Abstract
Human herpesvirus 6 (HHV-6) glycoproteins H and L (gH and gL, respectively) and the 80-kDa form of glycoprotein Q (gQ-80K) form a heterotrimeric complex that is found on the viral envelope and that is a viral ligand for human CD46. Besides gQ-80K, the gQ gene encodes an additional product whose mature molecular mass is 37 kDa (gQ-37K) and which is derived from a different transcript. Therefore, we designated gQ-80K as gQ1 and gQ-37K as gQ2. We show here that gQ2 also interacts with the gH-gL-gQ1 complex in HHV-6-infected cells and in virions. To examine how these components interact in HHV-6-infected cells, we performed pulse-chase studies. The results demonstrated that gQ2-34K, which is endo-β-N-acetylglucosaminidase H sensitive and which is the precursor form of gQ2-37K, associates with gQ1-74K, which is the precursor form of gQ1-80K, within 30 min of the pulse period. After a 1-h chase, these precursor forms had associated with the gH-gL dimer. Interestingly, an anti-gH monoclonal antibody coimmunoprecipitated mainly gQ1-80K and gQ2-37K, with little gQ1-74K or gQ2-34K. These results indicate that although gQ2-34K and gQ1-74K interact in the endoplasmic reticulum, the gH-gL-gQ1-80K-gQ2-37K heterotetrameric complex arises in the post-endoplasmic reticulum compartment. The mature complex is subsequently incorporated into viral particles.
Human herpesvirus 6 (HHV-6) is a betaherpesvirus related to human herpesvirus 7 (HHV-7) and human cytomegalovirus (HCMV) and is a human pathogen of emerging clinical significance. HHV-6 was first isolated from the peripheral blood lymphocytes of patients with lymphoproliferative disorders and AIDS (34). HHV-6 isolates can be categorized as two variants, A (HHV-6A) and B (HHV-6B), on the basis of their in vitro growth properties, DNA restriction site polymorphisms, antigenicity, and host cell tropism (1, 3-5, 45). HHV-6B is the causative agent of exanthem subitum (46).
Herpesviruses encode a number of glycoproteins that are present in the envelope of the virion and that play an important role in viral infection, including attachment, penetration, cell-cell spread, and the envelopment and maturation of nascent viral particles. A number of studies have reported a role for glycoprotein H (gH) and glycoprotein L (gL) in the membrane fusion events involved in herpesvirus entry and cell-cell spread. Monoclonal antibodies (MAbs) raised to gH neutralize the activity of infectious virus (6, 10, 12, 13, 22, 23, 37). Similarly, antibodies against gL can inhibit virus infectivity and cell fusion (19, 29, 30).
Previous studies showed that gH and gL form a heteromeric glycoprotein complex (11, 17, 18, 22, 33, 47). Intermolecular disulfide bridges are required for complex formation in betaherpesviruses HCMV and HHV-6 (2, 18, 38) but not in alphaherpesviruses herpes simplex virus type 1, varicella-zoster virus, and bovine herpesvirus 1 (8, 9, 17, 42). The Epstein-Barr virus (EBV) gH-gL complex includes a third glycoprotein, called gp42, which is encoded by the BZLF2 open reading frame (ORF) (21). The gCIII complex, which includes gH, gL, and glycoprotein O (gO) (UL74), has been demonstrated on the HCMV virion envelope (14, 15, 20).
Recently, the HHV-6A gH-gL complex was found to interact with one of the U100 gene products, an 80-kDa protein with complex N-linked oligosaccharides. Glycosidase digestion analysis showed that the U100 gene products exist as glycoproteins, including an 80-kDa form containing complex N-linked oligosaccharides and a 74-kDa form containing immature, high-mannose N-linked oligosaccharides. Based on these characteristics, the U100 gene products were designated as glycoprotein Q (gQ) (24) and the 80-kDa form was designated as gQ-80K. Furthermore, the gH-gL-gQ-80K complex of HHV-6A was identified as a viral ligand for human CD46 (27), which is a cellular receptor of HHV-6 (36). gH has been reported to be the viral component responsible for binding to CD46 (35).
In this study, we focused our attention on the analysis of this novel glycoprotein, gQ, which is unique to HHV-6 and HHV-7. To further analyze HHV-6 gQ, we performed Northern blotting and found in the gQ gene region another small transcript which encodes a protein of 214 amino acids. MAbs against the predicted protein recognized 37-kDa (gQ-37K) and 34-kDa (gQ-34K) forms in HHV-6A-infected cells. Interestingly, gQ-37K is expressed abundantly on the viral envelope and associates with gQ-80K, gH, and gL to form tetrameric complexes in HHV-6-infected cells and in virions. This novel finding is the first report for the herpesvirus family that heterotetrameric glycoprotein complexes which may play an important role in virus entry are expressed in virions.
MATERIALS AND METHODS
Cells and viruses.
T-cell lines (HSB-2 cells) were cultured in RPMI 1640 with 10% fetal calf serum. Umbilical cord blood mononuclear cells (CBMCs) were prepared as described previously (7). HHV-6A strains U1102 and GS were propagated in CBMCs, and the titers of the viruses were estimated by using HSB-2 cells. Cell-free HHV-6 was prepared as described previously (7). When HHV-6-infected CBMCs showed evidence of more than 80% infection in an immunofluorescence assay (IFA), the cells were lysed by freezing-thawing twice and spun at 1,500 × g for 10 min. The supernatant was used as cell-free virus (26). Partially purified virions were isolated as follows. HSB-2 cells were infected with HHV-6, and at 72 to 96 h postinfection, the cells were spun at 1,500 × g for 15 min at 4°C. The supernatant from the cells was concentrated by centrifugation at 20,000 rpm for 2 h at 4°C through a 20% sucrose cushion in an SW27 rotor (Beckman). Virions were collected from the bottom. Phosphonoformic acid (PFA), which inhibits viral DNA synthesis, was used to determine whether the gQ2 gene is an early or a late protein. For early protein, HSB-2 cells were infected with HHV-6A, cultured with medium supplemented with PFA (300 μg/ml), and harvested at 48 h postinfection.
Antibodies.
Hybridoma clones producing MAbs were established from the splenocytes of mice immunized with a purified recombinant protein as described previously (7). The MAbs, designated AgQ-182 and AgQ-178, were raised against recombinant protein AgQ2 (corresponding to the codons for amino acids 57 to 214 of gQ2) (Fig. 1A). Primers AgQF495bam (5′-ACCGGATCCGAAATTCTGTTTCAAAATGAAG-3′) and AgQHindR (5′-ACCAAGCTTTTAGTAATTTGTGTAATTTAATAAG-3′; underlining indicates restriction enzyme sites) were used to amplify inserts from HHV-6A cDNA (strain GS) for AgQ2. MAbs AgL-3 and AgL-4 were raised against recombinant protein AgL (corresponding to the codons for amino acids 39 to 250). Primers AgLbamF (5′-GGATCCGTAATAAACTGCACGAAATCC-3′) and ABgLecoRVR (5′-GATATCTTATGTGTTTCTAATCAGAAT-3′; underlining indicates restriction enzyme sites) were used to amplify inserts from HHV-6A DNA (strain U1102) for the gL gene.
FIG. 1.
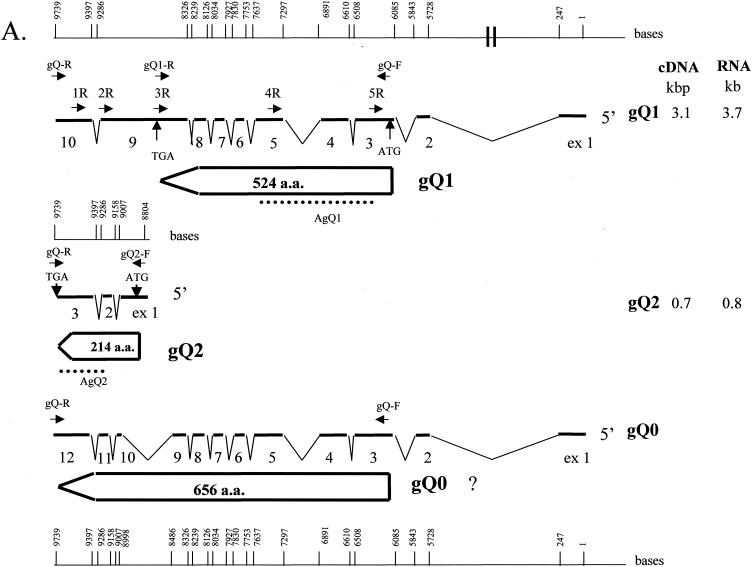
Organization of the transcription pattern of the HHV-6A U100 (gQ) gene region. (A) All exons and introns are drawn to scale. Numbering starts with the first nucleotide of the 5′ end of both transcripts gQ1 and gQ0. Numbers for splice sites apply to the last nucleotide of exon n and to the first nucleotide of exon n + 1. For special sequence motifs, the numbers apply to the first 5′ nucleotide. Thin lines represent introns; thick lines represent exons. The arrow-shaped boxes represent ORFs with ATG initiation codons and TGA termination codons. The dotted lines represent the positions of recombinant proteins (AgQ1 and AgQ2) used to produce MAbs. The single-stranded DNA probes (1R, 2R, 3R, and 4R) used for Northern blot analyses, the primers used for 5′ RACE (gQ-R, 1R, and 2R for the gQ2 transcript; gQR, 3R, and 5R for the gQ1 transcript), and the primers used for RT-PCR (gQ-F, gQ-R, gQ1-R, and gQ2-F) are indicated by small closed arrows. The sizes of the cDNA molecules and the corresponding mRNAs, identified by Northern blotting of transcripts gQ1 and gQ2, are noted on the right. The sequence of the gQ0 transcript of strain GS was from Pfeiffer et al. (31). a.a., amino acids. (B) Location of the gQ gene on the viral genome. The HHV-6 genome is shown with emphasis on the right terminal region. DRL and DRR, left and right direct repeats, respectively. (C) Amino acid (aa) sequences of the predicted products of the HHV-6A U100 genes, gQ1 and gQ2. The hydrophobic domain is underlined, and potential N-linked glycosylation sites are boxed. Numbers on the right denote amino acid residues.
