Summary
3-Aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine®) is a novel small molecule inhibitor of ribonucleotide reductase (RR) with clinical signs of activity in pancreatic cancer. Therefore, the Phase 2 Consortium (P2C) initiated a trial (two single stage studies with planned interim analysis) of 3-AP at 96 mg/m2 intravenously days 1–4 and 15–18 of a 28-day cycle in both chemotherapy-naive and gemcitabine-refractory (GR) patients with advanced pancreatic cancer. The primary endpoint was survival at six months (chemotherapy-naive) and four months (GR). Secondary endpoints were toxicity, response, overall survival, time to progression and mechanistic studies. Fifteen patients were enrolled including one chemotherapy-naïve and 14 GR. The chemotherapy-naïve patient progressed during cycle 1 with grade 3 and 4 toxicities. Of 14 GR patients, seven received two cycles, six received one cycle and one received eight cycles. Progression precluded further treatment in 11 GR patients. Additionally, one died of an ileus in cycle 1 considered related to treatment and two stopped treatment due to toxicity. Five GR patients had grade 4 toxicities possibly related to 3-AP and six GR patients had grade 3 fatigue. Toxicities and lack of meaningful clinical benefit prompted early study closure. Four-month survival in GR patients was 21% (95% CI: 8–58%). Correlative studies confirmed that 3-AP increased the percentage of S-phase buccal mucosal cells, the presence of multidrug resistance gene polymorphisms appeared to predict leukopenia, and baseline pancreatic tumor RR M2 expression was low relative to other tumors treated with 3-AP. In conclusion, this regimen appears inactive against predominantly GR pancreatic cancer. RR M2 protein may not have a critical role in the malignant potential of pancreatic cancer.
Keywords: Pancreatic cancer, Triapine, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, 3-AP, Ribonucleotide reductase
Introduction
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States [1] and has the lowest 5-year survival rate at 5% of any malignancy [1]. Gemcitabine, a nucleoside analog that inhibits DNA synthesis by blocking DNA polymerase and the M1 binding site of ribonucleotide reductase (RR), is standard of care for advanced pancreatic cancer [2]. Phase III trials of 5-FU, CPT-11, cisplatin, or oxaliplatin with gemcitabine did not improve overall survival (OS) compared to single-agent gemcitabine [3–7]. Although OS improved when combining gemcitabine with erlotinib (6.24 vs. 5.91 months) [8], this gain was minimal and underscores the need for novel treatments for pancreatic cancer.
3-Aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine®, Vion Pharmaceuticals, Inc., New Haven, CT) is a novel small molecule inhibitor of the M2 metal binding site of RR [9, 10]. RR contains a tyrosine free radical required for enzymatic reduction of ribonucleotides and 3-AP inactivates RR by neutralizing this free radical. Phase I trials with single-agent 3-AP showed solid tumor activity, including one of two patients with pancreatic cancer who experienced stable disease of three months duration and a 33% decrease in CA 19-9 [11–13]. Pharmacokinetics and tolerability data determined that a feasible regimen for phase II testing was 96 mg/m2/day as a 2-hour infusion for four days every two weeks. Toxicities seen in these trials included anemia, thrombocytopenia, leukopenia, hyperbilirubinemia, nausea, vomiting, asthenia, hypertension, dyspnea, elevated creatinine and low serum bicarbonate.
The multi-drug resistant (MDR) 1 gene product P-gp represents a mechanism for protection of mammalian cells against cytotoxic drugs [14, 15]. Rappa and colleagues [16] showed that when the human MDR1 gene is transfected into a L1210/VMDRC0.06 cell line, cells with the MDR1 gene are 2–3 fold more resistant to 3-AP, and accumulated less [14C]3-3-AP than the parent cell line without the MDR1 gene. This suggested that individuals with variants in the MDR1 gene may be more sensitive to treatment with 3-AP. Three major single nucleotide polymorphisms (SNPs) on the MDR1 gene include point mutations in exon 26 (C3435T), exon 12 (C1236T) and exon 21 (G2677T) [17]. A rarer variation of the G2677T SNP is characterized by a guanine-adenine point mutation (G2677A). Patients with SNPs at all three loci may experience a 40% reduction in P-gp function [18]. Therefore, we hypothesized that pancreas cancer patients with these MDR polymorphisms would more likely respond to 3-AP.
The activity of hydroxyurea, like that of 3-AP, is related to binding to the M2 submit of mammalian RR. Resistance to hydroxyurea in four strains of S. cerevisiae has been correlated with high levels of M2 mRNA and M2 protein [19]; in addition, high M2 protein levels are found in tumor cell lines made resistant to hydroxyurea [20–22]. Therefore, we hypothesized that high levels of M2 mRNA and M2 protein in pancreatic tumors at baseline would confer resistance to 3-AP.
