Abstract
We report a patient with dysfibrinogenemia treated with purified fibrinogen concentrate who had discrepant post-treatment laboratory values. The patient had mild bleeding symptoms and was diagnosed with dysfibrinogenemia based on fibrinogen activity of 51 mg/dL and antigen of 240 mg/dL. He was treated for an adenoidectomy with purified fibrinogen concentrate (RiaSTAP®) at a dose of 70 mg/kg. A discrepancy in post-treatment fibrinogen activity was observed between the hospital and reference laboratories. Investigation revealed differences in laboratory assay and calibration methods. Fibrinogen concentrate may be a treatment option for patients with dysfibrinogenemia, but laboratory technique is critical for fibrinogen measurement.
Keywords: dysfibrinogenemia, fibrinogen, genetics, hemorrhagic disorders, thrombotic disorders
Introduction
Fibrinogen is the soluble precursor to fibrin, the endpoint of the clotting cascade. Fibrinogen is composed of three polypeptide chains, Aα, Bβ and γ, each encoded by a gene located on chromosome 4; FGA, FGB and FGG, respectively [1]. The final structure is a large dimeric protein (Aα,Bβ,γ)2 [2]. When thrombin cleaves fibrinogen, fibrin strands are released and cross-linked by factor XIII for stability. Normally, plasma fibrinogen ranges between 150–350 mg/dL [3]. Dysfibrinogenemia results when fibrinogen is present in adequate levels but functionality is compromised. This is manifested as a discrepancy between the amount of total fibrinogen present and the amount of functional fibrinogen present; measured as immunoreactive and clottable fibrinogen, respectively [4].
There is large phenotypic variability amongst dysfibrinogenemias, with over half of patients in one study reported as asymptomatic, and the remaining patients nearly equally split between hemorrhagic and thrombotic type [5]. This phenotypic variability complicates treatment. We report on a patient with dysfibrinogenemia treated with purified fibrinogen concentrate prior to a surgical procedure, to demonstrate that this treatment can be used for dysfibrinogenemia. Management was further complicated by discrepant laboratory values of fibrinogen activity which may be an issue for clinicians treating these patients.
Methods
Informed consent was obtained for DNA sequencing following review of the protocol by the Institutional Review Boards of the associated institutions. Genomic DNA was extracted from peripheral blood using the Qiagen Blood Mini kit (Qiagen, Valencia, CA). All coding exons of FGA were amplified using flanking primers and bidirectional direct sequencing was performed using BigDye Terminator chemistry (Applied Biosystems, Foster City, CA). Sequence analysis was performed using Mutation Surveyor v4.0 software package (Softgenetics, State College, PA).
Fibrinogen measurements were performed in two different laboratories. The hospital laboratory uses the STA® fibrinogen assay to measure fibrinogen on plasma samples with the STA® Compact automated coagulation instrument (Diagnostica Stago, Parsippany, NJ). Calibration is performed using control plasmas provided along with the instrument. Fibrinogen is measured by the clotting method of Clauss in which in the presence of an excess of thrombin, the clotting time of diluted plasma is inversely proportional to the level of plasma fibrinogen. The clot is detected by the STA® Compact (Diagnostica Stago, Parsippany, NJ), which electromagnetically senses and registers the time when an oscillating steel ball within a cuvette with thrombin stops.
The reference laboratory also measures fibrinogen activity by Clauss methodology, using Dade® Thrombin Reagent on an automated Siemens BCS coagulometer (Dade Behring, Siemans Healthcare Diagnostics, Deerfield, IL) which detects clot formation optically. Calibration is performed with CRYOcheck normal reference plasma (PrecisionBioLogics, Dartmouth, Nova Scotia, Canada) which is in referenced to a SSC/ISTH secondary reference standard. Fibrinogen antigen was determined by radial immunodiffusion assay (Human Fibrinogen Bindarid®, Binding Site, Birmingham, UK) using calibrators provided by the kit manufacturer which are in turn referenced to NIBCS standard 98/612.
Case report
The proband is a 15-year-old male who presented to the hematology clinic for evaluation prior to an adenoidectomy. He was diagnosed with dysfibrinogenemia at an outside institution at five years of age after an episode of hematuria. Other bleeding symptoms included easy bruising, epistaxis, and oozing with loss of deciduous teeth. He had not experienced any major bleeding episodes and had no prior surgeries. An adenoidectomy had been recommended due to prominent adenoid tissue and a history of snoring and sleep difficulties.
