Abstract
Squamous cell carcinoma (SCC) and pre-malignant endobronchial lesions have been difficult to study in murine models. In this report, we evaluate the topical N-nitroso-tris-chloroethylurea (NTCU) murine SCC model, determine the extent to which resulting pre-malignant airway dysplasia develops, discuss clinicopathologic grading criteria in lesion progression, and confirm that immunohistochemical (IHC) staining patterns are consistent with those observed in human endobronchial dysplasia and SCC. Male and female FVB mice were treated biweekly with topical NTCU (4, 8, or 40mM) or vehicle for 32 weeks. Following sacrifice, squamous cell lesions were enumerated and categorized into the following groups: flat atypia, low-grade dysplasia, high-grade dysplasia, and invasive SCC. The 40mM NTCU concentration produced the entire spectrum of premalignant dysplasias and squamous cell carcinomas, but was associated with poor survival. Concentrations of 4mM and 8mM NTCU were better tolerated and produced only significant levels of flat atypia. Squamous origin of the range of observed lesions was confirmed with IHC staining for cytokeratin 5/6, p63, thyroid transcription factor-1 (TTF-1), and Napsin-A. This study demonstrates that topical application of high dose NTCU produces endobronchial pre-malignant lesions with classic squamous characteristics and should allow for improved pre-clinical evaluation of potential chemopreventive agents.
Keywords: lung cancer, NTCU, squamous cell carcinoma, endobronchial dysplasia
Introduction
Lung cancer is the leading cause of cancer deaths in males and females in the United States with over 200,000 incident cases and more than 150,00 deaths occurring in the United States in 2010 (1). Adenocarcinoma (AC) and squamous cell carcinoma (SCC) are the most common forms of non-small cell lung cancer (NSCLC) (2). SCC chemoprevention trials in humans have focused on endobronchial dysplasia as an endpoint (3;4) and until relatively recently, this field has lacked a reproducible animal model of airway dysplasia that could aid in the evaluation of chemopreventive agents prior to the initiation of clinical trials. Although multiple, well-characterized models of murine AC exist (initiator-promoter carcinogenesis (5), mutant KRAS and the use of strict carcinogens (6–8)), SCC models are fewer in number and poorly characterized (9;10).
Similar to other common cancers, a reproducible SCC model would advance pre-clinical studies and the understanding of pre-malignant biology. The N-nitroso-tris-chloroethylurea (NTCU) murine model presents an opportunity to expand current understanding of carcinogenesis in human smokers as it is has been reported to induce murine SCC and airway dysplasia (9). Recently, this model has been used to demonstrate both the inhibitory effects of both pioglitazone (11), a mixture of Chinese herbs (12), and pomegranate fruit extract (13) in the progression of SCC and lung tumorigenesis. The present study aims to better characterize this model in FVB/N mice, to improve upon the reported dosing schedule, and to compare immunohistochemistry (IHC) staining patterns of SCC lesions in mouse lung with human endobronchial dysplasia. We confirm the squamous origin of these lesions by performing IHC with markers standard for SCC and AC identification. In this report we show that high dose NTCU exposure results in an array of pre-malignant lesions, but lower doses only lead to earlier grade lesions (flat atypia).
Methods
NTCU administration
The FVB/N murine strain selection was based on previous reports of susceptibility to NTCU (9). Strains such as FVB and Balb/C classically exhibit an intermediate degree of sensitivity to lung carcinogens (14–16), and display many similarities to human AC (17–19). Male and female wild-type FVB/N mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and NTCU treatment was initiated at approximately 10 weeks of age. Mice were housed in a negative airflow biohazard caging system and received normal rodent diet (Modified Rodent LabDiet 5001, TestDiet, Richmond, IN) ad libitum under a 12-hour diurnal light-dark cycle. Topical application of NTCU (Toronto Research Chemicals, Inc., North York, Ontario) occurred biweekly for 32 weeks at concentrations of 4mM, 8mM and 40mM, diluted with acetone (Sigma-Aldrich, St. Louis, MO). Following dorsal coat shaving, a 25μl volume was applied to exposed skin. Control animals received acetone alone.
