Abstract
Background
The aim of this study was to compare plasma cortisol concentration during anesthesia of children with congenital heart disease who received dexmedetomidine (DEX) with those who received etomidate (ETO).
Material/Methods
We recruited 99 ASA physical status II–III pediatric patients scheduled for congenital heart disease (CHD) corrective surgery and divided into them into 3 groups. Group DEX received an infusion of DEX intravenously with a bolus dose of 0.5 μg·kg−1 within 10 min during anesthesia induction, followed by a maintenance dose of DEX 0.5 μg·kg−1·h−1. Group ETO received ETO intravenously with a bolus dose of 0.3 mg·kg−1 without a maintenance dose. Group CON received routine anesthetics as controls. The preset timepoints were: before anesthesia induction (T0), at the end of induction (T1), 30 min after anesthesia induction (T2), at the time of aortic and inferior vena catheterization (T3), and at 180 min (T4) and 24 h (T5) after anesthesia induction.
Results
The cortisol concentration decreased gradually after anesthesia induction in all groups, and returned to baseline values after 24 h. The cortisol concentration was significantly lower in Group ETO children than in Group DEX or group CON at T4.
Conclusions
The plasma concentrations of cortisol decreased in CHD children after the operation, but returned to baseline after 24 h of anesthesia induction. The adrenal cortex function inhibition induced by ETO in CHD children is longer and more serious than that induced by DEX (if any) during the preoperative period.
Keywords: Adrenal Insufficiency; Child; Dexmedetomidine; Etomidate; Heart Defects, Congenital
Background
As an imidazole derivative, etomidate (ETO) is an intravenous anesthetic. In 1983, Ledingham et al. [1,2] reported that ETO inhibits the synthesis and release of human adrenocortical hormone. The peak effect of inhibiting adrenocortical hormone after single injection of ETO appears at 6~8 h, and the hormone restores automatically at 24 h; after continuous infusion, inhibition of ETO lasts for over 24 h. McKee et al. [3] reported that cortisol replacement therapy might reduce mortality in intensive care patients after using ETO. The adverse effects of etomidate (ETO) include injection pain, nausea, vomiting, and inhibition of adrenal cortex function, which is the principal factor limiting its wide clinical application. Use of ETO in induction of emergency anesthesia is still controversial, particularly in patients with septic shock [4–7]. Although ETO continues to be used [8], several trials demonstrated the relationships among ETO administration, adrenal insufficiency caused by reversible inhibition of cortisol synthesis, and increased mortality [9–11]. However, these studies were limited by confounding factors, especially post-hoc or subgroup analyses, and the use of ETO in more severely ill patients [12]. Hemodynamic effects of ETO with a conventional dose for induction are much smaller than other anesthetics, which makes it an optimal drug for anesthesia induction in emergency departments, intubation of the trachea in a critical care setting, and congenital heart disease with hemodynamic instability in children. ETO is commonly used for anesthesia induction before cardiac surgery due to its relative cardiovascular stability. Hemodynamic alterations are common after cardiac surgery, thus a functional adrenal axis is essential. ETO administration can dramatically affect the hemodynamic status of such patients through the reduction of cortisol synthesis, particularly via systemic inflammatory status triggered by cardiopulmonary bypass (CPB) [13,14]. Dexmedetomidine (DEX) is a new and highly selective adrenergic receptor agonist with sedative, analgesic, and anxiolytic functions, which is widely used in various surgical anesthesias. Because DEX has an imidazole ring structure similar to ETO’s [4,5], we assumed that DEX and ETO would have a similar inhibition effect on adrenocortical hormone. We therefore compared the plasma cortisol concentration during anesthesia in children with congenital heart disease who received DEX with that in children receiving ETO.