Initial laboratory studies performed at the reference laboratory showed a fibrinogen activity of 51 mg/dL and fibrinogen antigen of 240 mg/dL, consistent with the diagnosis of dysfibrinogenemia. Thrombin time was predictably elevated (41.7 seconds, normal range 13.1–19.8) as was prothrombin time (18.6 seconds, normal range 11.4–13.6). Platelet count, hemoglobin and aPTT were within normal limits. Genetic testing revealed a heterozygous mutation in FGA (c.103C>T) that substitutes the arginine residue at position 16 of the mature fibrinogen alpha chain for cysteine (p.Arg35Cys; R16C in mature Aα chain). This mutation impairs fibrin polymerization and delays fibrinopeptide A release after thrombin cleavage, impairing thrombus formation and predisposing to hemorrhage [6,7]. This mutation can also predispose to thrombosis and fibrinolytic resistance due to intrinsic decreased susceptibility of the clot to proteolysis [8].
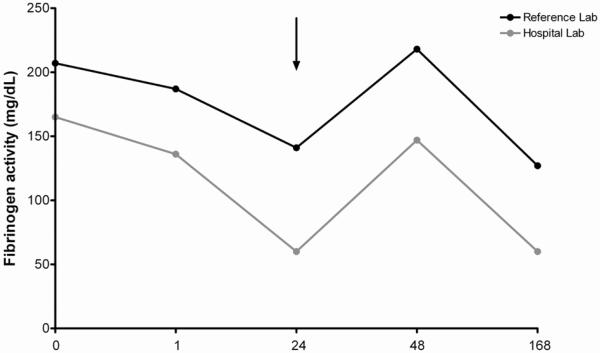
A discussion was held with the family regarding treatment options, and the decision made to treat with purified fibrinogen concentrate at a dose of 70 mg/kg of purified fibrinogen concentrate (RiaSTAP®) prior to surgery. Minimal bleeding was reported, with negligible estimated blood loss. A 24-hour post infusion fibrinogen activity level obtained at the hospital laboratory was <100 mg/dL, at which time cryoprecipitate infusion was performed to avoid late bleeding However, the same sample analyzed at the reference laboratory, resulted after treatment with cryoprecipitate, was greater than two times that of the hospital laboratory value. Subsequent results showed continued discrepancies between the two laboratories (table 1). No further treatment was administered and no bleeding complications were reported. Fibrinogen results are compared graphically in figure 1.
Table 1.
Comparison of fibrinogen activity and antigen levels
| Time (hours) 1 | Fibrinogen Activity (mg/dL) | Fibrinogen Antigen (mg/dL) | |
|---|---|---|---|
| Hospital Lab | Reference Lab | Reference Lab | |
| 0 | 165 | 207 | 438 |
| 1 | 136 | 187 | 438 |
| 242 | 60 | 141 | 361 |
| 48 | 147 | 218 | 435 |
| 168 | 60 | 127 | 435 |
Hours post infusion of fibrinogen concentrate.
Cryoprecipitate was infused after this measurement was obtained.
Figure 1. Comparison of fibrinogen activity measurements between reference laboratory and hospital laboratory.
Fibrinogen activity in mg/dL is plotted on the y axis for the reference laboratory (in black) and the hospital laboratory (in grey) for the given timepoints following infusion of purified fibrinogen concentrate. Surgery occurred immediately after the 1 hour timepoint. The arrow indicates time of cryoprecipitate administration.
Discussion
Treatment of fibrinogen disorders has advanced considerably in the past several years with the introduction of purified fibrinogen concentrate. Traditional therapies, including plasma and cryoprecipitate, carry the risk of viral transmission. They contain factor VIII, fibrinogen, von Willebrand factor and factor XIII, increasing the risk of thrombotic events. Finally, the volume required to achieve normal fibrinogen levels with plasma therapy puts patients at risk for volume overload. Fibrinogen concentrate is by definition highly concentrated, so more precise dosing can be achieved and the risk of viral transmission is negligible. [9]. A comprehensive review of fibrinogen replacement therapy conducted by Bornikova and colleagues reported on ten patients with fibrinogen disorders treated prior to surgical intervention [10]. They concluded that fibrinogen levels between 100–200 mg/dL were adequate to maintain hemostasis during surgery [10]. A recent review of patients from European treatment centers with fibrinogen defects corroborated these results [11].