The expression vectors were made by inserting the PCR products into the prokaryotic expression vector pQE30 (Qiagen) at the BamHI and HindIII restriction sites for AgQ2 and at the BamHI and SmaI restriction sites for AgL. AgQ2 and AgL had an N-terminal tag containing six histidine residues (MRGSHHHHHHGS). The recombinant proteins were expressed in Escherichia coli and purified under denaturing conditions as specified by Qiagen.
MAbs for HHV-6A gQ1—AgQ-119 (24), for early nuclear antigen of HHV-6, and OHV-2 (25), for HHV-6A glycoprotein B (gB)—87-Y-13 (39), and a mouse antiserum specific for HHV-6A gH (24) were generated in our laboratory as described previously. A MAb for HHV-6A gH—1D3—was generously provided by G. Campadelli-Fiume, University of Bologna, Bologna, Italy. Rabbit anticalreticulin antibody and Helix pomatia lectin (HPL)-fluorescein isothiocyanate (FITC) conjugates were purchased from Sigma. A Fluorotag FITC conjugation kit (Sigma) was used for conjugation of FITC to MAb AgQ-119.
Isolation of RNA and Northern blot hybridization.
HSB-2 cells were infected with GS or were mock infected for 72 h; poly(A)+ RNA was extracted by using an mRNA isolation kit (Takara) according to the manufacturer's protocol. For Northern blot hybridization, 5 μg of poly(A)+ RNA was subjected to electrophoresis on 1% agarose-formaldehyde gels, blotted onto Hybond-N+ nylon membranes (Amersham Biosciences), and hybridized to oligonucleotide probes as described elsewhere. The oligonucleotide probes used for hybridization were 5′ labeled by using a Mega label kit (Takara). The sequences of the oligonucleotides used for radiolabeling were as follows: 1R, CGGAATAAATCCGATATAAACGTTTCCATCTG; 2R, TCGACAAAACTTCTGGCCTTCATTTTGAAACAG; 3R, AGTGTACAAATGTATATGTAAACACAACAGG; 4R, TGTGAAGATGTTGTTAACCATCAATAATCCCGC; and 5R, CGTCGCCATCGAAAACGCGCACAAAAATATCAG.
RT-PCR.
Reverse transcription (RT)-PCR was carried out with poly(A)+ RNA extracted from HHV-6A (strain U1102 or GS)-infected HSB-2 cells and Superscript II reverse transcriptase (Invitrogen) essentially as indicated in the manufacturer's instructions. The primers used for RT-PCR were as follows: gQ-F, 5′-GAGACGAAGAATGGCAACCGCAAGG-3′; gQ-R, 5′-TCAGTAATTTGTGTAATTTAATAAG-3′; gQ1-R, TCAGGTCCTCACGACAGTAGGTT; and gQ2-F, TGCTAGATAATTATGCATTTC.
5′ RACE.
Rapid amplification of cDNA 5′ ends (5′ RACE) was performed by using a 5′ RACE system kit (Invitrogen) according to the manufacturer's protocol. Primers gQ-R, 1R, and 2R were used for gQ2 transcripts, and primers gQ-R, 3R, and 5R were used for gQ1 transcripts.
Preparation of pulse-chase and metabolically labeled proteins and immunoprecipitation experiments.
HSB-2 cells were infected with HHV-6A strain GS at a multiplicity of infection (MOI) of 0.1 or were mock infected. At 72 h postinfection, for pulse-chase labeling, the cells were incubated in methionine-free RPMI 1640 for 1 h and then pulse-labeled with Redivue Pro-mix L-35S in vitro cell labeling mix (400 μCi/ml; Amersham Pharmacia Biotech) for 30 min. The pulse-labeled cells were chased for 1, 2, and 4 h. At 72 h postinfection, for metabolic labeling, the cells were incubated in methionine-free RPMI 1640 for 1 h and then radiolabeled with Redivue Pro-mix L-35S in vitro cell labeling mix (20 μCi/ml) for 16 h. The cells were recovered and lysed in radioimmunoprecipitation (RIPA) buffer (0.01 M Tris-HCl [pH 7.4], 0.15 M NaCl, 1% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) for 30 min on ice. After centrifugation at 200,000 × g for 1 h, the supernatants were incubated with MAb AgQ-119- or AgQ-182-protein G-Sepharose (Amersham Pharmacia Biotech) complex at 4°C for 4 h. Immunocomplexes were washed with RIPA buffer to remove unbound proteins. Precipitated proteins were solubilized with sample buffer (32 mM Tris-HCl [pH 6.8], 1.5% SDS, 5% glycerol, 2.5% 2-mercaptoethanol) and separated by SDS-polyacrylamide gel electrophoresis (PAGE). The gels were fixed, dried, and exposed to Kodak X-Omat film.
Glycosidase digestion.
For endoglycosidase digestion, endoglycosidase H (endo H) and peptide N-glycosidase (PNGase F) were purchased from New England Biolabs. Lysed cells were resuspended in digestion buffer and digested with endo H or PNGase F as specified by the manufacturer.
Immunoblotting.
HHV-6A strain GS-infected and mock-infected cells were lysed in RIPA buffer. The lysed proteins were resolved by SDS-PAGE and electrotransferred to polyvinylidene difluoride (PVDF) membranes for immunoblotting. After being blocked, the membranes were incubated for 1 h with blocking buffer (10 mM Tris-HCl [pH 7.2], 0.15 M NaCl, 5% skim milk, 0.75% Tween 20) containing MAbs or antisera. The reactive bands were visualized by using a horseradish peroxidase-linked secondary conjugate and enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech).
Immunohistochemical analysis.
The IFA was performed as described previously (7). Specific immunofluorescence was observed with a confocal laser scanning microscope (Nikon TE300 Bio-Rad Radiance 2100 and Carl Zeiss LSM 510).
RESULTS
Transcriptional analysis of the U100 (gQ) gene.
The gQ gene has been mapped to a region within the direct repeats and at the right end of the UL segment of the virus genome but not to the contiguous sequence connecting these regions (Fig. 1B) (31).
Pfeiffer et al. identified the transcript of the HHV-6A U100 gene (31). The transcript that they reported is shown as the gQ0 transcript in Fig. 1A. Based on their cDNA sequence, we designed primers gQ-F and gQ-R, which amplified the gQ0 ORF (Fig. 1A). Two cDNAs, 2.0- and 2.5-kb species, were amplified by RT-PCR with the primer pair from the poly(A)+ RNA of strain GS-infected HSB-2 cells, and the amplified 2.5-kb cDNA was much more abundant. Sequencing showed that the 2.0- and 2.5-kb cDNAs corresponded to the gQ0 and gQ1 transcripts shown in Fig. 1A, respectively. The difference in sequences between the gQ1 and the gQ0 transcripts was that an intron that lay between exons 9 and 10 in gQ0 was not present in gQ1. Thus, the stop codon (TGA) appeared in exon 9 of the gQ1 cDNA, and a new, shorter gQ1 ORF was predicted from the cDNA sequence of the gQ1 transcript.
