Abstract
BACKGROUND
Opioid-induced side effects, such as pruritus, nausea, and vomiting are common and may be more debilitating than pain itself. A continuous low-dose naloxone infusion (0.25 µg/kg/h) ameliorates some of these side effects in many but not all patients without adversely affecting analgesia. We sought to determine the optimal dose of naloxone required to minimize opioid-induced side effects and to measure plasma morphine and naloxone levels in a dose escalation study.
METHODS
Fifty-nine pediatric patients (24 male/35 female; average age 14.2 ± 2.2 years) experiencing moderate to severe postoperative pain were started on IV patient-controlled analgesia morphine (basal infusion 20 µg/kg/h, demand dose 20 µg/kg, 5 doses/h) and a low-dose naloxone infusion (initial cohort: 0.05 µg/kg/h; subsequent cohorts: 0.10, 0.15, 0.25, 0.40, 0.65, 1, and 1.65 µg/kg/h). If 2 patients developed intolerable nausea, vomiting, or pruritus, the naloxone infusion was increased for subsequent patients. Dose/treatment success occurred when 10 patients had minimal side effects at a naloxone dose. Blood samples were obtained for measurement of plasma morphine and naloxone levels after initiation of the naloxone infusion, processed, stored, and measured by tandem mass spectrometry with electrospray positive ionization.
RESULTS
The minimum naloxone dose at which patients were successfully treated with a <10% side effect/failure rate was 1 µg/kg/h; cohort size varied between 4 and 11 patients. Naloxone was more effective in preventing pruritus than nausea and vomiting. Concomitant use of supplemental medicines to treat opioid-induced side effects was required at all naloxone infusion rates. Plasma naloxone levels were below the level of assay quantification (0.1 ng/mL) for infusion rates ≤0.15 µg/kg/h. At rates >0.25 µg/kg/h, plasma levels increased linearly with increasing infusion rate. In each dose cohort, patients who failed therapy had comparable or higher plasma naloxone levels than those levels measured in patients who did not fail treatment. Plasma morphine levels ranged between 3.52 and 172 ng/mL, and >90% of levels ranged between 10.2 and 61.6 ng/mL. Plasma morphine levels were comparable between patients who failed therapy and those patients who achieved symptom control.
CONCLUSIONS
Naloxone infusion rates ≥1 µg/kg/h significantly reduced, but did not eliminate, the incidence of opioid-induced side effects in postoperative pediatric patients receiving IV patient-controlled analgesia morphine. Patients who failed therapy generally had plasma naloxone and morphine levels that were comparable to those who had good symptom relief suggesting that success or failure to ameliorate opioid-induced side effects was unrelated to plasma levels.
In patients of all ages, opioids are the analgesics most frequently prescribed for the management of moderate to severe pain. Regardless of their method of administration, all opioids produce undesired side effects, including nausea and vomiting, pruritus, constipation, urinary retention, respiratory depression, cognitive impairment, opioid-induced hyperalgesia, dependence, and tolerance. Some of these side effects, such as nausea, vomiting, pruritus, and opioid-induced bowel dysfunction are common and can be so debilitating that patients would rather be in pain than experience the consequences of opioid therapy. Additionally, physicians are often reluctant to prescribe opioids because of these side effects and because of their fear of other less common, but more serious complications such as respiratory depression. Indeed, the amelioration or elimination of these side effects is increasingly one of the most important challenges in acute pain management.
Naloxone, a μ-opioid receptor antagonist, is effective at reducing and antagonizing both desired and undesired opioid effects. The coadministration of low-dose naloxone (0.25 µg/kg/h) with opioid analgesics has shown promise as a method of ameliorating undesired side effects, specifically pruritus, and nausea and vomiting, without impairing the quality of analgesia.1 Indeed, we previously demonstrated the success of this technique in children and adolescents receiving IV morphine to treat moderate to severe postoperative pain.2 Inclusion of a low-dose naloxone infusion decreased the incidence of pruritus from 77% to 20% and the incidence of nausea from 70% to 35% in children and adolescents receiving IV patient-controlled analgesia (PCA) after surgery.2 However, in that study, more than one-third of patients still experienced intolerable opioid-related side effects despite the use of low-dose naloxone. Because this prior study tested only 1 naloxone infusion rate, we did not know if either a smaller or larger dose might more effectively decrease the incidence of opioid-induced side effects in our patients, or if treatment failure could be explained in individual patients by sub-therapeutic naloxone or increased morphine plasma levels.
Thus, the primary purpose of this study was to use a dose escalation study method to determine the naloxone infusion rate that would most effectively reduce the incidence of intolerable opioid-induced side effects (failure rate <10%) without affecting analgesia or opioid analgesic requirements in pediatric patients receiving IV PCA morphine. Our secondary aim was to measure plasma levels of morphine and naloxone at each of the naloxone infusion rates used, to determine whether specific naloxone or morphine plasma levels correlated with therapeutic success or failure.
METHODS
After obtaining IRB approval, written parental informed consent, and, when applicable, written patient assent, patients older than 6 and younger than 18 years of age, with acute, moderate to severe postoperative pain were enrolled and studied. Exclusion criteria included patients who remained tracheally intubated postoperatively, who required preoperative benzodiazepine administration, who were unable to communicate verbally, or who were unable to initiate a bolus (demand) dose via the PCA device as a result of mental or physical disability. Additionally, patients who were allergic to opioids, were in any investigational drug trial within 1 month of the treatment day of the study, who had received opioids within 7 days of the study, or who had a parent with a psychiatric illness that impaired their ability to provide consent were also excluded. Surgical procedures included posterior spinal fusion and pectus excavatum repair. Patients were recruited by a study investigator before surgery, and the study protocol was instituted in the immediate postoperative period.
Although intraoperative general anesthetic management was not standardized, all patients enrolled in this study underwent general anesthesia during which they were routinely monitored, paralyzed with nondepolarizing muscle relaxants, and endotracheally intubated. After antagonism of neuromuscular blockade with neostigmine and atropine or glycopyrrolate, patients’ tracheas were extubated and patients were transported to either the pediatric postanesthesia care unit or the pediatric intensive care unit for recovery. Upon arrival, patients were started on IV PCA (CADD®-Solis; Smiths Medical, St. Paul, MN). The PCA pump cassette contained 100 mg morphine sulfate in 100 mL normal saline (1 mg/mL). The following routine settings were established: an initial loading dose of up to 100 µg/kg or more to achieve patient comfort, a maintenance basal infusion rate of 20 µg/kg/h, a demand dose of 20 µg/kg, a lockout time interval of 8 minutes, and a maximum of 5 doses per hour (maximum hourly morphine 0.12 mg/kg).