Material and Methods
Grouping
After getting approval of our Ethics Committee and written consent from the guardians, 99 pediatric patients were included according to references after calculation [17] and divided into 3 treatment groups by envelope-drawing method: group DEX, group ETO, and group CON (control group). Inclusion criteria were: patients who underwent surgery (including anesthesia) lasting less than 3 h, and those who had not used corticosteroids within the last 6 months and did not exhibit preoperative inadequate secretion of adrenocortical hormone. Exclusion criteria were: patients with allergy history or contraindications to drugs used in the study; those with postoperative use of corticosteroids; those with postoperative use of ETO or DEX; and those with shock, septicopyemia, or other diseases rendering use of ETO for induction inappropriate.
Clinical anesthesia process
Pediatric patients were divided into group DEX, group ETO, and group CON (control group) by envelope-drawing method. The patients were required to fast for 6 h, and were given 0.5 mg·kg−1 of midazolam orally at 30 min before surgery. After entering the operating room, electrocardiogram (ECG), pulse oximetry (SpO2), non-invasive blood pressure (NiBP) and other care monitors were connected to monitor basic indicators. We opened peripheral veins, gave induction by groups, and conducted endotracheal intubation after loss of consciousness. We connected the anesthesia ventilator and narcotic drugs and pressure-control ventilation (PCV) with tidal volume of 8~12 ml·kg−1, respiratory rate of 20~30 times/min (adjust settings according to the age and weight), and respiratory ratio set to 1:1.5 to 1:2. Subsequently, ventilatory parameters were adjusted based on concentration of end-tidal carbon dioxide (PetCO2), and PETCO2 was maintained at 35~45 mmHg. Finally, left radial artery and right jugular vein catheterization was performed.
Group DEX received midazolam 0.2 mg·kg−1, DEX 0.5 g·kg−1.10 min, and sufentanil 2 μg·kg−1 plus rocuronium 0.6 mg·kg−1. Group ETO received midazolam 0.2 mg·kg−1, ETO 0.3 mg·kg−1, and sufentanil 2 μg·kg−1 plus rocuronium 0.6 mg·kg−1. Group CON received midazolam 0.2 mg·kg−1 and sufentanil 2 g·kg−1 plus rocuronium 0.6 mg·kg−1. During the maintenance of anesthesia, group DEX received intraoperative continuous infusion of DEX 0.5 μg·kg−1·h−1 until the end of cardiopulmonary bypass (CPB). The 3 groups all inhaled 2% to 3% of sevoflurane and 2 L·min−1 of fresh gas, and received intravenous infusion of sufentanil 2.5 μg·kg−1·min−1 plus rocuronium 0.6 mg·kg−1·h−1 plus Propofol 2~3 mg·kg−1·h−1 to maintain the depth of anesthesia.
Blood collection and processing
At the timepoints of opening peripheral veins (T0), right after induction (T1), 30 min after intubation (T2), after the completion of superior and inferior vena cava catheterization (T3), 3 h after induction (T4), and 24 h after induction (T5), we drew 0.5 ml of venous blood. We centrifuged the specimens using cold-chain transportation at 3000 rmp and room temperature for 3 min, and placed 250 μl of plasma in a refrigerator at −70°C. We determined the total plasma concentration of CORT using the same batch of chemical immunoluminometric reagent (Accessory 8000-type immune luminescence analyzer, Beckman Coulter, Inc.) and completed determination of CORTISOL concentration within 2 months.
Data processing and analysis
EXCEL and SPSS 17.0 software were used for data analysis. Normally distributed measurement data were expresses as mean ± standard deviation. For comparisons of CORT concentration among groups at each timepoint, spherical test (Mauchly’s test of sphericity) was used to determine whether there was a correlation among repeatedly measured data. If there was no correlation, one-way analysis of variance (ANOVA) was used; if relevant, repeated measures analysis of variance was used. In addition, if the data were non-normally distributed, Wilcoxon test was used for the differences between groups; if comparison between groups was positive, the SNK poet hoc test was conducted. Chi-square test was used for case composition data among groups; P <0.05 was considered statistically significant.
Results
General data
The 99 pediatric patients who participated in the test were without significant differences in age, weight, height, and body surface area (BSA) (P> 0.05), and there was no significant difference in the case composition of the 3 groups (P> 0.05). Descriptive results for demographic data of pediatric patients in each group are shown in Tables 1 and 2.
