Abstract
Background
Recent clinical studies have linked polymorphisms in the xeroderma pigmentosum group D (XPD) gene, a key repair gene involved in nucleotide excision repair, to increased risk of hepatocellular carcinoma (HCC). However, the cellular effects of XPD expression in cultured HCC cells remain largely uncharacterized. Therefore, the aim of this study was to characterize the in vitro cellular effects of XPD expression on the HCC cell line HepG2.
Material/Methods
HepG2 cells were transfected as follows to create four experimental groups: pEGFP-N2/XPD plasmid (XPD) group, EGFP-N2 plasmid (N2) control group, lipofectamine™ 2000 (lipid) control group, and non-transfected (CON) control group. An MTT cell proliferation assay, Annexin V-APC apoptosis assay, colony formation assay, scratch wound migration assay, Transwell migration assay, and Western blotting of the autophagic proteins LC3 and p62 were conducted.
Results
XPD expression significantly inhibited HepG2 cell proliferation (p<0.05), significantly promoted HepG2 cell apoptosis (p<0.05), significantly inhibited HepG2 colony formation (p<0.05), significantly decreased HepG2 cells’ migratory ability (p<0.05), and significantly lowered HepG2 cells’ invasive capacity (p<0.05). Western blotting showed that XPD expression significantly increased LC3 expression (p<0.05) and significantly reduced p62 expression (p<0.05).
Conclusions
XPD expression serves as a tumor suppressor and dysregulates autophagic protein degradation in HepG2 cells in vitro. Further in vivo pre-clinical studies and clinical trials are needed to validate XPD’s potential as a tumor-suppressive gene therapy.
Keywords: Carcinoma, Hepatocellular; Hep G2 Cells; Xeroderma Pigmentosum Group A Protein
Background
Hepatocellular carcinoma (HCC) represents 90% of primary liver cancers and typically occurs secondary to chronic liver conditions, including hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, obesity, and alcoholism on a background of cirrhosis [1,2]. Currently, HCC is the sixth most common solid tumor and the third most lethal malignancy globally, with rising incidence in developing sub-Saharan Africa and East Asia, where approximately 85% of all HCC cases occur [1,2]. HCC is a major global public health issue that requires effective clinical management.
Although advances in diagnostic imaging technologies and surveillance programs have improved early detection of HCC, only 20% of HCC patients are amenable to curative therapy by liver transplant, surgical resection, or ablative therapy [2]. Moreover, HCC commonly recurs after curative therapy, with the prognosis for HCC patients with advanced-stage disease remaining rather poor [2]. Therefore, more efficacious therapies are still needed for HCC.
To address this challenge, targeted agents (e.g., sorafenib) have emerged as promising treatments for advanced HCC. Furthermore, new approaches such as gene therapy and immunotherapy have emerged as promising avenues for HCC therapy. In order to foster development of such advanced therapies, a better understanding of the molecular mechanisms underlying HCC development is needed.
To address this question, several recent clinical studies have attempted to link polymorphisms in the xeroderma pigmentosum group D (XPD) gene, a key repair gene involved in nucleotide excision repair, to increased risk of HCC development; however, the results from these studies have been inconsistent [3–7]. Moreover, the cellular effects of XPD expression in cultured HCC cells remain largely uncharacterized. Therefore, the aim of this study was to characterize the in vitro cellular effects of XPD expression on the HCC cell line HepG2.
Material and Methods
Culturing of HepG2 cell line
The HCC cell line HepG2 was obtained from American Type Culture Collection (ATCC, USA) and was cultured in our ‘standard medium’ consisting of DMEM (Gibco, USA), 10% fetal calf serum (Thermo Fisher Scientific, USA), 100 μg/ml streptomycin (Gibco, USA), and 100 U/ml penicillin (Gibco, USA)) at 5% CO2 and 37°C. The medium was changed every two days, and cells were passaged at 70–80% confluence.
Plasmid transfection of HepG2 cells
As previously described [8], a vacant vector plasmid pEGFP-N2 was purchased from Clontech (USA), from which the recombinant plasmid pEGFP-N2/XPD was constructed that was identified through PCR, endonuclease restriction, and sequencing.