Due to dampened survival rates in the initial 40mM treatment group (summarized in the results section), a behavioral index scoring system was developed to track the health of animals over time in all treatment groups: a. weight loss: 10–15% (3 points), >15% (euthanize); b. piloerection, disheveled appearance, blepharitis, fecal/urine changes (1 point); c. decreased activity, ataxia (1 point); d. inappetance, anorexia (1 point); and e. labored respiration (1 point). Animals of the 4mM and 8mM groups were weighed and scored at the time of each NTCU application throughout the course of the study. At any point during the study, animals reaching a score of three points were suspended from further treatments and monitored daily until a change in score was observed; animals exceeding three points were removed from the study and euthanized; animals showing improvement resumed treatments.
After 32 weeks of NTCU application animals were euthanized and tissues were collected. At the time of sacrifice, the right lower lobe was removed for prostaglandin metabolite level measurement. The remaining four lobes were insufflated, preserved in formalin, and used for lesion quantitation and IHC.
Lesion grading and quantitation
Lesion grading was performed under bright field microscopy on hematoxylin- and eosin-stained (H&E) tissue of ten animals from each of the four treatment groups (4mM, 8mM, 40mM, and acetone control). Three separate H&E slides were examined per animal, taken from a single paraffin block, each spaced 50μm apart. Lesion counts are reported per area of lung tissue as calculated using ImagePro Plus software (version 7.0, Media Cybernetics, Bethesda, MD).
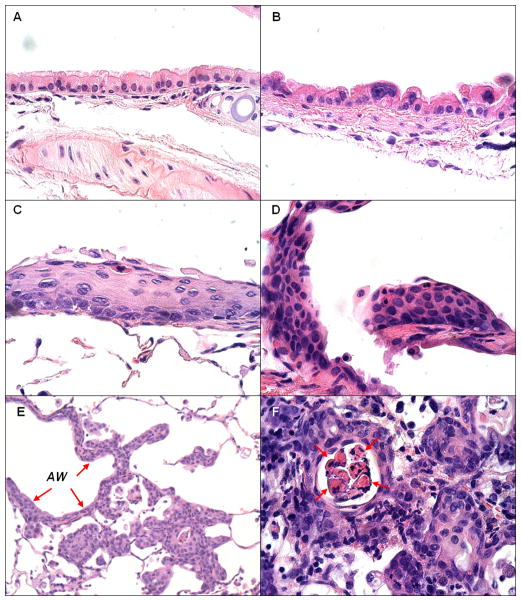
Endobronchial lesions were quantitated and classified by two independent, blinded observers (D.M., L.O.) using the following clinicopathologic grading criteria (photomicrographs contained in figure 1): a. flat atypia - bronchial epithelium composed of a single cell layer with normal bronchial epithelial cells interspersed between atypical cells that show enlarged and hyperchromatic nuclei, but generally maintaining the luminal cytoplasmic clearing and/or ciliation seen in normal cells; b. low-grade dysplasia - stratified non-ciliated squamous epithelial layer with maturation in upper layers as indicated by horizontal nuclear orientation and decreased nuclear-to-cytoplasmic (N:C) ratios. Atypia was confined to the lower one-third to one-half of the epithelium; c. high-grade dysplasia - stratified, non-ciliated squamous epithelial layer without maturation showing high N:C ratios throughout the epithelium, high degrees of nuclear pleiomorphism, variably-sized nuclei, hyperchromasia, a lack of orientation, occasional distinctive nucleoli, and atypia that extended into the upper half or involves the full thickness of the epithelium; and d. invasive squamous cell carcinoma - nests of cells with cytologic similarity to high-grade dysplasia but demonstrating invasion through the bronchial basement membrane. Tumor nests show evidence of squamous differentiation with distinct cell membranes or intercellular bridges, central keratinization in tumor nests, and/or the presence of dyskeratotic cells. Adenomas/adenocarcinomas were observed in all experimental groups at a low frequency, but not included in the analyses.
Figure 1.
