Abstract
Purpose
Resistance to antiangiogenic tyrosine kinase inhibitors such as sunitinib is an important clinical problem, but its underlying mechanisms are largely unknown. We analyzed tumor sunitinib levels in mice and patients and studied sensitivity and resistance mechanisms to sunitinib.
Experimental Design
Intratumoral and plasma sunitinib concentrations in mice and patients were determined. Sunitinib exposure on tumor cell proliferation was examined. Resistant tumor cells were derived by continuous exposure and studied for alterations in intracellular sunitinib accumulation and activity.
Results
Intratumoral concentrations of sunitinib in mice and patients were 10.9 ± 0.5 and 9.5 ± 2.4 μmol/L, respectively, whereas plasma concentrations were 10-fold lower, 1.0 ± 0.1 and 0.3 ± 0.1 μmol/L, respectively. Sunitinib inhibited tumor cell growth at clinically relevant concentrations in vitro, with IC50 values of 1.4 to 2.3 μmol/L. Continuous exposure to sunitinib resulted in resistance of 786-O renal and HT-29 colon cancer cells. Fluorescent microscopy revealed intracellular sunitinib distribution to acidic lysosomes, which were significantly higher expressed in resistant cells. A 1.7- to 2.5-fold higher sunitinib concentration in resistant cells was measured because of increased lysosomal sequestration. Despite the higher intracellular sunitinib accumulation, levels of the key signaling p-Akt and p-ERK 1/2 were unaffected and comparable with untreated parental cells, indicating reduced effectiveness of sunitinib.
Conclusion
We report that sunitinib inhibits tumor cell proliferation at clinically relevant concentrations and found lysosomal sequestration to be a novel mechanism of sunitinib resistance. This finding warrants clinical evaluation whether targeting lysosomal function will overcome sunitinib resistance.
Introduction
Resistance to antiangiogenic tyrosine kinase inhibitors (TKI) is a major clinical problem and mechanistic insight into the possible underlying mechanisms of resistance is limited (1). Sunitinib is an antiangiogenic TKI which prolongs progression-free and overall survival of patients with advanced renal cell carcinoma (RCC; refs. 2, 3) and of patients with gastrointestinal stroma cell tumors refractory to imatinib treatment (4). Recently, sunitinib has shown activity in patients with advanced pancreatic neuroendocrine tumors as well (5). Several distinct resistance mechanisms to sunitinib and other antiangiogenic TKIs have been proposed on the basis of preclinical studies including induction of alternative growth factor signaling as well as epithelial to mesenchymal transformation (EMT; 6–9), but these cannot fully explain the clinical observations of resistance. Acquired mutations in target kinases play an important role in TKI resistance such as to imatinib in chronic myeloid leukemia and to B-RAF inhibitors in melanoma, but altered pharmacokinetics and pharmaco-dynamics should be evaluated as potential resistance mechanisms as well (10). While sunitinib has been developed as an antiangiogenic agent, intended to primarily target endothelial and perivascular cells through its high-affinity binding to VEGF receptor (VEGFR) 2 and platelet-;derived growth factor receptor (PDGFR), it inhibits many other kinases (22). In fact, Karaman and colleagues (23) studied the affinity of TKIs to a panel of 317 kinases and found that sunitinib has a low selectivity for specific tyrosine kinases.
On the basis of this potential broader profile of kinase inhibition, we investigated whether an alternative mechanism of action may play a role in the antitumor activity of sunitinib rather than solely its antiangiogenic activity. We determined intratumoral concentrations of sunitinib and studied these clinically relevant concentrations on tumor cells and endothelial cells in vitro. In addition, we studied whether continuous sunitinib exposure would induce tumor cell resistance. We found that sunitinib resistance of tumor cells is mediated by lysosomal sequestration. This sunitinib resistance mechanism is transient as shown by recovery after drug-free culture and can be modulated by interference with lysosomal function. Therefore, this new resistance mechanism warrants further clinical translation.
Materials and Methods
Cell culture
The tumor cell lines 786-O, HT-29, DLD-1, HCT116, and MCF-7 were cultured in Dulbecco's Modified Eagle's Medium supplemented with 5% FBS. The 786-O and HT-29 cell lines were authenticated by the American Tissue Culture Collection. Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical cords. Endothelial colony–forming cells (ECFC) were isolated from the mononuclear cell fraction that was obtained from cord blood. To induce resistance, 786-O and HT-29 cancer cell lines were continuously exposed for more than 12 months to gradually increasing concentrations of sunitinib up to 6 (786-O) or 12 μmol/L (HT-29). Cells were maintained in a humidified incubator containing 5% CO2 at 37°C. Sunitinib was provided by Pfizer Global Pharmaceuticals and was prepared as 20 μmol/L stock solution in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored at 20°C.
Sunitinib measurements in murine samples, human samples, and in vitro
BALB/c female mice were injected with 5 × 105 Renca RCC tumor cells and treated with sunitinib (40 mg/kg/d) and sacrificed after 1 month. Experiments were approved by the Institutional Care and Use Committee at the Johns Hopkins Medical Institutions and in accordance with the NIH Guide for the care and use of laboratory animals.
