Abstract
Introduction
Overweight men with diabetes often report erectile dysfunction, but few studies have examined effects of weight loss on this problem.
Aim
This study examined 1-year changes in erectile function in overweight/obese men with type 2 diabetes participating in the Look AHEAD trial.
Methods
Participants in Look AHEAD were randomly assigned to a control condition involving diabetes support and education (DSE) or to Intensive Lifestyle Intervention (ILI) involving group and individual sessions to reduce weight and increase physical activity. Men from 5 of the clinical sites in Look AHEAD completed the International Index of Erectile Function (IIEF) at baseline (N = 372) and at 1 year (N = 306) (82%).
Main Outcome Measures
Changes in erectile function as reported on the EF subscale of the IIEF.
Results
At 1 year, the ILI group lost a greater percent of initial body weight (9.9% vs 0.6 %) and had greater improvements in fitness (22.7% vs 4.6%) than DSE. Erectile function improved more in ILI (17.3 ± 7.6 at baseline; 18.6 ± 8.1 at 1-year) than in DSE (18.3 ± 7.6 at baseline; 18.4 ± 8.0 at 1-year); p=.04, and p=.06 after adjusting for baseline differences. Using established norms for none (i.e., normal erectile function), and three grades (i.e., mild, moderate and severe) ED, 8% of men in ILI reported a worsening of erectile function from baseline to 1 year, 70% stayed in the same category, and 22% reported improvements. In contrast, 20% of DSE reported worsening, 57% stayed in the same category, and 23% improved (p=.006).
Conclusion
In this sample of older overweight/obese diabetic men, weight loss intervention was mildly helpful in maintaining erectile function.
Keywords: Diabetes, lifestyle, fitness, International Index of Erectile Function (IIEF), weight loss
INTRODUCTION
Erectile dysfunction (ED), defined as the consistent inability to achieve and/or maintain an erection, occurs commonly in middle-aged men, especially in those with diabetes (1-4). In prior studies, 39 – 51% of men with diabetes self-reported having ED (4). Several risk factors for ED have been defined including age, diabetes duration, and the presence of other cardiovascular risk factors (1, 5-8). Of particular note is that both obesity and sedentary lifestyle are important risk factors for ED (9-12). Compared to men with a body mass index (BMI) of <25 kg/m2, the odds of having ED are 1.5 fold higher in men with a BMI of 25-30 kg/m2 and 3 fold higher in men with a BMI >30 kg/m.2 (10). In addition, independent of BMI, the risk of ED is 30% lower in men who report >16 metabolic equivalents (MET) hours/week of exercise compared to sedentary men (7).
Given the effects of obesity and sedentary lifestyle on the risk of developing ED, lifestyle interventions could be expected to improve this condition. Esposito and colleagues (13) found evidence supporting this hypothesis in a randomized controlled trial. In this study, 110 obese men with ED, defined by a score of ≤ 21 on the erectile function (EF) subscale of the International Index of Erectile Function (14), who did not have hypertension, diabetes, or hyperlipidema, were randomly assigned to a control group or to a lifestyle intervention that focused on weight loss and physical activity. Over 2 years of intervention, the lifestyle group lost an average of 15 kg, compared to 2 kg in the control, and had greater increases in physical activity (195 vs. 84 minutes/week). Erectile function improved significantly more in the intervention group than in the control (p = .008), with scores increasing from 13.9 to 17.0 in the intervention group and from only 13.5 vs 13.6 in the control. Both weight loss and increases in physical activity were related to the improvement in EF scores.
To date, there has been no effort to replicate the results of this trial or to extend the findings to men with diabetes. Since neurovascular factors associated with diabetes may be important mediators of diabetic ED (15), lifestyle interventions may be less effective in this population. We report here results of an ancillary study that examined the effect of a lifestyle intervention on erectile function in men with diabetes. All men were participants in the Look AHEAD (Action for Health in Diabetes) study (16, 17), a multi-center clinical trial that is examining the effects of lifestyle intervention on cardiovascular morbidity and mortality in 5,145 overweight individuals with type 2 diabetes. In the present study, men from 5 sites of Look AHEAD who were sexually active at baseline were asked to complete the IIEF at baseline and 1 year. We compared 1-year changes in weight, physical fitness, and erectile function in men who were randomly assigned to a lifestyle intervention or control condition.
METHODS
Participants
The eligibility criteria for Look AHEAD and the baseline characteristics of the sample have been described in detail elsewhere (16, 18). Primary eligibility criteria included type 2 diabetes, age 45-74 years (changed to 55 – 74 in later years of recruitment), and a BMI ≥25 kg/m2 (≥27 kg/m2 for individuals using insulin). Participants were excluded if they had uncontrolled hyperglycemia (HbA1c >11%), hypertension (BP >160/100 mmHg), fasting triglycerides ≥600 mg/dl, a cardiovascular event within the past 3 months, significant pulmonary or renal disease, or conditions likely to limit adherence to the study protocol.