In vivo flow cytometry studies of hydroyxurea have demonstrated an accumulation of cells in early S phase due to a dose-dependent inhibition of hydroxyurea on DNA synthesis [23]. On the basis that hydroxyurea and 3-AP both inhibit RR M2, we hypothesized that 3-AP may also induce S-phase arrest.
Based on phase I activity of single-agent 3-AP in pancreatic cancer, the Phase 2 Consortium, an National Cancer Institute (NCI)-designated group of multiple cancer centers in the United States and Asia, including the Mayo Clinic, University of Wisconsin, and Johns Hopkins University, conducted a study to evaluate 3-AP in patients with chemotherapy-naive and gemcitabine-refractory (GR) advanced ductal adenocarcinoma of the exocrine pancreas. The primary endpoint was survival at six months (chemotherapynaive) and four months (GR). Secondary endpoints included evaluation of toxicity, response rate (RR), OS and time to progression (TTP). Secondary endpoints further included (1) correlation of MDR gene polymorphisms, baseline M2 mRNA levels, and baseline M2 protein expression with clinical outcome; and (2) evaluation of 3-AP for buccal mucosal cell S-phase arrest.
Patients and methods
Patient selection
Eligibility criteria included age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, a life expectancy ≥ 6 weeks, and measurable, biopsy-confirmed adenocarcinoma of the pancreas that was locally advanced, recurrent or metastatic. Enlargement of a previously irradiated lesion was required for it to be measurable. Eligible patients had, within seven days prior to registration, an absolute neutrophil count (ANC) ≥ 1500 mm3, platelet count ≥ 75,000/mm3, total bilirubin ≤ 1.5 times the institutional upper limit of normal (ULN), aspartate transaminase (AST) ≤ three times ULN and adequate renal function (a creatinine ≤ 1.5 times ULN or creatinine clearance > 60 mL/min/1.73 m2). Lastly, eligible patients had the capability to understand the investigational nature of the study and provide written informed consent. The Institutional Review Boards of participating institutions approved the study protocol prior to its implementation.
Patients were excluded if they were pregnant or breast feeding, were of childbearing age and unwilling to use contraception, had brain metastasis, or had an uncontrolled medical condition or a psychiatric illness that would limit protocol compliance. Concurrent anti-neoplastic therapy, hypersensitivity or severe allergic reaction to 3-AP or a related compound, use of anti-retroviral therapy and glucose-6-phosphate dehydrogenase deficiency (due to the risk for hemolytic anemia with 3-AP) [24] were exclusion criteria.
Pretreatment evaluation and follow-up studies
History, physical exam, ECOG performance status, and serum tests including CBC, total bilirubin, AST, alanine transaminase, creatinine, sodium, potassium, chloride, bicarbonate, albumin, calcium, alkaline phosphatase and CA 19-9 were obtained at baseline and at the beginning of each cycle. Other pre-registration studies included measurement of height, serum pregnancy testing for women of childbearing age, an electrocardiogram and glucose-6-phosphate dehydrogenase testing (in patients of African, Asian or Mediterranean origin/ancestry). In addition, on day 15 of each cycle, physical exam, review of systems and CBC were obtained. On day 1 of cycle 1, a methemoglobin level was obtained prior to treatment, at the end of the infusion and then at 2.0, 4.5 and 22.0 hours after 3-AP administration.
Treatment plan and dose modifications
3-AP was supplied by Vion Pharmaceuticals, Inc., and distributed by the Cancer Therapy Evaluation Program, the Division of Cancer Treatment and Diagnosis, NCI. Patients received 3-AP initially at 96 mg/m2 as a 2-hour infusion on days 1–4 and 15–18 of a 28-day cycle. Additional dose levels of 3-AP were 80 mg/m2/day (dose level—1) and 60 mg/m2/day (dose level—2). Dose reductions were based on toxicity (NCI Common Terminology Criteria for Adverse Events, version 3.0) and were permanent. Patients requiring more than two dose level reductions, or treatment delay greater than 14 days, were removed from treatment.
