Dear editor
MMP-9 has been involved in favoring cardiovascular disease, and evidence suggests that oxidative stress favors its release. Previous studies demonstrated that during both acute hyperglycemia and hypoglycemia an oxidative stress is produced in type 1 diabetes. On the other hand, GLP-1 has shown an antioxidant power. In this study an acute hyperglycemia and hypoglycemia, with or without the simultaneous infusion of GLP-1, were produced. During both hyperglycemia and hypoglycemia, an acute increase of MMP-9 and 8-iso-PGF2α plasma levels was observed. GLP-1 significantly reduced this increase. This study suggests that metalloproteinase-9 is activated by acute hyperglycemia and hypoglycemia because they generate an oxidative stress and that GLP-1 counteracts this effect.
Cardiovascular disease is a rising complication of type 1 diabetes.1 MMP-9 has been involved in favoring cardiovascular disease,2 and evidence suggests that MMP-9 is increased in the plasma of type 1 diabetic patients.3,4 Hyperglycemia seems to favor MMP-9 expression,5,6 possibly through the generation of an oxidative stress.6
Attention is paid today to the possibility that GLP-1 and GLP-1 receptor agonists can be used in the management of type 1 diabetes.7 We have recently shown that, in type 1 diabetes, GLP-1 protects endothelial function and reduces inflammation during both acute hyperglycemia and hypoglycemia.8 GLP-1 exerts this protection increasing intracellular antioxidant defenses and decreasing the oxidative stress generated by both hyperglycemia and hypoglycemia.8 Therefore, the aim of this study has been to evaluate the impact of both acute hyperglycemia and hypoglycemia on MMP-9 plasma levels and the possible protective effect of GLP-1.
Plasma samples regarding the experiments performed in type 1 diabetes were from the study previously published.8 A total of 30 type 1 diabetic patients (15 males and 15 females) participated in such study.8 For this new study, 30 healthy (17 males and 13 females), aged (24.0±2.3 versus [vs] 24.4±2.2 years, mean ± standard error) and body mass index (23.5±2.0 vs 23.7±2.2 kg/m2) matched controls were recruited. The assays for MMP-9 were performed at the same time.
The diabetic patients were divided into two groups.8 In group 1, two different experiments were planned for each subject in a randomized order: a period of 2 hours of hypoglycemia (2.9±0. mmol/L), with or without GLP-1 (0.4 pmol kg−¹ min−¹) infusion.8
Two different experiments were planned for each subject of group 2 in a randomized order: a period of 2 hours of hyperglycemic clamp (15 mmol/L), with or without GLP-1 (0.4 pmol kg−¹ min−¹) infusion.8
At baseline and after 1 and 2 hours, glycemia, plasma 8-iso-PGF2α (Cayman Chemical, Ann Arbor, MI, USA) and MMP-9 (R&D Systems, Inc., Minneapolis, MN, USA.) plasma levels were measured. For the statistics and more details about the study see Ceriello et al.8
Baseline 8-iso-PGF2α (67.5±4.8 vs 34.5±4.5 pg/mL, P<0.01), and MMP-9 (580.15±10.9 vs 290.10±12.4 ng/mL, P<0.01) were increased in diabetic patients compared to controls.