Table 1.
Description of demographic data.
| Index | Group | Means ± Standard Deviation | P value |
|---|---|---|---|
| Weight (kg) | ETO | 10.227±5.9447 | 0.527 |
| DEX | 8.905±5.0553 | ||
| CON | 9.235±4.852 | ||
| Height (cm) | ETO | 77.91±21.009 | 0.358 |
| DEX | 72.99±17.813 | ||
| CON | 75.23±19.25 | ||
| Months of age | ETO | 19.47±3.56 | 0.158 |
| DEX | 15.97±4.68 | ||
| CON | 18.24±5.32 | ||
| BSA (m2) | ETO | 0.457±0.198 | 0.432 |
| DEX | 0.412±0.166 | ||
| CON | 0.428±0.182 |
Table 2.
The distribution of 3 groups of operations.
| Group (n=33) | VSD | VSD, ASD | TOF |
|---|---|---|---|
| ETO | 12 | 11 | 10 |
| DEX | 13 | 14 | 6 |
| CON | 11 | 15 | 7 |
There was no significant difference in the case composition among the 3 groups, P>0.05.
Measurement methodology results of CORT
The correlation coefficient of assay of CORT was 0.967~0.973, with an average recovery rate of 98.3~109%; intra-day and inter-day variations of the 3 repeated measurements was 4.4~6.7% and 6.0~7.9%, respectively. The sensitivity was 0.4 μg·ml−1 (11 nmol·L−1).
CPB
The lowest temperature during CPB in group DEX was significantly higher than in group ETO and group CON (P<0.05). No significant differences were found in other indexes among groups (P>0.05). Despite the significant differences in the lowest temperature during CPB, the 3 groups all underwent surgery at room temperature and there were no significant differences in other data (Table 3).
Table 3.
Comparisons of CPB time and operation time among the 3 groups.
| Items | Group | Mean ± Standard Deviation | P value |
|---|---|---|---|
| CPB time (min) | ETO | 47.15±19.447 | 0.083 |
| DEX | 55.56±19.673 | ||
| CON | 53.32±19.870 | ||
| Aortic clamp time (min) | ETO | 27.97±15.791 | 0.121 |
| DEX | 35.19±13.703 | ||
| CON | 30.73±15.339 | ||
| Operation time (min) | ETO | 122.27±31.026 | 0.145 |
| DEX | 132.65±25.173 | ||
| CON | 134.24±28.476 | ||
| Lowest temperature (°C) | ETO | 31.44±2.13 | 0.024* |
| DEX | 33.75±3.47 | ||
| CON | 32.01±2.41 |
P<0.05, the lowest temperature during CPB in group DEX was significantly higher than those in group ETO and group CON.
Secretion of CORT
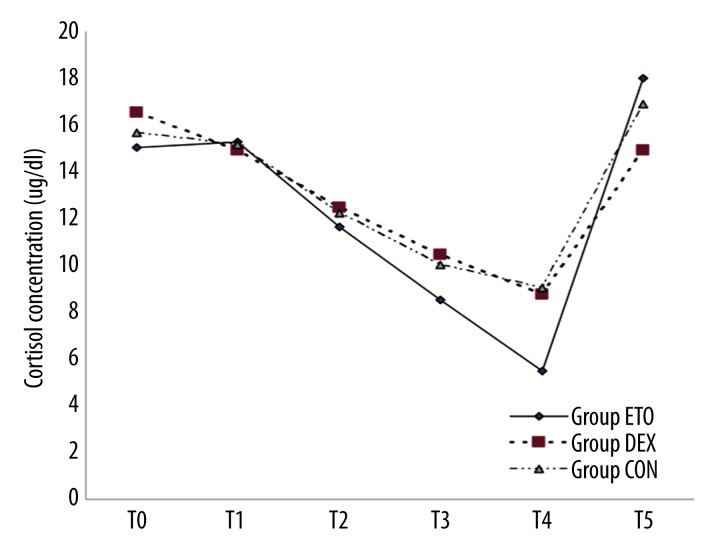
Concentration of CORT at each timepoint in the 3 groups was tested by sphericity test (Mauchly’s test of sphericity, P<0.01). The results showed that repeated measures analysis of variance should be used for processing, and corrected Greenhouse-Geisser value was selected. From the average secretion of CORT at different timepoints in each group (Table 4 and Figure 1), secretion of CORT in group ETO was significantly lower than in group DEX and group CON at T4 (P<0.05); at other timepoints, there were no significant differences among the 3 groups (P>0.05).