Prior to retreatment, toxicities had to resolve to ≤ grade 2. Dose level modifications for a non-hematologic grade 3 toxicity occurred as follows: no change if it resolved to ≤ grade 2 in ≤ 48 hours; reduction by one level if it resolved to ≤ grade 2 in > 48 but < 72 hours; and removal from treatment if it persisted ≥ 72 hours. The dose of 3-AP was reduced by one level for a grade 4 non-hematologic toxicity if it resolved to ≤ grade 2 in ≤ 48 hours. If it persisted ≥ 48 hours, the patient was removed from treatment. No change in dose level occurred for a grade 3 ANC or platelet count; however, the dose level was reduced by one for a grade 4 ANC or platelet count. Anemia was treated with growth factors and packed red blood cells transfusions rather than dose modification. Treatment was held for at least one week for any grade 4 toxicity, or grade 3 non-hematologic toxicity, not resolving to ≤ grade 2 within 24 hours.
Vital signs were monitored during, and for four hours after, infusion of 3-AP during the first cycle. Isolated hypoxia (pulse oximetry ≤ 92%) was managed with supplemental oxygen. However, the infusion was stopped when moderate or severe dyspnea occurred and the dose was reduced by one level. Patients developing a systolic blood pressure less than 85 mm Hg during infusion were removed from treatment. A transient 10–15% rise in methemoglobin levels was expected with 3-AP. Unless accompanied by hypoxia or symptoms, or failure of methemoglobinemia to decrease to less than 5% within 24 hours, treatment continued unmodified. Patients with methemoglobinemia > 15% for more than several hours were removed from treatment.
Disease assessment
Disease status was monitored using Response Evaluation Criteria in Solid Tumors [25]. Tumor assessment was performed at baseline and every cycle if measured by physical examination, or every other cycle if measured by imaging, utilizing identical methods. Patients with progressive disease (PD) were followed every six months until death.
Multi-drug resistance gene polymorphism analysis
Samples were obtained on day 1 of cycle 1 from all patients to test for the common MDR polymorphisms C1236T, G2677T/A and C3435T (Lafferty et al., submitted). Briefly, DNA was extracted from peripheral blood specimens by standard methods [26]. Gene fragments containing the SNP of interest were amplified by polymerase chain reaction (PCR) using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) in 40 μl reactions with 20 μl PCR Master Mix (Promega, Madison, WI), 14 μl nuclease-free water, 10–100 ng genomic DNA and 10 pmol reverse and forward primer (IDT Technology, Coralville, IA). PCR conditions were as follows: denaturation at 95°C for 5 minutes, denaturation for 50 cycles for 30 seconds at 95°C, 30 seconds of annealing at 52°C, extension for 30 seconds at 72°C, then an extension for 5 minutes at 72°C. SNP detection was performed by pyrosequencing using primers designed with SNP Primer Design Software Version 1.01 (Uppsala, Sweden). Biotinylated PCR product (35 μl) were immobilized on streptavidin-coated Sepharose beads (GE Healthcare Bio-Sciences, Piscataway, NJ) with binding buffer (10 mmol/L Tris–HCl, 2 mol/L NaCl, 1 mmol/L EDTA, and 0.1% Tween 20, pH 7.6). After incubation in ambient conditions with 10 minutes of agitation, strands were separated and treated with 70% ethanol, 0.2 mol/L NaOH (denaturation solution), and washing buffer (10 mmol/L Tris–Acetate, pH 7.6). The beads were released into wells with a 40 μl mixture of annealing buffer (20 mmol/L Tris–Acetate, 2 mmol/L Magnesium Acetate Tetrahydrate, pH 7.6) and 21 pmol of sequencing primer (IDT). Incubation occurred for two minutes at 80°C. Genotyping was performed with a PSQ 96 SNP Reagent Kit and PSQ 96MA system (Biotage AB, Uppsala, Sweden). Genotypes were resolved on the basis of peak height measurements using PSQ96 SNP Software, version 1.2 AQ.