Naloxone was administered by a continuous infusion pump “piggy-backed” into the patient’s IV catheter. The naloxone solution was prepared using a standard naloxone infusion consisting of 2 mg naloxone in 250 mL 0.9% saline (final concentration = 8 µg/mL).2 The initial naloxone infusion rate evaluated (cohort 1) was chosen to be 0.05 µg/kg/h. This dose was doubled for the second cohort (0.10 µg/kg/h). Subsequent infusion rates were defined as the sum of the prior 2 infusion rates (0.10, 0.15, 0.25, 0.40, 0.65, 1, and 1.65 µg/kg/h, respectively).
Every 4 hours while awake, patients were evaluated by their bedside nurse for the presence and severity of pain and opioid-related side effects. Subjective pain scores were assessed using self-report, either the Wong-Baker FACES™ scale,3 or, in older children, a numerical 0 to 10 scale.4 Patients were also assessed by a study nurse to determine side effect severity. They were asked to self-assess pruritus and nausea (0 = none, 1 = present but tolerable, 2 = severe, intolerable), and if they had vomited. In addition, bedside nursing flow sheets were scrutinized for documented episodes of nausea and/or vomiting. Vital signs, including arterial blood pressure, respiratory rate, and oxyhemoglobin saturation were monitored and recorded every 4 hours.
Use of “rescue” antiemetics and antipruritics, opioid consumption, and the occurrence of respiratory depression were recorded. Patients who developed opioid-induced side effects were treated symptomatically by a protocol-driven algorithm. Nausea and/or vomiting was treated with IV dolasetron 0.35 mg/kg (maximum dose 12.5 mg) every 8 hours or ondansetron 0.1 mg/kg (maximum dose 4 mg) every 4 hours as needed. IV diphenhydramine 1 mg/kg (maximum dose 50 mg) every 4 to 6 hours was used as a second-line antiemetic rescue therapy and the primary antidote to treat pruritus. If these maneuvers did not relieve the patient’s symptoms, the study was terminated, and the IV naloxone infusion was increased to 1 µg/kg/h. The amount of morphine used and the requirements for supplemental analgesia or symptomatic treatment during the 24-hour study period were recorded. Finally, if respiratory depression occurred (respiratory rate <8 breaths/min, oxygen saturation <90%, and the patient was unarousable), the IV PCA was to be turned off and the patient was ordered to receive naloxone 1 µg/kg IV as a bolus dose every minute until respiratory depression resolved.
Dose/treatment failure at any naloxone infusion rate occurred when 2 patients experienced intolerable side effects despite the naloxone infusion and the use of rescue medications. When this occurred, the naloxone infusion rate was increased for subsequent patients. Dose/treatment success and study completion occurred when 10 patients were successfully treated at a given dose with no more than 1 failure in that treatment cohort. The maximum number of patients in any cohort, therefore, was 11. After determining this minimum effective dose, the naloxone dose was increased 1 final time to determine whether a higher dose was associated with adverse events, such as reversal of analgesia as demonstrated by an increase in opioid consumption.
Serology
Blood samples (5 mL) were obtained from a dedicated indwelling IV catheter for measurement of plasma morphine, naloxone, and naloxone-3-glucoronide levels after initiation of the naloxone infusion, and at a later time point between 12 and 24 hours after the start of the infusion. Additionally, in patients who failed therapy, a blood sample was obtained before termination of the study. Blood samples were collected in EDTA-containing tubes and were processed within 30 minutes of collection by centrifugation for 10 minutes at 1500g. The plasma supernatant was stored at −20°C until subsequent analysis using a validated liquid chromatography/tandem mass spectrometry method developed by the Kimmel Cancer Center at Johns Hopkins University Analytical Pharmacology Core Laboratory. Briefly, salirasib was extracted from plasma using acetonitrile precipitation. Separation of morphine, naloxone, and naloxone-3-glucuronide and the internal standard, morphine- (N-methyl-d3), was achieved on a Waters XTerra® C-18 (3.5 µm, 150 × 2.1 mm internal diameter) analytical column using a mobile phase consisting of acetonitrile/2 mM ammonium acetate (65:35, v/v) containing 0.1% formic acid and an isocratic flow of 0.20 mL/min. The analytes were monitored by tandem mass spectrometry with electrospray positive ionization. Detection was performed by monitoring the ion transitions from m/z 286.0 → 152.0 for morphine, 328.1 → 310.0 for naloxone, 504.0 → 310.0 for naloxone-3-glucuronide, and 289.0 → 152.0 for the internal standard. The linear calibration curves were generated over the range of 5 to 500 ng/mL for morphine and 0.1 to 10 ng/mL for naloxone. The presence of naloxone-3-glucuronide was qualitatively assessed. Plasma samples that were diluted 1:10 (v/v) with pooled plasma were accurately quantified. The accuracy and within- and between-day precision met the acceptance criteria for bioanalytical assays.5 An analytical run was deemed acceptable if 75% of calibrators tested were within ≤15% from the nominal concentration (≤20% for the lower limit of quantification) and 66% of the quality controls tested were within ≤15% from the nominal concentration.
Data Analysis
Patient characteristics, efficacy, plasma naloxone and morphine levels, and side effect scores were summarized by cohort level and over all cohorts. Data are presented as mean ± SEM. Analyses between genders and dose categories on naloxone and morphine levels as well as pain scores were performed using regression analyses with robust estimates of standard errors. Similar analyses on side effects were performed with logistic regression.
RESULTS
Patient characteristics are displayed in Table 1. Overall, 59 patients participated in this study. There were 24 males and 35 females. One female and 11 males underwent repair of pectus excavatum, and the remaining 47 underwent posterior spinal fusion. Cohort size varied between 4 and 11 subjects. Thirteen patients were treatment failures (7 female, 6 male). The minimum naloxone infusion rate at which 10 patients were successfully treated with no more than 1 failure was 1 µg/kg/h. Increasing the naloxone dose to 1.65 µg/kg/h resulted in no patients failing treatment because of intolerable side effects.
Table 1.
Patient Demographics
| Naloxone infusion rate (µg/kg/h) |
No. of subjects (success/failure) |
Age (y) (mean ± SEM) |
Sex, male/female |
Posterior spine fusion/ pectus excavatum |
Cause of failure |
|---|---|---|---|---|---|
| 0.05 | 5 (3/2) | 12.7 ± 2.5 | 3/2 | 3/2 | P × 1, N/V and P × 1 |
| 0.10 | 5 (3/2) | 16.9 ± 1.3 | 3/2 | 1/4 | P × 2 |
| 0.15 | 11 (9/2) | 15.0 ± 2.1 | 5/6 | 10/1 | P × 2 |
| 0.25 | 4 (2/2) | 13.6 ± 2.0 | 0/4 | 4/0 | P × 1, N/V × 1 |
| 0.40 | 8 (6/2) | 15.1 ± 1.5 | 3/5 | 7/1 | P × 1, N/V × 1 |
| 0.65 | 5 (3/2) | 13.3 ± 1 | 3/2 | 2/3 | P × 2 |
| 1 | 11 (10/1) | 14.2 ± 2.5 | 7/4 | 9/2 | N/V × 1 |
| 1.65 | 10 (10/0) | 13.8 ± 2.3 | 0/10 | 10/0 | None P × 9 |
| Overall | 59 (46/13) | 14.4 ± 2.2 | 24/35 | 36/13 | N/V × 3 N/V and P × 1 |
N/V = nausea/vomiting; P = pruritus.