Table 4.
Secretion of CORT at different time point in the 3 groups.
| Group | T0 (μg·dl−1) | T1 (μg·dl−1) | T2 (μg·dl−1) | T3 (μg·dl−1) | T4 (μg·dl−1) | T5 (μg·dl−1) |
|---|---|---|---|---|---|---|
| ETO | 15.02±1.10 | 15.28±1.14 | 11.64±1.16 | 8.52±0.70 | 5.49±0.53 | 17.97±3.22 |
| DEX | 16.54±1.30 | 14.91±1.27 | 12.47±1.18 | 10.45±1.12 | 8.76±1.15 | 14.90±2.93 |
| CON | 15.67±1.31 | 15.13±1.18 | 12.23±1.25 | 10.02±1.01 | 9.04±1.01 | 16.87±2.01 |
| P-value | 0.178 | 0.228 | 0.320 | 0.153 | 0.013* | 0.482 |
Secretion of CORT of Group ETO was significantly lower than those of group DEX and group CON at T4 (P<0.05).
Figure 1.
CORTISOL concentration curve at different timepoints in the 3 groups. Opening peripheral veins (T0), right after induction (T1), 30 min after intubation (T2), after the completion of superior and inferior vena cava catheterization (T3), 3 h after induction (T4), and 24 h after induction (T5).
The concentration of CORT in each group gradually decreased over time, with an obvious decrease 3 h after induction, but it returned to the original baseline 24 h after induction (P>0.05). There was no significant difference between the concentration at T5 and the baseline concentration in group DEX, group ETO, and group CON (P>0.05). The comparison results among the 3 groups showed that ETO has greater inhibition in adrenal cortex function.
Secretion of CORT and its clinical results
There were no significant differences in ventilator support time, time in CICU, 24-h urine volume, and 24-h thoracic blood drainage volume among the 3 groups (P>0.05). We analyzed the correlation between CORT at each timepoint and the aforementioned corresponding clinical outcomes. Statistically, high concentrations of CORT decreased with reduction of urine volume or thoracic blood drainage volume, but clinically, this correlation may not be important after taking into account the differences of surgery types, surgical procedures, and growth and development (Table 5).
Table 5.
Descriptive statistics of clinical results in the 3 groups.
| Group | Mean | P-value | |
|---|---|---|---|
| Time in CICU (days) | ETO | 4.09±1.44 | 0.185 |
| DEX | 3.94±1.99 | ||
| CON | 4.12±1.27 | ||
| 24-h urine volume (ml) | ETO | 560.87±251.66 | 0.279 |
| DEX | 506.29±135.14 | ||
| CON | 523.84±178.2 | ||
| 24-h thoracic blood drainage volume (ml) | ETO | 85.84±25.34 | 0.369 |
| DEX | 81.42±27.75 | ||
| CON | 76.36±28.14 | ||
| Ventilator support time (h) | ETO | 10.52±2.37 | 0.098 |
| DEX | 12.11±3.86 | ||
| CON | 10.87±3.26 |
Discussion
After congenital heart surgery, the body is in a complex stress state of shock [18]. Because diagnostic criteria of relative adrenal insufficiency (RAI) vary, it is difficult to accurately determine the incidence rate of RAI after congenital heart surgery. Although the ACTH stimulation test can be used to assess adrenal function based on the hypothalamic-pituitary-adrenal axis reactivity, this approach is used primarily to assess reserve capacity of adrenal function rather than synthesis and secretion of adrenocortical hormone [19,20], and ethically, the ACTH stimulation test cannot verify whether RAI occurred.