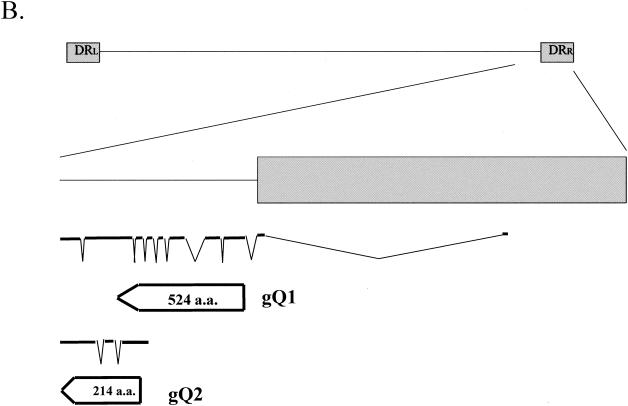
To further analyze gQ transcription, we performed Northern blot analysis to determine the patterns of HHV-6A gQ transcription. Antisense oligonucleotides from the gQ gene served as probes (Fig. 1A). Analysis of poly(A)+ RNA from infected cells probed with 1R and 2R, antisense oligonucleotides derived from exons 10 and 9 of the gQ1 transcript, respectively (exons 3 and 2 of the gQ2 transcript, respectively), revealed mRNAs that were approximately 5.6, 5.5, 3.7, 2.7, and 0.8 kb long; of these, the 3.7- and 0.8-kb mRNAs were most abundant (Fig. 2, panels 1R and 2R). In contrast, probes 3R and 4R, antisense oligonucleotides derived from exons 9 and 5 of the gQ1 transcript, respectively, detected mRNAs of approximately 5.6, 5.5, 3.7, 2.9, and 2.7 kb but not 0.8 kb (Fig. 2, panels 3R and 4R).
FIG. 2.
Transcription pattern of the U100 (gQ) region of HHV-6A strain GS with several probes. Five micrograms each of poly(A)+ RNA from mock-infected cells (Mock) and cells infected for 72 h with HHV-6A strain GS (GS) was subjected to Northern blot analysis. mRNA sizes are indicated on the right. The positions of the oligonucleotide probes used here are shown in Fig. 1A. β-Actin was used as an internal control for the detection of mRNA. 1R and 2R, antisense probes derived from the third and second exons of the gQ2 transcript, respectively, detected mRNAs of 5.6, 5.5, 3.7, 2.7, and 0.8 kb (panels 1R and 2R). In contrast, 3R and 4R, antisense probes derived from the ninth and fifth exons of the gQ1 transcript, respectively, detected mRNAs of 5.6, 5.5, 3.7, 2.9, and 2.7 kb but not the 0.8-kb mRNA (panels 3R and 4R).
To map the mRNAs more precisely, 5′ RACE was carried out to determine the 5′ end of each transcript by using the gQ-R primer in combination with 3R and 5R for the gQ1 transcript and in combination with 1R and 2R for the transcript corresponding to 0.8-kb mRNA. Probe 3R, used for the gQ1 transcript, was derived from the intron between exons 9 and 10 of the gQ0 transcript; therefore, it did not detect the gQ0 transcript. The 5′ RACE analyses showed that two species of major transcripts, gQ1 and gQ2, which represented different ORFs besides that of the gQ0 transcript, were expressed in the gQ gene region (Fig. 1A) and that the 5′ end of the gQ1 transcript was the same as that of the gQ0 transcript reported previously (31). Interestingly, the 5′ end of the gQ2 transcript was located in the gQ gene region, and a typical TATA box-like sequence, TATATTT, was found 33 bp upstream of the cap site. Exons 2 and 3 of the gQ2 transcript corresponded to exons 11 and 12 of the gQ0 transcript, respectively, and the gQ2 ORF was read in the same frame as the gQ0 ORF.
From the Northern blotting, RT-PCR, and 5′ RACE data, two additional species of major transcripts (transcripts gQ1 and gQ2), which encoded proteins besides the one reported previously (31), were identified. The gQ1 transcript may correspond to the 3.7-kb mRNA and the gQ2 transcript may correspond to the 0.8-kb mRNA detected by Northern blotting. The gQ0 transcript reported previously may correspond to the 3.7-kb mRNA because 3.7-kb mRNAs were detected as broad bands by Northern blotting. The gQ1 transcript encoded a protein of 524 amino acids, with a predicted molecular weight of 59,000; the gQ2 transcript encoded a protein of 214 amino acids, with a predicted molecular weight of 27,000; and the gQ0 transcript encoded a protein of 656 amino acids, with a predicted molecular weight of 75,000. The three proteins had a hydrophobic domain at the N terminus that could function as a signal peptide, but there was no hydrophobic domain at the C terminus of sufficient length to function as a membrane anchor (gQ1 and gQ2 in Fig. 1C).
Characterization of U100 (gQ) gene products in HHV-6A (strain GS)-infected cells and purified virions.
Two products of the gQ1 gene, the 80-kDa (gQ-80K) and 74-kDa (gQ-74K) proteins, were recognized by MAb AgQ-119 (24), which was raised against amino acids 3 to 422 of the gQ1 gene product, which was derived from the gQ1 transcript. To further analyze the gQ1 and gQ2 gene products, we raised MAbs AgQ-182 and AgQ-178 against amino acids 57 to 214 of the gQ2 gene product, which was derived from the gQ2 transcript. MAbs AgQ-182 and AgQ-178 specifically recognized the purified recombinant proteins to which they were raised (see Materials and Methods for details) and did not cross-react with purified recombinant gH, gL, or gQ1 (data not shown).
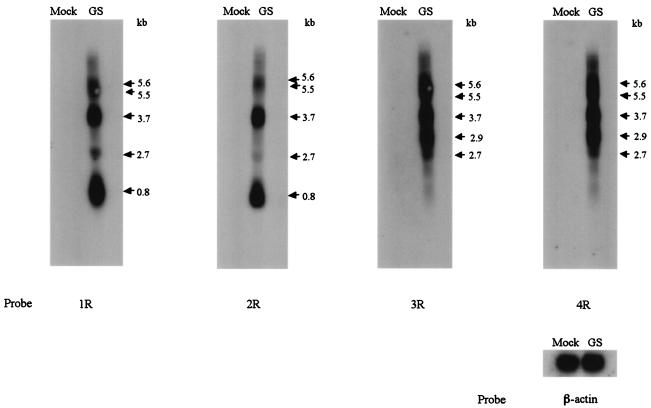
To test the specificity of these antibodies against gene products made in cells, we made use of the fact that our two primer pair sets (gQ-F and gQ1-R for gQ1 and gQ2-F and gQ-R for gQ2) amplified two different gQ ORFs. These two ORFs, gQ1 and gQ2, were inserted into the pCAGGS eukaryotic expression vector (28), and 293T cells were transfected with the resulting vectors, pCAGGS-gQ1 and pCAGGS-gQ2, respectively. The cells were harvested 48 h later and lysed with RIPA buffer, and the lysates were subjected to immunoblotting with anti-gQ1 and anti-gQ2 MAbs. The pCAGGS-gQ1 product was recognized as 73- to 74-kDa bands by MAb AgQ-119 (anti-gQ1) (Fig. 3A, panel a), and the pCAGGS-gQ2 product was recognized as 30- to 32-kDa (32-kDa) bands with faint 25- to 27-kDa (26-kDa) bands by MAb AgQ-182 (anti-gQ2) (Fig. 3A, panel b). Both MAbs for gQ1 and gQ2 recognized the product of the gQ0 ORF(data not shown).
FIG. 3.
Immunoblotting of recombinant gQ1- or gQ2-transfected 293T cells. 293T cells were transfected with plasmid pCAGGS-gQ1 (amplified by primers gQ-F and gQ1-R in gQ1 of Fig. 1A), plasmid pCAGGS-gQ2 (amplified by primers gQ2-F and gQ-R in gQ2 of Fig. 1A), or plasmid pCAGGS as a negative control. (A) The cells were lysed with RIPA buffer at 48 h posttransfection, resolved by SDS-PAGE with 10 or 15% polyacrylamide under reducing conditions, and electrotransferred to PVDF membranes. The blots were reacted with MAb AgQ-119 (for gQ1) (a) or MAb AgQ-182 (for gQ2) (b). (B) Lysates were digested with endo H (H) or PNGase F (F), resolved by SDS-PAGE with 15 or 7.5% polyacrylamide under reducing conditions, and electrotransferred to PVDF membranes. The blots were reacted with the MAb for gQ1 (a) or the MAb for gQ2 (b). The numbers beside the panels show molecular masses. WB, Western blotting.