M2 mRNA expression analysis
M2 mRNA expression analysis was performed on archived tumor tissue at baseline for all patients to correlate expression of M2 mRNA in their pancreatic adenocarcinoma cells with response to 3-AP. Standard paraffin imbedding was utilized on tumor from either primary or metastatic sites. Laser capture microdissection (LCM) with the SL μCut Laser Microdissection System (Molecular Machines & Industries, Glattbrug, Switzerland) was utilized to ensure the isolation of only tumor cells. After LCM, RNA extraction was performed using the Paradise Whole Transcript RT Reagent System (Arcturus Bioscience, Sunnyvale, CA) designed to use with formalin fixed paraffin embedded (FFPE) tissue scrapes. Caps were placed in a microcentrifuge tube containing proteinase K and incubated at 37°C for 16–20 hours. After centrifugation, the caps were removed and the RNA was isolated and treated with DNase following the manufacturer's instructions. The total RNA was resuspended and then treated with DNase. The RNA was quantified via NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). Total RNA extracted from FFPE tumor tissue was reverse transcribed using random primers following the manufacturer's instructions. M2 RNA was quantified by the TaqMan assay with primers designed by using Integrated DNA Technologies (Coralville, IA) maintaining an amplicon length < 100 bp. Quantitative real-time PCR (RT-PCR) was performed using the Bio-Rad iCycler IQ system (Hercules, CA). Due to limited sample supply we amplified the target gene and housekeeping gene in a single well. Each well contained 5 pmol/μL of the probes, 5 pmol/μL of the primers and 12.5 μL of iQ Multiplex Powermix (Bio-Rad) in a 25 μL final reaction mixture. The Multiplex Powermix was heat activated for 3 min at 95°C. Each of the 50 PCR cycles consisted of 15 seconds of denaturation at 95°C and hybridization of primers and probes for 45 seconds at 60°C. The normalized level of M2 expression was calculated using YWHAZ as the endogenous reference. Each sample was analyzed in triplicate.
M2 protein analysis by automated quantitative analysis
Immunohistochemistry (IHC) is the standard method for assessing protein expression in archived human tissue; however, IHC lacks standardization and is inherently subjective. Therefore, we used the Automated Quantitative Analysis (AQUA®) system (HisotRx, New Haven, CT), or “quantitative IHC,” which utilizes automated fluorescent image acquisition and data processing to allow for quantitation of data and standardization of analysis [27– 29]. Briefly, target compartments were localized using a fluorescently tagged (Alexa Fluor 555, Invitrogen, Carlsbad, CA) rabbit anti-cytokeratin antibody or anti-S100 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). 4,6-Diamidino-2-phenylindole was added to visualize nuclei. Target biomarker (anti-ribonucleotide reductase, M2) was visualized with a fluorescent chromogen (Alexa Fluor 488-tyramide; Invitrogen, Carlsbad, CA). The target biomarker was tagged and measured within the subcellular compartments by the PLACE algorithm. A Z-score, defined as [(AQUA® score)—(mean score of all AQUA® scores in other studies)]/standard deviation, was used to compare results from different experiments.
S-phase arrest in buccal mucosal cells
We examined the effects of 3-AP on cell cycle only in patients treated at the University of Wisconsin (UW) using buccal mucosa cells collected pre-infusion, at 2.0 and 4.5 hours after the administration of 3-AP on day 1 of cycle 1. Buccal mucosal cells are ideal for phase analysis because they have a higher turnover rate and a higher number of cells in S-phase than cells obtained from blood. Buccal scrapings were collected and fixed in 95% ice-cold ethanol and stored at −80°C until staining. Samples were centrifuged for 10 minutes at 4°C and the alcohol was removed. Ten ml of cold PBS were added to the pellet, the cells were re-suspended before centrifugation and the supernatant was discarded. Then, 500 ml of a staining solution (33 mcg/ml propidium iodide (Sigma), 1 mg/ml RNAse A (Sigma), and 0.1% Triton X) were added to cell pellets. Samples were incubated 30 minutes at 37°C and placed at 4°C for at least 2 hours before flow cytometric analysis. Samples were filtered with a 40 mm mesh cell filter prior to analysis. Data were acquired with CellQuest software (Becton Dickinson, San Jose, CA) on a FACS Calibur benchtop flow cytometer (also Becton Dickinson). S-phase percentage was determined with ModFit2.0 DNA analysis software (Verity Software House, Topsham, MA).
Statistical considerations
The primary endpoint of this trial was to evaluate the efficacy of 3-AP in two groups of patients with advanced adenocarcinoma of the pancreas. The first group consisted of chemotherapy-naive patients receiving 3-AP and the second group contained patients refractory to gemcitabine receiving 3-AP. Gemcitabine received more than six months prior to recurrence in the adjuvant setting was not considered prior therapy. This study was designed to employ two, single-stage designs to be conducted and analyzed independently, with planned interim analysis. Given that the median OS is estimated as 5.7 months in patients receiving first-line gemcitabine [2], and as 4 months in patients receiving 2nd line gemcitabine [30], it was considered clinically significant if we increased the rates alive to 65% in each of the two groups of patients. Therefore, the primary endpoint of this trial was to evaluate the six- and four-month survival rates for 3-AP as first line and second line treatment, in 48 and 106 evaluable patients, respectively.
