCV1-ORF2 fused with the C-terminal sequence of PCV2-ORF2 at amino acid positions 14 (r39), 47 (r140), 58 (r175), 63 (r192), 85 (r256), 110 (r327), and 142 (r427), respectively. (B) For the second set of constructs, the N-terminal sequence of PCV2-ORF2 is joined with the C-terminal sequence of PCV1-ORF2 at amino acid positions 165 (r464), 185 (r526 and r526F), 200 (r588 and r588F), and 224 (r652 and r652F). The F chimeras (r526F, r588F, and r652F) differ from their corresponding ORF2 chimeras in that they contained the last four amino acid residues of PCV2-ORF2 (PLKP) rather than those of PCV1-ORF2 (-LNK) at the C terminus. (C) The third set of PCV1/PCV2 ORF2 mosaic chimeras contains the sequence of PCV2-ORF2 encompassing residues 58 to 185 (r175/526), 47 to 200 (r140/588), 47 to 200 plus the last four amino acids at the C terminus (140/588F), 47 to 224 (r652), and 47 to 224 plus the last four amino acids at C terminus (140/652F), flanked by the remaining sequence of PCV1-ORF2. (D) The last set of the chimeras contains block(s) of PCV2-ORF2 sequences at residues 47 to 62 (r140/192), 185 to 200 (r526/588), 185 to 200 plus the last four amino acids at C terminus (r526/588F), or 47 to 62 plus 185 to 200 (rbox140) flanked by PCV1-ORF2 sequences. The results of IFA reactivities between each antibody and PK-15 cells transfected with each PCV construct were indicated next to each construct. IFA reactivities of the constructs in transfected Pk-15 cells were demonstrated by PCV2 convalescent-phase swine antiserum (S), rabbit hyperimmune antiserum (R) or MAbs (9H7, 12G3, 6H2, 3C11, 8F6, 3B7, and 4A10). +, a strong reactivity; +w, a weak reactivity; +I, an intermediate or moderate reactivity; −, no reactivity.
In vitro transfection.
Plasmids p31/31 and p31/15 and chimeras were excised with XhoI digestion to produce XhoI fragments containing the entire genomic sequence of PCV2 or PCV1/PCV2 chimeras (Fig. 1). One microgram of the purified XhoI fragments was self ligated, using T4 DNA ligase (Gibco BRL), and subsequently transfected into PK-15 cells, using Lipofectamine 2000 (Gibco BRL), according to the manufacturer's instructions. Briefly, 100 ng of DNA was incubated for 20 min with 0.8 ml of Lipofectamine 2000 in 50 μl of MEM. Fifty microliters of the mixture was applied onto PK-15 cells in each well of a 96-well plate. After incubation for 5 h at 37°C, 100 ml of MEM containing antibiotics and 10% FBS was added to each well and incubated at 37°C with 5% CO2. At 24 h posttransfection, the cells were fixed with absolute methanol and processed for IFA.
IFA.
PK-15 cells transfected with PCV2 clone or chimeras were incubated with a 1:50 dilution of a convalescent-phase PCV2 swine antiserum (13), a 1:1,000 dilution of an anti-PCV2 rabbit hyperimmune serum (23), or a 1:50 dilution of each MAb culture supernatant. PK-15 cells transfected with constructs p31/31 (PCV2) and p31/15 (PCV2 with PCV1-ORF2) as well as nontransfected cells were included as positive and negative controls for each transfection. After incubation at 37°C for 1 h, cells were stained with fluorescein isothiocyanate-labeled secondary antibodies of respective species (Sigma). At low dilutions, the PCV2 convalescent-phase swine antiserum recognized both PCV1 and PCV2 antigens in clones p31/31 and p31/15 and served as a confirmation for replication of PCV2 and PCV1/PCV2 chimeras. The anti-PCV2 rabbit hyperimmune serum is PCV2 specific and reacted with clone p31/31 but not with chimeric clone p31/15. The reactivities of each MAb to each PCV1/PCV2 ORF2 chimera by IFA were tested and compared with results for the controls.
RESULTS
Generation and characterization of monoclonal antibodies against PCV2 capsid protein.
Positive hybridomas secreting PCV2 MAbs were selected and recloned by the cell sorter method, and their supernatants were retested by IFA in PCV2-infected cells to confirm the reactivity of each MAb with PCV2. Seven clones of hybridomas secreting MAbs against the capsid protein of PCV2 were selected and characterized. Classes and subclasses of the MAbs were determined using a sandwich ELISA, and their immunoglobulin isotypes are summarized in Table 1. Clones 6H2, 9H7, and 12G3 secreted the IgG1 subclass of antibody, whereas clones 3C11 and 4A10 both have the IgG2a isotype. The hybridoma clones 8F6 and 3B7 secreted MAbs of IgG2B subclass and IgA class, respectively (Table 1). All seven hybridomas produced MAbs with a κ-light chain of immunoglobulin. These seven MAbs were subsequently used in this study for mapping the epitopes of PCV2 capsid protein.
TABLE 1.