Three patients diagnosed with advanced malignancies [malignant solitary fibrous tumor, colorectal cancer (CRC), and leiomyosarcoma] were treated with sunitinib in context of a clinical trial at a dose of 37.5 to 50 mg/d for at least 4 weeks. Tumors of these patients were biopsied and in parallel plasma samples were collected. The use of these samples was in accordance with the local ethical guidelines at VU University Medical Center. For the determination of sunitinib in tissue (tumor or skin), an amount of about 10 mg of snap frozen tissue was cut, put into a vial and weighed. A total of 200 μL of water was added, the sample was snap-frozen and freeze-dried overnight. For extraction, 200 μL of ice-cold 83% acetonitrile (ACN) was added and left on ice for 1 hour. After centrifugation, 50 μL of super-natant was transferred for subsequent liquid chromatography—tandem mass spectrometry (LC/MS-MS) analysis as reported previously for plasma and cell pellet homogenates (13). The data are expressed in micromole to allow comparison of tissue concentrations with the IC50 values in cell culture and are based on the conversion of 1 g tissue to 1 mL liquid (14).
Proliferation and clonogenic assays
For proliferation and clonogenic assays, cells were seeded and allowed to attach for 24 hours. After these 24 hours, sunitinib was added at different concentrations. For proliferation assays, a t = 0 measurement was carried out at the same time. Cell proliferation was studied 96 hours after sunitinib treatment. T = 0 and t = 96 hours measurements are carried out using MTT or by cell counts. Cell proliferation was calculated using the following formula:
% of proliferation = [(96 hours measurement of treated cells – 0 hours measurement)/(96 hours measurement of untreated cells – 0 hours measurement)] × 100%
Subtracting the measurement at the beginning of treatment (t = 0 measurement) might result in negative value, representing cell killing.
For clonogenic assays (15), medium was refreshed after 72 hours of sunitinib treatment. After 10 days in drug-free medium, colonies were fixed, stained with 10% Giemsa and counted. Proliferation and clonogenic assays were carried out in triplicate and repeated a minimum of 3 times independently. IC50 values of the parental and resistant cell lines were estimated in parallel in 4 independent experiments, by direct reading from the proliferation curve. Results were normalized to DMSO controls.
Western blot analysis
Cells were treated as indicated. The cells were lysed in M-PER Mammalian Protein Extraction Reagent (Pierce) supplemented with protease and phosphatase inhibitor cocktails (Pierce). Protein concentrations were determined by Micro BCA protein assay (Pierce). Samples containing 50 μg protein underwent electrophoresis on 8% to 12% SDS polyacrylamide gels and were subsequently transferred to PVDF membranes. Proteins were detected using the following antibodies (with catalogue numbers in parentheses): Akt (9272), phospho-Akt (on Ser473; 9271), ERK 1/2 (9102), phospho-ERK 1/2 (on Thr202 and Tyr204; 9101; Cell Signaling Technology), LAMP-1 (sc-20011), LAMP-2 (sc-18822; Santa Cruz Biotechnology), β-actin (A5441; Sigma-Aldrich). After incubation with IRDye (infrared dye)-labeled secondary antibodies (LI-COR Biosciences), membranes were scanned and analyzed with the Odyssey Infrared Imaging System and accompanying software program (LI-COR Biosciences; ref. 16).
Subcellular colocalization studies
Cells were incubated with sunitinib, Lysotracker Red DND-99 (Invitrogen) or Mitotracker Red FM (Invitrogen), Hoechst 33342 (Invitrogen) and bafilomycin A1 (LC laboratories) or ammonium chloride (NH4Cl; Sigma-Aldrich) as indicated. Viable cells were imaged in real time with a Zeiss Axiovert 200 Marianas inverted microscope (ZEISS) equipped with a motorized stage (stepper-motor z-axis increments, 0.1 μm), multiple fluorescence (FITC filter for sunitinib, Cy3 filter for Lysotracker or Mitotracker and DAPI filter for Hoechst nuclear stain), and a Cooke Sensicam cooled charge-coupled device camera (Cooke; 1,280 by 1,024 pixels) with true 16-bit capability at 63 × oil immersion objective. The acquisition protocols included three-dimensional optical sections in real time. Image acquisition and analysis was carried out under full software control (SlideBook 5.0.0.18; Intelligent Imaging Innovations). Three-dimensional optical sections were deconvoluted using the same software. Representative images from more than 3 independent experiments are shown.
Statistical analysis
Data are expressed as means ± SEM. When appropriate, results are shown as normalized data (percentage of DMSO controls). Statistical analyses were carried out using Student t test. A P value less than 0.05 was considered to be statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Intratumoral sunitinib concentrations are significantly higher than plasma concentrations
After 4 weeks of sunitinib treatment at a dose of 40 mg/kg/d, intratumoral sunitinib concentrations in the murine Renca RCC model were 10-fold higher than the corresponding steady-state plasma concentrations [mean ± SEM (range): 10.9 ± 0.5 (9.95–11.8) μmol/L vs. 1.0 ± 0.1 (0.84–1.2) μmol/L sunitinib; n = 3, respectively; P < 0.001; Fig. 1A). The intratumoral sunitinib concentrations in micromoles correspond to, in micrograms sunitinib per gram tissue: 4.33 ± 0.21 (3.96–4.69) μ/g. In normal skin tissue of these mice, sunitinib concentrations were comparable with intratumoral concentrations [mean (range): 7.4 (6.6–8.3) μmol/L, or in μg/g: 3.0 (2.6–3.3); n = 2). Subsequently, tumor biopsies from 3 patients undergoing sunitinib treatment were obtained. In line with the murine data, intratumoral concentrations in patients were 30-fold higher than plasma concentrations. Intratumoral concentrations of sunitinib in patients were 9.5 ± 2.4 (5.1–13.4) μmol/L, whereas their plasma concentrations were 0.3 ± 0.1 [(0.22–0.34) μmol/L; n 3; P < 0.05; Fig. 1A]. The intratumoral sunitinib concentrations in μmol/L correspond to, in micrograms sunitinib per gram tissue: 3.79 ± 1.67 (2.02–5.32) μg/g.