Normal, premalignant and invasive lesions: A. Normal epithelium - basally located nuclei and luminal cilia; B. Flat atypia - epithelium shows enlarged hyperchromatic nuclei but single cell layer and luminal cytoplasmic clearing with frequent maintenance of ciliation; C. Low grade dysplasia - stratified non-ciliated squamous epithelial layer with maturation in upper layers (horizontal orientation and decreased N:C ratios); D. High grade dysplasia - stratified non-ciliated squamous epithelial layer without maturation. Cells show high N:C throughout epithelium, hyperchromatic, variably sized nuclei with lack of orientation and occasional distinctive nucleoli; E. Invasive squamous cell carcinoma arising from airway with high grade dysplasia (AW, arrows); F. Invasive carcinoma - central keratinization in nests of tumor and dyskeratotic cells (arrows). magnification: A.–D., F. – 600X; E. – 200X
Immunohistochemistry
To determine the cellular origin of NTCU-induced lesions, representative lesions were identified from H&E slides and stained for the classic SCC markers cytokeratin 5/6 (CK5/6, 1:200, Biocare Medical, Concord, CA), and p63 (1:50, Biocare Medical), and the AC markers thyroid transcription factor 1 (TTF-1, 1:100, Biocare Medical) and Napsin-A (1:100, Biocare Medical). These markers are routinely employed to differentiate between SCC and AC in human tumor samples (20).
Tissue sections were deparaffinized and rehydrated in graded alcohol and placed in 1X Rodent Decloaking buffer (Biocare Medical) in a pressure cooker for 5 minutes at 125°C. Sections were processed with the MM-Polymer Kit (Biocare Medical) and incubated in primary antibodies at 37°C for one hour. Controls of antibody staining consisted of the application of the secondary antibody without the primary antibody, in which case no staining was detected. In order to differentiate between squamous cell carcinoma and adenocarcinoma, the antibodies CK5/6, p63, TTF-1 and Napsin-A were applied to both a known human squamous cell carcinoma and adenocarcinoma. Indication of squamous cell carcinoma was positive staining with CK 5/6 and p63; negative staining with TTF-1 and Napsin-A. The standard for determining lung adenocarcinoma is positive staining with TTF-1 and Napsin-A, and negative staining with CK5/6 and p63. All images were captured using an Olympus BX51 microscope with a DP72 digital camera via CellSens Entry image capture software (version 1.3, Olympus, Center Valley, PA).
Statistical analysis
Lesion quantitation is reported graphically as the mean ± SEM. Comparisons between cohorts were made using a paired, two-tailed Student’s t-test. Survival curves were established using the Kaplan-Meier method and compared using the log-rank test. Results were considered significant at p ≤ 0.05. All statistical analyses and associated graphics were generated using Graphpad Prism (version 4.02, Graphpad Software, Inc., LaJolla, CA)
Results
40mM NTCU severely reduces survival
While animals in the 40mM did exhibit progressive weight loss during NTCU treatment, weight loss was not observed in the 4mM and 8mM treatment groups (figure 2). We observed survival rates of 45.0% (18/40) in 40mM NTCU-treated animals, 77.8% (21/27) in 8mM, 92.3% (24/26) in 4mM, and 84.6% (22/26) in acetone control animals (figure 3). The log-rank test of survival curves of all cohorts revealed a significant difference in survival of the 40mM cohort compared to all other treatment groups (p < 0.05). A large initial drop in survival of the 40mM cohort was observed during the sixth week of treatment (day 39), after which the survival curve remained significantly different from other treatment groups. The survival rates in the acetone, 4mM, and 8mM cohorts did not significantly differ from each other. Based on the excessive toxicity with the 40mM dose, decisions were made to alter the planned twice weekly NTCU application, periodically suspending animals from treatment. The observed toxicity also prompted the investigation of NTCU doses reduced 5- and 10-fold (8mM and 4mM) and implementation of the behavioral index outlined in the methods section for these groups. Initially, the animals of the 40mM cohort did gain weight when treatment was withheld (arrows in supp. figure 1), but after the NTCU was completely stopped (arrowhead) due to excess mortality weight loss continued. This group was divided into two waves (supp. figure 1, panels A and B), with differing periods of suspensions throughout the study. The number of animals in each score category was tabulated (supp. table 1) and the scores used as endpoints in determining treatment suspension and termination in high-scoring animals.
Figure 2.