Reactivity of each MAb with the PCV2 virion and recombinant PCV2ORF2-AcMNPV antigens
| MAb | Isotype | IFA result for cells infected by:
|
Western blot resultd
|
|||
|---|---|---|---|---|---|---|
| PCV1a | PCV2b | rORF2c | PCV2 | rORF2 | ||
| 3B7 | IgA | <1:2 | 1:100 | + | − | − |
| 3C11 | IgG2a | <1:2 | 1:1,000 | + | − | − |
| 4A10 | IgG2a | <1:2 | 1:1,000 | + | − | − |
| 6H2 | IgG1 | <1:2 | 1:1,000 | + | − | − |
| 8F6 | IgG2b | <1:2 | 1:100 | + | − | − |
| 9H7 | IgG1 | <1:2 | 1:1,000 | + | +w | +w |
| 12G3 | IgG1 | <1:2 | 1:100 | + | − | − |
Negative at the lowest dilution tested in PK-15 cells infected with PCV1.
The highest dilution that showed positive signal in PK-15 cells infected by PCV2.
Sf9 insect cells infected with recombinant ORF2-AcMNPV virus and tested at a 1:20 dilution.
+w, very weak positive signal.
Specificity of MAbs to the capsid protein of PCV2.
The seven hybridoma clones reacted strongly and specifically with the PCV2-infected PK-15 cells. The reactivity of the hybridoma supernatants with PCV2- or PCV1-infected PK-15 cells was measured as a reciprocal of the highest dilution of the supernatants that produced a positive signal by IFA. All seven MAbs reacted with the PCV2 antigen in the infected cells and had a titer of at least 1:100 (Table 1). None of the hybridoma supernatants reacted with the PCV1-infected or uninfected PK-15 cells.
All seven MAbs also produced strong immunofluorescent staining in Sf9 cells infected with the recombinant baculovirus (AcMNPV.ORF2) expressing the PCV2-ORF2 capsid protein. The MAbs did not stain the cells infected with AcMNPV.wt or AcMNPV.PRSSV or uninfected Sf9 cells.
Reactivity of MAbs in Western blotting and ELISA.
The ability of each MAb to recognize a linear epitope on the capsid protein was tested by Western blot analysis. Cell lysates containing proteins from uninfected PK-15 cells, PCV2-infected PK-15 cells, Sf9 cells infected with AcMNPV.wt or recombinant AcMNPV.ORF2 viruses, and uninfected Sf9 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Western blot analyses showed that MAb 9H7 reacted very weakly with the 30-kDa protein on membranes containing antigens from purified PCV2 virion and AcMNPV.ORF2-infected Sf9 cells (Table 1). All six other MAbs did not react with the purified PCV2 virion or with the recombinant ORF2 protein (Table 1).
The MAbs were also tested in an ELISA format to determine if they react with native PCV2 particles. Serial twofold dilutions of biotinylated MAbs were allowed to react with purified PCV2 virion antigen in microtiter plates. The signals were detected using the Extravidin-peroxidase system. Among the seven MAbs tested, six (3B7, 3C11, 4A10, 6H2, 8F6, and 12G3) produced a positive signal at a dilution of 1:320, the highest dilution tested. MAb 9H7 had an ELISA titer of 1:160 (Table 2).
TABLE 2.
Optical densities for reactivities of MAbs with purified PCV2 particles in an ELISA
| MAb | OD405b at MAb dilution of:
|
|||||
|---|---|---|---|---|---|---|
| 1:10 | 1:20 | 1:40 | 1:80 | 1:160 | 1:320 | |
| 3B7 | >3.000 | 1.994 | 1.395 | 1.011 | 0.812 | 0.678 |
| 3C11 | 1.834 | 1.460 | 1.159 | 0.905 | 0.615 | 0.433 |
| 4A10 | 2.044 | 1.676 | 1.344 | 1.051 | 0.853 | 0.677 |
| 6H2 | 1.771 | 1.225 | 0.822 | 0.607 | 0.390 | 0.227 |
| 8F6 | 1.746 | 1.248 | 1.050 | 0.905 | 0.770 | 0.725 |
| 9H7 | 1.302 | 0.856 | 0.565 | 0.362 | 0.226 | 0.160 |
| 12G3 | 2.510 | 1.774 | 1.191 | 0.841 | 0.620 | 0.418 |
| Anti-CCVa | 0.183 | 0.158 | 0.152 | 0.153 | 0.062c | 0.065d |
A MAb from an irrelevant virus, canine coronavirus.
Optical density at 405 nm.
Plate background.
Conjugate background.
VN activity of MAbs.
Pooled supernatants from each hybridoma clone were tested for their ability to neutralize PCV2 strain ISU31 by a focus reduction assay. MAb 6H2 was found to possess VN activity. The other six MAbs had a low neutralizing activity (Table 3). The anti-PCV2 convalescent-phase swine serum that served as a positive control showed strong VN activity (Table 3).
TABLE 3.
Neutralizing activities of MAbs against PCV2
| Antibody | Avg no. of foci/field | % Foci reduction | VN activity |
|---|---|---|---|
| Anti-PRRSV MAb | 14.80 | 0 | − |
| Anti-PCV2 swine serum | 0.27 | 98.18 | + |
| 3B7 | 4.80 | 67.57 | − |
| 3C11 | 4.60 | 68.92 | − |
| 4A10 | 4.53 | 69.39 | − |
| 6H2 | 1.13 | 92.36 | + |
| 8F6 | 4.67 | 68.45 | − |
| 9H7 | 4.47 | 69.79 | − |
| 12G3 | 6.07 | 58.99 | − |
Reactivity of clones p31/31 and p31/15 with various antibodies.