The HepG2 cell line culture was trypsin-digested at its logarithmic phase (Shanghai Chemical Reagent Company, China) and re-suspended in our standard medium. The cell suspension (~5×104 cells) was seeded onto a six-well plate and incubated at 37°C in 5% CO2 until reaching ~30% confluence. Cells were transfected as follows to create four experimental groups: pEGFP-N2/XPD plasmid (XPD) group, EGFP-N2 plasmid (N2) control group, lipofectamine™ 2000 (lipid) control group, and non-transfected (CON) control group. An appropriate plasmid amount was added according to the multiplicity of infection (MOI). If there were no observable cytotoxic effects after 12 h, transfection was continued. Then, after 24 h, the standard medium was changed. Three days post-transfection, we checked for at least 80% transfection efficiency by GFP fluorescence; if the transfection efficiency was less than 80%, the transfection was re-performed. The four experimental groups were transferred onto a six-well plate for protein extraction.
MTT cell proliferation assay
A MTT assay was conducted according to the kit instructions (DH343-2, Beijing Ding Guo Biotechnology, China). Briefly, the four experimental groups were trypsin-digested and resuspended in our standard medium after achieving logarithmic growth phase. Cells were plated at equal cell density (2000 cell/100 μl per well) in five 96-well plates and incubated at 37°C and 5% CO2 for continuous detection over a five-day period. At the beginning of the second day, the culture was terminated by adding 10 μl MTT (5 mg/ml) to the original culture medium. After 4 h, the medium was aspirated out, and 100 μl DMSO (Shanghai Pharmaceutical Group, Shanghai, China) was added to each well to terminate the reaction. The plates were oscillated at room temperature for 10 min. Using a microplate reader (Biotek Elx800, USA), cell proliferation was measured using OD490 values. This experiment was performed in triplicate.
Annexin V-APC apoptosis assay
The four experimental groups were trypsin-digested and resuspended in our standard medium after achieving logarithmic growth phase. After washing the cells, the Annexin V-APC Apoptosis Detection Kit and propidium iodide (PI) (catalog# 88-8007, eBioscience, USA) was applied to assess apoptosis according to the kit instructions. Briefly, cells were re-suspended in 1× Binding Buffer at 1×106 cells/ml, and 100 μl of this suspension was added to each of the following tubes: (i) an empty tube, (ii) a tube containing Annexin V-FITC reagent (5 μl); (iii) a tube containing a PI reagent (10 μl); and (iv) a tube containing both Annexin V-FITC reagent (5 μl) and a PI reagent (10 μl). All four tubes were mixed gently in a dark room for 15 min at room temperature, then l× Binding Buffer (400 μl) was added to each tube. Flow cytometry was conducted after l h. This experiment was performed in triplicate.
Colony formation assay
The four experimental groups were trypsin-digested and resuspended in standard medium after achieving logarithmic growth phase. A hemocytometer was used to assess the cell count, and cells were seeded onto six-well plates with each experimental group being plated in three wells at a density of 800 cells/well. The cells were incubated and observed over a period of 14 days with half of the medium being changed every three days.
Cell colonies were photographed by fluorescence microscopy (MicroPublisher 3.3RTV; Olympus, Japan). The cells were washed with PBS and then fixed with paraformaldehyde (1 ml/well; Shanghai Sangon, China) for 30–60 min. All cell wells were washed with PBS and then stained with 500 μl Giemsa (ECM550 Chemicon) for 20 min. Then, the cells were washed with ddH2O several times and then left to dry. Under light microscopy, a digital camera was used to take photographs and obtain colony counts. This experiment was performed in triplicate.
Scratch wound migration assay
As previously described [9], a scratch wound of 1.0–1.5-mm width was made by scraping the cell monolayers of the four experimental groups with a sterile tip. The wells were immediately washed twice. Wounded cultures were maintained in our standard medium at 37°C in a humidified incubator with 5% CO2. At 24 h and 72 h post-scratch, the migratory distance traversed by the cells was assessed by inverted phase-contrast microscopy (IX-70; Olympus, Tokyo, Japan). This experiment was performed in triplicate.