Naloxone was more effective in preventing pruritus than nausea and vomiting (Table 2). The overall incidence of pruritus was 27%, whereas only 2 of 21 patients reported any pruritus at naloxone doses ≥1 µg/kg/h. Patients were next stratified into 3 naloxone dose ranges: low (0.05– 0.15 µg/kg/h), moderate (0.25– 0.65 µg/kg/h), and high (1–1.65 µg/kg/h). Naloxone infusion rates between 0.05 and 0.15 µg/kg/h were grouped together as a low-dose cohort based on the observation that most previous studies have considered 0.25 µg/kg/h as a low-dose infusion. Thus, grouping these doses together allowed us to examine whether doses lower than the previously studied low dose might be effective. Infusion rates of 1 and 1.65 µg/kg/h were cohorted together as high-dose naloxone because both doses led to treatment success. Comparing cohorts, we found that the odds of pruritus was decreased by 88% at high-dose as compared with low-dose infusions (P = 0.013). However, there was no difference in the incidence of nausea and vomiting among the 3 naloxone infusion cohorts. Overall, 47% of patients studied reported no nausea or vomiting, and 48% reported no nausea or vomiting at doses ≥1 µg/kg/h. The incidence of severe side effects (pruritus or nausea/vomiting score = 2) did trend toward lower levels as naloxone infusion rate was increased. Severe pruritus occurred in 19%, 12%, and 0% of patients, whereas severe nausea/vomiting occurred in 15%, 24%, and 4% of patients, respectively. However, this trend did not reach statistical significance. Concomitant use of supplemental medicines to treat opioid-induced side effects was required at all naloxone infusion rates. Whereas the likelihood of receiving diphenhydramine to treat opioid-induced side effects was comparable across all dose cohorts (48% vs 48% vs 39% for low-, moderate-, and high-dose cohorts, respectively), its use to specifically treat pruritus was significantly decreased in the high-dose naloxone cohort (P = 0.024). However, we found no difference in the use of rescue antiemetic therapy across dose cohorts (Table 2).
Table 2.
Efficacy of Naloxone in Ameliorating Pruritus and Nausea and Vomiting
| Naloxone infusion rate |
No. of patients studied (success/ failure) |
Pruritus score = 0 |
Pruritus score = 1 |
Pruritus score = 2 |
N/V score = 0 |
N/V score = 1 |
N/V score = 2 |
Use of rescue antipruritic therapy |
Use of rescue antiemetic therapy |
|---|---|---|---|---|---|---|---|---|---|
| Low | 21 (15/6) | 52% | 29% | 19% | 55% | 30% | 15% | 43% | 43% |
| Moderate | 17 (11/6) | 76% | 12% | 12% | 35% | 41% | 24% | 24% | 65% |
| High | 21 (20/1) | 90%a | 10% | 0% | 48% | 48% | 4% | 10%b | 57% |
| Overall | 59 (46/13) | 73% | 17% | 10% | 47% | 40% | 14% | 25% | 54% |
N/V = nausea/vomiting.
The odds of pruritus was decreased by 88% at high-dose as compared with low-dose naloxone infusions (P = 0.013).
The odds of receiving rescue therapy (diphenhydramine) to treat pruritus was decreased by 86% at high-dose as compared with low-dose naloxone infusions (P = 0.024).
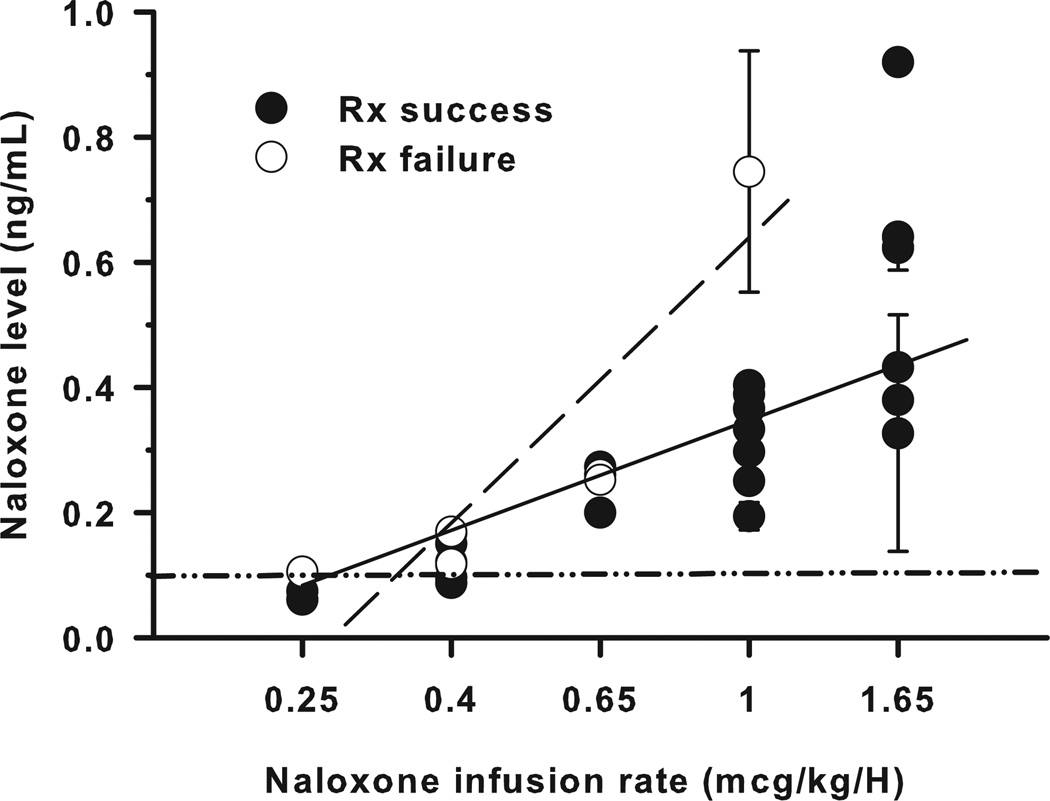
Naloxone-3-glucoronide, naloxone, and morphine plasma levels were measured for those patients who received naloxone infusions of ≥0.15 µg/kg/h. Naloxone-3-glucoronide levels were below the measurement limits of the assay (0.1 ng/mL) for all samples tested. Plasma naloxone levels were below the measurement limits of the assay at an infusion rate of 0.15 µg/kg/h, and generally below assay limits at an infusion rate of 0.25 µg/kg/h. At infusion rates >0.25 µg/kg/h, in patients who achieved adequate symptom control, average plasma levels increased linearly with increasing infusion rate (R2 = 0.76). At comparable naloxone infusion rates, patients who failed treatment had similar or higher plasma naloxone levels than mean plasma naloxone levels measured in those who did not fail treatment (comparison of slopes P = 0.009) (Fig. 1 and Table 3).