IFA reactivities of a convalescent-phase PCV2 swine antiserum with clones p31/31 and p31/15 are shown in Fig. 3. At a dilution of 1:50, the PCV2 convalescent-phase pig antiserum recognized the antigens produced by clones p31/31 (PCV2) and p31/15 (PCV2 with PCV1-ORF2) and thus served as a positive transfection control. The PCV2 convalescent-phase swine antiserum likely recognized the ORF1 proteins of both PCV1 and PCV2. The convalescent-phase swine antiserum produced strong and homogeneous intranuclear and cytoplasmic staining in cells transfected with PCV2 clone p31/31 (Fig. 3A). Within cells transfected with chimeric clone p31/15 (PCV2 with PCV1-ORF2), the positive signals appeared predominantly as small, dense, granular intranuclear inclusion bodies resembling the prereplication sites reported in herpes simplex viruses (27) (Fig. 3D). With the exception of the chimera rbox140, all other PCV1/PCV2 chimeras reacted with PCV2 convalescent-phase pig antiserum (Fig. 2), indicating that these PCV1/PCV2 chimeras are viable and replicating in PK-15 cells.
FIG. 3.
IFA reactivity with polyclonal antibodies or a representative MAb 3B7 in PK-15 cells transfected with PCV clone p31/31 or p31/15. The upper panel indicates reactivities of PCV2 convalescent-phase swine antiserum (A), rabbit hyperimmune antiserum (B), or MAb 3B7 (C) with cells transfected with PCV2 clone p31/31. The lower panel showed reactivities between clone p31/15 and the swine antiserum (D) and no reactivity between clone p31/15 and the rabbit hyperimmune serum (E) or MAb 3B7 (F).
IFA with anti-PCV2 rabbit hyperimmune serum and the seven MAbs produced strong signals in cells transfected with PCV2 clone p31/31, similar to that observed in IFA with the convalescent-phase swine antiserum (Fig. 3B and C). However, neither the rabbit serum nor MAbs reacted with chimeric clone p31/15 (Fig. 3E and F). These results confirmed that both the rabbit hyperimmune antiserum and all seven MAbs were specific to the PCV2 capsid protein and that the PCV2 convalescent-phase swine antiserum recognized both PCV1 and PCV2.
Epitope mapping with chimeras containing the N terminus of PCV1-ORF2 fused with the C terminus of PCV2-ORF2.
To identify the antigenic sites on the capsid protein of PCV2, a set of PCV1/PCV2 chimeras was constructed in which the N terminus of PCV2-ORF2 was replaced with the corresponding region of PCV1-ORF2 (Fig. 2A and 4). The PCV1/PCV2 chimeras, r39, r140, r175, r192, r256, r327, and r427, contained 14, 47, 58, 63, 85, 110, and 142 amino acid residues of PCV1-ORF2 recombined with the remaining amino acid sequence of PCV2-ORF2, respectively (Fig. 2A). The IFA reactivities of these chimeras, when transfected into PK-15 cells, with polyclonal antibodies and MAbs, are shown in Fig. 2. The swine antiserum, which likely recognized the ORF1 antigens of both PCV1 and PCV2, reacted strongly with all of the chimeras. The rabbit hyperimmune serum, which recognized only PCV2 capsid protein, reacted strongly with chimeras r39, r140, r175 and r192 but not with r256, r327, or r427.
FIG. 4.
Comparison between deduced amino acid sequences of PCV1-ORF2 (derived from PK-15 cells) and PCV2-ORF2 (from strain ISU31 used in the study). Both PCV1-ORF2 and PCV2-ORF2 contained 233 amino acid residues. Dots (.) represent an identical amino acid in both strains. Dashes (-) represent deletions. The recombination position of each chimera is indicated by an underscore (_) either preceded or followed by the name of each chimera. The chimeras are named according to the nucleotide position at the joining junction.
All seven MAbs yielded strong IFA signals with cells transfected with chimeras r39 and r140, similar to their reactivities with cells transfected with PCV2 clone p31/31. The IFA reactivities of the seven MAbs with r140 and other chimeras are shown in Fig. 5. When the next 11 amino acids (residues 47 to 57) of PCV2-ORF2 in chimera r140 were replaced with those of PCV1-ORF2 to produce a new chimera, r175, MAbs 3B7, 3C11, 4A10, 6H2, and 8F6 lost their reactivities with the r175 chimeric capsid protein. This indicates that amino acid residues 47 to 57 are important for the recognition of MAbs 3B7, 3C11, 4A10, 6H2, and 8F6. However, MAb 9H7 still reacted strongly with clone r175, indicating that the 57 amino acids at the N terminus of the capsid protein were not involved in binding of this MAb. A weak IFA reactivity between MAb 12G3 and chimera r175 was observed, suggesting that MAb 12G3 may recognize an epitope overlapping the joining region. Further replacement of residues 58 to 62 of PCV2-ORF2 with those of PCV1-ORF2 in chimera r192 completely abolished the binding of MAbs 9H7 and 12G3 to the chimeric capsid protein. Therefore, residues 58 to 62 are important for the recognition of MAb 9H7, and the binding site for MAb 12G3 encompassed residues 47 to 62.
FIG. 5.
Positive reactivity between each MAb and each PCV chimera. MAb 9H7 gives a strong positive signal with chimeras r140 (A), r175 (B), r526 (C), and r175/526 (D). MAb 12G3 reacts strongly with chimeras r140 (E) and r526 (F) but only weakly with r175 (G) and r175/526 (H). MAb 6H2 shows strong IFA reactivity with chimeras r140 (I), r588 (J), and r140/588 (K). MAb 8F6 also reacts strongly with chimeras r140 (L) and r588F (M). MAb 3C11 reacts strongly with chimera r140 (N), moderately with chimeras r588F (O) and r140/588F (P), and weakly with chimeras r588 (Q), r652 (R), and r140/588 (S). MAbs 4A10 and 3B7 have a similar IFA staining pattern: a strong reactivity of MAb 3B7 with chimera r140 (T) but weak reactivity with chimera r588F (U); a strong reactivity between MAb 4A10 and chimera r140 (V) but a weak reactivity with chimera r588F (W).
