Abstract
Background
The IκB kinase inhibitor of κB kinase epsilon (IKBKE) is overexpressed in several human cancers. Although IKBKE plays an important role in smoking-induced non-small cell lung cancer carcinogenesis, its role in squamous cell carcinoma of the lung (SCCL) remains unclear.
Material/Methods
IKBKE protein expression was assessed by immunohistochemistry in 288 paraffinized SCCL specimens (with adjacent squamous dysplastic and normal tissue). IKBKE mRNA expression was assessed by reverse transcription PCR in 66 fresh SCCL specimens (with adjacent squamous dysplastic and normal tissue). Separately, immortalized human bronchial epithelial cells were cultured in 7 groups: untreated control, ethanol-treated, and cigarette smoke condensate (CSC)-exposed for 10, 20, 30, 40, and 50 generations (P10, P20, P30, P40, and P50, respectively). Malignant transformation was assessed by serum resistance and colony formation assays. IKBKE protein and mRNA expression were detected by Western blotting and reverse transcription PCR, respectively.
Results
IKBKE protein expression showed a significant upward trend from normal bronchial epithelium to squamous cell dysplasia to SCCL. IKBKE protein expression in SCCL was significantly associated with smoking status, smoking index, degree of differentiation, and clinical stage. Current and former smokers displayed significantly higher IKBKE protein and mRNA expression than non-smokers. IKBKE protein and mRNA expression displayed a significant upward trend with the smoking index. P30, P40, and P50 CSC-exposed cells displayed malignant transformation with increasing IKBKE mRNA and protein expression from P20 through P50.
Conclusions
IKBKE upregulation is positively associated with SCCL and smoking indices as well as CSC-induced malignant transformation of human bronchial epithelial cells.
Keywords: Bronchial Neoplasms, Carcinoma, Epithelium
Background
Lung cancer has the highest-ranking incidence and mortality in the world and leads to more than 100 million deaths worldwide per annum [1,2]. Lung cancer and smoking status are closely related, with smoking estimated to increase lung cancer risk by a factor of 30 [2–5]. Specifically, malignant transformation of epithelial cells by tobacco-specific nitrosamines is 1 of the primary mechanisms underlying smoking-induced carcinogenesis in lung cancer [6]. For example, 1 key tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), has been shown to lead to malignant transformation of immortalized papillomavirus-infected bronchial epithelial cells [6,7]. Moreover, signaling pathways associated with abnormal cell proliferation – such as the Akt/mToR, MAPK, and JAK/STAT3 signaling pathways – have been shown to be dysregulated in bronchial epithelial cells of smokers with non-small cell lung cancer (NSCLC) [8]. Moreover, cigarette smoke extract (CSE)-treated macrophages have been shown to potentiate malignant transformation of human bronchial epithelial cells through signaling pathways involving PPAR-γ, ERK, and MUC1 [9,10]. Although all the molecular mechanism(s) are not yet fully elucidated, the investigation of signaling pathway dysregulation in lung cancer remains a hotbed of current research activity.
To that end, inhibitor of κB kinase epsilon (IKBKE, IKKɛ) is an important member of the IκB kinase (IKK) family of serine/threonine kinases, which includes IKKα, IKKβ, IKBKE, TBK1, and connexin IKKγ [11,12]. Inflammatory stimuli induce IKKα, IKKβ, and connexin IKKγ to form a complex with phosphorylated IκB-Ser32/Ser36 to induce IκB degradation and subsequent nuclear factor-kappaB (NF-κB) pathway activation [12]. Among these IKK proteins, only IKBKE has been shown to be frequently overexpressed in human cancers, including upregulation in breast cancer, ovarian cancer, prostate cancer, and NSCLC [8,13,14]. In NSCLC, IKBKE upregulation has been associated with smoking status, while blocking IKBKE activity increases NSCLC cell sensitivity to chemotherapy and induces their apoptosis [8]. Although IKBKE appears to play an important part in smoking-induced NSCLC carcinogenesis, its exact role in squamous cell carcinoma of the lung (SCCL) still remains unclear.
Therefore, in this study, through clinicopathological analysis of SCCL lesions (with adjacent squamous dysplastic and normal tissue) sampled from SCCL patients, we investigated the relationship between IKBKE expression, smoking status, and SCCL development. Moreover, through in vitro analysis of the effects of cigarette smoke condensate (CSC) on an immortalized human bronchial epithelial cell line, we assayed the effects of CSC exposure upon IKBKE expression and malignant transformation in bronchial epithelial cells.
Material and Methods
Ethics Statement
This study was approved by the Ethics Committee (IRB) of the First Affiliated Hospital at Bengbu Medical College (Bengbu, China). Written informed consents were obtained from all patients prior to specimen collection.