Figure 1.
Plasma naloxone levels measured after 5 half-lives of naloxone infusion for subjects who achieved adequate symptom control (Rx success, ●) and subjects who failed therapy (Rx failure, ○) are shown. In treatment responders, average plasma naloxone levels increased linearly with increasing infusion rate (straight line,  , R2 = 0.76). Patients who failed treatment (dashed line,
, R2 = 0.76). Patients who failed treatment (dashed line,  ) had higher average plasma naloxone levels than those who did not fail treatment (comparison of slopes, P = 0.009). The minimum level of assay quantification, 0.1 ng/mL, is represented by a dash-dot-dot line (
) had higher average plasma naloxone levels than those who did not fail treatment (comparison of slopes, P = 0.009). The minimum level of assay quantification, 0.1 ng/mL, is represented by a dash-dot-dot line ( ).
).
Table 3.
Plasma Naloxone and Morphine Levels and 24-Hour Consumption for Naloxone Infusion Cohorts and Subjects Who Failed Therapy
| Naloxone infusion rate (µg/kg/h) |
Equilibrateda naloxone level (ng/mL) |
Naloxone levels for treatment failuresb (time measured) |
Morphine level (ng/mL) |
Morphine levels for treatment failuresb (time measured) |
24-Hour morphine consumption (mg/kg) |
|---|---|---|---|---|---|
| 0.05 | N/A | N/A | N/A | N/A | 1.13 ± 0.22 |
| 0.10 | N/A | N/A | N/A | N/A | 1.16 ± 0.24 |
| 0.15 | BLQ | BLQ (15 h, 18 h) BLQ (15 h, 21 h) |
24.6 ± 2.9 | 16.0, 30.0, 14.0 (15 h, 18 h, 22 h) 19.0, 21, 24.9 (15 h, 21 h, 23 h) |
1.38 ± 0.19 |
| 0.25 | BLQ | BLQ (1 h) 0.11 (15 h) |
21.2 ± 2.0 | 24.2 (1 h) 18.4 (15 h) |
1.23 ± 0.05 |
| 0.40 | 0.10 ± 0.02 | 0.12 (15 h) BLQ, 0.17 (1.5 h, 22 h) |
30.9 ± 3.1 | 10.2 (15 h) 30.9, 37.6 (1.5 h, 22 h) |
1.54 ± 0.17 |
| 0.65 | 0.25 ± 0.02 | 0.17, 0.26 (1 h, 22 h) 0.25 (16 h) |
33.7 ± 4.8 | 83.1, 22.8 (1 h, 22 h) 41 (16 h) |
1.38 ± 0.28 |
| 1 | 0.37 ± 0.06 | 0.61, 0.88 (8 h, 13 h) | 29.4 ± 2.5 | 29.1, 34.2 (8 h, 13 h) | 1.44 ± 0.25 |
| 1.65 | 0.54 ± 0.07 | No failures | 33.8 ± 4.8 | No failures | 1.64 ± 0.17 |
| Overall | 31.8 ± 2.3 | 1.41 ± 0.08 |
Data are presented as mean ± SEM.
BLQ = below level of quantification; N/A = not available.
Equilibrated naloxone levels were calculated as the mean value measured after 5 half-lives of drug infusion (>400 minutes).
Data presented are individual naloxone and morphine levels measured in patients who failed treatment at time after start of naloxone infusion.
Average 24-hour morphine consumption was 1.41 ± 0.08 mg/kg/d and ranged from 1.13 ± 0.22 to 1.64 ± 0.63 mg/kg/d across cohorts (Table 3). Female subjects consumed significantly more morphine than males (1.57 ± 0.10 vs 1.17 ± 0.12 mg/kg/24 h, P < 0.025). However, observing each gender individually, we found no significant difference in 24-hour morphine consumption as a function of naloxone infusion dose cohort (Table 4).
Table 4.
Twenty-Four-Hour Morphine Consumption for Male and Female Subjects
| Naloxone infusion rate |
24-hour morphine consumption (mg/kg) |
|
|---|---|---|
| Males | Females | |
| Low | 1.03 ± 0.12 | 1.57 ± 0.21 |
| Moderate | 1.22 ± 0.06 | 1.51 ± 0.15 |
| High | 1.35 ± 0.36 | 1.62 ± 0.17 |
| Overall | 1.17 ± 0.12 | 1.57 ± 0.10* |
Data are presented as mean ± SEM.
Significantly different from overall males (P < 0.025).