Although chimera r192 (Fig. 2A) did not bind with any of the seven MAbs, it reacted strongly with the anti-PCV2 rabbit serum (data not shown), indicating that residues 63 to 84 of the capsid protein may also be involved in epitope formation. Chimeras r256, r327, and r427, in which 84, 109, and 141 amino acid residues at the N terminus of PCV2-ORF2 were replaced with those of PCV1-ORF2, respectively, did not react with the anti-PCV2 rabbit hyperimmune serum or any of the MAbs. Therefore, the chimeric capsid proteins of chimeras r256, r327, and r427 may not contain epitopes for PCV2-ORF2. This observation also indicated that residues 47 to 84 at the N terminus of the capsid protein are required for binding of MAbs and thus may contain immunoreactive epitopes.
Epitope mapping with chimeras containing the N terminus of PCV2-ORF2 joined with the C terminus of PCV1-ORF2.
A second set of PCV1/PCV2 chimeras containing the N terminus of PCV2-ORF2 joined with the remaining C terminus of PCV1-ORF2 was constructed for further epitope mapping (Fig. 2B). In chimeras r464, r526, r588, and r652, the C terminus of PCV2-ORF2 was replaced with the corresponding regions of PCV1-ORF2 at amino acid positions 165, 185, 200, and 224 (68, 48, 33, and 9 residues from the C termini), respectively (Fig. 2B). The results of IFA reactivities of various antibodies to these chimeric capsid proteins are summarized in Fig. 2B. The PCV2 convalescent-phase swine antiserum produced strong signals with all of the chimeras, indicating that the chimeras are infectious and replicating in PK-15 cells. The rabbit anti-PCV2 hyperimmune serum reacted strongly with chimeras r526, r588, and r652 but not with r464.
None of the 7 MAbs reacted to the chimera r464, in which the 68 amino acids at the C terminus of PCV2-ORF2 were replaced with the corresponding region of PCV1-ORF2 (Fig. 2B). Therefore, the replacement of amino acids 165 to 233 of PCV2-ORF2 with those of PCV1-ORF2 completely disrupted the binding of MAbs to the PCV2 capsid protein. Extending the C terminus of PCV2-ORF2 from amino acid position 165 in chimera r464 to position 185 in chimera r526 fully restored the reactivity of MAbs 9H7 and 12G3 with the chimeric capsid proteins, suggesting that these 21 residues (positions 165 to 185) were also involved in epitope formation for both MAbs. MAb 6H2 reacted weakly with chimera r526; however, when the length of PCV2-ORF2 was extended from amino acid position 185 in chimera r526 to position 200 in chimera r588, full reactivity of this MAb was restored. Thus, the epitope for MAb 6H2 at the C terminus included amino acid residues 165 to 200. MAb 3C11 reacted weakly with chimera r526 and r588. When the PCV2-ORF2 amino acid sequence was extended from position 200 in chimera r588 to position 224 in chimera r652, the reactivity of MAb 3C11 with r652 was only slightly increased. The IFA reactivity of each MAb with the chimeras is shown in Fig. 5.
Since some MAbs did not recognize chimeric capsid proteins produced by the second set of chimeras, the last three amino acids (-LNK) at the C termini of chimeras r526, r588, and r652 were replaced with the corresponding amino acid PLPK of PCV2-ORF2 (Fig. 4) to create the new chimeras r526F, r588F, and r652F, respectively (Fig. 2B). Both convalescent-phase swine serum and rabbit hyperimmune serum yielded strong signals in cells transfected with these three new chimeras. MAb 3C11 reacted with chimera r588F but not with chimera r526F or r652F (Fig. 2B). MAb 3C11 reacted moderately with chimera r588F and weakly with chimeras r526, r588, and r652: the signals were less intense compared to the reactivity of this MAb with the PCV2 clone p31/31 (Fig. 5). Hence, the epitope for MAb 3C11 possibly included amino acid residues 165 to 200 plus the four amino acids at the C terminus of the PCV2 capsid protein. The correct conformation of the capsid protein may be essential for the full reactivity of MAb 3C11 with this epitope.
In contrast to MAb 3C11, MAb 8F6 did not recognize chimeric capsid proteins in cells transfected with chimera r464, r526, r588, or r652. However, MAb 8F6 reacted strongly with chimera r588F, similar to its reaction with the PCV2 clone p31/31 (Fig. 2B and 5). Reactivity of MAb 8F6 with chimera r526F was not observed. These findings indicated that residues 185 to 200 as well as the four amino acids at the C terminus of the PCV2 capsid protein are important for the recognition of MAb 8F6. The absence of reactivity of MAb 8F6 with chimeras r652 and r652F may be due to improper folding of these two chimeric capsid proteins.