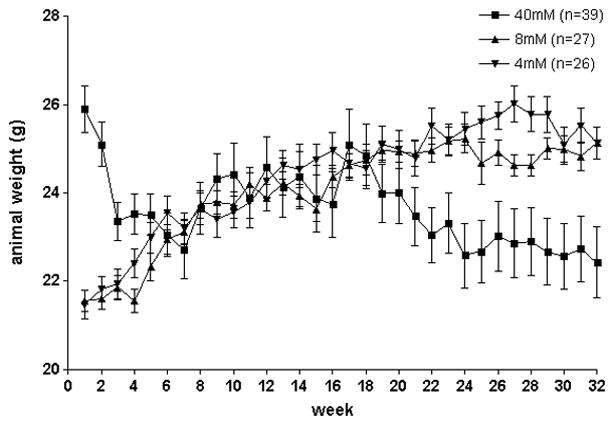
Animal weights for all treatment groups. The higher average weight of the animals of the 40mM group at the beginning of the study is a result of a 3-week age difference; animals of the 40mM groups began NTCU application at a mean age of 11 weeks and those of the other groups began application at a mean age of 8 weeks.
Figure 3.
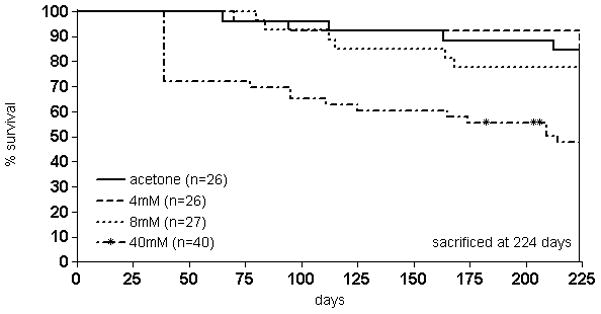
Kaplan-Meier survival curve in NTCU-treated animals. The long-rank test of survival curves of all cohorts revealed significant survival differences when comparing the 40 mM with all other treatment groups (p<0.05). The survival rates in the acetone, 4mM, and 8mM cohorts did not significantly differ from each other. Euthanized animals as well as those found dead were recorded as events rather than censored subjects. * NTCU treatment suspended.
NTCU induces flat atypia, endobronchial dysplasia, and invasive SCC
Histopathologic analysis of serial lung sections revealed a range of endobronchial squamous lesions, similar to those present in airways of smokers (21). These were classified as flat atypia, low-grade dysplasia, high-grade dysplasia, and invasive carcinoma (figure 1). A lesion similar to murine flat atypia has not been reported in human studies of smoking-related endobronchial damage. The 40mM NTCU dose induced the entire spectrum of pre-malignant dysplastic lesions, including invasive SCC in experimental animals (figure 4). However, it was associated with excessive mortality (figure 3). An analysis of differences in lesion number between the males and females of this group revealed a significantly higher number of low-grade and high-grade dysplasias in the females (supp. figure 2, p<0.05).
Figure 4.
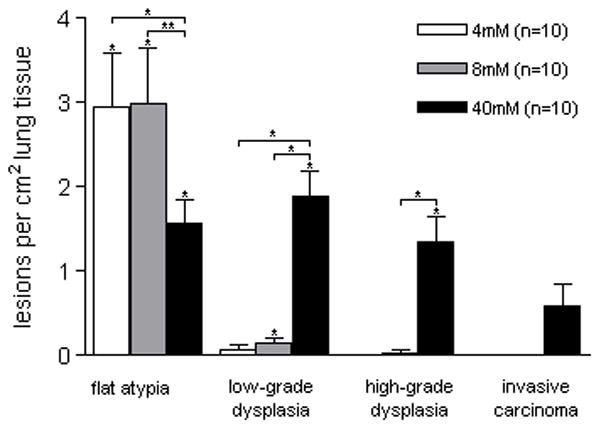
Multiplicity by lesion type. Significant flat atypia was detected in all treatment groups. Significant low-grade dysplasia was detected in middle- and high-dose groups, and high-grade dysplasia and SCC was only detected in the high-dose group. *p<0.05
Animals of the 4mM and 8mM groups did not develop high-grade dysplasia or invasive SCC. After 32 weeks of treatment they did, however, have large amounts of flat atypia (in fact, more than the animals of the 40mM group). Significant low-grade dysplasia was found only in animals that received 8mM and 40mM NTCU. Significant high-grade dysplasia and invasive carcinoma were only detected in the 40mM group (figure 4).