According to the results of the present study, after surgery, the concentration of CORT in CHD children gradually decreased and returned to baseline 24 h later. Chu et al. [21] reported that after surgery for CHD children, the total plasma concentrations of CORT gradually increased. Different findings may be caused by different cases, as the subject of the present study is mainly VSD and ASD patients, while nearly two-thirds of the children were diagnosed with severe cyanotic congenital heart disease in the study by Chu et al. [21], such as total anomalous pulmonary venous connection (TAPVC), pulmonary atresia (PA), transposition of great arteries (D-TGA), and double-outlet right ventricle (DORV). In the present study the surgery was completed at room temperature under CPB, while in their study the CPB temperature was significantly lower and CPB and aortic cross-clamp times were completely different.
Similar to our results, Ando et al. [22] found that the postoperative concentration of CORT gradually decreased, and the concentration of CORT in the control group returned to baseline at 48 h after surgery. They also found that, after using hydrocortisone, the plasma concentrations of CORT were always higher than that of the control group and the results of the 2 group crossed until 144 h later. For hydrocortisone, their results in the control group were very similar to our results. In the present study, we added dexamethasone 5 mg·kg−1 in the CPB priming solution. Because dexamethasone with 190 min of Tl/2α (elimination half-life in plasma) will be metabolized completely in 24 h, it could have affected our results only within 3 h after administration; therefore, we believed that it was not related to the cross of concentration of cortisol and plasma concentrations of dexamethasone 24 h later in this study. The inhibition of dexamethasone on hypothalamic-pituitary-adrenal axis can last up to 2.75 days, so it cannot be ruled-out that plasma concentrations of cortisol were influenced by the function of the HPA axis at 24 h after surgery. The elimination half-life of methylprednisolone in plasma was about 2.3~4 hours, which is close to that of dexamethasone, but its inhibition on the HPA axis is significantly weaker than that of dexamethasone (about one-tenth) and can last for 1.25~1.5 days. Moreover, considering that the plasma concentrations of ACTH in their study was always in the normal range, the effect of HPA axis can be excluded and the results of their control group may be closer to the real situation.
According to the results of the present study, after giving ETO and DEX or propofol, trends in CORT concentration curve were almost identical and were different only at T4. The difference shows in that CORT concentration restored more quickly after using DEX or propofol, indicating that the inhibition function of ETO on the adrenal cortex lasted longer and with greater efficacy than DEX (if any), which was similar to the result of Aho et al. in adults [23]. They found that after using 2.4 μg·kg−1 of DEX, plasma concentrations of CORT in patients were lower than that of the saline control group, showing that DEX can weaken endocrine system response to surgical stimulation El-Tahan et al. [24], after 0.2 μg·kg−1, 0.4 μg·kg−1, and 0.6 μg·kg−1 of DEX were used for general anesthesia in caesarean surgery, found that puerpera in the 0.4 μg·kg−1·h−1 and 0.6 μg·kg−1·h−1 groups had weaker response to surgical stimulation than in the saline control group and 0.20 μg·kg−1·h−1 group, and the plasma concentrations of CORT at 5 min after the induction, 1 h after childbirth, and 1 h after extubation were significantly lower than baseline vales. Maze et al. [25] found that, at 30 min after intramuscular injection of 80 μg·kg−1 of DEX into intravital beagles, the ACTH stimulation-induced increase of cortisol secretion was slightly lower than that of the control group (saline), with 4.1~34.2 μg·dl−1 in the control group and 2.2~27.5 μg·dl−1 in group DEX. At the same time, they claimed that, if experimental animals were changed to hybrid dogs, baseline of cortisol secretion within 3 h after administration of DEX would be significantly less than that of the saline control group, and ACTH stimulation-induced increase of cortisol secretion within 3 h was significantly less than the control group. Different species and diets may explain the differences above. However, according to the subsequent cytological study results, half the maximal inhibitory concentration (IC50) of DEX is 10−6 M; and when the concentration was over 10−7 M, the inhibition of DEX on the secretion of CORT in cells of rats was positively correlated with the increasing dose. This indicates