The reactivities of both MAbs 3B7 and 4A10 with this panel of PCV chimeras were similar to those of MAb 8F6 (Fig. 2B and 5). These two MAbs did not react with chimera r464, r526, r588, r652, r526F, or r652F, although they produced a weaker positive signal with cells transfected with chimera r588F than with PCV2 clone p31/31. The four residues at the C terminus of the PCV2 capsid protein may be partially responsible for MAb 3B7 and 4A10 recognitions, and the substitution of these four amino acids may partially restore the conformation of the epitopes. The weaker positive signal between the two MAbs with the chimera r588F could be due to improper formation of the tertiary structure of the epitope.
Epitope mapping with mosaic PCV1/PCV2 ORF2 chimeras.
To further confirm that the aforementioned amino acid residues at the C and N termini of the PCV2 capsid protein play roles in binding with the seven MAbs, a third set of chimeric PCV1/PCV2 ORF2 cassettes was produced (Fig. 2C). These mosaic chimeras contained the amino acid sequences of PCV2-ORF2, encompassing residues 58 to 185 in chimera r175/526; 47 to 200 in chimera r140/588; 47 to 200 plus the four amino acids at the C terminus for chimera r140/588F; 47 to 224 for chimera r140/652; and 47 to 224 plus the four amino acids at the C terminus for chimera r140/652F, flanked by the remaining sequence of PCV1-ORF2 (Fig. 2C and 4). The convalescent-phase swine antiserum produced strong signals for all of the mosaic chimeras, indicating that they are all viable and infectious (Fig. 2C). The polyclonal rabbit antiserum specific for the PCV2 capsid protein reacted strongly with chimeras r175/526, r140/588, r140/588F, r140/652, and r140/652F (Fig. 2C).
The results of reactivities between MAbs and these PCV1/PCV2 ORF2 mosaic chimeras are shown in Fig. 2C and 5. Strong reactivities of MAb 9H7 with chimeras r175/526, r140/588, r140/588F, r140/652, and r140/652F further confirmed that the binding site of this MAb resides between residues 58 and 185. MAb 12G3 reacted weakly with chimera r175/526 but strongly with chimeras r140/588, r140/588F, r140/652, and r140/652F, indicating that it recognized amino acid residues 47 to 185, possibly extending to residue 200. MAb 6H2 showed full reactivity with chimeras r140/588, r140/588F, r140/652, and 140/652F but had no reactivity with chimera r175/526, suggesting that the region spanning amino acid residues 47 to 200 is responsible for binding to this MAb. MAb 3C11 produced a moderate positive signal with chimera r140/588F, a weak positive signal with chimera r140/588, and no signal with chimera r175/526. Therefore, this epitope may contain amino acids residing between residues 47 and 200, as well as the last four residues at the C terminus. The absence of binding of MAb 3C11 to chimeras r140/652 and 140/652F implied that improper folding of these chimeric capsid proteins may have disrupted the epitope structure. The lack of reactivity observed among this set of ORF2 mosaic chimeras with MAb 8F6, 3B7, or 4A10 (Fig. 2C) may be due to the incorrect formation of three-dimensional structures of the mosaic chimeric capsid proteins, thus disrupting the epitope structure.
Attempts to further define the regions of the reacting epitopes.
In an attempt to further pinpoint the precise locations of the reacting epitopes, another set of chimeric ORF2 cassettes was constructed, which consisted of block(s) of PCV2-ORF2 sequences in the context of PCV1-ORF2 (Fig. 2D). These chimeras contained PCV2-ORF2 residues 47 to 62 in chimera r140/192, 185 to 200 in chimera r526/588, 185 to 200 plus the C-terminal four amino acids in chimera r526/588F, and 47 to 62 plus 185 to 200 for chimera rbox140. No IFA reactivity was detected using the polyclonal rabbit antiserum or the seven MAbs for chimera r526/588F (Fig. 2D), indicating that the amino acid residues at the C terminus (positions 185 to 200 plus PLPK) of the PCV2 capsid protein alone are not sufficient for recognition of these MAbs (Fig. 2D). The chimeras r140/192 and r526/588 showed reactivities with the convalescent-phase swine antiserum but did not react with the polyclonal rabbit antiserum or any of the MAbs. The chimera rbox140 failed to replicate in the PK-15 cells (Fig. 2D), since the transfected cells did not react with the convalescent-phase swine antiserum.
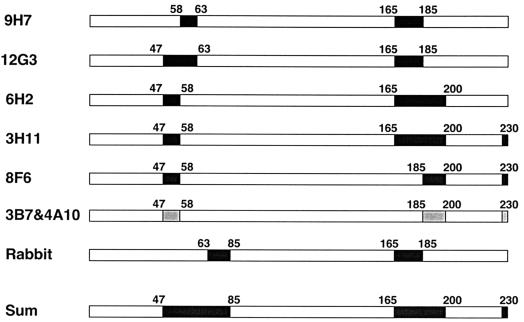
Figure 6 summarizes the immunoreactive regions of the PCV2 capsid protein that are essential for recognitions of MAbs and polyclonal rabbit antiserum as demonstrated in this study. The polyclonal rabbit serum recognized most on the PCV2 capsid. Since deletion of the 84 amino acids at the N terminus in chimera r526 or the 68 amino acids at the C terminus in chimera r464 of the PCV2 capsid protein completely abolished reactivity with the rabbit antiserum, these residues may be involved in epitope formation of the capsid protein. In addition, the regions spanning amino acid residues 47 to 62, 165 to 200, and 230 to 233 are essential for epitope recognition of the seven MAbs generated in this study.
FIG. 6.