Paraffinized specimen collection for immunohistochemistry
From January 2008 to July 2013, a total of 312 paraffinized SCCL specimens were collected from the Department of Pathology of the First Affiliated Hospital at Bengbu Medical College. A total of 17 cases were excluded due to an unknown history of smoking, and 7 additional cases were excluded due to substandard paraffinization. Therefore, 288 SCCL paraffinized specimens (from bronchoscopic biopsy [n=84] and surgical resection [n=204] including 108 matching cases of adjacent squamous cell dysplastic tissue specimens and 112 matching cases of normal epithelial control tissue specimens) were finally included in this study. Of these 288 SCCL cases, 84 patients had lymph node metastasis according to imaging data (e.g., chest computer tomography (CT), positron emission tomography-computer tomography (PET-CT), whole-body bone emission computed tomography (ECT), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), and lymph node puncture evaluation. All specimens were collected prior to radiotherapy, chemotherapy, targeted therapy, and/or immunotherapy. All relevant clinical and pathological data, including sex, age, smoking status, smoking index, lymph node metastasis, clinical stage, and histological grade (Table 1), were collected. According to the 2010 National Health Interview Surveys (NHIS) criteria [15], smokers were classified as “current smokers” (i.e., those who had smoked at least 100 cigarettes during their lifetime and, at the time of the interview, reported smoking within the past year), “former smokers” (i.e., those who reported smoking at least 100 cigarettes during their lifetime but had quit smoking for more than 1 year), and “non-smokers” (i.e., those who reported smoking less than 100 cigarettes during their lifetime).
Table 1.
IKBKE protein expression in human lung tissue specimens by immunohistochemistry.
| IKBKE | Normal bronchial epithelium | Squamous dysplasia | SCCL | χ2 | P |
|---|---|---|---|---|---|
| Positive (%) | 11 (9.8) | 45 (41.7) | 204 (70.8) | 125.108 | <0.001 |
| Negative (%) | 101 (90.2) | 63 (58.3) | 84 (29.2) |
Fresh specimen collection for reverse transcription PCR (RT-PCR)
From January 2012 to February 2014, we collected fresh, non-paraffinized specimens for RT-PCR. A total of 66 fresh SCCL specimens with adjacent normal epithelial tissues (diagnosed by hematoxylin and eosin (H&E)-stained biopsy) at our Respiratory Endoscopy Center (the First Affiliated Hospital, Bengbu Medical College) were collected and then immediately refrigerated at −80°C for later use. All specimens were collected prior to radiotherapy, chemotherapy, targeted therapy, and/or immunotherapy. All relevant clinical and pathological data, including sex, age, smoking status, smoking index, lymph node metastasis, clinical stage, and histological grade (Table 1), were collected.
Immunohistochemical staining of SCCL specimens
The paraffin-waxed specimens were sliced into 4-μm sections and analyzed by immunohistochemistry (EliVision Plus IHC Kit; Fuzhou Maixin Biotechnology Development Co., China). Briefly, specimens were deparaffinized with xylene and dehydrated with an ethanol series. Antigen retrieval was performed in a citrate buffer (pH 6.0) over 15 min at room temperature. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in absolute methanol at room temperature for 10 min. The primary antibody – a murine anti-human IKKi/IKKe polyclonal antibody (50 μl, diluted 1:200, Abcam, USA) – was applied overnight at 4°C. The negative control used 50 μl of PBS in lieu of the 50 μl of primary antibody. Following overnight freezing, specimens were warmed at 37°C for 30 min, washed with PBS 3 times for 5 min each, and then treated with 50 μl polymer enhancer for 20 min in a 37°C incubator. Specimens were washed with PBS 3 times for 5 min each and then treated with 50 μl polymerized horseradish peroxidase-anti-mouse immunoglobulin G (IgG) (DAB Kit; Maixin Biological Co., China) for 30 min in a 37°C incubator. Reaction products were visualized using 3, 3′-diaminobenzidine (DAB Kit; Maixin Biological Co., China). Sections were counterstained with H&E, dehydrated, and observed under light microscopy. The percentage of positive cells was calculated by dividing the number of positive tumor cells by the total number of counted tumor cells and multiplying by 100%.
In vitro cell culture of BEP2D Cells
Immortalized human bronchial epithelial cells (BEP2D) were graciously provided by Professor Harris at the National Cancer Institute (Bethesda, USA). BEP2D cells were exposed to either (i) nothing (control), (ii) 95% ethanol or (iii) CSC (at a dose of 0.001 cigarettes/ml) over the course of 10 passages (P10), 20 passages (P20), 30 passages (P30), 40 passages (P40), or 50 passages (P50). After recovery with LHC-8 serum-free medium (Biofluids, USA), the cell culture was incubated at 37°C and 5% CO2 under saturated humidity conditions. The culture medium was changed every 2–3 days, and cells were passaged by trypsin digestion (0.25% trypsin and 0.02% EDTA-2Na in PBS) every 5–6 days.
Serum resistance assay
After reaching the exponential growth phase, cultured cells were re-suspended with a gradient of LHC-8 into a single-cell suspension. After a precise cell count, cells were plated onto 60-mm dishes at a density of 300 cells/dish. Each group was plated on 6 parallel dishes, of which 3 dishes contained 10% fetal bovine serum (FBS) and 5.0 ml LHC-8 medium. Static culture was conducted for 6 days at 37°C and 5% CO2 under 95% humidity. Culture was terminated by anhydrous methanol fixation and Giemsa staining. After washing and drying, the number of colonies in each dish was counted. As previously described [6], plating efficiency (PE%) was calculated as (number of colonies/number of seeded cells) ×l00%, and serum resistance was assessed by calculating the ratio of PE (with serum) to PE (serum-free). These experiments were performed in triplicate.