that DEX may inhibit the secretory function of adrenocortical cells, and that less than 3 h may not be long enough to see the full picture of DEX inhibition of synthesis and release of adrenocorticotropic hormone. So far, there are few studies about whether DEX within the clinical dose range may lead to inhibition of adrenal function, and their results varied widely. Venn et al. [26] reported DEX had no inhibition on the adrenal cortex after considering propofol did not have pharmacological property of interfering with secretory function of adrenocortical cells. Therefore, they thought that short-term application (<24 h) of DEX would not affect the synthesis of corticosteroid hormones and ACTH, nor inhibit the hypothalamic-pituitary axis. The differences in the results between our study and the Venn et al. study may be caused by the following factors: 1) Different doses of DEX. A loading dose of 0.5 μg·kg−1 and 0.5 μg·kg−1·h−1 for maintenance infusion was used by us, while Venn et al. did not use a loading dose and maintenance infusion dose was 0.2~2.5 μg·kg−1·h−1 with a relatively larger range. 2) The 20 adults needing ICU sedation therapy after surgery in the study by Venn et al. had a wider diagnosis distribution, and most of them were older, so it is difficult to compare their surgical stimulation and trauma, as well as the response capability of patients to stress reaction. 3) The conclusion by Venn et al. that DEX does not influence the synthesis and secretion of adrenocorticotropic hormone came from comparisons with propofol. Considering that there were no significant differences in average plasma concentrations of cortisol in the 2 groups of patients and no inhibition of propofol on adrenocorticotropic hormone was found, they speculated that DEX did not influence the function of adrenal cortex. However, our conclusions were drawn from comparisons among ETO, DEX, and propofol.
We observed clinical results of the patients in the 3 groups and calculated their correlation with CORT concentration, but we cannot provide a unified and rational explanation of the results and actual situation. CORT concentration cannot completely reflect clinical results. Cardiovascular activity indexes of 2 groups were calculated and compared (INSTROPIC index = dopamine dose + dobutamine dose + milrinone dose×15 + epinephrine dose ×100 + norepinephrine ×100 + phenylephrine ×100, dose in the formula refers to μg·kg−1·min−1) [27], and the results demonstrated that there were no differences in use of cardiovascular active drugs among the 3 groups. Using infants after congenital heart surgery as the subjects, Gajarski et al. [28] found that the total plasma concentrations of cortisol in most children achieves a peak value at 2 h after surgery, function of the HPA axis remained intact, the body’s response to stress stimulation was maintained, and peak concentrations of cortisol had no correlation with deep hypothermic circulatory arrest (DHCA) time. It is impossible to forecast the demand for postoperative cardiovascular active drugs. They wondered whether the >15 ratio of ACTH to CORT was more suitable for evaluation of postoperative adrenal function, so as to guide the applications of early postoperative glucocorticoid. Murphy et al. [29] indicated that the decrease in cortisol secretion in newborns after intracardiac surgery and inhibition of release of hypothalamic ACTH cannot be simply attributed to the intraoperative use of dexamethasone that led to feedback inhibition of release of ACTH, and they believed that it was necessary to use postoperative glucocorticoid for newborns.
Free CORT in the plasma is the main form that plays a physiological role. About 90% of CORT combines with cortisol-binding globulin (CBG) and only free CORT will be active. Therefore, measuring the total concentrations of CORT cannot determine the true concentration of free CORT, and the plasma concentrations standard of free CORT is not yet clear in diagnosis of RAI [30–32]. Plumpton et al. [33] found that after children aged <3 years old with congenital heart disease underwent open-heart surgery under CPB, the total plasma concentrations of CORT and plasma concentrations of free CORT only showed a linear relationship within a certain range of concentrations. Wald et al. [34] also reported that simple use of the total plasma concentration of cortisol is not enough to determine the secretion and synthesis functions of adrenal cortex, and they found that continuous decline of CBG concentration and increase of free CORT concentration after CPB were closely related with clinical adverse events after the Coolen formula was used to calculate the concentration of free cortisol [35].