Participants in the sexual dysfunction ancillary study were recruited from 5 of the 16 Look AHEAD sites (University of Pennsylvania, The Miriam Hospital/Brown Medical School, Johns-Hopkins University, University of Alabama – Birmingham, and University of Tennessee – Memphis). These sites were selected on the bases of geographic and ethnic diversity of the study participants. Sites were asked to identify male participants who had been sexually active within the past 6 months or were in a committed relationship. Those who were interested in participating signed a separate consent form and were provided a modest incentive ($25 gift certificate) at study entry and at 1 year.
Procedures
Look AHEAD participants were randomly assigned within clinic to the Intensive Lifestyle Intervention (ILI) or to a control group that received diabetes support and education (DSE). Specific details of the intervention have been published previously (19). The ILI focused on changing diet and physical activity with a study goal of inducing a loss of 7% or more of initial weight during year 1. To accomplish this, participants attended weekly meetings during months 1-6 and were seen 3 times/month during months 7-12. Intervention included a combination of group and individual sessions, and was modeled on group behavioral weight loss programs and on approaches used successfully in the Diabetes Prevention Program (DPP). Calorie restriction was the primary approach to weight loss; participants were given calorie goals of 1200-1500 if <250 lb and 1500-1800 if >250 lb, with a maximum of 30% of calories from fat. Portion controlled diets, including liquid meal replacements and frozen entrees, were prescribed. The physical activity recommendation was to gradually increase activity to 175 minutes/week, using moderate intensity activities such as brisk walking.
The DSE group attended an initial diabetes education course and was invited during year 1 to three sessions that provided basic education about diet and physical activity, as well as opportunities for social support. Participants in both groups continued to receive their medical care from their own physicians.
Measures
All measures were completed at baseline and 1 year by assessors who were masked to the participants’ treatment group.
Sexual function
Sexual function was evaluated with the International Index of Erectile Function (IIEF) (14). The IIEF questionnaire is a validated measure of sexual function that has been used extensively in clinical trials and longitudinal studies. The IIEF provides standardized scores for sexual function over the past 6 months, assessing five specific domains – erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. Participants who omitted any questions were excluded from scores on that domain. Higher scores indicate more normal sexual function. The erectile function (EF) domain is the primary measure of erectile function. Using previously established norms from similarly aged men (20), severe erectile dysfunction was defined by scores of ≤10 on the EF domain; moderate by scores of 11-21; mild by scores of 22-25; and no erectile dysfunction by scores of ≥ 26.
Participants were also asked whether they were using any phosphodiesterase type 5 inhibitors (PDE-5i) such as sildenafil, tadalafil, or vardenafil (i.e., Viagra, Cialis or Levitra) at baseline and 1 year.
Weight, Height, and BMI
Weight and height were measured in duplicate using a digital scale and standard stadiometer. BMI was calculated as weight in kg/height in m2.
Fitness
Prior to randomization, participants completed a maximal graded exercise test, in which they walked on a motorized treadmill at a self-selected speed. The elevation of the treadmill was set at a 0° grade initially and increased by 1% every minute. Heart rate and rate of perceived exertion (RPE) were measured during the final 10 seconds of each exercise stage and at study termination. To enter the study, participants were required to achieve at least 85% of age-predicted maximal heart rate and a minimum of 4 metabolic equivalents (METS). Individuals on prescription medications that would affect heart rate during exercise (e.g., beta blockers) were required to achieve a RPE of at least 18 on the Borg 15-category scale (21) and a minimum of 4 METS.
At 1 year, a submaximal fitness test was performed on those who were physically able to complete this assessment. The test was terminated when the participant first achieved or exceeded 80% of age predicted maximal heart rate (HR max in beats/minute = 220 minus age). Change in fitness was computed as the difference in estimated METS between points during baseline and 1 year when >80% of age–predicted HR max was attained. For participants on beta blockers, the test was terminated when the participant first exceeded 16 on the 15-category RPE scale, and change in fitness was computed as the difference in estimated METS between points during the baseline and 1-year test when RPE >16 was attained (17).