All eligible patients who signed a consent form and initiated treatment were considered evaluable for the primary endpoint. Survival was defined as the time from registration to death due to any cause. Given that we were seeking to improve survival rates in both groups from 50% to 65%, the same Three-Outcome design [31] was applied to each group. A total of 50 patients were to be enrolled to each group. The treatment was considered (1) “promising” in a group if at least 30 patients survived until the time point of interest, (2) “inconclusive” and needing further Phase II testing if 29 patients survived until the time point of interest, or (3) “ineffective” if at most 22 patients survived until the time point of interest. An interim analysis was planned at the time the 28th patient was evaluable for a given group and 14 patients alive at the time point of interest was considered sufficient evidence to warrant expanding enrollment to the full 50 patients in that patient group. This study design has an 80% chance of concluding that the regimen is promising in a patient group and a 5% chance of finding the study as inconclusive in a specific patient group.
Secondary endpoints were to evaluate the toxicity and tolerability of 3-AP, evaluate TTP, OS, tumor response and correlative laboratory studies that would enhance understanding of 3-AP and its effects on cellular processes. TTP was defined as the time from registration to PD (last contact or death). Patients lost to follow-up were censored for progression and survival at their date of last disease assessment and contact, respectively. Patients that died without documentation of their disease status at death were considered to have had PD on the date of death. Standard statistical techniques were to be used including summary statistics, frequency tables, categorical data analysis, t-tests and non-parametric Wilcoxon rank-sum tests, Kaplan–Meier [32] methodology and Cox [33] proportional hazards models. All analyses were performed using SAS Version 9.0 (www.sas.com).
Results
Enrollment was suspended after 15 evaluable patients were treated and prior to planned interim analysis due to the inactivity of 3-AP and rate of grade ≥ 3 toxicities.
1st line (Chemotherapy-naïve) 3-AP
One chemotherapy-naive patient was enrolled. This 88-year-old male completed days 1–4 of cycle 1 treatment; however, he presented with fatigue, nausea, vomiting, anorexia and an ECOG performance status of 3 on day 15 of cycle 1. He required paracentesis and admission with a serum sodium of 116 mmol/L and a serum potassium of 7.1 mmol/L. This precluded further study treatment and he was considered to have PD. After discharge, he was soon readmitted with abdominal pain, ascites, hyponatremia, hyperkalemia, hypothyroidism, leukocytosis, acute renal failure and hyperlipidemia and died five days later from progressive respiratory insufficiency.
2nd line (Gemcitabine-refractory) 3-AP
Patient characteristics
Baseline characteristics of 14 GR patients enrolled from January 2005 to October 2005 are summarized in Table 1.
Table 1. Baseline characteristics of 14 enrolled gemcitabine-refractory patients.
| Characteristic | Frequency | |
|---|---|---|
|
|
||
| Number | Percent | |
| Age, years | ||
| Median | 64 | |
| Range | 51–76 | |
| Gender | ||
| Male | 8 | 58 |
| Female | 6 | 42 |
| ECOG Performance Status | ||
| 0 | 6 | 43 |
| 1 | 7 | 50 |
| 2 | 1 | 7 |
| Race | ||
| Caucasian | 13 | 93 |
| African American | 0 | 0 |
| Asian | 0 | 0 |
| Unknown | 1 | 7 |
| Prior Therapya | ||
| Gemcitabine | 13 | 93 |
| 5-Fluorouracil | 2 | 14 |
| Capecitabine | 1 | 7 |
| Radiotherapy | 4 | 27 |
| Surgery | 8 | 57 |
| Investigational | 0 | 0 |
| None | 0 | 0 |
| Prior Chemotherapy Regimensb | ||
| 0 | 1 | 7 |
| 1 | 11 | 79 |
| 2 | 1 | 7 |
| 3 | 1 | 7 |
| Metastatic sites | ||
| Liver | 9 | 64 |
| Abdomen/peritoneal | 2 | 14 |
| Lung | 3 | 21 |
ECOG Eastern Cooperative Cancer Group
Patients appear more than once in this category
Includes investigational regimens
3-AP administration and toxicity
Fourteen GR patients received a total of 28 cycles of 3-AP. Six patients received at most one cycle, seven patients received at most two cycles, and one patient received eight cycles of 3-AP. Patients received a median of 92% (range, 12% to 101%) and 72% (range, 13% to 101%) of the planned dose of 3-AP during cycles 1 and 2, respectively. The one patient to receive more than two cycles of treatment received greater than 80% of the planned dose during cycles 3 through 6. Six patients had at most one dose reduction within and across cycles and one patient had two dose reductions within and across cycles. Dose reductions were required for nonhematologic adverse events (three patients), methemoglobinemia (two patients), hypotension (one patient), grade ≥ 3 adverse event (one patient) and hypoxia (one patient). Two patients (14%) required a dose reduction for the day 15 dose of cycle 1 and 21% (three patients) did not receive the day 15 dose during cycle 1. One patient required a dose reduction for the day 15 dose of cycle 2 and one did not receive the day 15 dose during cycle 2. Two treatment delays were seen during cycles 2 and 7. The reasons for these delays were a hematologic adverse event (one patient) and scheduling conflicts (one patient). No patients experienced methemoglobinemia requiring close observation. All patients have completed treatment and due to the following reasons: progressive disease (11 patients), adverse event (two patients) and death (one patient).