Transwell migration assay
As previously described [9], the four experimental groups (1×105 cells per group) were plated in the top chamber with a membrane containing 8-μm diameter pores in 300 μl serum-free DMEM. Inserts were positioned in the bottom chamber with a 24-well plate containing DMEM with 30% FBS as a chemoattractant. After 12 h, 24 h, and 48 h of incubation, cells remaining on the insert’s top layers were removed by cotton swab. Cells on the lower surface of the membrane were fixed in 100% methanol for 2 min, followed by 1% toluidine blue staining solution in PBS. Inserts were washed several times in distilled water prior to air-drying. The membranes were photographed, and the average cell counts were determined from five random fields. This experiment was performed in triplicate.
Western blotting
The four experimental groups were removed from the incubator, and the medium was discarded. Cells were washed with PBS twice and then treated with pre-cooled 1×Lysis Buffer (i.e., protein electrophoresis loading buffer). After sufficient lysing by pipetting, the cell lysate was quickly transferred into a sample tube and placed on ice for 10–15 min. Then, the lysate was centrifuged at 12000 g for 5 min (4°C) then transferred into a boiling water bath for 10 min. The lysate was re-centrifuged at 12000 g for 1 min (4°C) then placed at −80°C.
Electrophoresis was performed at 80 V for 2 h; post-electrophoresis, the proteins were transferred to PVDF membrane at 4°C (300 mA for 150 min). The PVDF membrane was blocked with 5% skim milk in TBST solution at room temperature overnight at 4°C. The PVDF membrane was incubated with primary antibodies against the microtubule-associated protein light chain 3 (LC3) and p62 at room temperature overnight at 4°C. The membrane was washed in TBST three times (10 min each time). Then, the PVDF membrane was incubated with the appropriate secondary antibody at room temperature for 2 h. The PVDF membrane was washed in TBST three times (10 min each time). Proteins were visualized using the Amersham™ ECL Plus Western Blotting Detection System (GE Healthcare, UK).
Statistical analysis
Statistical analyses were performed with the GraphPad Prism and GraphPad InStat software (GraphPad Software, La Jolla, CA, USA). Bar graphs represent the means of the data from triplicate experiments with error bars indicating standard deviations (SDs). Comparisons between groups were performed using Student’s t-test with a p-value of less than 0.05 deemed significant.
Results
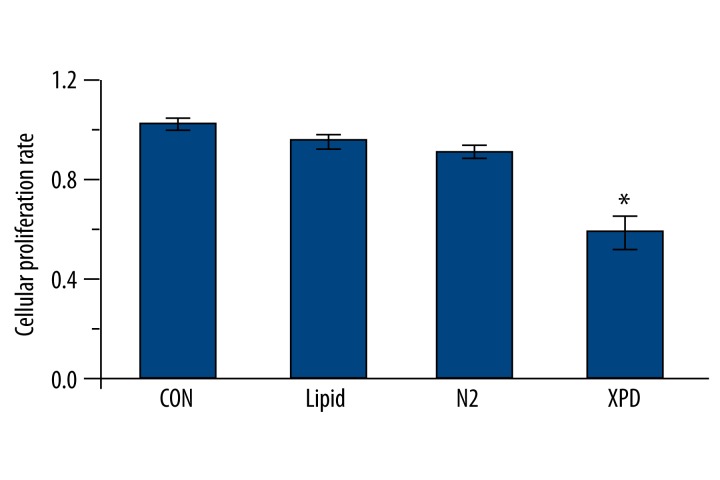
The MTT results show that the OD value of the XPD group was significantly lower than that of the three control groups (p<0.05; Figure 1). Among the three control groups, the differences were not statistically significant (p>0.05; Figure 1). These findings suggest that XPD expression significantly inhibits HepG2 cell proliferation.
Figure 1.
MTT assay. The OD value of the XPD group was significantly lower than that of the other groups (* p<0.05). Among the three control groups, the differences were not statistically significant (p>0.05).