Previously, Mori et al. reported that the gQ gene products were glycosylated in infected cells (24). The predicted gQ1 and gQ2 gene products have some potential N-linked glycosylation sites, as shown in Fig. 1B. Therefore, to examine whether the products of the gQ gene were glycosylated in our recombinant expression system, we treated cell lysates with endo H, which removes immature, high-mannose N-linked oligosaccharides but not mature, complex oligosaccharides, and with PNGase F, which removes both high-mannose and complex oligosaccharides. As shown in Fig. 3B, the 74-kDa bands of gQ1 shifted in electrophoretic mobility to approximately 59 kDa after either endo H or PNGase F treatment (Fig. 3B, panel a). However, for gQ2, the 32- and 26-kDa bands shifted to approximately 24- and 21- to 23-kDa bands after endo H treatment and shifted to approximately 21- to 23-kDa bands after PNGase F treatment (Fig. 3B, panel b). Thus, the forms of gQ1 observed in transfected cells were endo H sensitive, and some forms of gQ2 were endo H resistant, indicating that the gQ1 and gQ2 products are glycosylated in transfected cells when expressed alone by a recombinant expression vector.
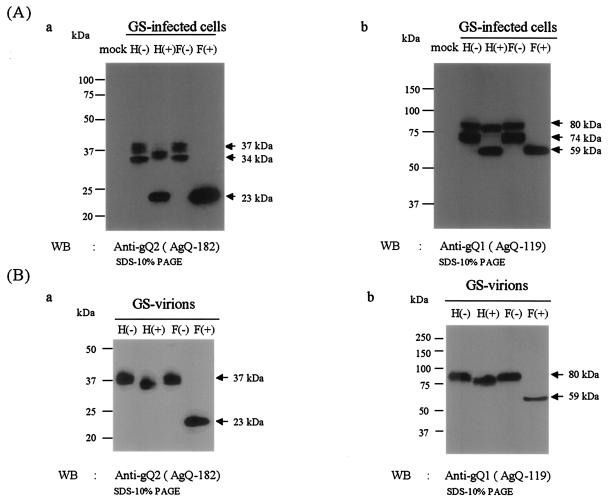
To characterize the products of the gQ gene derived from the gQ2 transcript in HHV-6A-infected cells, strain GS-infected cells were harvested 4 days postinfection and lysed. Lysates from HSB-2 cells infected with GS or mock infected were immunoblotted with anti-gQ2 MAb AgQ-182 or AgQ-178. Both clones specifically reacted with broadly migrating protein species: staining was seen at between 32 and 34 kDa and broad staining was seen at between 35 and 37 kDa in GS-infected cell lysates (Fig. 4A, anti-gQ2).
FIG. 4.
Immunoblotting of HHV-6A strain GS-infected HSB-2 cells and purified virions. Lysates were digested with endo H (H) or PNGase F (F), resolved by SDS-PAGE with 10% polyacrylamide under reducing conditions, and electrotransferred to PVDF membranes. The blots were reacted with MAb AgQ-182 (for gQ2) or MAb AgQ-119 (for gQ1). The numbers beside the panels show molecular masses. (A) HSB-2 cells were infected with strain GS at an MOI of 1.0 or mock infected. The cells were lysed with RIPA buffer at 96 h postinfection. (B) Purified GS virions were lysed with RIPA buffer and digested with endo H (H) or PNGase F (F). WB, Western blotting.
To examine whether the products of the gQ2 transcript were glycosylated in strain GS-infected cells, lysates of GS-infected cells were digested with endo H and PNGase F. The digested proteins were analyzed by SDS-PAGE under reducing conditions. As shown in Fig. 4A, the 35- to 37-kDa proteins shifted in electrophoretic mobility to approximately 34 to 35 kDa after endo H digestion and to 21 to 23 kDa after PNGase F digestion, while the 32- to 34-kDa proteins shifted to 21 to 23 kDa after either endo H or PNGase F treatment. The results indicated that the 35- to 37-kDa protein (gQ-37K) contained complex N-linked oligosaccharides and that the 32- to 34-kDa protein (gQ-34K) contained immature high-mannose N-linked oligosaccharides. Lysates of GS-infected cells were also immunoblotted with anti-gQ1 MAb AgQ-119, and the endo H-resistant 80K and endo H-sensitive 74K proteins were detected in the membrane as previously reported (24) (Fig. 4A, anti-gQ1). Accordingly, we hypothesized that gQ-37K as well as gQ-80K is packaged into HHV-6 particles.
To compare the steady-state pool of gQ present in infected cells to the final form found in virions, partially purified virions were lysed and immunoblotted with anti-gQ2 MAb AgQ-182. As expected, the gQ found in virions was largely endo H resistant, with a moderate shift in electrophoretic mobility (Fig. 4B, anti-gQ2). Our results showed that whereas gQ-37K was abundant in virions, as was gQ-80K, gQ-34K was not incorporated into virions (Fig. 4B).
These results showed that glycoproteins gQ-80K and gQ-74K were recognized by MAbs against gQ1, that glycoproteins gQ-37K and gQ-34K were recognized by MAbs against gQ2, and that these proteins were detected in HHV-6A-infected cells and in virions. Therefore, we designated glycoproteins gQ-80K and gQ-74K as gQ1 and glycoproteins gQ-37K and gQ-34K as gQ2. The proteins that corresponded to products of the gQ0 ORF shown in Fig. 1A were not detected in HHV-6A-infected cells and virions.
Expression of HHV-6A gQ1 and gQ2 in HSB-2 cells infected with strain GS.
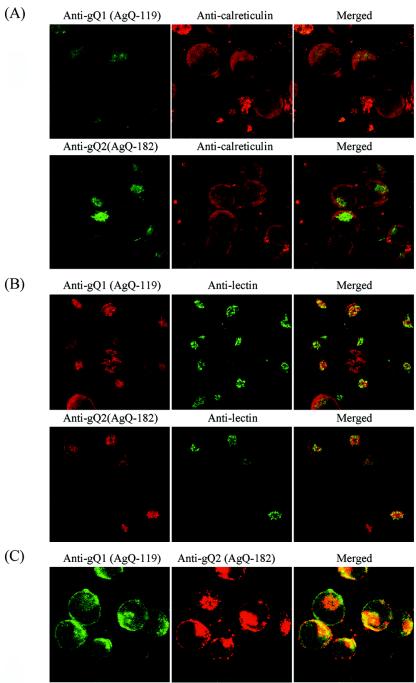
To examine the cellular localization of several gQ glycoproteins, an IFA was performed with GS-infected HSB-2 cells at 72 h postinfection (Fig. 5). Although there was a significant overlap of signals when the infected cells were doubly labeled with anti-gQ1 MAb AgQ-119 and an antibody for calreticulin, an endoplasmic reticulum (ER) marker, little overlap was observed when anti-gQ2 MAb AgQ-182 was used in conjunction with the anticalreticulin antibody (Fig. 5A). These results indicate that the gQ1 glycoproteins were localized in the ER but that the gQ2 glycoproteins were localized largely in the post-ER compartment. We performed additional colocalization studies with HPL, which binds terminal unsubstituted GalNAc and recognizes intermediate forms of glycoconjugates after the O-linked addition of the sugar in cis-Golgi cisternae (41). HPL labeling showed colocalization of the gQ2 and gQ1 glycoproteins in the Golgi apparatus (Fig. 5B). To further confirm that gQ1 and gQ2 colocalized in the same areas, GS-infected HSB-2 cells were labeled with anti-gQ1 MAb AgQ-119-FITC conjugates and anti-gQ2 MAb AgQ-182 at same time. As shown in Fig. 5C, gQ1 and gQ2 colocalized in Golgi apparatus-derived areas. No specific labeling was found for mock-infected cells (data not shown).
FIG. 5.
Confocal microscope analyses of strain GS-infected HSB-2 cells. Strain GS-infected HSB-2 cells were harvested 3 days postinfection and then fixed and stained for IFA. (A) MAbs AgQ-119 (for gQ1) and AgQ-182 (for gQ2) were detected with FITC-goat anti-mouse immunoglobulin G (IgG), and the anticalreticulin antibody was detected with rhodamine isothiocyanate (RITC)-swine anti-rabbit IgG. (B) MAbs AgQ-119 (for gQ1) and AgQ-182 (for gQ2) were detected with RITC-rabbit anti-mouse IgG, and lectin was detected with HPL-FITC conjugates. (C) gQ1 was detected with AgQ-119-FITC conjugates, and AgQ-182 (for gQ2) was detected with RITC-rabbit anti-mouse IgG. Specific immunofluorescence was observed with a confocal laser scanning microscope (Nikon TE300 Bio-Rad Radiance 2100 for panels A and B and Carl Zeiss LSM 510 for panel C).
These observations provide evidence for the accumulation of gQ2 in a Golgi apparatus-derived compartment of HHV-6-infected cells and the accumulation of gQ1 in both the ER and the Golgi apparatus.