The presence of flat atypia did not appear to have a clear relationship with the development of SCC in the 40mM group (figure 5). The presence of high-grade dysplasia, however, was associated with a significant increase in SCC, (p<0.05). While the presence of low-grade dysplastic lesions was associated with increased high-grade dysplasia, the relationship only trended toward significance (p=0.10).
Figure 5.
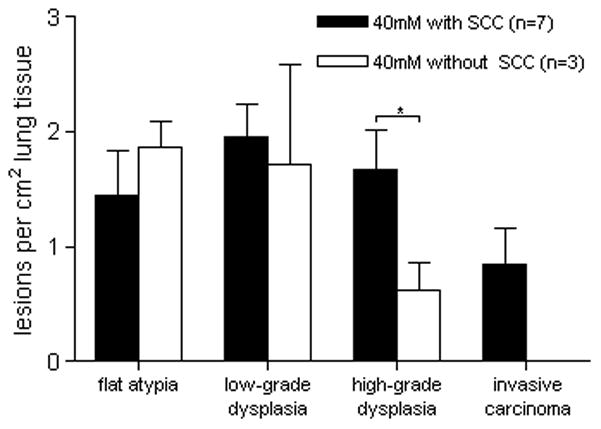
Multiplicity by lesion type in animals receiving 40mM NTCU based on the development of SCC. The number of high-grade dysplasia lesions present in animals with SCC is significantly higher than in animals with no SCC. *p<0.05
As a result of necessary treatment suspensions, 10 animals in the 40mM group were available for lesion enumeration(6 received 33 doses and 4 received 46 doses). While only significant regarding flat atypia, lesion multiplicity was higher in all lesion categories of the 40mM animals that received more doses of NTCU over the course of the study (figure 6). No squamous lesions of any type were detected in acetone control animals.
Figure 6.
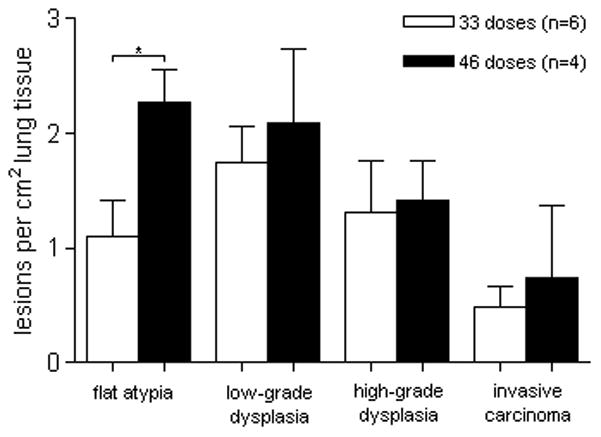
Multiplicity by lesion type and the number of doses given to the 40mM cohort. Lesion multiplicity was higher in all categories of animals that received 46 doses of NTCU over the course of the study compared to animals that received 33 doses. *p<0.05
Adenomas/adenocarcinomas were observed in all of the treatment groups at the following rates: 40mM, 22.2% (4/18); 8mM, 19.0% (4/21); 4mM, 8.3% (2/24); and acetone, 9.1% (2/22). The majority of animals with an AC had a single tumor (11/12). Pulmonary tumors were confirmed as AC via examination of H&E-stained tumor sections and positive IHC for TTF-1 and Napsin-A (shown in supplementary Figure 3).