We cannot fully determine the function of the adrenal cortex system because we did not conduct an ACTH stimulation test, and we did not measure plasma concentrations of ACTH, plasma concentration of free CORT, or plasma concentrations of CBG.
Conclusions
After open-heart surgery for children with congenital heart disease, the plasma concentrations of cortisol and secretion of adrenocorticotropic hormone decreased significantly and then returned to baseline after 24 h of anesthesia induction. ETO has greater inhibition on secretion of CORT, indicating that the efficacy of DEX’s inhibition of the adrenal cortex is lower than that of ETO.
Footnotes
Source of support: Departmental sources
References
- 1.Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet. 1983;321:1270. doi: 10.1016/s0140-6736(83)92712-5. [DOI] [PubMed] [Google Scholar]
- 2.Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology. 2011;114:695–707. doi: 10.1097/ALN.0b013e3181ff72b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee J, Finlay WI. Cortisol replacement in severely stressed patients. Lancet. 1983;321:484. doi: 10.1016/s0140-6736(83)91489-7. [DOI] [PubMed] [Google Scholar]
- 4.Annane D. ICU physicians should abandon the use of etomidate. Intensive Care Med. 2005;31:325–26. doi: 10.1007/s00134-005-2560-1. [DOI] [PubMed] [Google Scholar]
- 5.Jackson WL., Jr Should we use etomidate as an induction agentfor endotracheal intubation in patients with septic shock? A criticalappraisal. Chest. 2005;127:1031–38. doi: 10.1378/chest.127.3.1031. [DOI] [PubMed] [Google Scholar]
- 6.Wagner RL, White PF. Etomidate inhibits adrenocorticalfunction in surgical patients. Anesthesiology. 1984;61:647–51. doi: 10.1097/00000542-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield R, Noble DW. Etomidate and fatal outcome – even asingle bolus dose may be detrimental for some patients. Br JAnaesth. 2006;97:116–17. doi: 10.1093/bja/ael124. [DOI] [PubMed] [Google Scholar]
- 8.Jaber S, Jung B, Corne P, et al. An intervention to decrease complicationsrelated to endotracheal intubation in the intensive careunit: a prospective, multiple-center study. Intensive Care Med. 2010;36:248–55. doi: 10.1007/s00134-009-1717-8. [DOI] [PubMed] [Google Scholar]
- 9.Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidateon adrenal responsiveness and mortality in patients withseptic shock. Intensive Care Med. 2009;35:1868–76. doi: 10.1007/s00134-009-1603-4. [DOI] [PubMed] [Google Scholar]
- 10.Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal functionin sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–18. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 11.Kenyon CJ, McNeil LM, Fraser R. Comparison of the effects of etomidate, thiopentone and propofol on cortisol synthesis. Br JAnaesth. 1985;57:509–11. doi: 10.1093/bja/57.5.509. [DOI] [PubMed] [Google Scholar]
- 12.Ray DC, Hay AW, McKeown DW. Induction drug and outcome ofpatients admitted to the intensive care unit after emergencylaparotomy. Eur J Anaesthesiol. 2010;27:481–85. doi: 10.1097/EJA.0b013e3283333a61. [DOI] [PubMed] [Google Scholar]
- 13.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatoryresponse to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–52. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Serrano CV, Jr, Souza JA, Lopes NH, et al. Reduced expressionof systemic proinflammatory and myocardial biomarkersafter off-pump versus on-pump coronary artery bypasssurgery: a prospective randomized study. J Crit Care. 2010;25:305–12. doi: 10.1016/j.jcrc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Pediatr Drugs. 2008;10:49–69. doi: 10.2165/00148581-200810010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Su F, Hammer GB. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10:55–66. doi: 10.1517/14740338.2010.512609. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Wu T. Sample size calculation and power analysis of time-averaged difference. J Moder App Statist Meth. 2005;4:434–45. [Google Scholar]