Colony formation assay
After reaching the exponential growth phase, cultured cells were re-suspended with a gradient of LHC-8 into a single cell suspension. After a precise cell count, cells were plated onto 60-mm dishes at a density of 300 cells/dish with 5 ml pre-warmed medium at 37°C. The dishes were gently agitated to disperse the cells. The plates were incubated for 6 days at 37°C and 5% CO2 under a saturated humidity environment, and the medium was changed once after 3 days. Culture was terminated by anhydrous methanol fixation and Giemsa staining. After washing and drying, the number of colonies in each dish was counted. A cell count of greater than 12 was considered a colony. The colony-forming efficiency (CFE%) was calculated as previously described [6,16]: (number of colonies/number of seeded cells) ×100%. These experiments were performed in triplicate.
RT-PCR
RT-PCR was performed on both the 66 fresh SCCL specimens and the BEP2D cultured cells. Total RNA was extracted using a TRIzol kit (Tiangen Biotech, China), and synthesis of cDNA was conducted according to the reverse transcriptase kit instructions (Promega, USA). Synthetic IKBKE primers (upstream 5′-CAGGGCTTGGCTACAACGAG-3-′, downstream 5′-GATGTCCAGGAGGTCAGATGC-3′) and β-actin primers (upstream 5′-TGACGTGGACATCCGCAAAG-3′, downstream 5′-CTGGAAGGTGGACAGCGAGG-3′) were used for PCR amplification as follows: 95°C denaturation for 2 min followed by 35 cycles of 95°C denaturation for 30 s, β-actin annealing (57°C) for 30 s, IKBKE annealing (68.4°C, 63.8°C) for 30 s, and 72°C extension for 25 s. After PCR amplification, extension was performed at 72°C for 10 min. Images were processed using AlphaView software (v3.2.2.0) with the relative expression of the target mRNA calculated as the target protein: β-actin absorbance ratio. These experiments were performed in triplicate.
Western blotting
Western blotting was performed on BEP2D cells. All cells in each experimental group were pooled, and the whole-cell protein was extracted with RIPA lysis buffer. The protein extracts were run on an 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane, and then treated with a standardized blocking buffer solution (Pik-day Institute of Biotechnology, Shenzhen, China) for 2 h according to the manufacturer’s instructions. The membranes were incubated overnight with murine anti-human IKBKE and anti-human β-actin antibodies (1:800 and 1:400, respectively) at 4°C in a standardized antibody incubation solution (Pik-day Institute of Biotechnology, Shenzhen, China) according to the manufacturer’s instructions. A horseradish peroxidase-conjugated goat anti-mouse secondary antibody (diluted 1: 4000) was applied as a secondary antibody (Boster Biological Engineering, Wuhan, China). Protein immunoreactivity was detected using ECL Reagent (Millipore, USA) according to the manufacturer’s instructions with β-actin as an internal control. Images were processed using AlphaView software (v3.2.2.0) with the relative expression of the target protein calculated as the target protein: β-actin optical density (OD) ratio. These experiments were performed in triplicate.
Statistical snalysis
Statistical analysis was performed using SPSS v17.0 (IBM, USA). All experimental data were expressed as means ± standard deviations (±s). An independent Student’s t-test or analysis of variance (ANOVA) was used to compare continuous variables between groups, while a χ2 test was used for comparison of dichotomous variables with χ2 analysis for linear trend where appropriate. Two-sided p-values of less than 0.05 were deemed statistically significant.
Results
IKBKE protein expression in human lung tissue specimens & its relationship with clinicopathological parameters
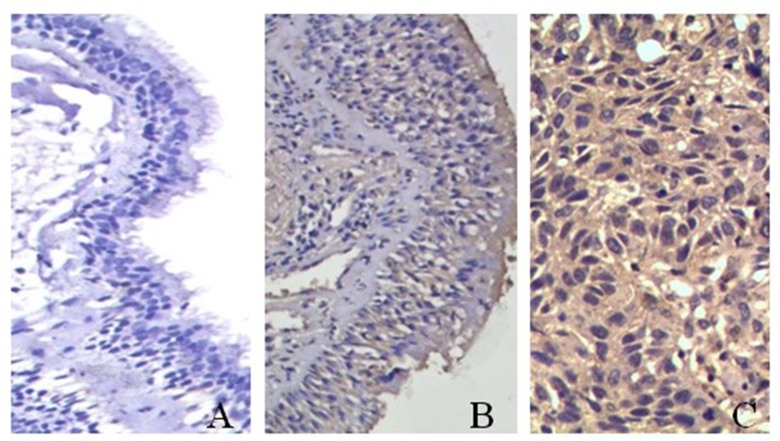
IKBKE protein expression – which was mainly localized in the cytoplasm – was highly expressed in SCCL cells but not in normal bronchial epithelial cells (Figure 1). The IKBKE+ protein expression rates were significantly different across normal bronchial epithelium (9.8%, 11/112), squamous cell dysplasia (41.7%, 45/108), and SCCL (70.8%, 204/288; p<0.001) with a statistically significant upward trend in IKBKE+ protein expression (χ2trend=124.804, ptrend<0.05; Table 1).