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 14.0 for Windows) and SAS 9.1 for Windows. Those who completed both baseline and year-1 assessments were compared to those who did not complete year-1 assessments on relevant baseline variables and demographic characteristics using analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables. Similar analyses were conducted to compare ILI and DSE participants on baseline predictor variables and demographic characteristics and on changes in weight and fitness over 1 year. Repeated measures analysis of variance was used to examine EF scores at baseline and 1 year, followed by an analysis of covariance (ANCOVA) that controlled for baseline EF score and compared ILI versus DSE participants on change in erectile function. Similar analyses were done within the subgroup of men with EF scores ≤ 21 at baseline (indicating erectile dysfunction) and those with EF scores ≥22. Categorical analyses were done with chi square analyses. Regression analysis was used to examine predictors of change in EF from baseline to 1 year, using baseline values for EF, METS, weight, age, hypertension medication use at baseline (yes/no), self-reported neuropathy at baseline (yes/no), clinic, treatment group, and percent weight change and percent METS change from baseline to 1 year. Similar analyses were conducted including history of cardiovascular disease at baseline (CVD) (yes/no) and levels of HbA1c, blood pressure and lipids at baseline, and changes in these risk factors from baseline to 1 year. Generalized Estimating Equations (GEE) methodology, using Proc GENMOD in SAS, was used to compare change in PDE-5i use status between baseline and 1 year. A repeated measures ANOVA was used to examine the effects of PDE-5i use and treatment group on EF score and to assess the effect of different levels of weight reduction on changes in EF.
RESULTS
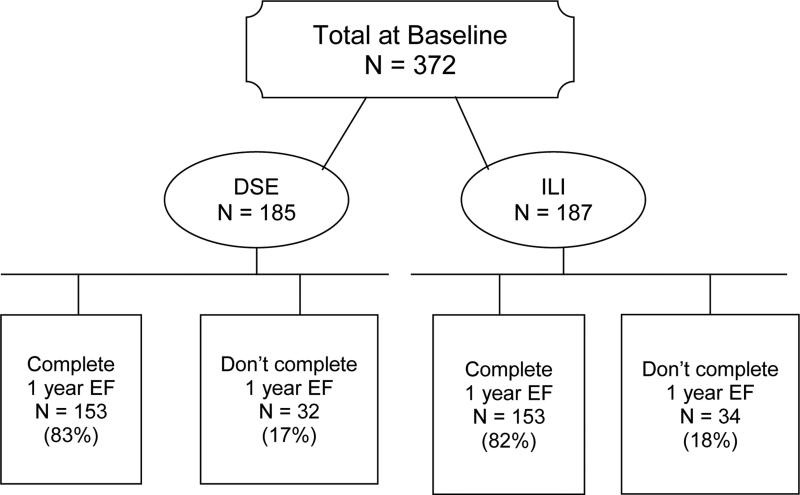
Erectile function (EF) domain scores were assessed on 372 men at baseline (187 in ILI and 185 in DSE). Of these, 306 (82%) completed the same measure at 1 year (Fig 1). The completion rate was comparable in the ILI and DSE groups (81.8% vs. 82.7%, p = .82). Those who completed the 1-year measure did not differ from non-completers on age, race, income, marital status, diabetes duration, or on baseline EF scores, weight, BMI or fitness (all p-values > .15). Baseline characteristics of the 306 completers are shown in Table 1, with no significant differences between DSE and ILI participants. At baseline, severe, moderate, mild, and no ED were reported by 22%, 29%, 25% and 24% of the DSE participants compared to 25%, 35%, 12%, and 27% of ILI (P = .05). However, the groups were similar (P = .08) on the proportion of participants with baseline EF ≤ 21 (51% of DSE and 61% of ILI) vs. > 21 (49% of DSE vs 39% of ILI).
Figure 1.
Flow chart of participants in the Sexual Dysfunction substudy.
Table 1.
Baseline Characteristics of Men Who Completed Sexual Function Study (N=306)1
| Total Sample (N=306) | ILI (N=153) | DSE (N=153) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (yrs) | 60.5 | 6.5 | 60.7 | 6.5 | 60.3 | 6.6 |
| Weight (kg) | 109.9 | 18.0 | 110.6 | 18.4 | 109.2 | 17.7 |
| BMI | 35.3 | 5.4 | 35.6 | 5.5 | 35.1 | 5.2 |
| Diabetes duration (yrs) | 7.7 | 6.9 | 7.9 | 7.0 | 7.5 | 6.9 |
| EF | 17.8 | 7.6 | 17.3 | 7.6 | 18.3 | 7.6 |
| METS | 5.5 | 1.6 | 5.6 | 1.6 | 5.4 | 1.6 |
| HbA1c | 7.3 | 1.2 | 7.3 | 1.1 | 7.4 | 1.3 |
| Systolic BP | 129.2 | 15.7 | 129.9 | 16.3 | 128.4 | 15.1 |
| Diastolic BP | 73.0 | 8.8 | 72.8 | 8.7 | 73.3 | 8.9 |
| Cholesterol | 182.5 | 37.4 | 180.8 | 38.0 | 184.1 | 36.8 |
| HDL | 39.0 | 8.5 | 39.8 | 7.9 | 38.2 | 8.9 |
| LDL | 108.0 | 30.8 | 107.7 | 30.3 | 108.2 | 31.4 |
| Testosterone (total) | 455.6 | 203.4 | 469.2 | 197.6 | 441.9 | 209.0 |
| % using insulin | 16.7% | 18.3% | 15.1% | |||
| Ethnicity | ||||||
| White | 77.8% | 79.7% | 75.8% | |||
| African American | 16.0% | 15.0% | 17.0% | |||
| Other | 6.2% | 5.3% | 7.2% | |||
Sample varies slightly across measures. All differences between ILI and DSE were non-significant (p > .09);
METS = Metabolic equivalents
Table 2 shows that participants in the ILI lost significantly more weight at year 1 than those in DSE (9.9 vs 0.6%, respectively; p<0.001). In addition, among the 269 men who had repeat fitness measures, those in the ILI group also had significantly greater changes in fitness level (22.7 vs 4.6%, respectively; p<0.001). The intervention group also had significantly greater changes in HbA1c, systolic and diastolic blood pressure and high-density-lipoprotein cholesterol (HDL-C), confirming findings from the larger sample in the Look AHEAD trial (17).