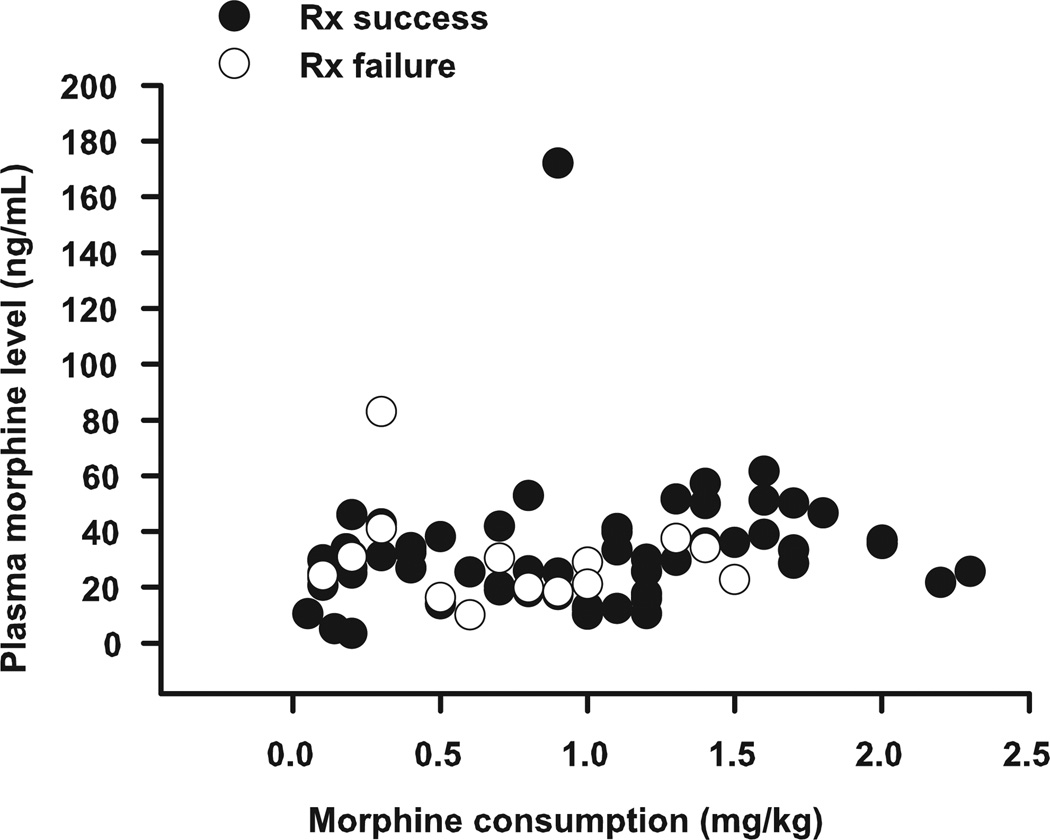
Whereas the overall average plasma morphine level was 31.8 ± 2.3 ng/mL (Table 3), interindividual variability was moderately high with morphine levels ranging between a minimum of 3.52 and a maximum of 172 ng/mL (Fig. 2). However, >75% of measured plasma morphine levels decreased between 16 and 46 ng/mL, whereas >90% ranged between 10.2 and 61.6 ng/mL. Comparing plasma morphine levels between responders and treatment failures, we found that at the time the second plasma morphine level was measured (>12 hours after initiation of PCA and naloxone infusion), mean plasma morphine levels did not differ significantly between the 2 groups (34.7 ± 5.1 vs 29.2 ± 3.2 ng/mL for responders and treatment failures, respectively). In addition, the slopes of the lines correlating morphine plasma level and morphine consumption were approximately equal to zero, suggesting that morphine level was generally stable and independent of morphine consumption for patients in both groups. Finally, beyond the initial postoperative period, the highest plasma morphine level measured in any patient who failed therapy was 41 ng/mL.
Figure 2.
Plasma morphine level as a function of morphine consumption for subjects who achieved adequate symptom control (Rx success, ●) and subjects who failed therapy (Rx failure, ○). At the time the second plasma morphine level was measured, plasma morphine level was independent of morphine consumption for patients in both groups, and average plasma morphine levels did not differ between groups (34.7 ± 5.1 vs 29.2 ± 3.2 ng/mL for responders and treatment failures, respectively).
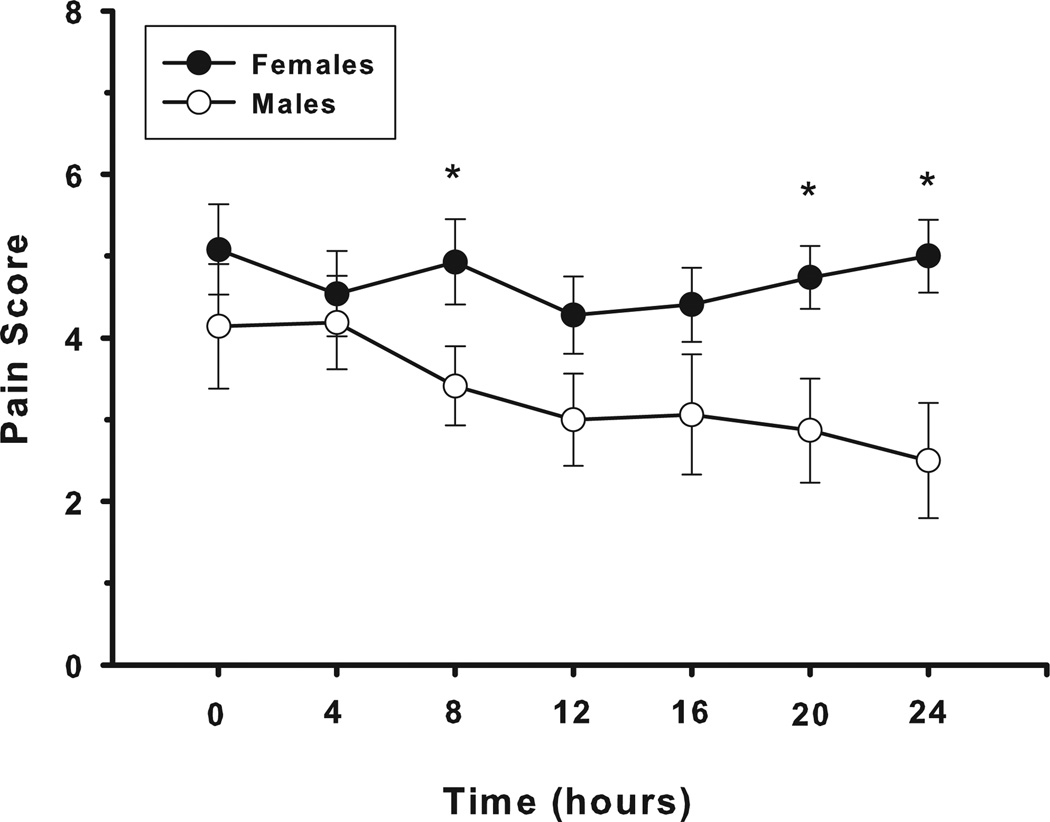
Comparing pain scores over the first 24 hours after surgery in those patients who did not fail treatment, we found that females reported significantly higher pain scores than males at 8, 20, and 24 hours after the start of the naloxone infusion (4.9 ± 0.5 vs 3.4 ± 0.5, P = 0.039, 4.7 ± 0.4 vs 2.9 ± 0.6, P = 0.023, and 5.0 ± 0.5 vs 2.5 ± 0.7, P = 0.002, respectively) (Fig. 3). However, we did not observe any difference in analgesia in female patients over time as the naloxone infusion rate was increased. In male patients, pain scores trended lower over time in the low- and moderate-dose infusion rate groups, but not in the highdose group. This difference was statistically significant at 24 hours, but this observation is limited by the fact that pain scores were obtained on less than half of all patients at this time point.
Figure 3.
Average self-reported pain scores for male (○) and female (●) subjects who achieved adequate symptom control. Female subjects reported significantly (*) higher pain scores than male subjects at 8, 20, and 24 hours after initiation of therapy (P = 0.039, P = 0.023, and P = 0.002, respectively).