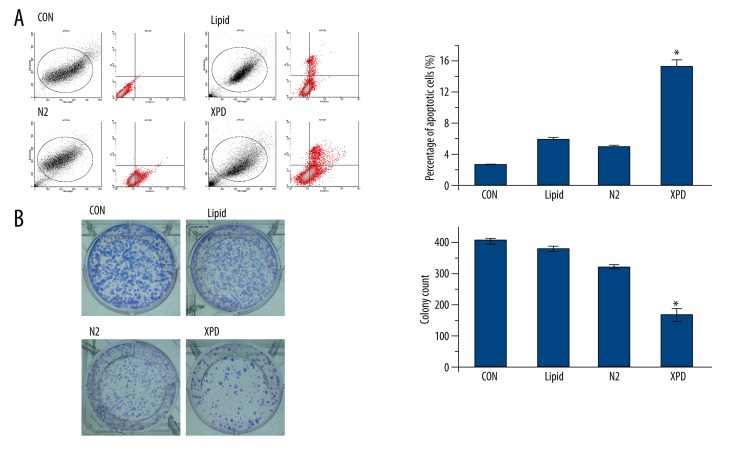
The apoptosis rate of the XPD group was significantly higher than that of the three control groups (p<0.05; Figure 2A). Among the three control groups, the differences were not statistically significant (p>0.05; Figure 2A). These findings suggest that XPD expression significantly promotes HepG2 cell apoptosis.
Figure 2.
Apoptosis and colony formation assays. (A) The apoptosis rate of the XPD group was significantly higher than that of the three control groups (* p<0.05). Among the three control groups, the differences were not statistically significant (p>0.05). (B) Colony formation was significantly lower in the XPD group than that of the three control groups (* p<0.05). Among the three control groups, the differences were not statistically significant (p>0.05).
Colony formation was significantly lower in the XPD group than that of the three control groups (p<0.05; Figure 2B). Among the three control groups, the differences were not statistically significant (p>0.05; Figure 2B). These findings suggest that XPD expression significantly inhibits HepG2 colony formation.
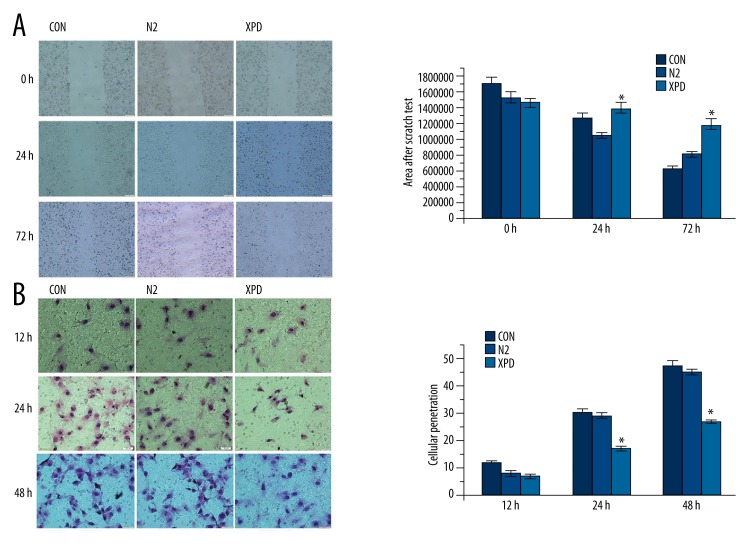
In the scratch wound migration assay, the XPD group showed a significantly decreased migratory ability compared to the N2 and CON groups as evidenced by significantly greater scratch areas after 72 h (p<0.05; Figure 3A). The difference between the XPD group and the N2 group was statistically significant at 24 h (p<0.05; Figure 3A); however, the difference between the XPD group and the CON group was not statistically significant at 24 h (p>0.05; Figure 3A). The differences between the N2 and CON groups were not statistically significant at either time point (p>0.05; Figure 3A). The Transwell migration assay revealed no significant differences among the XPD, N2, and CON groups after 12 h (p>0.05; Figure 3B). But after 24 h and 48 h, the XPD group showed significantly lower invasive capacity relative to the N2 and CON groups (p<0.05; Figure 3B). The differences between the N2 and CON groups were not statistically significant at any time point (p>0.05; Figure 3B).