Figure 1.
High intratumoral concentrations of sunitinib inhibit tumor cells directly. A, intratumoral and plasma concentrations of sunitinib in mice with RCC tumors (n = 3) and patients with advanced malignancies (n = 3), treated with sunitinib at a dose of 40 mg/kg/d for 1 month (mice) or 37.5 to 50 mg/d for at least 4 weeks (patients). B, proliferation (MTT assay) of tumor cell lines incubated with increasing sunitinib concentrations. Sunitinib inhibited proliferation of tumor cells at clinically relevant intratumoral concentrations. C, sunitinib inhibition of colony formation of tumor cell lines. D, proliferation of endothelial cells incubated with increasing concentrations of sunitinib while grown in complete medium. HUVECs were cultured in M199 medium supplemented with 10% FBS and 10% human serum, whereas ECFCs were cultured in endothelial basal medium (EBM-2) supplemented with 10% FBS, VEGF, epidermal growth factor (EGF), insulin—like growth factor (IGF), and FGF. E, Western blot analysis of (phosphorylated) ERK 1/2 and Akt in 786-O and HT-29 cell lines treated with 2 μmol/L sunitinib for 1 hour, compared with DMSO controls. Results are shown as means ± SEM. *, P < 0.05; ***, P < 0.001.
Sunitinib directly inhibits tumor cells at intratumoral concentrations
In a panel of RCC, CRC, and breast cancer cell lines, sunitinib inhibited the proliferation at clinically achievable intratumoral drug concentrations, with inhibitory concentrations of 50% (IC50) ranging between 1.4 and 2.3 μmol/L (Fig. 1B). These results were confirmed in a clonogenic assay in a subset of cancer cell lines (Fig. 1C). In addition, sunitinib inhibited the proliferation of HUVECs and ECFCs grown in complete medium at similar concentrations (Fig. 1D). Western blot analysis of tumor cell lysates revealed that sunitinib reduced phosphorylation of extra-cellular signal—regulated kinase (ERK) 1/2 and Akt, which are 2 downstream signaling proteins of sunitinib targets (17, 18; Fig. 1E). No inhibition of Akt phosphorylation was observed in 786-O cells in accordance with a mutated PTEN status in this cell line causing constitutive activation of the Akt pathway (19).
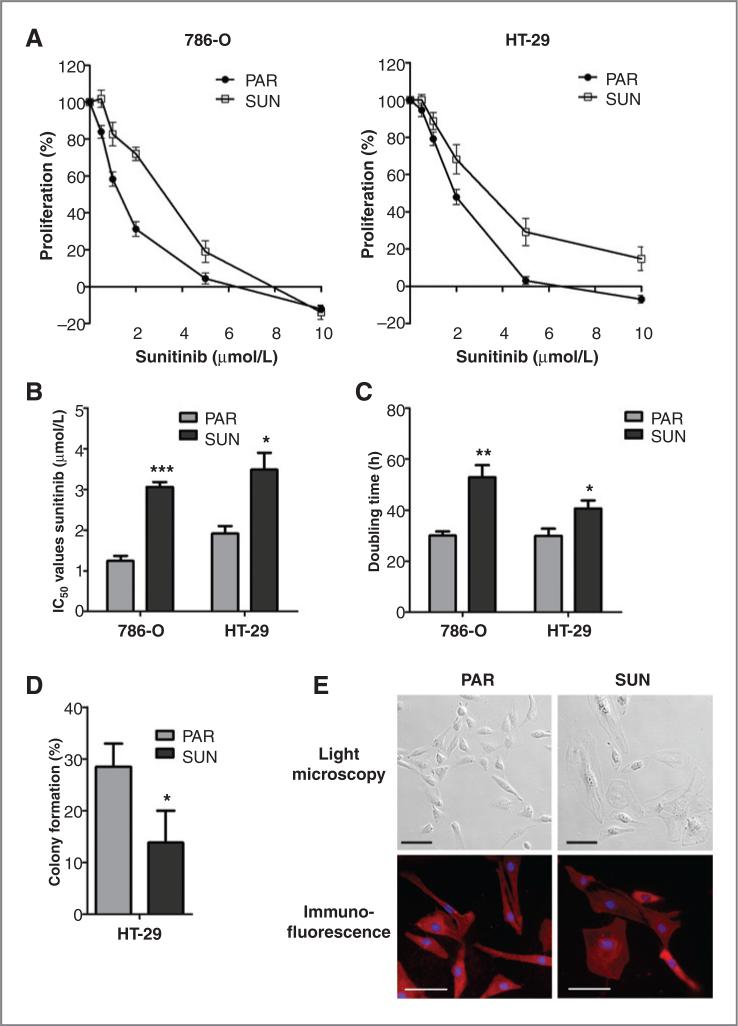
Prolonged sunitinib exposure results in tumor cell resistance in vitro
786-O and HT-29 cells were cultured for more than 12 months at gradually increasing sunitinib concentrations. This prolonged drug exposure resulted in tumor cell resistance, which is reflected by a continuation of growth at sunitinib concentrations above 6 μmol/L, whereas at these concentrations, cell death is induced in the parental 786-O and HT-29 cells (Fig. 2A). IC50 values were increased in the sunitinib-resistant cells compared with the parental cells from 1.2 to 3.1 μmol/L in 786-O cells (P < 0.001) and from 1.9 to 3.5 μmol/L in HT-29 cells (P < 0.05; Fig. 2B). In addition, resistant cells had a reduced growth rate (Fig. 2C) and a reduced clonogenic capacity (HT-29; Fig. 2D). Morphologic examination by light microscopy and immunofluorescent staining with β-actin and Hoechst revealed that a subpopulation of the resistant cells was more flattened, had enlarged nuclei, and/or were multinuclear compared with their parental cells (Fig. 2E). No signs of increased senescence characteristics were found, as mean cell volume and expression of pH-dependent β-galactosidase activity of the resistant cell population was comparable with parental cells (Supplementary Fig. S1A and S1B).