- 18.Güvener M, Korun O, Demirtürk OS. Risk factors for systemic inflammatory response after congenital cardiac surgery. J Card Surg. 2015;30(1):92–96. doi: 10.1111/jocs.12465. [DOI] [PubMed] [Google Scholar]
- 19.Peeters B, Boonen E, Langouche L, Van den Berghe G. The HPA axis response to critical illness: new study results with diagnostic and therapeutic implications. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.11.012. pii: S0303-7207(14)00368-2. [DOI] [PubMed] [Google Scholar]
- 20.Balbão VMP, Costa M, Castro M, Carlotti APCP. Evaluation of adrenal function in critically ill children. Clin Endocrinol (Oxf) 2014;81(4):559–65. doi: 10.1111/cen.12444. [DOI] [PubMed] [Google Scholar]
- 21.Zhu BX, Jia B, Chen ZG, et al. Effect factors of adrenal insufficiency after operation undergoing cardiopulmonary bypass in children with congenital heart disease. J App Clin Pediatr. 2007;22:813–14. 24. [Google Scholar]
- 22.Ando M, Park I-S, Wada N, Takahashi Y. Steroid supplementation: a legitimate pharmacotherapy after neonatal open heart surgery. Ann Thorac Surg. 2005;80:1672–78. doi: 10.1016/j.athoracsur.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Aho M, Lehtinen AM, Erkola O, et al. Intramuscularly administered dexmedetomidine attenuates hemodynamic and stress hormone responses to gynecologic laparoscopy. Anesth Analg. 1992;75:932–39. [PubMed] [Google Scholar]
- 24.El-Tahan MR, Mowafi HA, Al Sheikh IH, et al. Efficacy of dexmedetomidine in suppressing cardiovascular and hormonal responses to general anaesthesia for caesarean delivery: a dose-response study. Int J Obstet Anesth. 2012;21:222–29. doi: 10.1016/j.ijoa.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Maze M, Virtanen R, Daunt D, et al. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg. 1991;73:204–8. doi: 10.1213/00000539-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth. 2001;86:650–56. doi: 10.1093/bja/86.5.650. [DOI] [PubMed] [Google Scholar]
- 27.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 28.Gajarski RJ, Stefanelli CB, Graziano JN, et al. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr Crit Care Med. 2010;11:44–51. doi: 10.1097/PCC.0b013e3181a64743. [DOI] [PubMed] [Google Scholar]
- 29.Murphy TWG, Homewood J, McCabe A, et al. Evidence of cortisol suppression in neonates after major cardiac surgery: is supplementation necessary? Pediatric Anesthesia. 2006;16:1297. [Google Scholar]
- 30.Roth-Isigkeit AK, Dibbelt L, Schmucker P. Blood levels of corticosteroid-binding globulin, total cortisol and unbound cortisol in patients undergoing coronary artery bypass grafting surgery with cardiopulmonary bypass. Steroids. 2000;65:513–20. doi: 10.1016/s0039-128x(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 31.Vogeser M, Felbinger TW, Kilger E, et al. Corticosteroid-binding globulin and free cortisol in the early postoperative period after cardiac surgery. Clin Biochem. 1999;32:213–16. doi: 10.1016/s0009-9120(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 32.Bright GM. Corticosteroid-binding globulin influences kinetic parameters of plasma cortisol transport and clearance. J Clin Endocrinol Metab. 1995;80:770–75. doi: 10.1210/jcem.80.3.7883829. [DOI] [PubMed] [Google Scholar]
- 33.Plumpton KR, Anderson BJ, Beca J. Thyroid hormone and cortisol concentrations after congenital heart surgery in infants younger than 3 months of age. Intensive Care Med. 2010;36:321–28. doi: 10.1007/s00134-009-1648-4. [DOI] [PubMed] [Google Scholar]
- 34.Wald EL, Preze E, Eickhoff JC, Backer CL. The effect of cardiopulmonary bypass on the hypothalamic-pituitary-adrenal axis in children. Pediatr Crit Care Med. 2011;12:190–96. doi: 10.1097/PCC.0b013e3181f36d17. [DOI] [PubMed] [Google Scholar]
- 35.Coolens J-L, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26:197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]