Figure 3.
Migration assays. (A) In the scratch wound migration assay, the XPD group showed a significantly decreased migratory ability compared to the N2 and CON groups as evidenced by significantly greater scratch areas after 72 h (* p<0.05). The difference between the XPD group and the N2 group was statistically significant at 24 h (* p<0.05); however, the difference between the XPD group and the CON group was not statistically significant at 24 h (p>0.05). The differences between the N2 and CON groups were not statistically significant at either time point (p>0.05). (B) In the Transwell migration assay, there were no significant differences among the XPD, N2, and CON groups after 12 h (p>0.05). But after 24 h and 48 h, the XPD group showed significantly lower invasive capacity relative to the N2 and CON groups (* p<0.05). The differences between the N2 and CON groups were not statistically significant at any time point (p>0.05).
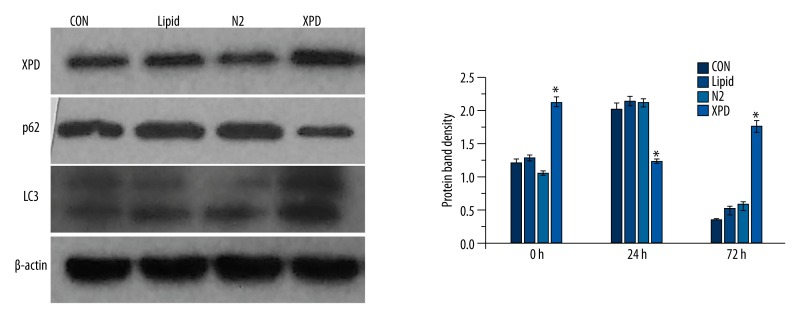
Western blotting revealed that XPD expression in the XPD group was significantly increased (p<0.05). Notably, in the XPD group, LC3 expression was significant increased (p<0.05), while p62 expression was significantly reduced (p<0.05), as compared to the three control groups. Among the three control groups, differences in protein expression were not statistically significant (p>0.05; Figure 4).
Figure 4.
Western blotting. XPD expression in the XPD group was significantly increased (* p<0.05). Notably, in the XPD group, LC3 expression was significant increased (* p<0.05), while p62 expression was significantly reduced (* p<0.05), as compared to the three control groups. Among the three control groups, differences in protein expression were not statistically significant (p>0.05).
Discussion
The XPD gene encodes a helicase subunit of the transcription factor II H (TFIIH) complex, which is involved in basal transcription and nucleotide excision repair [10]. XPD polymorphisms or changes in XPD expression have been associated with a wide array of malignancies [3,11–18]. With specific respect to HCC, recent studies have tied several unique XPD polymorphisms (e.g., Lys751Gln, Arg399Gln, and Asp312Asn) to increased HCC susceptibility [3–5,19].
To better understand XPD’s role in HCC, we investigated the in vitro cellular effects of XPD expression in HCC cells through transfection of the XPD gene into the HCC cell line HepG2. We found that, relative to controls, XPD expression significantly inhibited HepG2 cell proliferation, significantly promoted HepG2 cell apoptosis, significantly inhibited HepG2 colony formation, significantly decreased HepG2 cells’ migratory ability, and significantly lowered HepG2 cells’ invasive capacity (Figures 1–3). These combined findings indicate that XPD expression serves as a tumor suppressor in HepG2 cells in vitro, which is consistent with other previous in vitro studies on HCC cell lines [8,20]. A previous study by Wang et al. demonstrated that XPD expression induces p53 overexpression while suppressing c-myc and cdk2 expression in HepG2 cells [20]. Moreover, Ding et al. showed that XPD expression upregulates p21 and Bax expression while downregulating Bcl-2 and HBx expression through the p53 pathway [8]. These pathways may play a role in XPD’s tumor suppressive effects in HCC. On this basis, XPD may show promise as a potential tool for gene therapy in HCC. Further in vivo pre-clinical studies and clinical trials are needed to validate XPD’s potential as a tumor-suppressive gene therapeutic.