Figure 1.
IKBKE protein expression in human lung tissue specimens by immunohistochemistry. IKBKE protein expression in (A) normal bronchial epithelium (Elivision, ×200), (B) squamous cell dysplasia (Elivision, ×200), and (C) squamous cell carcinoma of the lung (SCCL) (Elivision, ×400).
IKBKE+ protein expression in SCCL was significantly associated with smoking status, smoking index, tumor grade, and clinical stage (p<0.05), but was not significantly associated with sex, age, or nodal metastasis status (p>0.05; Table 2). Current smokers (88.6%) and former smokers (45.3%) displayed significantly higher IKBKE+ protein expression rates than non-smokers (31.3%, χ2=73.126, p<0.001; Table 2). By smoking index, IKBKE+ protein expression rates were 84.2% in patients with greater than or equal to 40 pack-years, 79.2% in patients with 20–39 pack-years, 70.6% in patients with 1–19 pack-years, and 33.3% in patients with zero pack-years (Table 2). On this basis, IKBKE+ protein expression displayed a statistically significant upward trend with the smoking index (χ2trend=33.499, ptrend<0.001; Table 2).
Table 2.
Clinicopathological parameters & percentages of IKBKE-positive cells in human lung tissue specimens.
| Parameters | n | IKBKE protein expression status | P | |
|---|---|---|---|---|
| Positive (%) | Negative (%) | |||
| Sex | ||||
| Male | 188 | 132 (70.2) | 56 (29.8) | 0.751 |
| Female | 100 | 72 (72.0) | 28 (28.0) | |
| Age (yrs) | ||||
| ≥60 | 172 | 124 (72.1) | 48 (27.9) | 0.567 |
| <60 | 116 | 80 (69.0) | 36 (31.0) | |
| Smoking status | ||||
| Non-smoker | 48 | 15 (31.3) | 33 (68.2) | |
| Former smoker | 64 | 33 (45.3) | 31 (18.7) | 0.000* |
| Current smoker | 176 | 156 (88.6) | 20 (11.4) | |
| Pack-years (smoking index) | ||||
| 0 | 48 | 16 (33.3) | 32 (66.7) | |
| 1–19 | 68 | 48 (70.6) | 20 (29.4) | |
| 20–39 | 96 | 76 (79.2) | 20 (20.8) | 0.000* |
| ≥40 | 76 | 64 (84.2) | 12 (15.8) | |
| Tumor grade | ||||
| I | 92 | 75 (81.5) | 17 (18.5) | |
| II | 120 | 93 (77.5) | 27 (22.5) | 0.000* |
| III | 76 | 36 (47.4) | 40 (52.6) | |
| Nodal metastasis | ||||
| Negative | 128 | 92 (71.9) | 36 (28.1) | 0.728 |
| Positive | 160 | 112 (70.0) | 48 (30.0) | |
| Clinical stage | ||||
| I–II | 164 | 125 (76.2) | 39 (24.8) | 0.021* |
| III–IV | 124 | 79 (63.7) | 45 (36.3) | |
P<0.05
IKBKE mRNA expression in human lung tissue specimens & its relationship with clinicopathological parameters
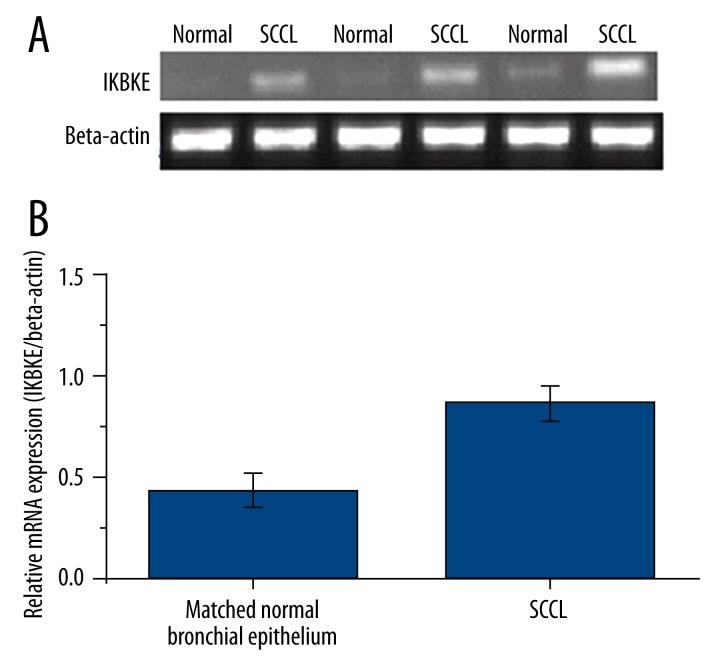
IKBKE mRNA expression was significantly higher in SCCL as compared to matched normal bronchial epithelium (Figure 2). IKBKE mRNA expression in current smokers (1.1864±0.2340) was significantly higher than former smokers and non-smokers (F=86.299, p<0.001) with former smokers (0.7420±0.1903) being higher than non-smokers (0.1991±0.1596, p<0.05; Table 3). By smoking index, IKBKE mRNA expression was 1.3408±0.2182 in patients with greater than or equal to 40 pack-years, 1.0518±0.1547 in patients with 20–39 pack-years, 0.7725±0.221 in patients with 1–19 pack-years, and 0.2127±0.1782 in patients with zero pack-years (p<0.05; Table 3). IKBKE mRNA expression increased with the smoking index (F=67.966, p<0.001; Table 3).