Table 2.
Changes in Weight and Fitness from Baseline to 1 Year for Men in the Sexual Function Study
| Variable | Intensive Lifestyle Intervention (ILI) | Diabetes Support and Education (DSE) | Comparison of ILI vs. DSE | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | F | P | |
| Weight loss (kg) | 153 | −11.1 | 9.4 | 150 | −0.7 | 4.5 | 153.2 | <.001 |
| % Weight loss | 153 | −9.9 | 7.8 | 150 | −0.6 | 3.9 | 169.4 | <.001 |
| MET change | 138 | 1.1 | 1.3 | 131 | 0.2 | 1.1 | 45.9 | <.001 |
| % MET change | 138 | 22.7 | 26.7 | 131 | 4.6 | 19.1 | 40.5 | <.001 |
| HbA1c change | 148 | −0.7 | 1.0 | 144 | −0.3 | 1.1 | 11.8 | .001 |
| Systolic BP change | 153 | −7.5 | 16.3 | 150 | −1.5 | 14.9 | 11.4 | .001 |
| Diastolic BP change | 153 | −4.7 | 7.9 | 150 | −1.0 | 7.6 | 17.4 | <.001 |
| Cholesterol change | 148 | −10.1 | 34.0 | 144 | −8.3 | 31.4 | 0.2 | .631 |
| HDL change | 148 | 3.7 | 6.6 | 144 | 1.0 | 5.8 | 13.3 | <.001 |
| LDL change | 148 | −6.9 | 25.3 | 144 | −5.4 | 28.5 | 0.2 | .621 |
MET = Metabolic equivalent; BP = blood pressure; HDL = high density lipoprotein; LDL = low density protein
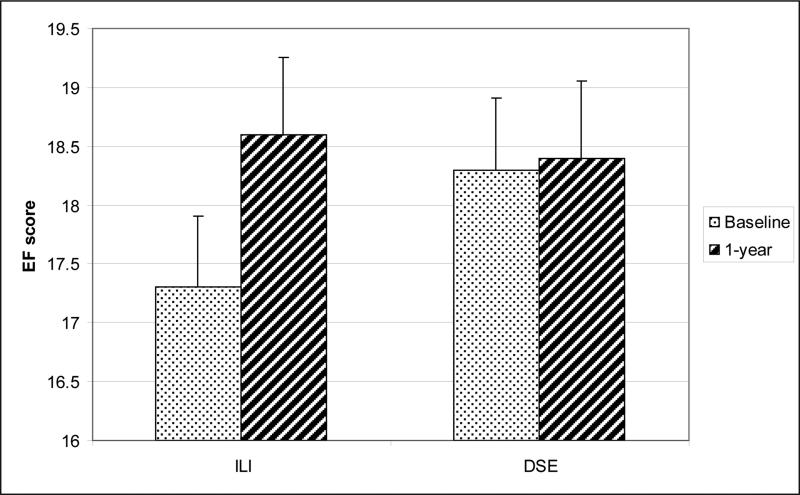
Repeated measures analysis of variance indicated a significant difference between groups for changes in EF domain scores (interaction of Group X Time, p = .035), as scores increased from 17.3 ± 7.6 at baseline to 18.6 ± 8.1 at 1 year in the ILI group, while scores remained stable in the DSE group (18.3 ± 7.6 at baseline and 18.4 ± 8.1 at 1 year) (Fig 2). After adjusting for baseline differences in EF score, there was only a trend for men in the ILI group to have greater increases in EF than men in DSE (+ 1.3 ± 4.7 vs. + 0.03 ± 5.7; p = 0.06). Results were similar using intent to treat analyses (in which drop-outs were considered to have had no changes in EF) or omitting men (N=67) who reported using beta blockers at baseline.
Figure 2.
Scores on the Erectile Function (EF) subscale of the International Index of Erectile Function at baseline and Year 1 for men in the Intensive Lifestyle Intervention (ILI) or in the control group given Diabetes Support and Education (DSE).