Autophagy appears to play a significant role in tumor cell metastasis [21]. LC3 and p62 are two key proteins involved in autophagic protein degradation, wherein p62 plays a role in the formation of inclusion bodies and then links these bodies to the autophagic machinery via directly interacting with LC3 [22]. Research on ~1400 tumors from 20 cancer types has revealed that punctate LC3 expression is a common feature of malignancy, with elevated LC3 expression positively associated with invasiveness and metastatic activity [21,23]. In particular, although HCC metastasis display higher LC3 expression as compared to primary HCC tumors, LC3 expression has not been associated with invasiveness, migratory ability, or detachment of HCC cells [21]. Moreover, p62 accumulation via impaired autophagy has also been associated with HCC tumorigenesis [24]. Here, through Western blotting, XPD expression was associated with significantly increased LC3 expression and significantly reduced p62 expression. These findings indicate that XPD expression dysregulates autophagic protein degradation in HepG2 cells in vitro; however, the question as to whether XPD expression affects the metastatic potential of HCC cells requires further in vivo investigation.
There are several limitations to this study. First, only one HCC cell line – HepG2 – was investigated in this study, and all experiments were performed in vitro. Future in vivo studies are needed to validate our findings. Second, although we show that XPD expression functions as a tumor suppressor in HepG2 cells, we did not examine the molecular mechanism(s) underlying these effects, as these issues have been addressed in previous studies [8,20]. Third, although we showed that XPD expression dysregulates autophagic protein degradation in HepG2 cells, we did not examine the molecular mechanism(s) by which XPD affects LC3 and p62 expression.
Conclusions
XPD expression serves as a tumor suppressor and dysregulates autophagic protein degradation in HepG2 cells in vitro. XPD may show promise as a potential tool for gene therapy in HCC. Further in vivo pre-clinical studies and clinical trials are needed to validate XPD’s potential as a tumor-suppressive gene therapy.
Footnotes
Source of support: This work was supported the National Natural Science Foundation of China (Grant No. 81360363, for Dr. JF Zheng; 81360074 for Dr. JX Zhang; 81260360 for Dr. WH Guo) and Educational Commission of Jiangxi Province of China (Grant No. GJJ13171)
Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None.
References
- 1.Cheung R. Epidemiology and radiotherapy of hepatocellular carcinoma. Int J Cancer Clin Res. 2014;1:2. [Google Scholar]
- 2.Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol Res. 2015;45:59–74. doi: 10.1111/hepr.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L-Y, Jin X-P, Niu W, et al. Association of XPD and XRCC1 genetic polymorphisms with hepatocellular carcinoma risk. Asian Pac J Cancer Prev. 2012;13:4423–26. doi: 10.7314/apjcp.2012.13.9.4423. [DOI] [PubMed] [Google Scholar]
- 4.Yuan T, Deng S, Liu H, et al. Relationship between XRCC1 and XPD polymorphisms and the risk of the development of hepatocellular carcinoma: A case-control study. Exp Ther Med. 2012;4:285–90. doi: 10.3892/etm.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Q, Li S, Lao X, et al. Association between XPD Lys751Gln and Asp312Asn polymorphisms and hepatocellular carcinoma risk: A systematic review and meta-analysis. Medicine. 2014;93:e330. doi: 10.1097/MD.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R-C, Mou S-H. Polymorphisms of excision repair gene XPD Lys751Gln and hOGG1 Ser326Cys might not be associated with hepatocellular carcinoma risk: a meta-analysis. Tumor Biol. 2013;34:901–7. doi: 10.1007/s13277-012-0625-7. [DOI] [PubMed] [Google Scholar]