Fourteen GR patients were evaluable for toxicities and those considered at least possibly related to 3-AP appear in Table 2. Five patients had grade 4 toxicities, including neutropenia (four patients), leukopenia (one patient), thrombocytopenia (one patient) and hypophosphatemia (one patient). One patient died within two weeks of initiating study treatment. This patient completed the initial four days of cycle 1 and was admitted to the hospital after experiencing grade 2 nausea and grade 3 vomiting, transferred to the intensive care unit and intubated for grade 4 dyspnea. The patient experienced worsening ascites and an ileus not amenable to surgery and considered related to 3-AP. The ventilator was stopped and the patient died on day 19.
Table 2. Maximum severity of toxicity (Grade ≥ 3) for 14 gemcitabine-refractory patients receiving a total of 28 cycles of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone per patient.
| Classificationa | Frequency | ||
|---|---|---|---|
|
|
|||
| Cycle 1 (n=14) | Cycle 2 (n=8) | Overall (n=14) | |
| Neutropenia | 4 | 3 | 6 |
| Leukopenia | 3 | 1 | 4 |
| Infection | 2 | 2 | |
| Hypokalemia | 1 | 1 | |
| Fatigue | 2 | 3 | 5 |
| Febrile Neutropenia | 2 | 2 | |
| Anemia | 1 | 2 | 3 |
| Thrombocytopenia | 1 | 1 | 2 |
| Hypophosphatemia | 1 | 1 | |
| Hypoxia | 1 | 1 | 2 |
| Hypotension | 1 | 1 | |
| Ileus | 1b | 1b | |
| Thrombosis | 1 | 1 | |
| Dehydration | 1 | 1 | |
| Total Bilirubin | 1 | 1 | |
| Limb Edema | 1 | 1 | |
Based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0
Grade 5 event
Patient outcome
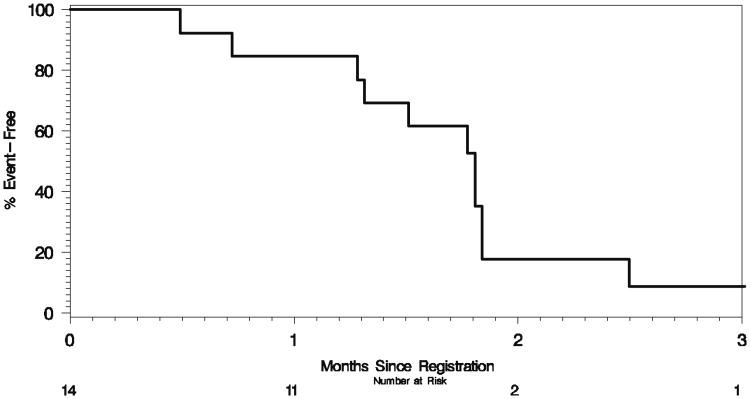
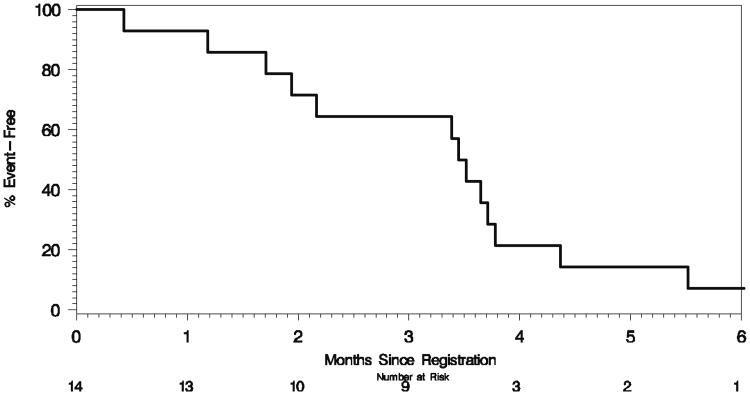
Fourteen GR patients were considered evaluable for tumor response to 3-AP. There were no responses and two patients demonstrated SD. PD precluded further treatment in 11 patients. One patient died while on study and two were removed due to toxicity. With a median follow up of 3.5 months (range, 1.9–3.8 months), one patient is alive and 13 have died. The estimated 4-month median survival rate in GR patients was 21% (95% CI: 8–58%). The distributions of TTP and OS are contained in Figs. 1 and 2, respectively, with details summarized in Table 3.