Analyses were also conducted to examine changes in the categories of erectile dysfunction by treatment arm. In DSE, 20% reported worsening in their category of ED (e.g. moving from moderate erectile dysfunction at baseline to severe dysfunction at 1-year), 57% stayed in the same category, and 23% reported improvements in category. The distribution in ILI differed significantly (p=.006), with only 8% reporting worsening, 70% staying in the same category, and 22% reporting improvements. Likewise, if only two categories are used (≤ 21 and ≥ 22), as in Esposito (13), there were marked differences in the percent of men who showed worsening in erectile function over time (0% in ILI and 19% in DSE, p<.001), but no differences between arms in the percent of men who improved (19% of DSE and 18% of ILI). Finally, using an increase of 2 or 3 units as a criterion for “clinically meaningful changes” we found no significant differences in the proportion of men reporting improvements (p > 0.4), but a significantly smaller percentage of men in ILI compared to DSE reported worsening of 2 units or more (18.3% vs. 28.8%, p = .03). Thus the weight loss intervention was effective in mitigating the worsening in erectile function seen in the DSE condition.
A total of 64 men (37 in DSE and 27 in ILI) had total testosterone levels < 300 ng./dl at baseline. Analyses comparing changes in EF for men with testosterone < 300 ng./dl vs. > 300 ng./dl indicated no significant differences by baseline testosterone levels and no significant interactions between baseline testosterone and treatment group.
We also conducted regression analyses predicting changes in EF scores using baseline variables (EF score, age, weight, fitness, clinic), treatment group, and percent changes in weight and fitness. The overall model had an R2 of .13 (p<.001). Table 4 shows that the strongest predictor of changes in EF was baseline EF score (p ≤ 0.001), with greater improvement in those with lower EF scores at baseline. Weight change also was significantly associated with changes in EF scores (p = 0.01) and change in METS approached significance (p=.09). When weight change and fitness changes were in the model, there was no significant effect of treatment arm. None of the other variables added to the model including history of cardiovascular disease, baseline HbA1c, baseline levels of cvd risk factors, baseline use of PDE's, or changes in HbA1c or cvd risk factors were related to outcome..
Table 4.
Regression Model Predicting Changes in Erectile Function (EF)1
| Beta | t | P | |
|---|---|---|---|
| Baseline EF Score | −.183 | −3.997 | < .001 |
| Age | −.043 | −.726 | .468 |
| Baseline weight (kg) | −.019 | −.973 | .331 |
| Baseline METS | −.114 | −.473 | .636 |
| Baseline HT medication (yes/no) | −.565 | −.654 | .514 |
| Baseline Neuropathy (yes/no) | −.098 | −.096 | .924 |
| Randomization arm | .722 | .891 | .374 |
| % weight change | −.148 | −2.596 | .010 |
| % METS change | −.028 | −1.730 | .085 |
Adjusted for clinic
MET = Metabolic equivalent; HT = hypertension
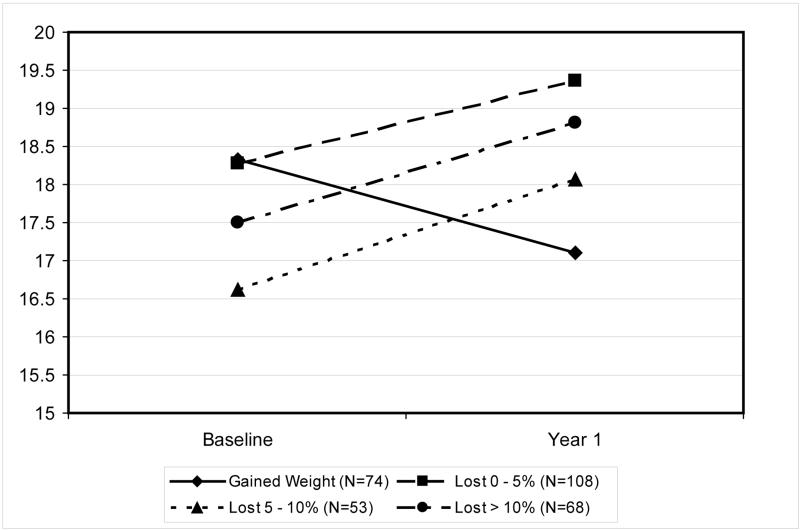
To further understand the effect of weight loss on EF, we collapsed the intervention and control group and examined the effect of amount of weight loss on EF scores. Men who gained weight (N = 74) were compared to those who lost 0 – 4.9% (N = 108), 5 – 10% (N = 53) or > 10% (N = 68) of their body weight. The 4-group interaction (Fig 3) was highly significant (p=.006), due to the fact that men who gained weight reported significant worsening in erectile function scores over time, and those who lost weight, of any magnitude, reported significant improvements in EF scores. There was, however, no evidence that greater amounts of weight loss were associated with greater improvements in EF (i.e., dose response effects).