NTCU-induced lesions express squamous cell IHC markers
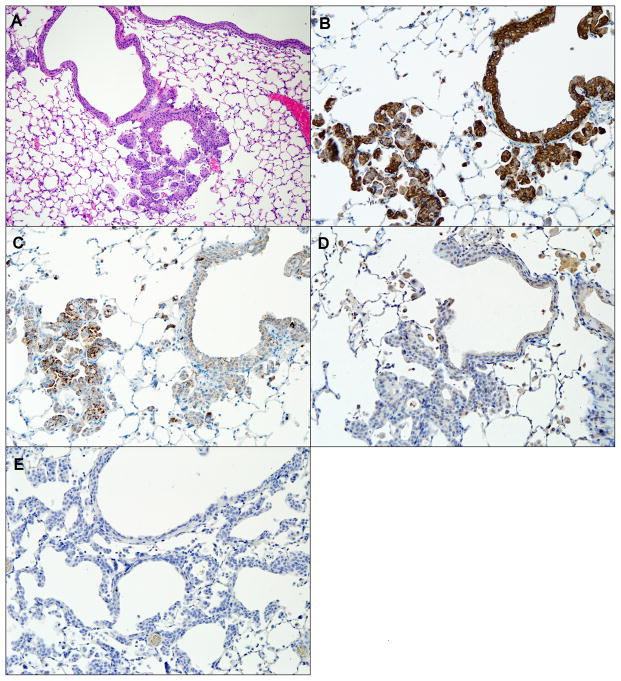
To better characterize the origin of squamous lesions, IHC was performed for a panel of markers commonly used to classify human lung cancer, including cytokeratin 5/6 (CK5/6), p63, TTF-1, and Napsin-A. CK5/6 are intermediate-size basic keratins that are expressed in squamous and non-keratinizing epithelia (22), and are routinely used to identify dysplasias and carcinomas of stratified epithelia (23). p63, the murine homologue of the tumor suppressor p53, is essential in airway epithelial development (24) and can be used as an aid in SCC diagnosis (25). TTF-1 is a transcription factor expressed in type II pneumocytes, Clara cells (26;27), and ciliated epithelial cells in the lung (28). TTF-1 is often used in conjunction with p63 in differentiating SCC from AC or small-cell carcinoma of the lung (29). Napsin-A is an aspartic proteinase involved in the maturation of surfactant protein B (30). TTF-1 and Napsin-A both positively stain AC and negatively stain SCC, the routine use of which improves diagnostic accuracy of human lung cancer specimens (31;32). All of the observed murine SCC lesions consistently stained positive for CK5/6 and p63, and negative for TTF-1 and Napsin-A, analogous to human endobronchial lesions (figure 7).
Figure 7.
A H/E-stained invasive SCC of airway (A), and immunohistochemical staining of the same airway for CK5/6 (B), p63 (C), TTF-1 (D), and Napsin-A (E). CK5/6 and p63 staining is positive, while TTF-1 and Napsin-A staining is negative, indicating squamous cell origin of dysplastic tissue. magnification: A. – 100X, B.–E. – 200X.
Discussion
In this report we summarize the effects of chronic NTCU administration in three separate doses on central airway dysplasia rates in FVB/N mice. While the application of high dose (40mM) NTCU resulted in the induction of a range of endobronchial lesions, including invasive SCC, it was associated with excessive mortality (55%) and modification of the treatment protocol. Ambrosini and colleagues report similar mortality rates (53%), as well as remarkable weight loss observed in animals receiving high dose NTCU (33). Cachexia is the progressive loss of fat and muscle despite adequate nutrition, and it is associated with increased mortality. Most animal models of cachexia involve tumor cell injections and then animals are monitored for tumor-free body weight, tumor mass and repeated muscle/fat pad measurements over time. We did not make these measurements during our study, but we can clearly show weight loss in the 40mM group (and this was the main reason for treatment being withheld).
While the 4mM and 8mM doses were better tolerated, they did not result in advanced lesions (high-grade dysplasia or invasive SCC). Additionally, these lower NTCU doses led to larger numbers of a newly appreciated lesion we term flat atypia. IHC results of representative samples showed classic squamous staining patterns (figure 7), consistent with those observed in human endobronchial dysplasia and SCC.
While multiple different pathologies were observed in the 40mM group, only flat atypia was detected in animals of the 4mM group at a significantly higher rate than control animals. In the 8mM group, significant flat atypia and low-grade lesions were detected, but the latter at a much lower frequency compared to the 40mM group. Of note, flat atypia was found at significantly higher rates in the 4mM and 8mM groups, raising the question whether these early lesions may have progressed had the duration of the experiments been extended. While histologically distinct from normal airway, flat atypia is not a strongly-associated precursor to cancer. It may represent a reactive cytological response to NTCU application. When comparing animals with and without SCC, the presence of SCC correlated with higher numbers of high-grade dysplastic lesions (but not low-grade or flat atypia). The relationship between the frequency of high-grade dysplastic lesions and the presence of SCC lends support to the theory that these are truly pre-malignant lesions that are associated with the development of SCC.