Fig. 1. Kaplan–Meier plot of time to progression in 14 gemcitabine-refractory patients treated with 3-aminopyridine-2-carboxaldehyde thiosemicarbazone.
Fig. 2. Kaplan–Meier plot of overall survival in 14 gemcitabine-refractory patients treated with 3-aminopyridine-2-carboxaldehyde thiosemicarbazone.
Table 3. Patient outcome for gemcitabine-refractory patients treated with 3-aminopyridine-2-carboxaldehyde thiosemicarbazone.
| Outcome | Estimate (95% CI)a |
|---|---|
| Best Objective Response | |
| Evaluable | 13 |
| CR | 0% (0–23%) |
| PR | 0% (0–23%) |
| SD | 15% (3–43%) |
| PD | 85% (50–94%) |
| Time to Progression | |
| Progressions | 12 |
| Median | 1.8 mos. (1.3–1.8 mos.) |
| Percent Progression-Free | |
| 3 mos. | 9% (1 –57%) |
| 4 mos. | 9% (1 – 57%) |
| 6 mos. | 9% (1 – 57%) |
| Survival | |
| Deaths | 13 |
| Median | 3.5 mos. (1.9–3.8 mos.) |
| Percent Alive | |
| 3 mos. | 64% (44–95%) |
| 4 mos. | 21% (8–58%) |
| 6 mos. | 7% (1–47%) |
| 9 mos. | 7% (1–47%) |
| 12 mos. | 7% (1–47%) |
| 15 mos. | 7% (1–47%) |
Abbreviations: CR complete response, PR partial response, SD stable disease, PD progressive disease, mos. Months
Kaplan–Meier method
Multi-drug resistance gene polymorphism analysis
Since there were no patients with a response to therapy, we evaluated the relationship between MDR polymorphisms and toxicity. MDR1 polymorphism frequencies were consistent with expected frequencies and were in Hardy– Weinberg equilibrium: C1236T (CC: 0.43, CT: 0.50, TT: 0.07), G2677T (GG: 0.27, GT: 0.53, GA: 0.07, TT: 0.13), and C3435T (CC: 0.20, CT: 0.60, TT: 0.20). Correlations for toxicity were based on genotype; however, the allele frequencies were taken into account in the analysis. The frequency of the T allele in C1236T and G2677T was associated with leukopenia (p<0.0001, chi-square test).
M2 mRNA expression analysis
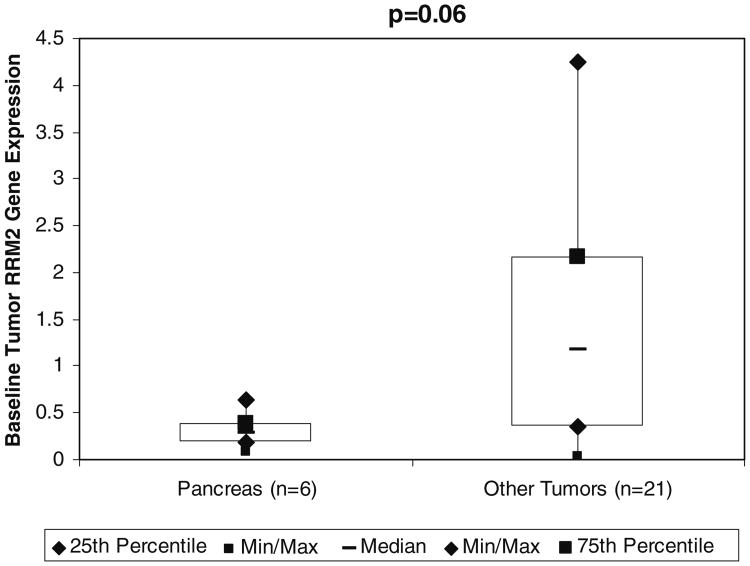
RR M2 mRNAwas evaluated in archived tumor samples by RT-PCR. There were six biopsies from pancreatic cancer patients in this study (two from primary sites and four from metastatic sites) available for mRNA analysis and 21 biopsies from non-pancreatic tumors from contemporaneous 3-AP studies at UW. The latter included one primary bladder cancer, one primary cervical cancer, one primary and three metastatic cholangiocarcinomas, two primary colon cancers, three primary esophageal cancers, one primary gastric cancer, three primary melanomas, one primary mesothelioma, one primary non-small cell lung cancer (NSCLC), two primary prostate cancers, one primary sarcoma and one primary small cell lung cancer (SCLC). Seven patients with pancreatic cancer (in the current study), two with breast cancer and one with lymphoma did not have sufficient material for RNA analysis. Additionally two patients with an unknown primary were excluded from this analysis because they could not be categorized as not having pancreatic cancer with certainty. We found a trend toward higher RR M2 gene expression in individuals with non-pancreatic tumors (median 1.17; range, 0.03 to 4.25) relative to those with pancreatic tumors (median 0.29, range 0.09 to 0.64, p=0.06, Wilcoxon rank-sum test, two-sided) (Fig. 3). This may suggest that relative to other tumors tested, our patients' pancreatic tumors express little RR M2 mRNA, which is potential reason for the observed inactivity of 3-AP in the current study.