DISCUSSION
In this prospective, dose finding study in children and adolescents being treated with IV PCA morphine after major surgery, we found that a naloxone infusion of ≥1 µg/kg/h significantly reduced, but did not eliminate, the incidence of opioid-induced side effects, and that this infusion rate was more effective than lower doses studied. We also found that patients who failed therapy generally had plasma naloxone and morphine levels that were comparable to those who had good symptom relief. This finding suggests that a specific naloxone plasma level is not correlated with therapeutic success or failure and should not be a target of therapy. Finally, comparing all doses studied here, we could not demonstrate either an opioidsparing effect or a significant increase in opioid consumption in our study patients.
Opioids are the analgesics most frequently prescribed for the management of moderate to severe pain and, regardless of the method of administration, produce undesired side effects. Some of these side effects, such as nausea, vomiting, and pruritus are common and often so debilitating that patients would rather be in pain than experience them. Crain and Shen6 in a series of laboratory experiments demonstrated that when administered in combination with opioids, ultralow-dose opioid antagonists may decrease opioid-induced side effects, such as hyperalgesia and tolerance, and improve pain control. Possible explanations for this effect include the hypothesis that at very low doses, opioid antagonists inhibit μ-opioid receptor excitatory G-protein complexes (Gs) while leaving the inhibitory G-protein receptors (Gi) available for pain control.6,7 Subsequently, consistent with these results, Gan et al.1 showed that administration of a naloxone infusion of 0.25 µg/kg/h in combination with IV PCA morphine attenuated opioid-induced side effects and significantly reduced 24-hour opioid consumption in women after abdominal hysterectomies. However, results from other trials have been more variable, with some showing no improvement. From our review of the literature, we believe that one possible explanation for the failure of opioid antagonist prophylaxis in these studies may relate to how the opioid antagonist was prepared and administered. In some trials in which the antagonist was ineffective, morphine and naloxone were mixed together in saline and delivered via a PCA pump.8–10 Thus, patients received only small doses of naloxone intermittently when the PCA pump was triggered. How much naloxone was administered and how long it remained at its effector sites varied within and between patients. However, in studies in which an antagonist was effective, the opioid antagonist was often either a long-acting drug, such as nalmefene,11 or a shorter-acting drug, naloxone, administered as an independent, continuous infusion as was done here.1,2 Of note, however, opioid sparing has been inconsistently found even when the antagonist has been administered continuously.
In our previous prospective study, a continuous 0.25 µg/kg/h naloxone infusion significantly ameliorated pruritus and, to a lesser degree, nausea and vomiting in two-thirds of the children and adolescents studied.2 Why it failed in one-third of patients, however, was unclear. Because we did not know whether a higher or lower infusion rate might be more effective or might, conversely, be unsuccessful, we undertook this dose finding study. We found that plasma naloxone levels increased in a linear fashion with increasing infusion rate, and that naloxone infusion rates ≥1 µg/kg/h reduced the treatment failure rate to <10%. Moreover, patients who failed therapy had comparable or higher plasma naloxone levels than those levels measured in patients who did not fail treatment, suggesting that a strategy to target IV infusions to achieve an effective plasma level would fail.
Other investigators have reported side effect amelioration at naloxone doses as low as 0.006 to 0.065 µg/kg/h, with worsening of analgesia at higher doses.8,9,12 Although we also found side effect amelioration at lower doses in our study, higher doses (≥1 µg/kg/h) were more consistently effective. A possible explanation for these different results may simply be related to how IV PCA was provided in this study. Specifically, all pediatric patients in our institution are treated with a continuous basal opioid infusion. The use of a continuous infusion may be associated with increased total opioid consumption13 as well as opioid-related side effects including respiratory depression.14–16 Although the use of a continuous basal opioid infusion is not universal in pediatric pain management, it is more frequently used2,14,17 than in adult practice.1,8,9,12,16 Our findings that a higher naloxone infusion rate more effectively reduced pruritus in children receiving basal/bolus IV PCA than the lower doses reported to be effective in adult patients receiving bolus-only PCA are similar to those reported in a small pilot study of patients with sickle cell anemia who received morphine via continuous infusion in combination with naloxone.18
Maxwell et al.2 found in their placebo-controlled trial that low-dose naloxone was more effective in ameliorating pruritus than nausea and vomiting. Our results support this. Indeed, in our study, increasing the naloxone infusion rate did not affect the overall incidence of gastrointestinal side effects as opposed to pruritus. This may be explained in part by the fact that pruritus is a more purely opioid-related side effect than nausea and vomiting. Postoperative nausea and vomiting are due only in part to the impact of opioids on central nervous system vomiting centers and gut motility.19 After surgery, other factors including the release of neurogenic, hormonal, inflammatory, and pharmacologic mediators can also contribute to disturbed gastrointestinal motility.20 As a result, nausea and vomiting may not be effectively reversed by opioid antagonism alone and will require, as in our treatment algorithm, other antiemetics, such as serotonin (5-HT3) receptor antagonists and/or antihistamines.
Although we did find moderate variability in plasma morphine levels between patients, success or failure of low-dose naloxone could not be explained by these differences. Plasma morphine levels ranged between a minimum of 3.52 and a maximum of 172 ng/mL, and >90% of levels ranged between 10.2 and 61.6 ng/mL, well within the range reported to be therapeutic in patients during or after surgery (between 10 and 65 ng/mL).21–23 However, levels were comparable between patients who failed therapy and those patients who achieved symptom control. Only 1 patient who developed intractable symptoms demonstrated an increased plasma morphine level (83.1 ng/mL). However, that level was obtained within 1.5 hours of the completion of surgery, at a time when the patient may have been in the midst of being “loaded” with opioid. A subsequent level was almost 4-fold lower and consistent with levels measured in other patients who had consumed similar amounts of morphine, suggesting that a diminished capacity to metabolize opioid was not the cause of treatment failure in this case.
In our initial data analysis, we did observe a trend toward increased opioid consumption as naloxone infusion rate increased. Further examination of our data suggested that this finding was attributable in part to gender-based differences within cohorts. Therefore, we next focused on our female patients because they were our largest and most homogeneous population subgroup. Combining naloxone infusion cohorts into low, moderate, and high infusion rate groups, we found that total daily opioid consumption did not increase significantly with increasing naloxone infusion rate in our female subjects. Consumption varied by only 7% between moderate- and high-dose naloxone groups and by only 3% between low- and high-dose groups. Thus, although this study was not designed or powered to detect whether opioid sparing did occur, this result suggests that little if any opioid sparing could have occurred in the cohorts receiving the lowest naloxone infusion rates. Similarly, at the higher infusion rates, we did not observe a need for significantly higher opioid doses to combat opioid antagonism. These findings are similar to those reported by Darnell et al.24 who studied low-dose naloxone infusions in combination with fentanyl infusions in critically ill children in an intensive care unit setting and did not observe a difference in opioid consumption.