Schematic diagram of immunoreactive epitopes on the PCV2 capsid protein responsible for binding of polyclonal and monoclonal antibodies. Each bar represents a full-length PCV2 capsid protein. Filled boxes within bars are the regions important for epitope recognitions of MAbs. All seven MAbs recognized different but overlapping conformational epitopes comprised of amino acid residues that reside in both N- and C-terminal regions of the PCV2 capsid protein. The regions recognized by seven MAbs are as follows: amino acids 58 to 62 and 165 to 185 for MAb 9H7; amino acids 47 to 62 and 165 to 185 for MAb 12G3; amino acids 47 to 57 and 165 to 200 for MAb 6H2; amino acids 47 to 57, 165 to 200, and 230 to 233 for MAb 3C11; and amino acids 47 to 57, 185 to 200, and 230 to 233 for MAbs 8F6, 3B7, and 4A10. Reactivities between the anti-PCV2 rabbit hyperimmune antiserum and PCV chimeras reveal an additional immunoreactive region within amino acids 63 to 84 on the capsid protein. The collective reactivities (Sum) of seven MAbs and the rabbit hyperimmune antiserum with the PCV chimeras indicates that the immunodominant epitopes encompassed amino acids 47 to 84, 165 to 200, and the last four amino acids at the C terminus of the PCV2 capsid protein.
DISCUSSION
The seven MAbs generated in this study all reacted with PCV2-infected PK-15 cells, with recombinant ORF2 protein expressed in insect cells by IFA, and with purified PCV2 particles by ELISA. Western blot analyses and PCV1/PCV2-ORF2 chimera studies indicated that the MAbs recognize conformational epitopes on the PCV2 capsid protein. Our results showed that the immunodominant epitopes of the PCV2 capsid protein likely located within amino acid residues 47 to 84, 165 to 200, and the last four amino acids of the capsid protein. The epitope for MAb 9H7 likely comprises residues 58 to 62 at the N terminus and residues 165 to 185 at the C terminus of the capsid protein. MAb 12G3 recognizes an epitope that resides within amino acids 47 to 62 and 165 to 185 of the capsid protein. Furthermore, amino acid sequences spanning residues 47 to 57 and residues 165 to 200 of the capsid protein may be involved in constituting the epitope recognized by MAb 6H2. An epitope recognized by MAb 3C11 resides from residues 47 to 57, 165 to 200, and 230 to 233. MAb 8F6 likely recognizes an epitope comprising amino acids 47 to 57, 185 to 200, and the C-terminal last four amino acids of the capsid protein.
The region essential for binding of MAbs 3B7 and 4A10 appears to be similar to that for MAb 8F6. MAbs 3B7 and 4A10 may recognize the same epitope of similar epitopes, since the patterns of their IFA reactivities with all chimeras are indistinguishable. However, it is unlikely that MAb 8F6 and MAb 3B7 (or 4A10) would bind to a similar epitope, since MAb 8F6 produced a very strong signal with chimera r588F whereas MAbs 3B7 and 4A10 both reacted only weakly with this chimera. Analyses of the reactivities of the PCV1/PCV2 chimeras suggests that the amino acid sequences from residues 47 to 62 and residues 165 to 200 as well as the C-terminal last four amino acids of the capsid protein were likely in close proximity to form a cluster of conformational epitopes on the surface of a PCV2 virion.
MAbs 8F6, 3B7, and 4A10 reacted with chimera r140 or r588F, and thus, they were expected to react with chimera r140/588F. On the contrary, all these three MAbs failed to react with cells transfected with chimera r140/588F. These negative results could be due to the incorrect formation of the three-dimensional structures of chimeric capsid proteins, thus disrupting the epitope structure.
The first 46 residues at the N terminus of the PCV2 capsid protein are likely not involved in the formation of conformational epitope(s), since full reactivities with anti-PCV2 rabbit hyperimmune antiserum and with the seven MAbs were observed in chimera r140 (the first 46 amino acids were replaced with the ORF2-PCV1 sequence). This region contains residues rich in basic amino acids (11, 21-22) and thus may be involved in the formation of the interior surface of the virion and interact with the negative charges of genomic DNA during virus assembly, as reported for many icosahedral viruses (29). Indeed, sequence analyses revealed that this stretch of basic amino acid-rich region at the N termini of the PCV capsid proteins and those of other members of the Circoviridae (psittacine beak and feather disease virus and chicken anemia virus) and plant viruses with a circular DNA genome (nanoviruses and geminiviruses) resembles that of a sobemovirus, southern bean mosaic virus, which has DNA-binding activity in this lysine-arginine-rich region (24). Three-dimensional structure of southern bean mosaic virus demonstrated that the basic amino acids at the N terminus of the capsid protein were in close contact with packaged genomic DNA in the native virion (14). However, this does not imply that the 46 amino acid residues at the N terminus of the PCV2 capsid protein are not immunogenic, since a linear epitope was found between residues 25 and 43 using synthetic peptides (18).