Figure 2.
Acquired resistance to sunitinib in tumor cells. To induce resistance, 786-O and HT-29 cancer cell lines were continuously exposed for more than 12 months to gradually increasing concentrations of sunitinib up to 6 (786-O) or 12 μmol/L (HT-29). A, proliferation assay showing reduced drug sensitivity of sunitinib-resistant (SUN) cancer cells compared with their parental (PAR) cells. B, IC50 values of sunitinib were increased in the resistant 786-O and HT-29 cells, compared with their parental cells. C, sunitinib-resistant 786-O and HT-29 cells had a reduced cell growth (higher doubling time), compared with their parental cells. D, sunitinib-resistant HT-29 cells had a reduced clonogenic capacity, compared with parental cells. E, morphologic changes in sunitinib-resistant 786-O cells. For immunofluorescent staining, cells were incubated with β-actin antibody, followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibody and Hoechst. Scale bars are 100 μm. Results are shown as means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
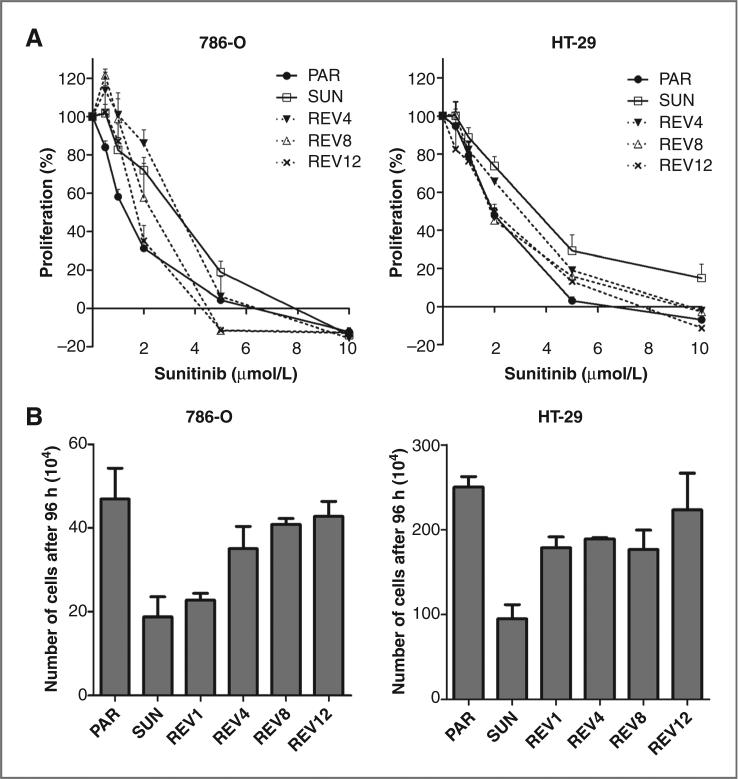
Sunitinib resistance is transient
To determine whether in vitro resistance is transient or irreversible, sunitinib-resistant 786-O and HT-29 cells were cultured in drug-free medium for a period of up to 12 weeks. Sunitinib sensitivity was gradually restored and these revertant 786-O and HT-29 cells reached the original drug sensitivity after 12 weeks (Fig. 3A). Concomitantly, their growth rate and cell morphology restored to that of their parental cells (Fig. 3B).
Figure 3.
Transient resistance to sunitinib in vitro. To recover cells from continuous sunitinib exposure, 786-O- and HT-29– resistant cells were cultured in sunitinib-free medium for a period of up to 12 weeks. A, sensitivity of revertant cells after 4, 8, and 12 weeks of drug-free culture conditions. Cells recovering from continuous sunitinib exposure gradually regained sensitivity to the level of parental cells. B, growth rate of revertant cells after 1, 4, 8, and 12 weeks of drug-free culture conditions. PAR, parental cells; SUN, sunitinib-resistant cells; REV, revertant cells after 1 week (REV1); 4 weeks (REV4), 8 weeks (REV8), and 12 weeks (REV12) in drug-free medium. Results are shown as means ± SEM.