The toxicity we observed at the highest dose, coupled with the length of treatments, translated into fewer animals completing the protocol. We speculate that a more intermediate dose, for example 20mM NTCU applied twice a week, or a higher dose (40mM) applied weekly may prove to be a better model. These experiments are currently in progress.
Treatment suspensions among animals of the 40mM cohort presented an opportunity to observe the effects of differential dosing over the course of our 32-week study and to determine if there was a threshold NTCU dose required to induce SCC. Our experimental animals fell into two distinct dosing groups, those that received 33 doses (n=6), and those that received 46 doses (n=4). In this comparison (figure 6), a threshold dose (a minimum NTCU concentration necessary for SCC induction) was not discernible, and it remains to be seen if optimal NTCU dosing (dose strength and number of applications) can reveal such a threshold. While our study did show that endobronchial lesions (mostly flat atypia) are inducible through a topical chemical application that does not adversely affect animal survival, advanced SCC was only observed with higher NTCU concentrations. Improvements also remain to be made in the development of more standardized dosing schedules and whether animals receiving lower doses should be maintained longer. The use of small-animal imaging has been reported (11;33). Advanced animal imaging may help to determine the optimal timing for tumor development, but the resolution to detect precancerous lesions (pre-SCC) is thus far insufficient.
Experimental animals also developed lung adenomas at a low but constant rate, and this is consistent with previous reports of spontaneous alveolar and bronchiolar carcinomas in the lungs of aged male (45.0%) and female (52.8%) FVB/N mice (34). There are no known reports of spontaneously occurring endobronchial dysplasia in mice and multiple AC models employed in our laboratory have failed to induce the dysplastic lesions seen in NTCU treated mice.
Overall, the NTCU SCC model improves pre-clinical modeling of endobronchial dysplasia, a main target of many current and future human lung cancer chemoprevention trials. Here we report on well tolerated NTCU dosing schedules with adequate pre-malignant lesion frequency. The NTCU model will improve our ability to test novel agents prior to initiating human trials and improve our understanding of the molecular events accompanying the development and progression of pre-malignant lesions.
Supplementary Material
Acknowledgments
Supported by Department of Veterans Affairs Merit Review Program (RLK) and NCI Grant K08-CA131483 (SPM).
Footnotes
No potential conflicts of interest are disclosed.
Reference List
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4(12):1524–1529. doi: 10.1097/JTO.0b013e3181ba3634. [DOI] [PubMed] [Google Scholar]
- 3.Lam S, McWilliams A, LeRiche J, Macaulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 4.Kelly K, Kittelson J, Franklin WA, Kennedy TC, Klein CE, Keith RL, et al. A randomized phase II chemoprevention trial of 13-CIS retinoic acid with or without alpha tocopherol or observation in subjects at high risk for lung cancer. Cancer Prev Res (Phila Pa) 2009;2(5):440–449. doi: 10.1158/1940-6207.CAPR-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malkinson AM, Koski KM, Evans WA, Festing MF. Butylated hydroxytoluene exposure is necessary to induce lung tumors in BALB mice treated with 3-methylcholanthrene. Cancer Res. 1997;57(14):2832–2834. [PubMed] [Google Scholar]
- 6.Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15(24):3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirvish SS. The carcinogenic action and metabolism of urethan and N-hydroxyurethan. Adv Cancer Res. 1968;11:1–42. doi: 10.1016/s0065-230x(08)60386-3. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang Z, Yan Y, Lemon WJ, LaRegina M, Morrison C, et al. A chemically induced model for squamous cell carcinoma of the lung in mice: histopathology and strain susceptibility. Cancer Res. 2004;64(5):1647–1654. doi: 10.1158/0008-5472.can-03-3273. [DOI] [PubMed] [Google Scholar]