Variation in measured fibrinogen activity is of concern, particularly at low levels. In this case, a level <100 mg/dL at the hospital laboratory precipitated additional treatment although upon subsequent investigation, fibrinogen was likely within normal ranges. Instrumentation and standardization differences may account for this discrepancy. We believe the reference lab values are accurate for two reasons; first, the instrument used to detect fibrinogen activity uses a plasma standard every time an assay is run which increases reliability. Secondly, the laboratory result obtained post treatment was within the range one would expect after infusion. However, it is difficult to say with absolute certainty which value is correct.
This report highlights the difficulty of treating patients with dysfibrinogenemia. Phenotypic variability and the lack of official treatment recommendations in the United States make appropriate therapeutic intervention challenging. As illustrated, discrepancies between different lab assays put patients at risk for potentially unnecessary and harmful therapy. Standardized methods of measuring fibrinogen activity level and reliable laboratory assays are critical for both diagnosis and treatment. It is imperative that laboratories validate their reference plasma for their instrument and maintain accurate calibration, particularly for values that fall outside the normal range. Further treatment studies are necessary to establish treatment guidelines and optimize care of these patients.
Acknowledgements
Special thanks to Judy Peot and Jane Kegel of the Children's Hospital of Wisconsin coagulation laboratory and the Clinical Hemostasis Laboratory of the BloodCenter of Wisconsin.
Footnotes
Conflict of Interest Statement None of the authors have any conflicts of interest to disclose.
References
- 1.Chung DW, Harris JE, Davie EW. Nucleotide sequences of the three genes coding for human fibrinogen. Adv Exp Med Biol. 1990;281:39–48. doi: 10.1007/978-1-4615-3806-6_3. [DOI] [PubMed] [Google Scholar]
- 2.Doolittle RF, Spraggon G, Everse SJ. Three-dimensional structural studies on fragments of fibrinogen and fibrin. Curr Opin Struct Biol. 1998;8:792–798. doi: 10.1016/s0959-440x(98)80100-0. [DOI] [PubMed] [Google Scholar]
- 3.Acharya SS, Dimichele DM. Rare inherited disorders of fibrinogen. Haemophilia. 2008;14:1151–1158. doi: 10.1111/j.1365-2516.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 4.de Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35:356–366. doi: 10.1055/s-0029-1225758. [DOI] [PubMed] [Google Scholar]
- 5.Santacroce R, Cappucci F, Pisanelli D, et al. Inherited abnormalities of fibrinogen: 10-year clinical experience of an italian group. Blood Coagul Fibrinolysis. 2006;17:235–240. doi: 10.1097/01.mbc.0000224841.48463.be. [DOI] [PubMed] [Google Scholar]
- 6.Hanss M, Biot F. A database for human fibrinogen variants. Ann N Y Acad Sci. 2001;936:89–90. doi: 10.1111/j.1749-6632.2001.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 7.Flood VH, Al-Mondhiry HA, Farrell DH. The fibrinogen aalpha R16C mutation results in fibrinolytic resistance. Br J Haematol. 2006;134:220–226. doi: 10.1111/j.1365-2141.2006.06129.x. [DOI] [PubMed] [Google Scholar]
- 8.Flood VH, Nagaswami C, Chernysh IN, et al. Incorporation of fibrin molecules containing fibrinopeptide A alters clot ultrastructure and decreases permeability. Br J Haematol. 2007;138:117–124. doi: 10.1111/j.1365-2141.2007.06630.x. [DOI] [PubMed] [Google Scholar]
- 9.Manco-Johnson MJ, Dimichele D, Castaman G, et al. Pharmacokinetics and safety of fibrinogen concentrate. J Thromb Haemost. 2009;7:2064–2069. doi: 10.1111/j.1538-7836.2009.03633.x. [DOI] [PubMed] [Google Scholar]
- 10.Bornikova L, Peyvandi F, Allen G, et al. Fibrinogen replacement therapy for congenital fibrinogen deficiency. J Thromb Haemost. 2011;9:1687–1704. doi: 10.1111/j.1538-7836.2011.04424.x. [DOI] [PubMed] [Google Scholar]
- 11.Peyvandi F, Palla R, Menegatti M, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: Results from the european network of rare bleeding disorders. J Thromb Haemost. 2012;10:615–621. doi: 10.1111/j.1538-7836.2012.04653.x. [DOI] [PubMed] [Google Scholar]