Figure 3.
Scores on the Erectile Function subscale of the International Index of Erectile Function at baseline and Year 1 for men who gained weight or lost 0 – 4.9%, lost 5 – 10%, or lost > 10% over this time.
Analyses were also conducted to assess changes in PDE-5i user status. In the ILI group, PDE-5i's were used by 32.4% and 38.7% of participants at baseline and 1 year respectively. For the DSE group, 39.2% and 38.3% of men reported their use at these time points. There were no significant effects of time, group, or time x group on use (yes/no) of these medications (all p's > .65) and no evidence of an interaction between use of these medications, time, and intervention group on EF scores.
DISCUSSION
In this sample of older, overweight/obese men with type 2 diabetes, weight loss intervention produced small improvements in erectile function relative to those in the control. The benefit of weight loss seemed to be due primarily to helping to preserve performance over the year of the study, with no difference between arms seen in the number of men reporting improvements. Thus lifestyle intervention may have a role in preserving erectile function over time in overweight/obese men with type 2 diabetes.
Weight loss has numerous benefits for men with diabetes (17, 22, 23), including improvements in glycemic control, blood pressure, and HDL-cholesterol, which are confirmed in the present sample. Men who were randomly assigned to intensive lifestyle intervention achieved a nearly 10% change in their body weight and had marked improvements in their fitness. However, despite these changes in weight and fitness, on average IIEF scores increased only slightly more in the ILI group than in DSE, and there were no differences in the proportion of men who experienced clinically significant improvements in erectile function. In contrast there were marked differences in the percent that experienced worsening in erectile function, with approximately 20% of DSE reporting worsening and only 8% of ILI.
The findings from this study complement those reported by Esposito et al. (13, 24, 25), and suggest that weight loss may have different effects on EF in different study populations. The original Esposito trial (13) involved men who were overweight, but had none of the health complications typically associated with obesity including hypertension, hypercholesterolemia, and - most importantly - diabetes. Other studies by Esposito (24, 25) have included men with metabolic syndrome but these participants were relatively young, with an average age of 45 years. In contrast, in the present study, men averaged 60 years of age; all men had diabetes with a mean duration of over 7 years; 75% were on hypertensive medications and 50% on lipid lowering drugs. The differences in the populations studied may well have contributed to the different findings.
Regression analyses that examined variables associated with changes in erectile function in the present study produced several interesting findings. First, these analyses suggested that the strongest predictor of improvements in EF was baseline levels of EF, with men who had impaired EF scores at baseline showing the greatest improvements. Thus reported changes may be strongly influenced by regression to the mean.
Second, these analyses indicated that, independent of treatment group, weight change was associated with improved erectile function. However, again, the effect remained modest from a clinical perspective – a 10% reduction in body weight was associated with a 1.5 point increase in EF scores – about half the amount usually considered a clinically meaningful change (i.e., 3 point change). Moreover, analyses that compared men who gained weight over the year-long trial, with those who lost 1-4.9%, 5-10%, or >10% of their initial body weight, indicated that men who gained weight had worsening in their erectile function. Weight loss, of any magnitude, seemed only to protect against this decrease in function. Greater amounts of weight loss were not associated with greater improvements in erectile function, suggesting a possible threshold or ceiling effect. The apparently beneficial effects of weight loss compared to weight gain could be due to differences between men who subsequently gain or lose, or to physiological or psychological changes that accompany these weight changes.
The third finding from the regression analysis was that despite large improvements in fitness (averaging over 20% in the intervention subjects), changes in fitness were only slightly, and not significantly, associated with changes in ED. This finding contrasts with the results from Esposito (13) which suggested that both weight loss and fitness had independent effects on erectile function. In a prior publication (26) describing baseline findings for the men in the Look AHEAD erectile function study, we reported that almost half of the men reported mild or moderate ED and that 25% reported complete or severe ED. Erectile function was related to age, duration of diabetes, HbA1c levels, and cardiovascular risk factors, confirming prior studies (1, 4, 11). Body mass index was not related to ED at baseline, perhaps owing to the fact that all participants were overweight to varying degrees. However, cardiorespiratory fitness was related to ED and was shown to have a protective effect. Perhaps more intensive exercise interventions would have greater impact on improving erectile function.
Strengths of this study include the use of a randomized controlled trial and high participant retention (82%). Moreover, no significant differences between completers and noncompleters were observed. Changes in erectile function were assessed with a standardized instrument that has been shown to be valid and reliable (27) and changes in weight and fitness were assessed with objective measures. All assessments were completed by staff masked to the participants’ treatment assignment. The changes in weight and fitness in the ILI group were representative of the current state-of-the art for behavioral weight control programs. Although only 5 of the 16 Look AHEAD sites participated in this study, these sites were selected a priori to include participants from diverse ethnic backgrounds. Although the use of only five centers is a limitation of this study, the fact that both the baseline characteristics of these participants and their overall weight losses and changes in cvd risk factors were similar to those reported in the main trial (17, 18) suggest that these centers were representative of the total study population.