Figure 2.
IKBKE mRNA expression in human lung tissue specimens by RT-PCR. IKBKE mRNA expression in (A) normal bronchial epithelium and (B) squamous cell carcinoma of the lung (SCCL). All data are expressed as means ± standard deviations from triplicate experiments.
Table 3.
Cigarette smoking parameters & IKBKE mRNA expression in human lung tissue specimens.
| Group | n | IKBKE mRNA expression (mean ±s) | F | P |
|---|---|---|---|---|
| Smoking status | ||||
| Never | 11 | 0.1991±0.1596 | ||
| Former | 19 | 0.7420±0.1903 | 86.299 | <0.001 |
| Current | 36 | 1.1864±0.2340*# | ||
| Pack-years | ||||
| 0 | 11 | 0.1991±0.1596 | ||
| 1–19 | 17 | 0.7725±0.221a | ||
| 20–39 | 25 | 1.0518±0.1547a,b | 67.966 | <0.001 |
| ≥40 | 13 | 1.3408±0.2182a,b,c | ||
Compared with non-smokers, p<0.05;
Compared with former smokers, p<0.05;
Compared with 0 pack-years, p<0.05;
Compared with 1–19 pack-years, p<0.05;
Compared with the 20–39 pack-years, p<0.05.
In vitro serum resistance in cultured BEP2D cells
Under exponential growth conditions, the cell growth of ethanol-treated cells was inhibited relative to control cells (Table 4). In contrast, the CSC-exposed cells at P30, P40, and P50 displayed significant resistance to serum-induced growth inhibition with P40 cells displaying the most significant serum resistance (p<0.05; Table 4).
Table 4.
Serum resistance across experimental groups of cultured BEP2D cells.
| Group | Plating efficiency (%) | Serum resistance | |
|---|---|---|---|
| No serum (x̄±s) | 10% serum (x̄±s) | ||
| BEP2D | 11.28±0.68 | 2.55±0.13 | 0.228 |
| Ethanol-treated | 12.95±1.34 | 1.81±0.39 | 0.139 |
| P10 | 13.21±0.52 | 3.84±0.71 | 0.291 |
| P20 | 16.74±0.27 | 4.35±0.98 | 0.259 |
| P30 | 28.94±3.66 | 19.87±2.37 | 0.687* |
| P40 | 32.59±4.18 | 22.52±2.37 | 0.691* |
| P50 | 39.85±1.98 | 23.59±3.11 | 0.592* |
P<0.05 as compared with the control group.
In vitro cell cloning efficiency in cultured BEP2D Cells
For all generations, the CFE%’s for CSC-exposed cells were significantly higher than both the ethanol-treated and control cells (p<0.05, Table 5). The CFE%’s for CSC-exposed cells showed an increasing trend with an increasing number of generation with the highest CFE% being at P50 (20.16±3.71; Table 5).
Table 5.
Clone formation efficiencies across experimental groups of cultured BEP2D cells.
| Group | Clones (x̄±s) | Clone formation efficiency (%) (x̄±s) |
|---|---|---|
| Control | 9±2 | 0.09±0.02 |
| Ethanol-treated | 12.9±3 | 0.13±0.03 |
| P10 | 26.8±10 | 0.27±0.10* |
| P20 | 45±19 | 0.45±0.19* |
| P30 | 1430±304 | 14.30±3.04* |
| P40 | 1760±229 | 17.60±2.29* |
| P50 | 2016±371 | 20.16±3.71* |
P<0.05 as compared with the control group.