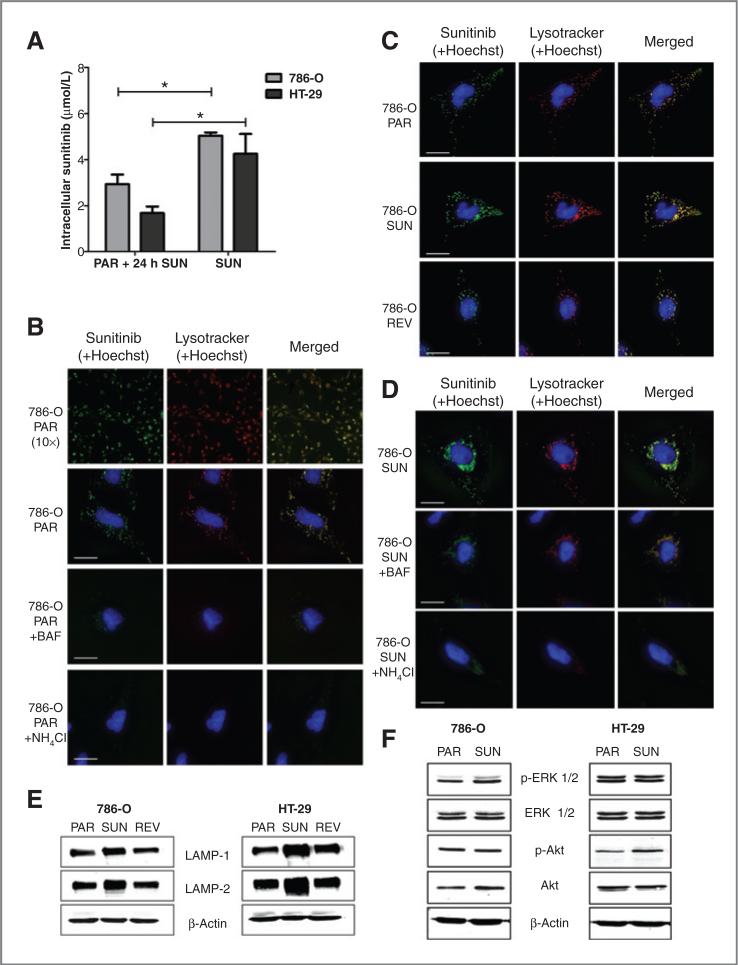
Sunitinib is sequestered in lysosomes
A classical mechanism of drug resistance includes increased drug efflux, resulting in decreased intracellular drug concentration in the resistant cells (10). To investigate whether a decreased intracellular drug concentration was involved in the sunitinib-resistant tumor cells, we analyzed the intracellular sunitinib accumulation. In contrast to expectations from classical drug resistance studies, pharmacologic analysis revealed that the intracellular sunitinib concentrations were significantly higher in the resistant tumor cells than in the parental cells incubated with 5 μmol/L sunitinib for 24 hours. Intracellular concentrations in the resistant 786-O and HT-29 cells were 5.04 ± 0.11 and 4.25 ± 0.86 mmol/L sunitinib (mean ± SEM), respectively (Fig. 4A). These concentrations in the resistant cells are 1.7-fold (P < 0.05) and 2.5-fold (P < 0.05) higher than the parental 786-O and HT-29 cells, in which intracellular concentrations of 2.93 ± 0.42 and 1.68 ± 0.29 μmol/L sunitinib were measured, respectively (Fig. 4A). These intracellular concentrations in the resistant cells are up to 1,000-fold the sunitinib concentration in the conditioned medium. The intracellular concentration of sunitinib calculated as the amount of drug per gram of cellular protein are 23.8 ± 0.4 and 13.0 ± 2.6 ng sunitinib/μg protein in the resistant 786-O and HT-29 cell, respectively, and to 12.2 ± 1.3 and 5.9 ± 0.8 ng sunitinib/μg protein in the parental 786-O and HT-29 cell, respectively.
Figure 4.
Lysosomal sequestration of sunitinib. A, intracellular sunitinib concentration of parental cells, incubated for 24 hours with 5 μmol/L sunitinib, and resistant cells, continuously incubated with sunitinib. B and C, confocal images after incubation with sunitinib (green), 75 nmol/L Lysotracker (red), and 0.5 μg/mL Hoechst (blue) for 60 minutes. Sunitinib and Lysotracker were found to highly colocalized (yellow). B, first and second rows, 786-O parental cells (PAR), treated with 5 μmol/L sunitinib for 60 minutes, 10× and 63× magnification, respectively; third row, 786-O PAR, treated with 50 nmol/L bafilomycin A1 (BAF) for 90 minutes, starting 30 minutes before addition of sunitinib, Lysotracker, and Hoechst; fourth row, 786-O PAR, treated with 10 μmol/L NH4Cl for 90 minutes, starting 30 minutes before addition of sunitinib, Lysotracker, and Hoechst. Scale bars are 25 mm. C, top, 786-O PAR, treated with 5 μmol/L sunitinib for 60 minutes; middle, 786-O sunitinib-resistant cells (SUN), continuously treated with sunitinib; bottom, 786-O revertant cells (REV), cultured for 12 weeks in sunitinib-free medium, and subsequently retreated as the parental cells. Scale bars are 25 μm. D, top, 786-O sunitinib-resistant cells (SUN), continuously treated with sunitinib; middle, 786-O SUN, treated with 50 nmol/L bafilomycin A1 (BAF) for 90 minutes; bottom, 786-O SUN, treated with 10 μmol/L NH4Cl for 90 minutes. Scale bars are 25 μm. E, Western blot analysis of LAMP-1 and LAMP-2 of parental, sunitinib-resistant, and revertant 786-O and HT-29 cells. F, Western blot analysis of (phosphorylated) ERK 1/2 and Akt of parental and sunitinib-resistant 786-O and HT-29 cells. Results are shown as means ± SEM. *, P < 0.05.