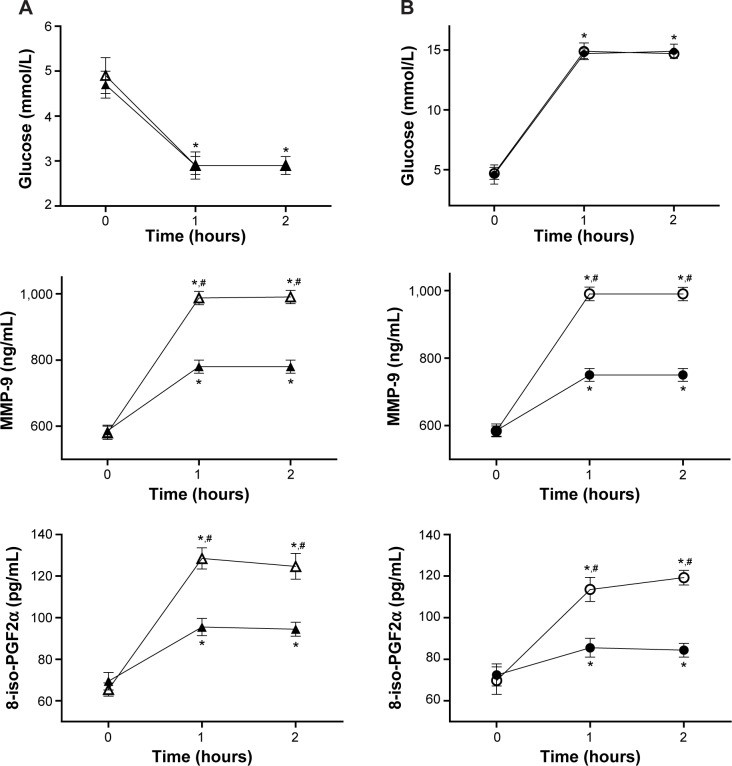
After 2 hours of hypoglycemia, 8-iso-PGF2α and MMP-9 significantly increased, compared to basal values (Figure 1). When hypoglycemia was accompanied by the simultaneous infusion of GLP-1, all these phenomena were significantly attenuated (Figure 1). Similar results were obtained in the hyperglycemic clamp experiments. After 2 hours of hyperglycemia, 8-iso-PGF2α and MMP-9 increased, compared to basal values (Figure 1). When hyperglycemia was accompanied by the simultaneous infusion of GLP-1, all these phenomena were significantly attenuated (Figure 1).
Figure 1.
MMP-9 and plasma 8-iso PGF2α variations during acute hypoglycemia (A) and acute hyperglycemia (B), with or without GLP-1.
Notes: (A) Glycemia, 8-iso-PGF2α, and MMP-9 in type 1 diabetes (n=15) during hypoglycemia and hypoglycemia plus GLP-1 infusion. White triangle: hypoglycemia; black triangle: hypoglycemia + GLP-1; *P<0.01 vs basal; #P<0.01 vs hypoglycemia + GLP-1. (B) Glycemia, 8-iso-PGF2α, and MMP-9 in type 1 (n=15) diabetes during hyperglycemia and hyperglycemia plus GLP-1 infusion. White circle: hyperglycemia; black circle: hyperglycemia + GLP-1; *P<0.01 vs basal; #P<0.01 vs hyperglycemia + GLP-1. Copyright © American Diabetes Association. From Diabetes Care®, Vol 36, 2013; 2346–2350. Reprinted with permission from The American Diabetes Association.8
In this study, for the first time, we report that both acute hypoglycemia and hyperglycemia induce an increase of MMP-9 and that GLP-1 can counterbalance this effect. Our data suggest that MMP-9 is activated by acute hyperglycemia and hypoglycemia because they generate an oxidative stress. The data of 8-iso-PGF2α support this hypothesis. GLP-1 counteracts the effects of both hyperglycemia and hypoglycemia, probably because of its antioxidant activity.8
Currently, there is huge interest in the use the GLP-1 receptor agonists for the therapy of type 1 diabetes.7 Our data suggest an additional benefit of this use.
Footnotes
Disclosure
The authors do not have any conflicts of interest to disclose.
References
- 1.Schnell O, Cappuccio F, Genovese S, Standl E, Valensi P, Ceriello A. Type 1 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2013;12:156. doi: 10.1186/1475-2840-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 3.Shiau MY, Tsai ST, Tsai KJ, Haung ML, Hsu YT, Chang YH. Increased circulatory MMP-2 and MMP-9 levels and activities in patients with type 1 diabetes mellitus. Mt Sinai J Med. 2006;73(7):1024–1028. [PubMed] [Google Scholar]
- 4.Jacqueminet S, Ben Abdesselam O, Chapman MJ, et al. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta. 2006;367(1–2):103–107. doi: 10.1016/j.cca.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Setianto BY, Achmad AF, Purnomo LB. Serum matrix metalloproteinase-9 levels in acute coronary syndrome patients with and without hyperglycemia. Acta Med Indones. 2014;46(2):83–89. [PubMed] [Google Scholar]
- 6.Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity role of oxidative stress. Circ Res. 2001;88(12):1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 7.Tölle S. GLP-1-analoga in der therapie des diabetes mellitus typ 1 [GLP-1 analogues in treatment of type 1 diabetes mellitus] Dtsch Med Wochenschr. 2014;139(42):2123–2126. doi: 10.1055/s-0034-1387304. German. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36(8):2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]