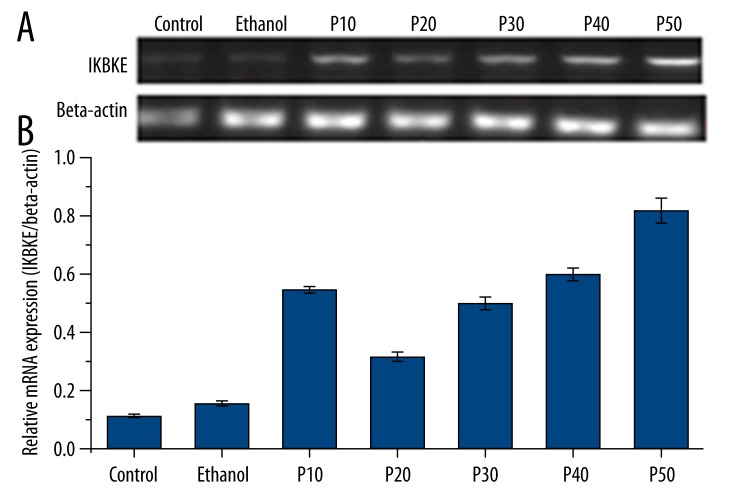
In vitro IKBKE mRNA & protein expression in cultured BEP2D Cells
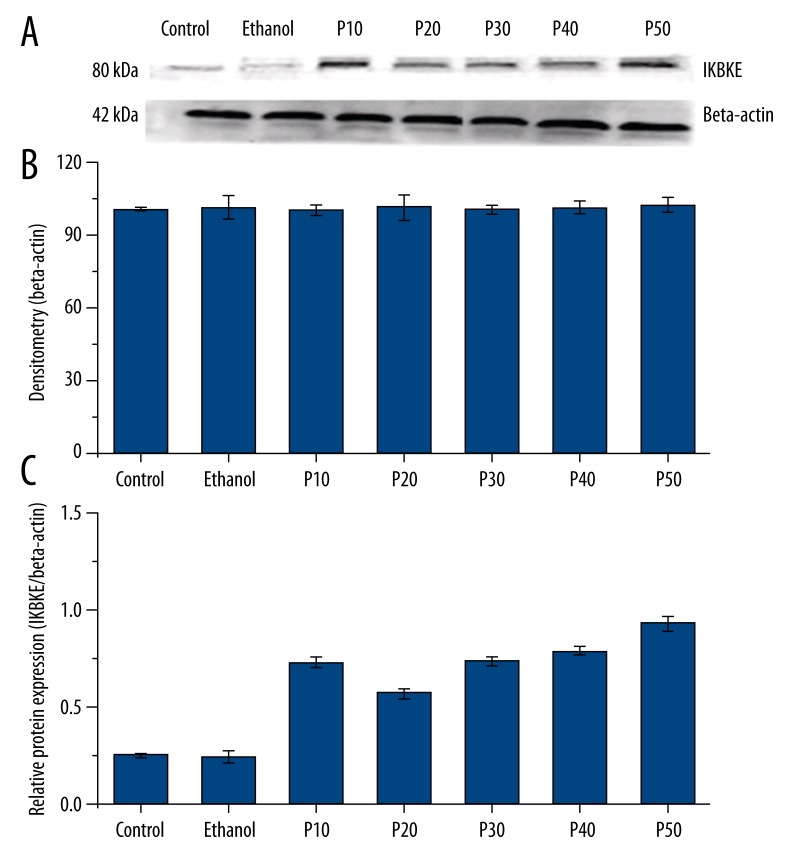
IKBKE mRNA expression between the 2 non-CSC-exposed groups (control, ethanol-treated) and the CSC-exposed groups (P10, P20, P30, P40, and P50) were significantly different (F=117.052, p<0.05; Figure 3). However, there was no significant difference in IKBKE mRNA expression between the control group and the ethanol-treated group, both of which displayed lower IKBKE protein expression relative to all CSC-exposed groups (p>0.05; Figure 3). Although the P10 cells expressed significantly higher mRNA expression than P20 cells (p<0.05), IKBKE mRNA expression gradually increased from P20 through P50 with no significant differences in mRNA expression above P30 (p>0.05; Figure 3). The pattern of protein expression findings derived from Western blotting precisely matched the pattern of mRNA expression findings (F=141.70, p<0.05; F=61.17, p<0.05; Figure 4).
Figure 3.
IKBKE mRNA expression in cultured BEP2D cells by RT-PCR. (A) Ethidium bromide staining showing IKBKE and β-actin mRNA bands. (B) Relative IKBKE mRNA expression with respect to β-actin (internal control). All data are expressed as means ± standard deviations from triplicate experiments.
Figure 4.
IKBKE protein expression in cultured BEP2D cells by western blotting. (A) Visualization of Western blot showing IKBKE and β-actin protein bands. (B) Densitometric analysis showing relatively equal values for β-actin across all groups. (C) Relative IKBKE protein expression with respect to β-actin (internal control). All data are expressed as means ± standard deviations from triplicate experiments.
Discussion
In this study, we showed that IKBKE protein expression was significantly upregulated in SCCL and squamous dysplasia relative to normal bronchial epithelial tissue and was positively correlated with tumor grade and clinical stage. Moreover, IKBKE mRNA expression was significantly increased in SCCL relative to normal bronchial epithelial tissue. Similar to our findings, Boehm et al. showed that IKBKE protein is significantly upregulated in breast cancer tumors relative to normal breast parenchyma [17]. In addition, Guo et al. work in ovarian cancer patients demonstrated that IKBKE protein expression is more frequently upregulated in ovarian primary tumors with alterations in IKBKE protein expression being associated with later-stage and higher-grade ovarian tumors and lower overall survival [18]. Moreover, Hildebrandt et al.’s work in clear cell renal cell carcinoma (ccRCC) patients showed that IKBKE is significantly upregulated in ccRCC tumors compared with adjacent normal tissues, and IKBKE upregulation is associated with a significantly increased risk of death and significantly reduced overall survival duration in these ccRCC patients [19]. Additionally, Guan et al. demonstrated that IKBKE mRNA and protein expression are significantly upregulated in human glioma tumors but showed no significant differences in IKBKE expression across different glioma grades [20]. Clearly, IKBKE upregulation can be positively linked to several human tumor types.