In interpreting the findings of this study, it is important to note that this is the first trial investigating the effects of lifestyle intervention in men with type 2 diabetes. Men with diabetes may have impaired neurogenic and endothelium-mediated relaxation of smooth muscle, in addition to advanced glycation end products (AGE's) and dysregulation of nitric oxide synthase by the l-arginine mechanism (15, 28). These pathophysiological changes could limit the reversibility of diabetic ED. It is of interest to note that even the 22 men who started using PDE-5i's reported non-significant increases in EF scores (17.1 at baseline and 19.4 at 1 year; p=.14). Since these data contrast with prior studies which indicate that these medications are effective in men with diabetes (29), it may be that the small sample limited the significance of the changes, or that the long duration of diabetes and/or their obesity made these men less responsive to these medications, and perhaps similarly, less responsive to lifestyle intervention. While baseline testosterone levels did not affect the reported changes in erectile function we will be conducting further analyses to examine the effect of weight loss on these parameters. This study examined the effects of weight loss over a period of one year. Thus we cannot exclude the possibility that a longer period of sustained weight loss might improve erectile function.
CONCLUSION
This study found that weight loss in men with diabetes and obesity may help to preserve function or prevent worsening in ED over time. However, there was little evidence in our study that weight loss was effective in actually improving erectile function. An important unanswered question is whether weight loss and improved fitness are effective in restoring or clinically improving erectile function in men with ED who are overweight or obese, but do not have diabetes. A randomized trial to evaluate this question is clearly needed.
Table 3.
Changes in erectile function for men with EF ≤ 21 at baseline (indicative of moderate or severe sexual dysfunction) compared to men with EF > 22 at baseline (indicative of mild or no sexual dysfunction)
| DSE | ILI | Significance | ||
|---|---|---|---|---|
| EF ≤ 21 | ||||
| N | 78 | 93 | ||
| Pre | 11.8 ± 4.8 | 12.0 ± 4.8 | ||
| 1 year | 13.3 ± 7.3 | 13.9 ± 7.2 | NS | |
| % changing to > 22 | 19% | 18% | NS | |
| EF ≥ 22 | ||||
| N | 75 | 60 | ||
| Pre | 25.1 ± 1.7 | 25.4 ± 1.7 | ||
| 1 year | 23.6 ± 4.9 | 25.8 ± 1.5 | .003 | |
| % changing to ≤ 21 | 19% | 0% | .001 |
ACKNOWLEDGEMENTS
This report is based on data collected within the Look AHEAD study. All investigators and staff involved in the baseline and 1-year results of Look AHEAD are listed in prior publications (Refs 14 – 15 cited above). The specific clinical sites and investigators involved in the sexual dysfunction ancillary study are identified below.
Sexual Dysfunction Research Group
New England Research Institutes (NERI) Raymond C. Rosen, PhD1
Robert Wood Johnson Medical School Isaias Noel C. Gendrano III, MPH, Stephen H. Schneider, MD3
Clinical Sites
The Johns Hopkins Medical Institutions Frederick L. Brancati, MD, MHS1; Jeff Honas, MS2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jeanne Charleston, RN; Kathy Horak, RD
The University of Tennessee Health Science Center
University of Tennessee East. Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Stephanie Connelly, MD, MPH3; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA
University of Tennessee Downtown. Abbas E. Kitabchi, PhD, MD1; Helen Lambeth, RN, BSN2; Debra Clark, LPN; Andrea Crisler, MT; Gracie Cunningham; Donna Green, RN; Debra Force, MS, RD, LDN; Robert Kores, PhD; Renate Rosenthal PhD; Elizabeth Smith, MS, RD, LDN; and Maria Sun, MS, RD, LDN; and Judith Soberman, MD3
University of Pennsylvania Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2;Stanley Schwartz, MD3; Gary D. Foster, PhD3; Robert I. Berkowitz, MD3; Henry Glick, PhD3; Shiriki K. Kumanyika, PhD, RD, MPH3; Johanna Brock; Helen Chomentowski; Vicki Clark; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Louise Hesson, MSN; Stephanie Krauthamer-Ewing, MPH; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, MS, RD; Leslie Womble, PhD, MS; Nayyar Iqbal, MD
The Miriam Hospital/Brown Medical School Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases: Barbara Harrison, MS; Van S. Hubbard, MD PhD; Susan Z.Yanovski, MD
National Heart, Lung, and Blood Institute: Lawton S. Cooper, MD, MPH; Jeffrey Cutler, MD, MPH; Eva Obarzanek, PhD, MPH, RD
Centers for Disease Control and Prevention: Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Funding and Support
The Sexual Dysfunction substudy of Look AHEAD was funded by a grant from the National Institutes of Health awarded to Dr. Raymond Rosen (R01 DK60438). The Look AHEAD study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women's Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056 44) and NIH grant (DK 046204); and the University of Washington / VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; Frederic C. Bartter General Clinical Research Center (M01RR01346)
The following organizations have committed to make major contributions to Look AHEAD: Federal Express; Health Management Resources; Johnson & Johnson, LifeScan Inc.; Optifast-Novartis Nutrition; Roche Pharmaceuticals; Ross Product Division of Abbott Laboratories; Slim-Fast Foods Company; and Unilever.