The 187 amino acids (from residue 47 to the C terminus) within the ORF2 protein may be important for capsid formation of PCV2 virions. In many spherical DNA and RNA viruses, about 200 amino acid residues at the C termini of the capsid proteins fold into an eight-stranded β-barrel to form the core of the capsid, and each strand connects to another with an α-helix or loop constituting the exterior surface of a protomer (1, 26, 29, 35, 37). The minimum number of residues required for the formation of the β-barrel domain is approximately 150 (29). The 187 residues in PCV2 capsid protein involving in reacting with antibodies are also within this range. The portion of the amino acid sequences of PCV2-ORF2 within this region possibly constitutes the exterior surface of the PCV2 virion, since it contains sequences essential for recognition by polyclonal antibodies as well as MAbs. This finding is in agreement with the results of a published study using PEPSCAN and anti-PCV2 swine sera to identify epitopes on the PCV2 capsid protein (18). Reactivity between the polyclonal antibodies and the synthetic peptides revealed that at least three immunodominant epitopes resided from residues 65 to 87, 113 to 147, and 157 to 183, respectively. Furthermore, few amino acid residues at the C terminus of the PCV2 capsid protein may be exposed on the exterior surface of the virion, as found in some RNA viruses (29), since they were required for the reactivities of MAbs 3B7, 4A10, and 8F6. Structural analyses of the PCV2 capsid protein using cryoelectron microscopy and x-ray crystallography are required to confirm these assumptions.
We showed in this study that in general, the chimeric PCV1/PCV2 ORF2 cassettes help maintain the structure of the chimeric capsid protein and its conformational epitopes to some extent. The IFA reactive signals of the PCV2 chimeras in transfected cells with MAbs were produced solely from PCV2-ORF2 product, since all seven MAbs reacted only with PCV2 but not with PCV1. The PCV1/PCV2 chimera system reported in this study is also useful in other studies for elucidating the pathogenesis and replication of PCV2 (9-10). The MAbs generated in this study should be very useful for further in-depth studies of the molecular biology and structure of the PCV2 capsid protein and for virus-cell interaction studies. The antigenic sites on the PCV2 capsid protein identified in this study should provide valuable information for further in-depth mapping and structural analyses of the PCV2 capsid protein.
Acknowledgments
This work was supported by Thailand Research Fund (grant TRG4580023) and in part by a grant from the U.S. Department of Agriculture’s National Research Initiative Competitive Grant Program (USDA-NRI 2004-35204-14213).
REFERENCES
- 1.Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of murine parvovirus, minute virus of mice. Structure 6:1369-1381. [DOI] [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, J. P. Cassidy, G. A. C. Reilly, B. A. Dair, W. A. Ellis, and M. S. McNulty. 1995. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 44:49-64. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. M., F. McNeilly, S. Kennedy, B. Daft, E. G. Clarke, J. A. Ellis, D. M. Haines, B. M. Meehan, and B. M. Adair. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig. 10:3-10. [DOI] [PubMed] [Google Scholar]
- 4.Allan, G. M., E. McNeilly, S. Kennedy, B. Meehan, D. Moffett, F. Malone, J. Ellis, and S. Krakowka. 2000. PCV-2-associated PDNS in Northern Ireland in 1990. Vet. Rec. 146:711-712. [PubMed] [Google Scholar]
- 5.Cheung, A. K. 2003. The essential and nonessential transcription units for viral protein synthesis and DNA replication of porcine circovirus type 2. Virology 313:452-459. [DOI] [PubMed] [Google Scholar]
- 6.Choi, C., C. Chae, and E. G. Clark. 2000. Porcine postweaning multisystemic wasting syndrome in Korean pigs: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Investig. 12:151-153. [DOI] [PubMed] [Google Scholar]
- 7.Clark, E. G. 1997. Post-weaning multisystemic wasting syndrome, p. 499-501. In Proceedings of the 28th Annual Meeting of the American Association of Swine Practitioners. American Association of Swine Practitioners, Quebec City, Canada.
- 8.Ellis, J., L. Hassard, E. Clark, J. Harding, G. Allan, P. Willson, J. Strokappe, K. Martin, F. McNeilly, B. Meehan, D. Todd, and D. Haines. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44-51. [PMC free article] [PubMed] [Google Scholar]
- 9.Fenaux, M., P. G. Halbur, G. Haqshenas, R. Royer, P. Thomas, P. Nawagitgul, M. Gill, T. E. Toth, and X. J. Meng. 2001. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 76:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenaux, M., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2003. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weaning pigs. J. Virol. 77:11232-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamel, A. L., L. L. Lin, and G. P. S. Nayar. 1998. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72:5262-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding, J. C. 1997. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation, p. 503. In Proceedings of the 28th Annual Meeting of the American Association of Swine Practitioners. American Association of Swine Practitioners, Quebec City, Canada.
- 13.Harms, P. A., S. D. Sorden, P. G. Halbur, S. R. Bolin, K. M. Larger, I. Morozov, and P. S. Paul. 2001. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and PRRSV. Vet. Pathol. 38:528-539. [DOI] [PubMed] [Google Scholar]
- 14.Hermodson, M., C. Abad-Zapatero, S. S. Abdel-Meguid, S. Pundak, M. G. Rossmann, and J. H. Tremaine. 1982. Amino acid sequence of southern bean mosaic virus coat protein and its relation to three-dimensional structure of the virus. Virology 119:133-149. [DOI] [PubMed] [Google Scholar]
- 15.Krakowka, S., J. A. Ellis, F. McNeilly, D. Gilpin, B. Meehan, K. McCullough, and G. Allan. 2002. Immunologic features of porcine circovirus type 2 infection. Viral Immunol. 15:567-582. [DOI] [PubMed] [Google Scholar]
- 16.Kuipel, M., G. W. Stevenson, S. K. Mittal, E. G. Clark, and D. M. Haines. 1998. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet. Pathol. 35:303-307. [DOI] [PubMed] [Google Scholar]
- 17.Lukert, P., G. F. de Boer, J. L. Dale, P. Keese, M. S. McNulty, J. W. Randles, and I. Tischer. 1995. The Circoviridae, p. 166-168. In F. A. Murphy, C. M. Fauquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Martelli, M. A. Mayo, and M. D. Summers (ed.), Virus taxonomy. Sixth report of the International Committee on Taxonomy of Viruses. Springer-Verlag, New York, N.Y.