With respect to lung cancer, previous research has revealed that IKBKE expression may be positively linked to smoking-induced lung carcinogenesis [8]. Specifically, Guo et al. demonstrated that IKBKE is upregulated in NSCLC cells by 2 components of cigarette smoke (i.e., nicotine and nicotine-derived nitrosamine ketone) through the signal transducer and activator of transcription 3 (STAT3) pathway [8]. Although Guo et al. work focused on nicotine’s effects upon IKBKE expression in NSCLC cells, here we sought to better define the relationship between smoking, IKBKE expression, and SCCL (as opposed to NSCLC). We found that IKBKE mRNA and protein expression was more prominently expressed in current smokers over former smokers and non-smokers with higher smoking indices (i.e., a greater number of pack-years) associated with increased IKBKE mRNA and protein expression. Similarly, the expression of several other genes that have been previously linked to carcinogenesis – such as p53, p73, and MafB – have also shown significant correlations with smoking indices [21–23]. These combined findings suggest that IKBKE upregulation may play a role in the development of smoking-induced SCCL.
On a cellular level, previous studies in various cancer cell lines have shown positive associations between IKBKE upregulation and malignant transformation [12,17,24] as well as cell survival, cell growth, and chemoresistance [8,18,24,25]. Here, we found that IKBKE mRNA and protein upregulation were positively associated with CSC-induced malignant transformation of bronchial epithelial cells in vitro (as evidenced by their increased serum resistance and CFE%) with higher IKBKE mRNA and protein expression associated with longer CSC exposure. Accordingly, similar findings have been shown in other cancer cell lines. For example, Boehm et al. showed that IKBKE protein is significantly overexpressed in breast cancer cell lines in vitro and that silencing of IKBKE expression induced cell death in these cell lines (likely through a NF-κB-based mechanism) [17]. In addition, Guo et al. demonstrated that IKBKE is more frequently overexpressed in human ovarian cancer cell lines in vitro, with IKBKE overexpression promoting cisplatin resistance and IKBKE silencing rescuing cisplatin resistance [18]. Also, Guan et al. demonstrated that IKBKE mRNA and protein expression were significantly upregulated in glioma cell lines in vitro. Moreover, they showed that ectopic IKBKE overexpression inhibited cellular apoptosis (likely through inducing Bcl-2 expression and NF-κB activation), while IKBKE silencing increased the sensitivity of glioma cells to apoptotic triggers [20]. Clearly, IKBKE upregulation can be positively linked to malignant transformation in several human cancer cell lines.
Mechanistically, IKBKE is well-established to function as an oncogene in several human cancers [17,26]. However, its precise mechanism(s) of action remains under investigation. Guo et al.’s work in breast cancer cells previously demonstrated that IKBKE can functionally replace the AKT oncogene in the process of malignant transformation by directly phosphorylating Akt-Thr308 and Ser473, with IKBKE expression largely correlating with Akt activation [12]. Moreover, Guo et al. later showed that IKBKE’s activation of Akt inhibits the function of the cell growth inhibitor FOXO3a in breast cancer and NSCLC cells through SerS644 phosphorylation [24]. In addition, Kim et al. showed that IKBKE functions as a downstream effector of the Ras oncogene in NSCLC cells and mediates Ras-based activation of NF-κB and Ras-based tumorigenic activity [27]. As we did not investigate the mechanism(s) underlying IKBKE upregulation in human bronchial epithelial cells here, further molecular studies on the precise signal transduction mechanisms associated with CSC-induced malignant transformation of bronchial epithelial cells are needed.
This study has several limitations. First, although this study provides novel evidence supporting IKBKE’s positive relationship with smoking-related SCCL, this study does not explain IKBKE’s role in the carcinogenesis of smoking-induced SCCL. Second, our serum resistance and cloning efficiency assays only demonstrate malignant transformation of bronchial epithelial cells in vitro; therefore, this study lacks in vivo data regarding the relationships between CSC exposure, IKBKE expression, and the degree of malignant tumorigenesis. In future experiments, we plan to employ a nude mouse model to investigate these questions. Third, we did not investigate IKBKE’s effect upon the chemoresistance of cultured bronchial epithelial cells. Future studies should assess the role of IKBKE overexpression and silencing in chemoresistance to cisplatin, carboplatin, gemcitabine, and other chemotherapeutic regimens in cultured bronchial epithelial cells [28]. Fourth, this study did not investigate the mechanism(s) underlying IKBKE upregulation in human bronchial epithelial cells in vitro nor did we assess the downstream effect(s) of such upregulation. Therefore, further investigation on plausible upstream and downstream signal transduction mechanisms associated with IKBKE and CSC-induced malignant transformation of bronchial epithelial cells (such as Akt activation or NFκB activity [12,27]) is needed to address this question.