Interestingly, however, we did observe a significant difference in 24-hour opioid consumption in female compared with male study participants. While this finding may be gender-based, it may also reflect unappreciated differences between the patients enrolled in this study, or may be related to differences in the distribution of surgical procedures between female and male subjects. Although we did not find a significant association between type of surgery and opioid consumption, it is important to note that a larger percentage of female as compared with male patients underwent posterior spinal fusion repair. It is possible that severity of pain varied between the 2 procedures over the course of the observation period and this could have affected cumulative opioid consumption.
In addition to higher opioid consumption, female subjects also reported significantly higher pain scores than male subjects at multiple times during the observation period. However, we did not observe any differences over time in analgesia in female patients as a function of naloxone infusion rate. In male patients, however, pain scores did trend lower over time in the low and moderate infusion rate groups, but not in the high-dose group. Whether this association, which is suggestive of a differential sensitivity to low-dose opioid antagonism between males and females, would achieve significance if a larger, more homogeneous group of male postoperative patients was studied is unknown.
Although we cannot be certain that gender was, or was not, a critical factor in these observed differences, it has been reported that females may demonstrate increased pain sensitivity compared with males.25 Furthermore, gender-related differences in analgesic sensitivity and opioid consumption have also been described.26 A possible explanation for gender-related differences in pain perception involves sex hormone variability.27 In this study, few, if any, prepubescent subjects were enrolled. Thus, hormonal differences may have affected our findings. Finally, it should be noted that in other studies, differences between male and female subjects have also been related to differences in treatment regimen. For example, some studies suggest that women experience better pain relief than men in response to butorphanol, a weak μ agonist, but strong κ agonist.28 Focusing on μ agonists, such as morphine, Chia et al.29 reported that female patients had a lower postoperative opioid requirement, whereas others have concluded that females experience more intense postoperative pain and require more opioid to experience a similar degree of analgesia.30,31
This study has several limitations. Because it was primarily designed to be a dose finding study, sample groups at each infusion rate tended to be small, nonhomogeneous, and of variable size. Hence, we could not always discern differences between individual groups and instead had to evaluate dose ranges to increase group size and power. Even so, group makeup may have limited our ability to discern statistical significance in some settings. For example, although our female population was very homogeneous in terms of type of surgery, male patients were divided almost evenly between pectus surgery and spine surgery, which limited our ability to clearly distinguish differences based on gender versus surgery performed.
In addition, intraoperative analgesic and antiemetic management was not standardized across patients. This approach may have resulted in differences in early opioid consumption and side effect profiles. However, in general, side effect management failure occurred >8 hours after institution of IV PCA and naloxone, at a time when patients were receiving a standardized pain and side effect management regimen.
It is also possible that unrecognized differences in concentrations of morphine’s active metabolite morphine- 6-glucuronide may have had a role in the differences in analgesia or side effect profiles observed. Given the limitations in the amount of blood that could be obtained from our study subjects, we were unable to measure plasma morphine metabolites along with plasma morphine, naloxone, and naloxone metabolites. Therefore, we chose to focus primarily on naloxone’s primary metabolite, naloxone-3-glucuronide, because we were concerned that its presence might affect our observed response. When administered enterally, naloxone-3-glucuronide has been shown to act as an active metabolite, reversing morphine-associated delays in gastrointestinal transit time.32,33 Hence, differences in naloxone metabolism related to genetic polymorphisms could have had a role in explaining the variable responses observed among patients. However, we found that plasma naloxone-3-glucuronide levels were below the limit of quantification in all patients studied, making this possibility unlikely.
A number of side effects that are associated with opioid administration, such as urinary retention, constipation, development of tolerance, and respiratory depression could not be evaluated in this study. Given the nature of the surgery performed in our study population, the majority of our patients had bladder catheters and poor bowel function in the observational immediate postoperative period. Additionally, because patients were treated with IV therapy for only 2 to 3 days, we could not discern the development of tolerance. Finally, our study sample size was too small to observe any difference in the development of respiratory depression, an event that occurs rarely in our clinical population.
In conclusion, although some patients achieved symptom relief at all doses studied, we found that naloxone infusion rates ≥1 µg/kg/h significantly reduced, but did not eliminate, the incidence of opioid-induced side effects, primarily pruritus, in children and adolescents after major surgery. This effect was not associated with a significant increase in opioid consumption or impairment of analgesia. Patients who failed therapy generally had plasma naloxone and morphine levels that were comparable to those who had good symptom relief, suggesting that absolute plasma drug levels do not have a prominent role in treatment failure.
ACKNOWLEDGMENTS
The Importance of FAER Funding to Career Development.
When I finished my residency/fellowship in pediatric anesthesia and critical care medicine (1981), newborns undergoing surgery were anesthetized with paralysis, oxygen, and a “whiff” of vapor. We weren’t cruel; rather, the newborn undergoing emergency surgery was thought to be akin to the gunshot wound to the chest, because of the widely held beliefs that the newborn could not hemodynamically tolerate anesthesia and did not experience pain. I was sure that there must be an alternative, and based on the adult work of Ted Stanley and a preliminary report by George Gregory in the newborn, thought that high-dose fentanyl anesthesia might be the answer. But was it safe and would it work in the newborn? Using a FAER grant and under the guidance of my mentor, Dr. Richard Traystman, I studied the hemodynamic and central nervous system effects of high-dose fentanyl alone and in combination with other anesthetic agents (nitrous oxide, ketamine, barbiturates) in a newborn lamb model. These studies led to several discoveries and ultimately helped change how anesthesia is delivered to newborn infants today. Furthermore, it was also clear that pain, anxiety, and discomfort (both physical and psychological) were not limited to the newborn and did not begin and end with the induction and conclusion of surgery. This understanding helped launch my academic career and allowed me to start the pediatric pain service and set up a research laboratory at the Johns Hopkins University, which have served as the source of extensive clinical and translational research as well as practice policy. None of this would have been possible without my FAER starter grant.
—Myron Yaster, MD.
While also a pediatric anesthesiologist with an interest in pediatric acute pain management, my FAER Research Starter grant focused on molecular mechanisms of cachexia. The goal of the research was to identify candidate genes responsible for the development of cachexia in a murine cancer cachexia model. At the time that this work was undertaken in 1998, cDNA expression arrays were just being developed and the human genome had not been sequenced. How times have changed! Receipt of a FAER grant sent me forward on a path that has allowed me to expand my laboratory skills with a focus on molecular biology, genomics, and epigenetics at a time when these disciplines and their relevance to clinical practice have undergone a dramatic transformation.
—Constance L. Monitto, MD.
Funding: Richard J. Traystman Endowed Chair.
Footnotes
DISCLOSURES
Name: Constance L. Monitto, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Constance L. Monitto has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Sabine Kost-Byerly, MD.
Contribution: This author helped design the study, conduct the study, and write the manuscript.
Attestation: Sabine Kost-Byerly has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Elizabeth White, RN.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Elizabeth White has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Carlton K. K. Lee, PharmD.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Carlton Lee has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Michelle A. Rudek, PharmD, PhD.