- 10.Lijinsky W, Reuber MD. Neoplasms of the skin and other organs observed in Swiss mice treated with nitrosoalkylureas. J Cancer Res Clin Oncol. 1988;114(3):245–249. doi: 10.1007/BF00405829. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, James M, Wen W, Lu Y, Szabo E, Lubet RA, et al. Chemopreventive effects of pioglitazone on chemically induced lung carcinogenesis in mice. Mol Cancer Ther. 2010;9(11):3074–3082. doi: 10.1158/1535-7163.MCT-10-0510. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang Z, Garbow JR, Rowland DJ, Lubet RA, Sit D, et al. Chemoprevention of lung squamous cell carcinoma in mice by a mixture of Chinese herbs. Cancer Prev Res (Phila Pa) 2009;2(7):634–640. doi: 10.1158/1940-6207.CAPR-09-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan N, Afaq F, Kweon MH, Kim K, Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67(7):3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 14.Tuveson DA, Jacks T. Modeling human lung cancer in mice: similarities and shortcomings. Oncogene. 1999;18(38):5318–5324. doi: 10.1038/sj.onc.1203107. [DOI] [PubMed] [Google Scholar]
- 15.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, et al. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci U S A. 2007;104(47):18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkinson AM. The genetic basis of susceptibility to lung tumors in mice. Toxicology. 1989;54(3):241–271. doi: 10.1016/0300-483x(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 17.Malkinson AM. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp Lung Res. 1998;24(4):541–555. doi: 10.3109/01902149809087385. [DOI] [PubMed] [Google Scholar]
- 18.Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32(3):265–279. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 19.Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52(9 Suppl):2670s–2676s. [PubMed] [Google Scholar]
- 20.Terry J, Leung S, Laskin J, Leslie KO, Gown AM, Ionescu DN. Optimal immunohistochemical markers for distinguishing lung adenocarcinomas from squamous cell carcinomas in small tumor samples. Am J Surg Pathol. 2010;34(12):1805–1811. doi: 10.1097/PAS.0b013e3181f7dae3. [DOI] [PubMed] [Google Scholar]
- 21.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40(2):90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40(5):403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 23.Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985;52(3):243–256. [PubMed] [Google Scholar]
- 24.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287(1):C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 25.Wang BY, Gil J, Kaufman D, Gan L, Kohtz DS, Burstein DE. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol. 2002;33(9):921–926. doi: 10.1053/hupa.2002.126878. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270(14):8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Whitsett JA, Stripp BR. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim Biophys Acta. 1997;1350(3):359–367. doi: 10.1016/s0167-4781(96)00180-7. [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10(1):60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Wang B, Gil J, Sabo E, Miller L, Gan L, et al. p63 and TTF-1 immunostaining. A useful marker panel for distinguishing small cell carcinoma of lung from poorly differentiated squamous cell carcinoma of lung. Am J Clin Pathol. 2003;119(5):696–702. doi: 10.1309/P5AB-R5KQ-89RN-JTFH. [DOI] [PubMed] [Google Scholar]
- 30.Ueno T, Linder S, Na CL, Rice WR, Johansson J, Weaver TE. Processing of pulmonary surfactant protein B by napsin and cathepsin H. J Biol Chem. 2004;279(16):16178–16184. doi: 10.1074/jbc.M312029200. [DOI] [PubMed] [Google Scholar]
- 31.Stoll LM, Johnson MW, Gabrielson E, Askin F, Clark DP, Li QK. The utility of napsin-A in the identification of primary and metastatic lung adenocarcinoma among cytologically poorly differentiated carcinomas. Cancer Cytopathol. 2010 doi: 10.1002/cncy.20108. [DOI] [PubMed] [Google Scholar]
- 32.Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol. 2010;41(1):20–25. doi: 10.1016/j.humpath.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosini V, Nanni C, Pettinato C, Fini M, D’Errico A, Trepidi S, et al. Assessment of a chemically induced model of lung squamous cell carcinoma in mice by 18F-FDG small-animal PET. Nucl Med Commun. 2007;28(8):647–652. doi: 10.1097/MNM.0b013e32823f9ffa. [DOI] [PubMed] [Google Scholar]
- 34.Huang P, Duda DG, Jain RK, Fukumura D. Histopathologic findings and establishment of novel tumor lines from spontaneous tumors in FVB/N mice. Comp Med. 2008;58(3):253–263. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.