Conclusions
IKBKE mRNA and protein expression are positively associated with SCCL and smoking indices, suggesting that IKBKE upregulation may be involved in smoking-induced SCCL carcinogenesis. Moreover, IKBKE mRNA and protein expression were positively associated with CSC-induced malignant transformation of human bronchial epithelial cells, suggesting that IKBKE upregulation may be involved in smoking-induced malignant transformation of bronchial epithelial cells.
Footnotes
Source of support: This work was funded by the National Natural Science Foundation of China (grant no. 81172213), the Natural Science Foundation in Higher Education of Anhui (KJ2012B107), and the National Natural Science Foundation of Anhui Province (grant no. 1408085MH144)
Conflicts of interest
None.
References
- 1.Chen W, Zhang S, Zou X. Evaluation on the incidence, mortality and tendency of lung cancer in China. Thoracic Cancer. 2010;1:35–40. doi: 10.1111/j.1759-7714.2010.00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Kim J-H, Kim SK, et al. Lung cancer in never smokers: change of a mindset in the molecular era. Lung Cancer. 2011;72:9–15. doi: 10.1016/j.lungcan.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Instit. 1996;88:1550–59. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 4.Wu D, Pang Y, Wilkerson M, et al. Gene-expression data integration to squamous cell lung cancer subtypes reveals drug sensitivity. Br J Cancer. 2013;109:1599–608. doi: 10.1038/bjc.2013.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walser T, Cui X, Yanagawa J, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5:811–15. doi: 10.1513/pats.200809-100TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, Calaf GM, Hei TK. Malignant transformation of human bronchial epithelial cells with the tobacco specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Cancer. 2003;106:821–26. doi: 10.1002/ijc.11319. [DOI] [PubMed] [Google Scholar]
- 7.Klein-Szanto A, Iizasa T, Momiki S, et al. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc Natl Acad Sci USA. 1992;89:6693–97. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Kim D, Gao J, et al. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non-small cell lung cancer. Oncogene. 2012;32:151–59. doi: 10.1038/onc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Xu X, Padilla MT, Li B, et al. MUC1 in Macrophage: Contributions to cigarette smoke–induced lung cancer. Cancer Res. 2014;74:460–70. doi: 10.1158/0008-5472.CAN-13-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List K, Szabo R, Molinolo A, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao H, Hu Y, Wang P, et al. Down-regulation of Notch receptor signaling pathway induces caspase-dependent and caspase-independent apoptosis in lung squamous cell carcinoma cells. APMIS. 2012;120:441–50. doi: 10.1111/j.1600-0463.2011.02825.x. [DOI] [PubMed] [Google Scholar]
- 12.Guo J-P, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–98. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18:R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- 14.Clément J-F, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–99. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults – United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–19. [PubMed] [Google Scholar]
- 16.Ke Y, Reddel RR, Gerwin BI, et al. Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation. 1988;38:60–66. doi: 10.1111/j.1432-0436.1988.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 17.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Guo J-P, Shu S-K, He L, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175:324–33. doi: 10.2353/ajpath.2009.080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt MA, Tan W, Tamboli P, et al. Kinome expression profiling identifies IKBKE as a predictor of overall survival in clear cell renal cell carcinoma patients. Carcinogenesis. 2012;33(4):799–803. doi: 10.1093/carcin/bgs018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan H, Zhang H, Cai J, et al. IKBKE is over-expressed in glioma and contributes to resistance of glioma cells to apoptosis via activating NF-κB. J Pathol. 2011;223:436–45. doi: 10.1002/path.2815. [DOI] [PubMed] [Google Scholar]
- 21.Tokuchi Y, Hashimoto T, Kobayashi Y, et al. The expression of p73 is increased in lung cancer, independent of p53 gene alteration. Br J Cancer. 1999;80:1623–29. doi: 10.1038/sj.bjc.6690572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Shibata Y, Kimura T, et al. Immunohistochemical staining for transcription factor MafB in alveolar macrophages is correlated with spirometric measures of airflow limitation in smokers. Respirology. 2011;16:124–30. doi: 10.1111/j.1440-1843.2010.01886.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanaoka T, Nakayama J, Mukai J, et al. Association of smoking with apoptosis-regulated proteins (Bcl-2, Bax and p53) in resected non-small-cell lung cancers. Int J Cancer. 2001;91:267–69. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1030>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Guo J-P, Tian W, Shu S, et al. IKBKE phosphorylation and inhibition of FOXO3a: A mechanism of IKBKE oncogenic function. PloS One. 2013;8:e63636. doi: 10.1371/journal.pone.0063636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Xie X, Zhang D, Zhao B, et al. IκB kinase ɛ and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Ac Sci USA. 2011;108:6474–79. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Geng H, Cheng SH, et al. KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas. Cancer Res. 2010;70:6516–26. doi: 10.1158/0008-5472.CAN-09-4566. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Guo J, Challa S, et al. IKBKE is a key mediator of Ras activation of NF-κB and Ras oncogenic function. Cancer Res. 2014;74:4416. [Google Scholar]
- 28.Stinchcombe TE. Targeted therapies for locally advanced or metastatic squamous cell carcinoma of the lung. Curr Treat Options Oncol. 2013;14:568–79. doi: 10.1007/s11864-013-0256-2. [DOI] [PubMed] [Google Scholar]