To further define the mechanism of sunitinib resistance in the tumor cells, we analyzed the subcellular distribution of sunitinib, to investigate whether sunitinib accumulates in specific subcellular compartments. Taking advantage of the autofluorescent properties of sunitinib, fluorescence microscopy revealed that sunitinib is specifically sequestered in subcellular compartments. This sequestration was significantly higher in resistant cells than in the parental cells. Consistent with the physicochemical properties of sunitinib, which is a hydrophobic (logP = 5.2) weak base (pKa, acid dissociation constant, = 8.95), it appeared that sunitinib predominantly colocalized with lysosomal staining upon treatment of 786-O and HT-29 cells in combination with a viable lysosomal-specific probe (Lysotracker Red DND-99; 786-O: Fig. 4B, first and second rows in second column; HT-29: data not shown). Lysosomal accumulation of sunitinib was also present in HUVECs (Supplementary Fig. S2). We studied whether the subcellular localization of sunitinib can be disturbed by interfering with the function of acidic lysosomes. 786-O cells were coincubated with bafilomycin A1, a specific inhibitor of vacuolar type H+-ATPase (V-ATPase), which abolishes acidification of lysosomes (20), and ammonium chloride, a lysosomotropic weak base, which rapidly increases lysosomal pH (21). These incubations resulted in a significantly reduced sequestration of sunitinib within lysosomes (Fig. 4B, third and fourth rows in second column), confirming acidic lysosomal localization of sunitinib. Alternative sequestration in mitochondria was ruled out by the absence of colocalization of sunitinib with Mitotracker Red FM staining (Supplementary Fig. S3).
Lysosomal sequestration is involved in sunitinib resistance
Comparison of parental and sunitinib-resistant 786-O and HT-29 cells indicated that the lysosomal capacity of resistant cells was substantially increased (Fig. 4C, middle). In addition, Fig. 4D shows that incubation of resistant 786-O cells with bafilomycin or ammonium chloride largely decreased the lysosomal fluorescence. Western blot analysis indeed showed increased expression of the major lysosome-associated membrane proteins (LAMP) 1 and LAMP-2 (22) in the resistant 786-O and HT-29 cells compared with their parental cells (Fig. 4E). Supportive for the increased sequestration in lysosomes of sunitinib as a mechanism of resistance includes similar phosphorylation levels of ERK 1/2 and Akt in resistant cells compared with untreated parental cells (Fig. 4F), despite the high intracellular sunitinib concentrations. In addition, after 12 weeks of drug-free culture of the sunitinib-resistant cells, these revertant cells had normalized their lysosomal capacity and LAMP expression to the level of the parental cells (Fig. 4C, bottom, and E). During this same period, sunitinib sensitivity was also restored to that of the parental cells (Fig. 3A), supporting the role of lysosomes in sunitinib resistance.
Discussion
On the basis of the pharmacokinetic properties of sunitinib with a high apparent volume of distribution (>2,000 L), the majority of the drug is distributed into various tissues as reflected by yellow discoloration of the skin in patients, whereas only a relatively small fraction of the drug remains in the circulation. To gain quantitative insight into the distribution of sunitinib in normal tissues and tumors, we determined sunitinib concentrations in skin and tumor tissues of a murine RCC model and in tumor biopsies from patients during sunitinib treatment. We found that intratumoral sunitinib concentrations in mice and patients were at least 10-fold higher than plasma concentrations. These plasma concentrations in mice and patients were similar to previously published observations (13, 23). Together, (pre) clinical intratumoral concentrations indicated that sunitinib plasma pharmacokinetics are unlikely to provide adequate insight into the antitumor mechanisms operative at the tumor tissue level.
While sunitinib has been developed as an antiangiogenic agent, intended to primarily target endothelial and perivascular cells through its high affinity binding to VEGFR2 and PDGFR, it inhibits many other kinases (11). Karaman and colleagues (12) studied the affinity of TKIs to a panel of 317 kinases and found that sunitinib has a low selectivity for specific tyrosine kinases. Given the fact that high intratu-moral sunitinib concentrations in patients may not only inhibit angiogenesis but may also target tumor cells directly, we studied the activity of sunitinib in vitro. We found that sunitinib inhibits tumor and endothelial cells at intratu-moral concentrations achieved in tumors of patients, explaining clinical observations that sunitinib treatment not only results in tumor stabilization due to its antiangiogenic activity, but also can cause partial or complete clinical tumor responses.