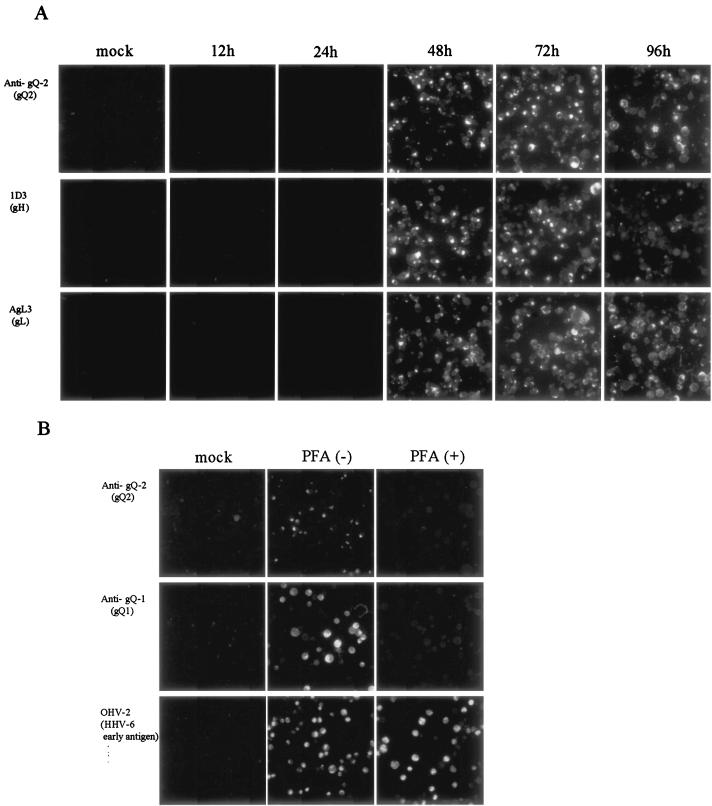
As shown in Fig. 6, the expression of gQ2 was first detected at 48 h postinfection and was strong at 72 h postinfection. The patterns of expression of gH and gL were similar, indicating that the gQ2 glycoproteins behave as late proteins. To confirm that the gQ2 glycoproteins behave as late proteins, the expression of gQ2 in strain GS-infected HSB-2 cells treated with PFA was examined. GS-infected HSB-2 cells were maintained for 48 h in culture medium either with or without PFA. Neither the gQ2 proteins nor the gQ1 proteins were detected in cells treated with PFA, although the early proteins recognized by MAb OHV-2 were detected in cells treated with PFA, indicating that the gQ2 and gQ1 proteins belong to the late class of HHV-6 proteins.
FIG. 6.
IFA of HHV-6A-infected HSB-2 cells at various times postinfection. (A) HSB-2 cells were infected with strain GS at an MOI of 0.1 or mock infected. The cells were harvested at 12, 24, 48, 72, or 96 h postinfection and stained with MAb AgQ-182 (for gQ2), 1D3 (for gH), or AgL3 (for gL). (B) HSB-2 cells were infected with strain GS at an MOI of 0.1 in the presence (+) or absence (−) of PFA. The cells were harvested at 48 h postinfection and stained with MAb AgQ-182 (for gQ2), AgQ-119 (for gQ1), or OHV-2 (for early antigen of HHV-6).
Immunoprecipitation of strain GS-infected cells.
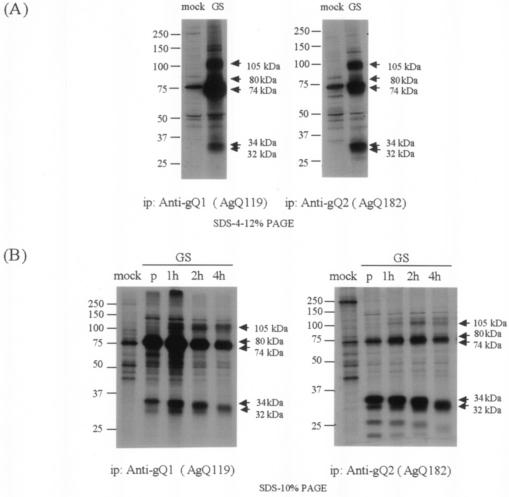
Here we found that two different kinds of glycoproteins, gQ1 and gQ2, were expressed from the gQ region. To investigate the processing of gQ1 and gQ2, lysates from metabolically [35S]methionine-labeled GS-infected cells were immunoprecipitated with MAb AgQ-119 (anti-gQ1) or AgQ-182 (anti-gQ2) (Fig. 7A). As reported previously, proteins of about 105 kDa (corresponding to gH) and 80 and 74 kDa (corresponding to gQ1) were precipitated from the lysates when an anti-gQ1 MAb was used (24). In addition, the 32- to 34-kDa proteins were coimmunoprecipitated (Fig. 7A, anti-gQ1). The major proteins precipitated by the anti-gQ2 MAb were about 32 to 34 kDa; bands of 105, 74, and 80 kDa (faint) were also seen when the anti-gQ2 MAb was used (Fig. 7A, anti-gQ2). These results indicate that the anti-gQ2 MAb may coimmunoprecipitate gH and the 74-kDa and faint 80-kDa glycoproteins that are recognized by the anti-gQ1 MAb and that gQ2 may associate with gQ1 and gH.
FIG. 7.
Immunoprecipitation of [35S]methionine-labeled strain GS-infected cells. (A) Mock- and HHV-6A strain GS-infected HSB-2 cells were metabolically labeled with [35S]methionine for 16 h, lysed, and immunoprecipitated (ip) with MAb AgQ-119 (for gQ1) or AgQ-182 (for gQ2). The immunoprecipitates were resolved by SDS-PAGE (4 to 12% Tris-glycine gel; Invitrogen). (B) Mock- and HHV-6A strain GS-infected HSB-2 cells were pulse-labeled with [35S]methionine for 30 min and chased for 1, 2, or 4 h. The lysates were immunoprecipitated with AgQ-119 (gQ1) or AgQ-182 (gQ2), and the immunoprecipitates were resolved by SDS-10% PAGE. Lanes: p, 30-min pulse; 1h, 1-h chase; 2h, 2-h chase; 4h, 4-h chase.
To investigate the processing of these glycoproteins, pulse-chase labeling experiments were carried out under reducing conditions with anti-gQ1 and anti-gQ2 MAbs. As shown in Fig. 7B, both gQ1-74K and gQ2-34K bands were labeled by the end of a 30-min pulse by both MAbs (Fig. 7B). The faint 105K band, which corresponded to gH, and gQ1-80K were detectable after a 1-h chase, and the amounts of these glycoproteins increased after a 4-h chase. The pulse-chase and metabolic labeling experiments indicated that gQ1-74K associates with gQ2-34K within 30 min after their synthesis.
Detection of gH-gL-gQ1-gQ2 complexes in strain GS-infected cells.
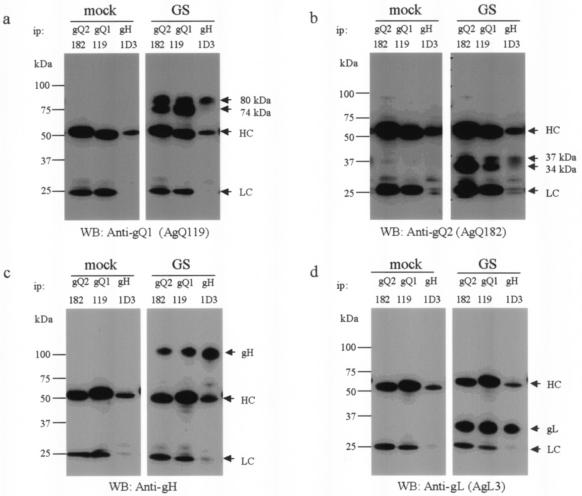
Mori et al. reported that gQ-80K, which can be digested with PNGase F but not endo H, is the third component of the gH-gL glycoprotein complex on the viral envelope (24). Here we found that the gQ1 glycoproteins were associated with the gQ2 glycoproteins. Next, to confirm this finding and to investigate whether the gQ2 glycoproteins associate with the gH-gL-gQ1-80K complex, lysates from GS-infected cells were used for immunoprecipitation with MAb for gQ2 or gQ1 or anti-gH MAb 1D3, followed by immunoblotting with the anti-gQ1, anti-gQ2, or anti-gL MAb or the anti-gH Ab (Fig. 8). As expected, the anti-gQ2 MAb immunoprecipitated gQ1-74K, gQ1-80K, gH, and gL (Fig. 8a, c, and d, ip: gQ2). Interestingly, only endo H-resistant gQ2-37K and not gQ2-34K was precipitated by the anti-gH MAb (Fig. 8b, ip: gH), although endo H-resistant gQ2-37K was not abundant, whereas the anti-gQ1 MAb coprecipitated both the gQ2-34K and the gQ2-37K glycoproteins (Fig. 8b, ip: gQ1). The anti-gH MAb precipitated only gQ1-80K and not gQ1-74K, as described previously (Fig. 8a, ip: gH) (24).