All other Look AHEAD staffs are listed alphabetically by site.
Footnotes
Conflict of Interest: None
Principal Investigator
Program Coordinator
Co-Investigator
REFERENCES
- 1.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, McKinlay JB. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts Male Aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 2.Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res. 2005;17:391–8. doi: 10.1038/sj.ijir.3901333. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hunayan A, Al-Mutar M, Kehinde EO, Thalib L, Al-Ghorory M. The prevalence and predictors of erectile dysfunction in men with newly diagnosed with type 2 diabetes mellitus. BJU Int. 2007;99:130–4. doi: 10.1111/j.1464-410X.2006.06550.x. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: The Rancho Bernardo Study. J Am Coll Cardiol. 2004;43:1405–11. doi: 10.1016/j.jacc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: Results of the 'Cologne Male Survey'. Int J Impot Res. 2000;12:305–11. doi: 10.1038/sj.ijir.3900622. [DOI] [PubMed] [Google Scholar]
- 7.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: Results from the Health Professionals Follow-up study. Ann Intern Med. 2003;139:161–8. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Teles AG, Carreira M, Alarcao V, Sociol D, Aragues JM, Lopes L, Mascarenhas M, Costa JG. Prevalence, severity, and risk factors for erectile dysfunction in a representative sample of 3,548 portuguese men aged 40 to 69 years attending primary healthcare centers: results of the Portuguese erectile dysfunction study. J Sex Med. 2008;5(6):1317–24. doi: 10.1111/j.1743-6109.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 9.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? Urology. 2000;56:302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 10.Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, Thomas S, Bosch JL. Correlates for erectile and ejaculatory dysfunction in older Dutch men: A community-based study. J Am Geriatr Soc. 2001;49:436–42. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosen RC, Wing R, Schneider S, Gendrano N., 3rd Epidemiology of erectile dysfunction: the role of medical comorbidities and lifestyle factors. Urol Clin North Am. 2005;32:403–17, v. doi: 10.1016/j.ucl.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6(Suppl 3):254–61. doi: 10.1111/j.1743-6109.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: A randomized controlled trial. JAMA. 2004;291:2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 14.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 15.Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, Steers WD, Van Den Eeden SK, McVary KT. Urologic complications of diabetes. Diabetes Care. 2005;28:177–85. doi: 10.2337/diacare.28.1.177. [DOI] [PubMed] [Google Scholar]
- 16.Look AHEAD Research Group Look AHEAD: Action for Health in Diabetes: Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the Look AHEAD Trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, Wing R. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–15. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Look AHEAD Research Group The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen R, Riley A, Wagner G, Osterloh I, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A linguistically and culturally validated multidimensional scale for assessment of male erectile dysfunction (MED). International Journal of Impotence Research. 1996;8:108. [Google Scholar]
- 21.Borg GV. Physical Performance and Perceived Exertion. Gleerup; Lund, Sweden: 1962. [Google Scholar]
- 22.Wing RR. Weight loss in the management of type 2 diabetes. In: Gerstein HHR, editor. Evidence Based Diabetes Care. Decker, Inc.; Ontario, Canada: 2001. [Google Scholar]
- 23.Maggio C, Pi-Sunyer FX. The prevention and treatment of obesity: Application to type 2 diabetes. Diabetes Care. 1997;20:1744–1766. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Ciotola M, Giugliano F, et al. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med. 2009 Jan;6(1):243–250. doi: 10.1111/j.1743-6109.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 25.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004 Sep 22;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 26.Rosen R, Wing RR, Schneider T, Wadden TA, Foster G, Smith-West D, Kitabchi A, Brancati B, Maschak-Carey B, Bahnson J, Nendrano III N, Look AHEAD Research Group Erectile dysfunction in type 2 diabetic men: Relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med. 2009;6(5):1414–1422. doi: 10.1111/j.1743-6109.2008.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen RC, Althof SE, Giuliano F. Research instruments for the diagnosis and treatment of patients with erectile dysfunction. Urology. 2006;68:6–16. doi: 10.1016/j.urology.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 28.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 29.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]