- 18.Mahe, D., P. Blanchard, C. Truong, C. Arnauld, P. Le Cann, R. Cariolet, F. Madec, E. Albina, and A. Jestin. 2000. Differential recognition of ORF2 protein from type 1 and type 2 porcine circoviruses and identification of immunorelevant epitopes. J. Gen. Virol. 81:1815-1824. [DOI] [PubMed] [Google Scholar]
- 19.Mankertz, A., J. Mankertz, K. Wolf, and H. Buhk. 1998. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol. 79:381-384. [DOI] [PubMed] [Google Scholar]
- 20.Meehan, B. M., J. L. Creelan, M. S. McNulty, and D. Todd. 1997. Sequence of porcine circovirus DNA: affinities with plant circovirus. J. Gen. Virol. 78:221-227. [DOI] [PubMed] [Google Scholar]
- 21.Meehan, B. M., F. McNeilly, D. Todd, S. Kennedy, V. A. Jewhurst, J. A. Ellis, L. E. Hassard, E. G. Clark, D. M. Haines, and G. M. Allan. 1998. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 79:2171-2179. [DOI] [PubMed] [Google Scholar]
- 22.Morozov, I., T. Sirinarumitr, S. D. Sorden, P. G. Halbur, M. K. Morgan, K. Yoon, and P. S. Paul. 1998. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 36:2535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nawagitgul, P., I. Morozov, S. R. Bolin, P. A. Harms, and S. D. Sorden. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81:2281-2287. [DOI] [PubMed] [Google Scholar]
- 24.Niagro, F. D., A. N. Forsthoefel, R. P. Lawther, L. Kamalanathan, B. W. Ritchie, K. S. Lartimer, and P. D. Lukert. 1998. Beak and feather disease virus and porcine circovirus genomes: intermediate between the geminiviruses and plant circoviruses. Arch. Virol. 143:1723-1744. [DOI] [PubMed] [Google Scholar]
- 25.Onuki, A., K. Abe, K. Togashi, K. Kawashima, A. Taneichi, and H. Tsunemitsu. 1999. Detection of porcine circovirus from lesions of a pig with wasting disease in Japan. J. Vet. Med. Sci. 61:1119-1123. [DOI] [PubMed] [Google Scholar]
- 26.Prasad, B. V. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 27.Roizman, B., and D. M. Knipe. 2001. Herpes simplex and their replication, p. 2399-2459. In D. M Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott William & Wilkins, Philadelphia, Pa.
- 28.Rosell, C., J. Segales, J. A. Ramos-Vara, J. M. Folch, G. M. Rodriguez-Arrioja, C. O. Duran, M. Balasch, J. Plana-Duran, and M. Domingo. 2000. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet. Rec. 146:40-43. [DOI] [PubMed] [Google Scholar]
- 29.Rossmann, M. G., and J. E. Johnson. 1989. Icosahedral RNA virus structure. Annu. Rev. Biochem. 58:533-573. [DOI] [PubMed] [Google Scholar]
- 30.Segales, J., M. Sitjar, M. Domingo, S. Dee, M. Del Pozo, R. Noval, C. Sacristan, A. De las Haras, A. Ferro, and K. S. Latimer. 1997. First report of post-wening multisystemic wasting syndrome in pigs in Spain. Vet. Rec. 141:600-601. [PubMed] [Google Scholar]
- 31.Sorden, S. D. 2000. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 8:133-136. [Google Scholar]
- 32.Thomson, J., S. Bill, G. Allan, F. McNeilly, and C. McVicar. 2000. PDNS, PMWS and porcine circovirus type 2 in Scotland. Vet. Rec. 146:651-652. [PubMed] [Google Scholar]
- 33.Tischer, I., H., Gelderblom, W. Vettermann, and M. A. Koch. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64-66. [DOI] [PubMed] [Google Scholar]
- 34.Tischer, I., W. Mields, D. Wolff, M. Vagt, and W. Griem. 1986. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 91:271-276. [DOI] [PubMed] [Google Scholar]
- 35.Tsao, J., M. S. Chapman, M. Agbandje, W. Keller, K. Smith, H. Wu, M. Luo, T. J. Smith, M. G. Rossmann, R. W. Compans, and C. R. Parrish. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251:1456-1464. [DOI] [PubMed] [Google Scholar]
- 36.Wellenberg, G. J., S. Pesch, F. W. Berndsen, P. J. G. M. Steverink, W. Hunneman, T. J. K. Van der Vorst, N. H. M. T. Peperkamp, V. F. Ohinger, R. Schippers, J. T. Van Oirschot, and M. F. de Jong. 2000. Isolation and characterization of porcine circovirus type 2 from pigs showing signs of post-weaning multisystemic wasting syndrome in the Netherlands. Vet. Q. 22:167-172. [DOI] [PubMed] [Google Scholar]
- 37.Wu, H., and M. G. Rossmann. 1993. The canine parvovirus empty capsid structure. J. Mol. Biol. 233:231-244. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Y., D. S. Rameshwer, and P. S. Paul. 1998. Monoclonal antibodies against conformationally dependent epitopes on porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 63:125-136. [DOI] [PubMed] [Google Scholar]