FIG. 8.
Detection of gH-gL-gQ1-gQ2 complexes in strain GS-infected HSB-2 cells. Mock- or strain GS-infected HSB-2 cell lysates were immunoprecipitated (ip) with anti-gQ1 MAb AgQ-119, anti-gQ2 MAb AgQ-182, or anti-gH MAb 1D3 and subjected to SDS-PAGE (4 to 12% Tris-glycine gel; Invitrogen) under reducing conditions. The gels were electrotransferred to PVDF membranes and probed with MAb for gQ1 (a), MAb for gQ2 (b), anti-gH monospecific serum (c), or MAb for gL (d). HC, immunoglobulin heavy chain of the immunoprecipitating antibody; LC, immunoglobulin light chain of the immunoprecipitating antibody. WB, Western blotting.
The pulse-chase analysis and these analyses suggested a stepwise association in the formation of the gH-gL-gQ1-gQ2 complex. First, gQ1-74K and gQ2-34K may form a complex, which is followed by their interaction with gL, gH, or gH-gL to form the gH-gL-gQ1-gQ2 complex. Subsequently, the mature form of the gH-gL-gQ1-gQ2 complex is assembled. Sensitivity to endo H is indicative of ER or cis-Golgi localization, and endo H resistance is evidence of glycoproteins whose N-linked glycans are processed to a complex form in the Golgi complex. Therefore, the rapid initial association of gQ1-74K and gQ2-34K occurs in the ER, and the mature tetrameric complex, gH-gL-gQ1-80K-gQ2-37K, is probably formed in the post-ER compartment.
Detection of the gH-gL-gQ1-80K-gQ2-37K complex on the viral envelope.
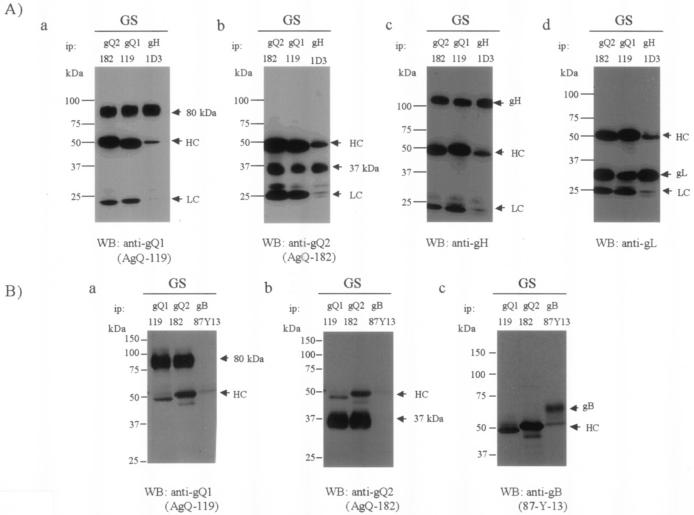
Because the gQ2-37K glycoprotein was detected in mature virions, we examined whether the gH-gL- gQ1-80K-gQ2-37K complex was incorporated into viral particles. Lysates containing purified GS virions were immunoprecipitated with anti-gQ1, anti-gQ2, and anti-gH Abs and immunoblotted with these antibodies. gQ1-80K, gH, and gL were coimmunoprecipitated by the anti-gQ2 MAb (Fig. 9A, panels a, c, and d, ip: gQ2), and gQ2-37K was immunoprecipitated by the anti-gH and anti-gQ1 MAbs (Fig. 9A, panel b). A MAb against HHV-6 gB was used as a negative control to demonstrate the specificity of the associations in the tetrameric complex. Lysates containing purified GS virions were immunoprecipitated with anti-gQ1, anti-gQ2, and anti-gB MAbs and immunoblotted with these antibodies. gQ1-80K was coimmunoprecipitated by the anti-gQ2 MAb (Fig. 9B, panel a, ip: gQ2) but not by the anti-gB MAb (Fig. 9B, panel a, ip: gB), and gQ2-37K was immunoprecipitated by the anti-gQ1 MAb (Fig. 9B, panel b, ip: gQ1) but not by the anti-gB MAb (Fig. 9B, panel b, ip: gB). gB was not coimmunoprecipitated by either the anti-gQ1 MAb or the anti-gQ2 MAb (Fig. 9B, panel c, ip: gQ1 or gQ2). These results support the idea that gQ2-37K is packaged into viral particles as part of the gH-gL-gQ1-80K- gQ2-37K complex. However, this novel virion-associated complex was not abundant in infected cells, indicating that it may arise late in the secretory pathway.
FIG. 9.
Detection of the gH-gL-gQ1-80K-gQ2-37K complex in purified virions. (A) Purified GS virion lysates were immunoprecipitated (ip) with MAb AgQ-119 (gQ1), AgQ-182 (gQ2), or 1D3 (gH) and subjected to SDS-PAGE (4 to 12% Tris-glycine gel; Invitrogen) under reducing conditions. The gels were electrotransferred to PVDF membranes and probed with MAb for gQ1 (a), MAb for gQ2 (b), anti-gH monospecific serum (c), or MAb for gL (d). (B) Purified GS virion lysates were immunoprecipitated (ip) with MAb AgQ-119 (gQ1), AgQ-182 (gQ2), or 87-Y-13 (gB) and subjected to SDS-10% PAGE under reducing conditions. The gels were electrotransferred to PVDF membranes and probed with MAb for gQ1 (a), MAb for gQ2 (b), or MAb for gB (c). HC, immunoglobulin heavy chain of the immunoprecipitating antibody; LC, immunoglobulin light chain of the immunoprecipitating antibody. WB, Western blotting.
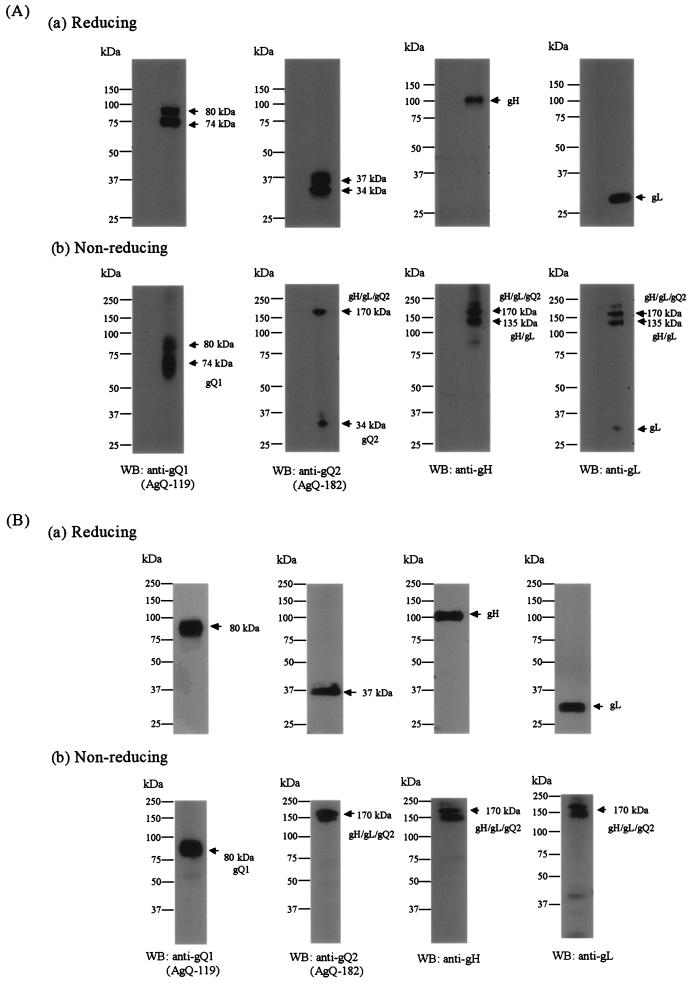
To examine whether the complex was dependent on disulfide bond formation, strain GS-infected cell lysates were immunoblotted with anti-gQ1, anti-gQ2, anti-gH, and anti-gL Abs under reducing conditions (Fig. 10A, panel a) and nonreducing conditions (Fig. 10A, panel b). Under nonreducing conditions, the Abs for gQ2, gH, and gL detected similar high-molecular-mass proteins, of approximately 170 kDa (Fig. 10A, panel b, WB: anti-gQ2, anti-gH, and anti-gL). In addition, gQ2-34K and the 32-kDa form of gL were also detected in the membrane independent of any other molecules, indicating that HHV-6A gH, gL, and gQ2 interact to form a disulfide bond-dependent complex of about 170 kDa, and some gQ2-34K and gL proteins might exist independent of the complex. Surprisingly, the anti-gQ1 MAb did not detect similar bands, even under nonreducing conditions (Fig. 10A, panel b, WB: anti-gQ1); these results indicate that, in contrast to earlier results, gQ1 might have a non-disulfide-bond-dependent association with the gQ2 complex. Moreover, Abs for gH and gL but not for gQ2 detected similar high-molecular-mass proteins, of approximately 135 kDa (Fig. 10A, panel b, WB: anti-gH and anti-gL), indicating that the 135-kDa proteins might be part of an HHV-6A gH-gL heterodimer complex that is held together by disulfide bonds.