Previous reports indicated that sunitinib antitumor activity could not be mediated by direct antitumor activity, first because no activity of sunitinib at assumed relevant plasma concentrations on tumor cells in vitro was seen and second because endothelial cells were several fold more sensitive to sunitinib treatment than tumor cells in previous reports (24). By gaining more insight into the pharmacokinetics of sunitinib, we here show that plasma concentrations do not truly reflect physiologic intratumoral pharmacokinetics of sunitinib due to its high volume of tissue distribution and by our finding that sunitinib concentrations are 10-fold higher in (tumor) tissue than plasma levels. Second, while tumor cell proliferation experiments are mostly carried out with a fixed concentration of bovine serum (5%–20% of the growth medium), for endothelial cells experimental conditions are being varied extensively. Mostly for specific proliferation assays, endothelial cells are being starved and stimulated at low serum level (0.1%–1%) with the addition of a specific growth factor, such as VEGF or basic fibroblast growth factor (bFGF; refs. 24, 25). Although these specific conditions are important to study specific growth factor pathway signaling modifications, vascular cells in the in vivo situation or in patients are not only being stimulated by one specific growth factor. Vascular cells in vivo are stimulated by multiple growth factors and proteins, due to tumor release of several growth factors, extracellular matrix proteins and platelet releasates, similar to the proteins present in serum (26, 27). Therefore, in our experimental set up we determined the sensitivity of endothelial cells in vitro under full growth stimulating conditions for endothelial cells and found under these conditions similar sunitinib sensitivity of endothelial cells as for tumor cells. These results further support our findings that sunitinib may not only inhibit angiogenesis but has a direct antitumor effect in vivo and in patients as well.
Furthermore, we found that sunitinib inhibits key signaling pathways in our cancer cell lines similar to what was previously reported by Yang and colleagues (28). While possible primary targets of the multitargeted agent sunitinib on tumor cells are multiple and remain to be studied, we reasoned that p-Akt and p-ERK would be a rational first choice as read-out of sunitinib activity, because they are 2 major downstream proteins of several known targets of sunitinib. In addition, they are major downstream junctions in many signaling pathways controlling cancer cell proliferation and/or apoptosis. Indeed, we found decreased p-Akt and p-ERK in the sensitive, but not the continuously exposed cells. This inhibitory action on signaling pathways is in support of the activity profile of sunitinib at higher doses due to its affinity to other kinases as previously reported by Karaman and colleagues (12). In preliminary studies using a peptide-based kinase activity array, we indeed confirmed that multiple kinases are inhibited by sunitinib in tumor cells (data not shown).
One of the major clinical problems with antiangiogenic TKIs including sunitinib is their intrinsic and acquired drug resistance (1, 17, 29). On the basis of preclinical studies, the upregulation of VEGF production as well as other compensatory cytokines and growth factors such as HGF/cMET, IL-8, PlGF, SDF-1, or erythropoietin and epithelial EMT have been suggested to play a role in sunitinib resistance (6–9, 30–32). On the basis of our findings that sunitinib inhibits tumor cell proliferation directly, we investigated the possible role of tumor cells in sunitinib resistance. We found that continuous exposure to sunitinib resulted in resistance of tumor cells by an adaptive tumor cell mechanism identified as an increased intracellular lysosomal sunitinib sequestration. The resistance factors were moderate, but were not due to variation in the MTT assays, which were highly consistent in 4 independent experiments per cell line. Moreover, the continuously exposed cells kept growing at concentrations (5 μmol/L for 786-0 and 10 μmol/L for HT-29) that kill the parental cells. Because this form of adaptive resistance to sunitinib might not be the result of a stable mutation in its target such as often occurs with TKIs in cells addicted to one oncogenic target receptor, we decided to investigate other possibilities. We reasoned that sunitinib might preferentially accumulate in acidic lysosomes, because sunitinib is a hydrophobic (logP 5.2) weak base (pKa as = 8.95; 4) is described for some other substances. Because sunitinib is a hydrophobic compound, it can easily cross plasma membranes and other intracellular membranes. In addition, sunitinib is a weak base with a pKa of 9, implying that decreasing the pH below 9, sunitinib will be increasingly protonated and thereby losing its ability to cross membranes. At a pH of 5, all sunitinib will be protonated. Therefore, upon entering an acidic organelle such as a lysosome, sunitinib becomes protonated and cannot cross membranes anymore. To test this hypothesis we studied co-localization of sunitinib and acidic lysosomes. Indeed, we found clear colocalization of acidic lysosomes by staining of lysosomal-associated membrane proteins and sunitinib due to its auto-fluorescence. No colocalization with other intracellular organs could be detected. In addition, subsequent disturbance of the lysosomal function resulted in sunitinib release from lysosomes. Subsequently, we found that resistant cells are able to sequester sunitinib from their cytoplasmic compartment into acidic lysosomes to a higher extent contributing to a decreased growth inhibitory activity. Despite higher intra-cellular concentration of sunitinib in resistant cells, intra-cellular kinase activity was comparable with untreated parent cells. This result supports that sequestration of sunitinib in acidic lysosomes reduces its cytoplasmic availability and provides a novel mechanism of resistance that has not been previously reported. This mechanism of resistance may play a role for other agents, including TKIs, as well and it may reflect a natural defense mechanism of cells.