Contribution: This author helped design the study and analyze the data.
Attestation: Michelle A. Rudek has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Carol Thompson, MS, MBA.
Contribution: This author helped analyze the data and write the manuscript.
Attestation: Carol Thompson has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Myron Yaster, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Myron Yaster has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Peter J. Davis, MD.
The authors declare no conflicts of interest.
REFERENCES
- 1.Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87:1075–1081. doi: 10.1097/00000542-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell LG, Kaufmann SC, Bitzer S, Jackson EV, Jr, McGready J, Kost-Byerly S, Kozlowski L, Rothman SK, Yaster M. The effects of a small-dose naloxone infusion on opioid-induced side effects and analgesia in children and adolescents treated with intravenous patient-controlled analgesia: a double-blind, prospective, randomized, controlled study. Anesth Analg. 2005;100:953–958. doi: 10.1213/01.ANE.0000148618.17736.3C. [DOI] [PubMed] [Google Scholar]
- 3.Wong DL, Baker C. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 4.McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore: Mosby; 1993. [Google Scholar]
- 5.Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies: conference report. Eur J Drug Metab Pharmacokinet. 1991;16:249–255. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- 6.Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence. Pain. 2000;84:121–131. doi: 10.1016/s0304-3959(99)00223-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-lowdose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and G signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Cepeda MS, Africano JM, Manrique AM, Fragoso W, Carr DB. The combination of low dose of naloxone and morphine in PCA does not decrease opioid requirements in the postoperative period. Pain. 2002;96:73–79. doi: 10.1016/s0304-3959(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda MS, Alvarez H, Morales O, Carr DB. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid requirement but decreased incidence of opioid side effects. Pain. 2004;107:41–46. doi: 10.1016/j.pain.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Sartain JB, Barry JJ, Richardson CA, Branagan HC. Effect of combining naloxone and morphine for intravenous patient-controlled analgesia. Anesthesiology. 2003;99:148–151. doi: 10.1097/00000542-200307000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Joshi GP, Duffy L, Chehade J, Wesevich J, Gajraj N, Johnson ER. Effects of prophylactic nalmefene on the incidence of morphine-related side effects in patients receiving intravenous patient-controlled analgesia. Anesthesiology. 1999;90:1007–1011. doi: 10.1097/00000542-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Yeh YC, Lin TF, Wang CH, Wang YP, Lin CJ, Sun WZ. Effect of combining ultralow-dose naloxone with morphine in intravenous patient-controlled analgesia: the cut-off ratio of naloxone to morphine for antiemesis after gynecologic surgery. J Formos Med Assoc. 2008;107:478–484. doi: 10.1016/S0929-6646(08)60156-4. [DOI] [PubMed] [Google Scholar]
- 13.Dal D, Kanbak M, Caglar M, Aypar U. A background infusion of morphine does not enhance postoperative analgesia after cardiac surgery. Can J Anaesth. 2003;50:476–479. doi: 10.1007/BF03021059. [DOI] [PubMed] [Google Scholar]
- 14.Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait AR, Malviya S. The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth Analg. 2008;107:70–75. doi: 10.1213/ane.0b013e318172fa9e. [DOI] [PubMed] [Google Scholar]
- 15.Doyle E, Harper I, Morton NS. Patient-controlled analgesia with low dose background infusions after lower abdominal surgery in children. Br J Anaesth. 1993;71:818–822. doi: 10.1093/bja/71.6.818. [DOI] [PubMed] [Google Scholar]
- 16.Fleming BM, Coombs DW. A survey of complications documented in a quality-control analysis of patient controlled analgesia in the postoperative patient. J Pain Symptom Manage. 1992;7:463–469. doi: 10.1016/0885-3924(92)90132-2. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KL, Yaster M, Kost-Byerly S, Monitto CL. A national survey of American pediatric anesthesiologists: patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth Analg. 2010;110:754–760. doi: 10.1213/ANE.0b013e3181ca749c. [DOI] [PubMed] [Google Scholar]
- 18.Koch J, Manworren R, Clark L, Quinn CT, Buchanan GR, Rogers ZR. Pilot study of continuous co-infusion of morphine and naloxone in children with sickle cell pain crisis. Am J Hematol. 2008;83:728–731. doi: 10.1002/ajh.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med. 2009;10:654–662. doi: 10.1111/j.1526-4637.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 20.Kraft MD. Methylnaltrexone, a new peripherally acting muopioid receptor antagonist being evaluated for the treatment of postoperative ileus. Expert Opin Investig Drugs. 2008;17:1365–1377. doi: 10.1517/13543784.17.9.1365. [DOI] [PubMed] [Google Scholar]
- 21.Lynn AM, Nespeca MK, Bratton SL, Shen DD. Ventilatory effects of morphine infusions in cyanotic versus acyanotic infants after thoracotomy. Paediatr Anaesth. 2003;13:12–17. doi: 10.1046/j.1460-9592.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- 22.Bouwmeester NJ, van den Anker JN, Hop WCJ, Anand KJS, Tibboel D. Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth. 2003;90:642–652. doi: 10.1093/bja/aeg121. [DOI] [PubMed] [Google Scholar]
- 23.Dahlström B, Bolme P, Feychting H, Noack G, Paalzow L. Morphine kinetics in children. Clin Pharmacol Ther. 1979;26:354–365. doi: 10.1002/cpt1979263354. [DOI] [PubMed] [Google Scholar]
- 24.Darnell CM, Thompson J, Stromberg D, Roy L, Sheeran P. Effect of low-dose naloxone infusion on fentanyl requirements in critically ill children. Pediatrics. 2008;121:e1363–e1371. doi: 10.1542/peds.2007-1468. [DOI] [PubMed] [Google Scholar]
- 25.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 26.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesthesiology. 2008;107:83–93. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 27.Riley JL, III, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 28.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 29.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49:249–255. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 30.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 31.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Netzer P, Sendensky A, Wissmeyer MP, Baumeler S, Batista C, Scheurer U, Krause T, Reber P, Brenneisen R. The effect of naloxone-3-glucuronide on colonic transit time in healthy men after acute morphine administration: a placebo-controlled double-blinded crossover preclinical volunteer study. Aliment Pharmacol Ther. 2008;28:1334–1341. doi: 10.1111/j.1365-2036.2008.03855.x. [DOI] [PubMed] [Google Scholar]
- 33.Reber P, Brenneisen R, Flogerzi B, Batista C, Netzer P, Scheurer U. Effect of naloxone-3-glucuronide and N-methylnaloxone on the motility of the isolated rat colon after morphine. Dig Dis Sci. 2007;52:502–507. doi: 10.1007/s10620-006-9563-9. [DOI] [PubMed] [Google Scholar]