FIG. 10.
Detection of gH-gL-gQ1-gQ2 complexes in strain GS-infected cells and purified GS virions by immunoblotting under nonreducing conditions. Strain GS-infected HSB-2 cells (A) and purified GS virions (B) were solubilized and subjected to SDS-10% PAGE under reducing (a) or nonreducing (b) conditions, followed by immunoblotting. The membranes were probed with AgQ-119 (gQ1), AgQ-182 (gQ2), anti-gH monospecific serum, or AgL3 (gL). WB, Western blotting.
To further examine whether the complex was dependent on disulfide bond formation, lysates of purified GS virions were immunoblotted with Abs for gQ1, gQ2, gH, and gL under reducing conditions (Fig. 10B, panel a) and nonreducing conditions (Fig. 10B, panel b). Under nonreducing conditions, MAbs for gQ2, gH, and gL detected similar high-molecular-mass proteins, of approximately 160 to 190 kDa; these results indicate that HHV-6A gH, gL, and gQ2 interact to form a disulfide bond-dependent complex (Fig. 10B, panel b, WB: anti-gQ2, anti-gH, and anti-gL), like that seen in HHV-6-infected cells (Fig. 10A, panel b). As shown in Fig. 10A, the MAb for gQ1 did not label bands similar to those labeled by the other Abs under nonreducing conditions (Fig. 10B, panel b, WB: anti-gQ1).
DISCUSSION
A previous study demonstrated that the gQ1 glycoprotein, one of the U100 gene products, is the third component of the gH-gL complex. Here we found that a 37-kDa gQ glycoprotein, another novel U100 gene product, also associates with gH, gL, and gQ-80K to form a heterotetrameric complex on the viral envelope. Interestingly, two kinds of gQ glycoproteins, gQ-80K and gQ-37K, were encoded by two distinct transcripts that were different from one reported previously (31). Furthermore, the results of Northern blotting and 5′ RACE indicated that the 5′ ends of these transcripts were in different positions, indicating that these two transcripts could be regulated by distinct promoters, even though both transcripts are encoded by the gQ region.
As shown in Fig. 1A and 2, the gQ1 transcript may correspond to the 3.7-kb mRNA and gQ2 may correspond to the 0.8-kb mRNA detected by Northern blotting. The size of the 3.7-kb mRNA was higher than that of the cDNA that we identified and expected. One possibility is that the 3′ untranslated region (UTR) of 3.1-kbp cDNA shown in Fig. 1A is longer than the cDNA expected. As shown in Fig. 2, the 2.9-kb mRNA was detected by Northern blotting with probes 3R and 4R but not probes 1R and 2R. The 3′ UTR of the 2.9-kb transcript may be shorter than the 3′ UTR of the 3.7-kb transcript, because the 2.9-kb transcript was not detected with either probe 1R or probe 2R; therefore, the products of the 2.9-kb transcript may be same as the products of the 3.7-kb transcript.
One large transcript includes the gQ1 ORF, whose products correspond to gQ-74K and gQ-80K, and the other, small transcript includes the gQ2 ORF, whose products are gQ-34K and gQ-37K. Based on these data, we have designated gQ-74K and gQ-80K as gQ1and gQ-34K and gQ-37K as gQ2. In this study, we used HHV-6A strains GS and U1102 to analyze the gQ1 and gQ2 genes. Both homologs are also encoded by HHV-6B, but it is unknown whether they are expressed in HHV-6B-infected cells.
In pulse-chase studies, the 34-kDa gQ2 bands migrated at about 32 kDa by 4 h of chase. The change in the molecular mass of the gQ2 glycoproteins from 34 to 32 kDa may suggest the addition of terminal sialic acid residues.
Anti-gQ1 and anti-gQ2 MAbs also immunoprecipitated 74- to 75-kDa bands from mock-infected cell lysates (Fig. 7), but similar bands detected in GS-infected cells were more abundant than those in mock-infected cells, indicating that the MAbs cross-reacted with proteins of about 75 kDa in the mock-infected cells. In pulse-labeling, the 74K-band of gQ1 that was precipitated by the gQ2 MAb was faint (Fig. 7B, anti-gQ2, GS-p) when immunoprecipitation of GS-infected cell lysates was performed with the anti-gQ2 MAb; this result indicated that the amount of gQ1 that interacted with gQ2 during pulse-labeling was small. However, the fact that gQ1 interacted with gQ2 during pulse-labeling was confirmed by the results of the immunoprecipitation with the anti-gQ1 MAb (Fig. 7B, anti-gQ1, GS-p).
The precursor form of gQ1-80K, gQ1-74K, which was detected in GS-infected cells, corresponds to the product of the gQ1 ORF, because when the gQ1 ORF was expressed in 293T cells, the molecular mass of its product was approximately 74 kDa (Fig. 3A, panel a). Furthermore, the products of gQ2 detected in GS-infected cells were indicated to be derived from the gQ2 ORF. When the gQ2 ORF was expressed alone in 293T cells, the molecular mass of its major product was approximately 32 kDa (Fig. 3A, panel b). gQ2-34K detected in GS-infected cells was not detected in the recombinant expression system. The molecular mass of the PNGase F-treated gQ2 product in GS-infected cells was 23 kDa, the same as the products detected in the gQ2 recombinant expression system when PNGase F was used for digestion (Fig. 3B, panel b); this result indicated that the molecular mass of unmodified gQ2 form is 23 kDa and that gQ2-34K detected in virus-infected cells is derived from the product of the gQ2 ORF. We confirmed that the sizes of PNGase F-treated gQ2 products detected in GS-infected cells and recombinant gQ2-transfected cells were the same by testing samples in the same gel. The proteins that correspond to the products from the gQ0 transcript shown in Fig. 1A were not detected in HHV-6-infected cells or in virions, indicating that the transcript may not be translated in HHV-6-infected cells or, even if it is expressed as proteins, the expression may occur at a very low level.
This study demonstrated that HHV-6 gQ1 and gQ2, the components of the tetrameric complex, fail to undergo the proper posttranslational processing seen in HHV-6 infected cells when expressed alone (Fig. 3A). These observations suggested a requirement for either a viral function or a virus-induced cellular function that facilitates biosynthesis.
It is known that herpesvirus gH homologs do not traffic correctly when expressed alone, but coexpression with gL allows the proper processing of gH (6, 32, 38). Similarly, HCMV gO does not traffic correctly when expressed alone (40). As described above, HHV-6A gQ1 and gQ2 also do not traffic correctly when expressed alone. Experiments are under way to analyze the posttranslational modifications of gQ1 and gQ2 after the in vitro constitution of the gH-gL-gQ1-gQ2 complex.
To date, several heterotrimeric viral glycoprotein complexes have been demonstrated, including the EBV gH-gL-gp42 complex (21, 43, 44), the HCMV gH-gL-gO complex (15, 16, 40), and the HHV-6 gH-gL-gQ complex. In the case of EBV, gp42 appears to be necessary for the infection of peripheral blood B lymphocytes but not cells of epithelial origin (21). Recently, Mori et al. showed that HHV-6 gH-gL-gQ-80K is a viral ligand of human CD46, which is the cellular receptor of HHV-6 (27). In this study, we found that HHV-6 forms a tetrameric glycoprotein complex on the viral envelope. Furthermore, we recently found that the tetrameric complex gH-gL-gQ1-gQ2 associates with human CD46 (unpublished data), indicating that the association of gQ2-37K with gH-gL-gQ1-80K may be important for the binding of CD46 by the complex and in the subsequent increase of entry in the virus entry process or in the virus infection cycle.