- 7.Long XD, Ma Y, Zhou YF, et al. XPD codon 312 and 751 polymorphisms, and AFB1 exposure, and hepatocellular carcinoma risk. BMC Cancer. 2009;9:400. doi: 10.1186/1471-2407-9-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding H, Xu J-j, Huang Y, et al. XPD could suppress growth of HepG2. 2.15 and down-regulate the expression of hepatitis B virus x protein through P53 pathway. Biochem Biophys Res Commun. 2012;419:761–67. doi: 10.1016/j.bbrc.2012.02.097. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Wang G, Shan JL, et al. MicroRNA-224 is upregulated in HepG2 cells and involved in cellular migration and invasion. J Gastroenterol Hepatol. 2010;25:164–71. doi: 10.1111/j.1440-1746.2009.05971.x. [DOI] [PubMed] [Google Scholar]
- 10.Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–58. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M-S, Liu C-y, Su L, et al. Polymorphisms in the nucleotide excision repair genes, ERCC1 and ERCC2/XPD and carcinogen DNA adducts in human lung. Cancer Research. 2012;72:4479. [Google Scholar]
- 12.Tengström M, Mannermaa A, Kosma V-M, et al. MnSOD rs4880 and XPD rs13181 polymorphisms predict the survival of breast cancer patients treated with adjuvant tamoxifen. Acta Oncol. 2014;53:769–75. doi: 10.3109/0284186X.2014.892210. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Pant MC, Singh HS, Khandelwal S. Associated risk of XRCC1 and XPD cross talk and life style factors in progression of head and neck cancer in north Indian population. Mutat Res. 2012;729:24–34. doi: 10.1016/j.mrfmmm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Xue H, Lu Y, Lin B, et al. The effect of XPD/ERCC2 polymorphisms on gastric cancer risk among different ethnicities: a systematic review and meta-analysis. PloS One. 2012;7:e43431. doi: 10.1371/journal.pone.0043431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrzad J, Hashemi M, Jamshidi M, et al. Decreased expression of DNA repair genes (XRCC1 and XPD/ERCC2) in colorectal cancer in Iranian patients. International Journal of Biosciences (IJB) 2014;4:197–205. [Google Scholar]
- 16.Duan X-L, Gong H, Zeng X-T, et al. Association between XPD Asp312Asn polymorphism and esophageal cancer susceptibility: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:3299–303. doi: 10.7314/apjcp.2012.13.7.3299. [DOI] [PubMed] [Google Scholar]
- 17.Avan A, Pacetti P, Reni M, et al. Prognostic factors in gemcitabine-cisplatin polychemotherapy regimens in pancreatic cancer: XPD-Lys751Gln polymorphism strikes back. Int J Cancer. 2013;133:1016–22. doi: 10.1002/ijc.28078. [DOI] [PubMed] [Google Scholar]
- 18.Chang C-H, Wang R-F, Tsai R-Y, et al. Significant association of XPD codon 312 single nucleotide polymorphism with bladder cancer susceptibility in Taiwan. Anticancer Res. 2009;29:3903–7. [PubMed] [Google Scholar]
- 19.Yue A-M, Xie Z-B, Guo S-P, et al. Implication of polymorphisms in DNA repair genes in prognosis of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:355–58. doi: 10.7314/apjcp.2013.14.1.355. [DOI] [PubMed] [Google Scholar]
- 20.Wang H-y, Xiong G-f, Zhang J-x, et al. The role of XPD in cell apoptosis and viability and its relationship with p53 and cdk2 in hepatoma cells. Med Oncol. 2012;29:161–67. doi: 10.1007/s12032-011-9818-y. [DOI] [PubMed] [Google Scholar]
- 21.Peng Y-F, Shi Y-H, Shen Y-H, et al. Promoting colonization in metastatic HCC cells by modulation of autophagy. PloS One. 2013;8:e74407. doi: 10.1371/journal.pone.0074407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 23.Lazova R, Camp RL, Klump V, et al. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18:370–79. doi: 10.1158/1078-0432.CCR-11-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hur CJ, Kim JM, Yang KH, et al. Poster Session: PS 0950; Liver: The autophagy-related marker P62 is useful for diagnosis in human hepatocellular carcinoma. Institute of Medicine Conference Proceedings. 2014;2014:562. [Google Scholar]