Lysosomal sequestration as a mechanism of drug resistance is reversible, as drug-free culture of resistant tumor cells resulted in normalization of their lysosomal capacity and recovery of drug sensitivity. Transient in vitro resistance of tumor cells supports clinical resistance data that were recently reported. While resistance to sunitinib is acquired during treatment in almost all patients (29), it is transient in some of them after treatment interruption and subsequent rechallenge (33). The latter is also supported by our recent finding with a tumor-derived cell line from a progressive skin metastasis (9).
Although in vitro prolonged primary endothelial cell culture experiments are not easily feasible, our results indicate that continuous clinical exposure to sunitinib may cause an increased lysosomal capacity in various cell types and provide a general mechanism of resistance. Whether this novel resistance mechanism is unique for sunitinib or whether this is a general mechanism of TKI resistance remains to be studied. Other TKIs either have a relatively high pKa (pazopanib and dasatinib) or a high lipophilicity (gefitinib and nilotinib), whereas sunitinib is both a weak base and hydrophobic. In conclusion, this novel mechanism of resistance warrants further clinical investigation either by re-treatment with sunitinib after prior tumor progression in patients or by concomitant treatment with clinically available drugs that interfere with lysosomal function, such as chloroquine (34).
Supplementary Material
Translational Relevance.
In this study, we obtained more insight in the clinical antitumor activity of and resistance to sunitinib. On the basis of intratumoral concentrations and in vitro experiments, we envision that clinical resistance to sunitinib is transient and rechallenging with sunitinib after an alternative treatment regimen may be beneficial for patients. In addition, on the basis of our results, we hypothesize that treatment with clinical available drugs interfering with lysosomal function, such as chloroquine, may potentiate the antitumor activity of sunitinib. Whether this mechanism of resistance mediated by acidic lysosomes will also play a role in other (antiangiogenic) tyrosine kinase inhibitors warrants further examination.
Acknowledgments
Grant Support
This study was supported by a research grant of Pfizer Global Pharmaceuticals and by an AEGON Research Fellowship Program. YGA is the recipient of a visiting professor fellowship from the Royal Netherlands Academy of Arts and Sciences (KNAW) and from the Netherlands Organization for Scientific Research (NWO).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Michaelson MD, Rosenberg J, Bukowski RM, Curti BD, George DJ, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178:1883–7. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JV, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 5.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 6.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 7.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–71. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doberstein K, Wieland A, Lee SB, Blaheta RA, Wedel S, Moch H, et al. L1-CAM expression in ccRCC correlates with shorter patients survival times and confers chemoresistance in renal cell carcinoma cells. Carcinogenesis. 2011;32:262–70. doi: 10.1093/carcin/bgq249. [DOI] [PubMed] [Google Scholar]
- 9.Hammers HJ, Verheul HM, Salumbides B, Sharma R, Rudek M, Jaspers J, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9:1525–35. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broxterman HJ, Gotink KJ, Verheul HM. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resist Updat. 2009;12:114–26. doi: 10.1016/j.drup.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Fabian MA, Biggs WH, III, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 12.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 13.Honeywell R, Yarzadah K, Giovannetti E, Losekoot N, Smit EF, Wal-raven M, et al. Simple and selective method for the determination of various tyrosine kinase inhibitors used in the clinical setting by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1059–68. doi: 10.1016/j.jchromb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Holdhoff M, Supko JG, Gallia GL, Hann CL, Bonekamp D, Ye X, et al. Intratumoral concentrations of imatinib after oral administration in patients with glioblastoma multiforme. J Neurooncol. 2010;97:241–5. doi: 10.1007/s11060-009-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 16.Mathews ST, Plaisance EP, Kim T. Imaging systems for westerns: chemiluminescence vs. infrared detection. Methods Mol Biol. 2009;536:499–513. doi: 10.1007/978-1-59745-542-8_51. [DOI] [PubMed] [Google Scholar]
- 17.Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–6. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75:3327–31. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–35. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 24.Huang D, Ding Y, Li Y, Luo WM, Zhang ZF, Snider J, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–62. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 25.Verheul HM, Lolkema MP, Qian DZ, Hilkes YH, Liapi E, Akkerman JW, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. 2007;13:5341–7. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- 26.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiological and pathological angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouaib S, Kieda C, Benlalam H, Noman MZ, Mami-Chouaib F, Ruegg C. Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Crit Rev Immunol. 2010;30:529–45. doi: 10.1615/critrevimmunol.v30.i6.30. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010;8:35–45. doi: 10.1158/1541-7786.MCR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–5. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 30.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vroling L, van der Veldt AA, de Haas RR, Haanen JB, Schuurhuis GJ, Kuik DJ, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis. 2009;12:69–79. doi: 10.1007/s10456-009-9133-9. [DOI] [PubMed] [Google Scholar]
- 32.Ellis LM, Hicklin DJ. Resistance to targeted therapies: refining anti-cancer therapy in the era of molecular oncology. Clin Cancer Res. 2009;15:7471–8. doi: 10.1158/1078-0432.CCR-09-1070. [DOI] [PubMed] [Google Scholar]
- 33.Zama IN, Hutson TE, Elson P, Cleary JM, Choueiri TK, Heng DY, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer. 2010;116:5400–6. doi: 10.1002/cncr.25583. [DOI] [PubMed] [Google Scholar]
- 34.Fehrenbacher N, Jaattela M. Lysosomes as targets for cancer therapy. Cancer Res. 2005;65:2993–5. doi: 10.1158/0008-5472.CAN-05-0476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.