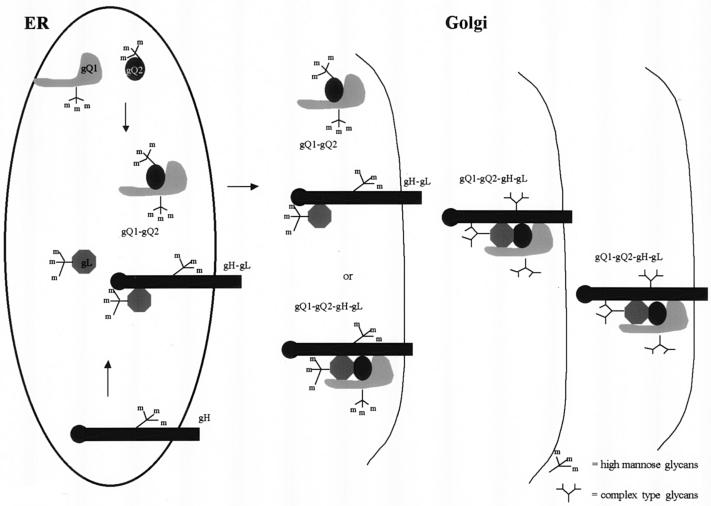
The results obtained here yielded a model for the biosynthesis of the gH-gL-gQ1-80K-gQ2-37K complex (Fig. 11). Shortly after or coincident with their translation in the ER, gQ1-74K and gQ2-34K associate to form a heterodimeric complex. Within 1 h after gQ1-gQ2 dimer formation, the gQ1-74K-gQ2-34K form associates with gL or gH-gL to form the gH-gL-gQ1-gQ2 complex. Given the presence of high-mannose N-linked oligosaccharides on the glycoproteins, we believe that the formation of the tetrameric complex precursor likely occurs in the post-ER compartment, because when lysates from GS-infected cells were precipitated with anti-gH, little gQ1-74K or gQ2-34K was found. By 4 h of chase, the mature gH-gL-gQ1-gQ2 complex had probably formed. The processing of the precursor to the mature form of the complex likely occurs in the Golgi compartment, since the mature gH-gL-gQ1-gQ2 complex contains mainly complex-type glycans, which are found on gQ1-80K and gQ2-37K. Once these post-ER processing steps have been completed, the mature gH-gL-gQ1-gQ2 complex is competent to traffic to compartments that will insert it into the HHV-6 envelope. Although this model provides a basic framework for understanding the intracellular processing of the gQ1-gQ2-based complex, more detailed analyses will be required to answer questions concerning the interactions of gH, gL, gQ1, and gQ2.
FIG. 11.
Model of the intracellular processing of the gH-gL-gQ1-gQ2 tetrameric complex in HHV-6-infected cells. First, gQ1-74K and gQ2-34K associate to form a heterodimeric complex in the ER. After gQ1-gQ2 dimer formation, gQ1-74K-gQ2-34K associates with gL or with gH-gL to form a gH-gL-gQ1-gQ2 complex in the post-ER compartment. Once these post-ER processing steps have been completed, the mature gH-gL-gQ1-gQ2 heterotetrameric complex is competent to traffic to compartments that will insert it into the HHV-6 envelope.
Acknowledgments
This study was supported in part by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science of Japan and a grant-in-aid for specially promoted research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We thank Kazuhiko Takeda (International Reagents Corporation, Kobe, Japan) for helpful discussions and Yun Bao Jiang (Osaka University) for technical assistance.
REFERENCES
- 1.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. A., D. X. Liu, and U. A. Gompels. 1996. Definition of a human herpesvirus-6 betaherpesvirus-specific domain in glycoprotein gH that governs interaction with glycoprotein gL: substitution of human cytomegalovirus glycoproteins permits group-specific complex formation. Virology 217:517-526. [DOI] [PubMed] [Google Scholar]
- 3.Aubin, J. T., H. Collandre, D. Candotti, D. Ingrand, C. Rouzioux, M. Burgard, S. Richard, J. M. Huraux, and H. Agut. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., S. Guerrini, X. Liu, and L. Foa-Tomasi. 1993. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J. Gen. Virol. 74:2257-2262. [DOI] [PubMed] [Google Scholar]
- 5.Chandran, B., S. Tirawatnapong, B. Pfeiffer, and D. V. Ablashi. 1992. Antigenic relationships among human herpesvirus-6 isolates. J. Med. Virol. 37:247-254. [DOI] [PubMed] [Google Scholar]
- 6.Cranage, M. P., G. L. Smith, S. E. Bell, H. Hart, C. Brown, A. T. Bankier, P. Tomlinson, B. G. Barrell, and T. C. Minson. 1988. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J. Virol. 62:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhepakson, P., Y. Mori, Y. B. Jiang, H. L. Huang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol. 83:847-854. [DOI] [PubMed] [Google Scholar]
- 8.Duus, K. M., and C. Grose. 1996. Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH. J. Virol. 70:8961-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duus, K. M., C. Hatfield, and C. Grose. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210:429-440. [DOI] [PubMed] [Google Scholar]
- 10.Foa-Tomasi, L., A. Boscaro, S. di Gaeta, and G. Campadelli-Fiume. 1991. Monoclonal antibodies to gp100 inhibit penetration of human herpesvirus 6 and polykaryocyte formation in susceptible cells. J. Virol. 65:4124-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forghani, B., L. Ni, and C. Grose. 1994. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 199:458-462. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gompels, U., and A. Minson. 1986. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology 153:230-247. [DOI] [PubMed] [Google Scholar]
- 14.Gretch, D. R., B. Kari, L. Rasmussen, R. C. Gehrz, and M. F. Stinski. 1988. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J. Virol. 62:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 19.Klupp, B. G., W. Fuchs, E. Weiland, and T. C. Mettenleiter. 1997. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J. Virol. 71:7687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, D. X., U. A. Gompels, L. Foa-Tomasi, and G. Campadelli-Fiume. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12-22. [DOI] [PubMed] [Google Scholar]
- 23.Montalvo, E. A., and C. Grose. 1986. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology 149:230-241. [DOI] [PubMed] [Google Scholar]
- 24.Mori, Y., P. Akkapaiboon, X. Yang, and K. Yamanishi. 2003. The human herpesvirus 6 U100 gene product is the third component of the gH-gL glycoprotein complex on the viral envelope. J. Virol. 77:2452-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori, Y., P. Dhepakson, T. Shimamoto, K. Ueda, Y. Gomi, H. Tani, Y. Matsuura, and K. Yamanishi. 2000. Expression of human herpesvirus 6B rep within infected cells and binding of its gene product to the TATA-binding protein in vitro and in vivo. J. Virol. 74:6096-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori, Y., T. Seya, H. L. Huang, P. Akkapaiboon, P. Dhepakson, and K. Yamanishi. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 76:6750-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori, Y., X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 29.Novotny, M. J., M. L. Parish, and P. G. Spear. 1996. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology 221:1-13. [DOI] [PubMed] [Google Scholar]
- 30.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer, B., B. Thomson, and B. Chandran. 1995. Identification and characterization of a cDNA derived from multiple splicing that encodes envelope glycoprotein gp105 of human herpesvirus 6. J. Virol. 69:3490-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen, L., M. Nelson, M. Neff, and T. C. Merigan, Jr. 1988. Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology 163:308-318. [DOI] [PubMed] [Google Scholar]
- 33.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 35.Santoro, F., H. L. Greenstone, A. Insinga, M. K. Liszewski, J. P. Atkinson, P. Lusso, and E. A. Berger. 2003. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 278:25964-25969. [DOI] [PubMed] [Google Scholar]
- 36.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, J. A., J. C. Chow, J. Baker, N. Avdalovic, S. Yuan, D. Au, M. S. Co, M. Vasquez, W. J. Britt, and K. L. Coelingh. 1993. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J. Virol. 67:489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 39.Takeda, K., T. Okuno, Y. Isegawa, and K. Yamanishi. 1996. Identification of a variant A-specific neutralizing epitope on glycoprotein B (gB) of human herpesvirus-6 (HHV-6). Virology 222:176-183. [DOI] [PubMed] [Google Scholar]
- 40.Theiler, R. N., and T. Compton. 2002. Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells. J. Virol. 76:2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torrisi, M. R., M. Gentile, G. Cardinali, M. Cirone, C. Zompetta, L. V. Lotti, L. Frati, and A. Faggioni. 1999. Intracellular transport and maturation pathway of human herpesvirus 6. Virology 257:460-471. [DOI] [PubMed] [Google Scholar]
- 42.van Drunen Littel-van den Hurk, S., S. Khattar, S. K. Tikoo, L. A. Babiuk, E. Baranowski, D. Plainchamp, and E. Thiry. 1996. Glycoprotein H (gII/gp108) and glycoprotein L form a functional complex which plays a role in penetration, but not in attachment, of bovine herpesvirus 1. J. Gen. Virol. 77:1515-1520. [DOI] [PubMed] [Google Scholar]
- 43.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt, L. S., N. Balachandran, and N. Frenkel. 1990. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J. Infect. Dis. 162:852-857. [DOI] [PubMed] [Google Scholar]
- 46.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1:1065-1067. [DOI] [PubMed] [Google Scholar]
- 47.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]