Fig. 3.
Ribonucleotide reductase M2 mRNA evaluation by real time-polymerase chain reaction following laser capture microdissection in patients with pancreatic cancer and other solid tumors at the University of Wisconsin-Madison. Abbreviations: RR M2, ribonucleotide reductase M2
M2 protein analysis by automated quantitative analysis
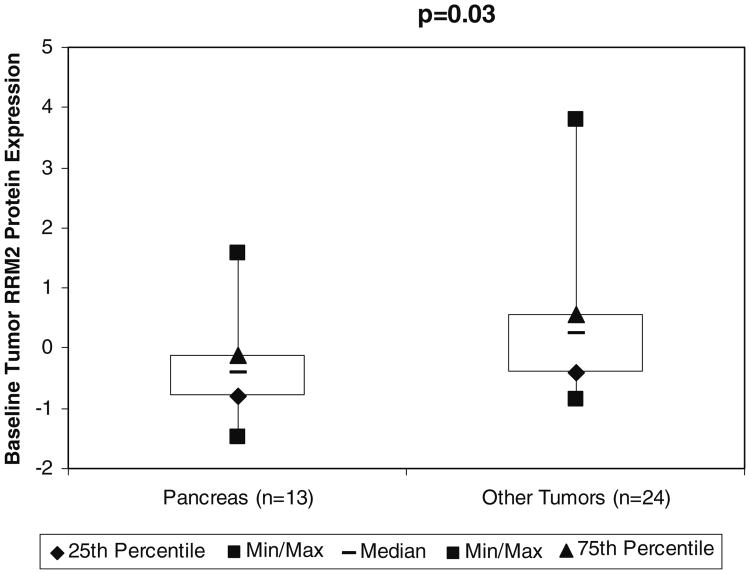
Archived tumor biopsies were evaluated for protein expression and localization of RR M2 by quantitative IHC (i.e., AQUA®). There were 13 samples from subjects with pancreatic cancer in the current study (four from primary sites and nine from metastatic sites) and 24 samples from patients in contemporaneous studies of 3-AP with tumors other than pancreatic cancer at UW. These included one primary bladder, two metastatic breast cancers, one primary cervical cancer, one primary and three metastatic cholangiocarcinomas, two primary colon cancers, three primary esophageal cancers, one primary gastric cancer, one lymphoma from a metastatic site, three primary melanomas, one primary mesothelioma, one primary NSCLC, two primary prostate cancers, one primary sarcoma and one primary SCLC. Two individuals with unknown primaries in other 3-AP studies were excluded from this analysis as they could not be categorized as not having pancreatic cancer with certainty. We found that the RR M2 expression was significantly higher in non-pancreatic tumors (median Z score 0.25, range −0.84 to 3.8) relative to pancreatic tumors (median Z score −0.41, range −1.49 to 1.5, p=0.03, Wilcoxon rank-sum test, two-sided). Similar to our experiments with RR M2 mRNA, these experiments demonstrated that relative to other solid tumors tested, our pancreatic cancer patients' tumors express little RR M2 protein, which may be a reason for the inactivity of 3-AP observed in the latter (Fig. 4).
Fig. 4.
Baseline ribonucleotide reductase M2 (RR M2) protein expression in patients with pancreatic cancer and other solid tumors at the University of Wisconsin evaluated by automated quantitative analysis and expressed as a Z-score. Abbreviations: RR M2, ribonucleotide reductase M2
Flow cytometric analysis of cell cycle arrest in buccal mucosal cells
Table 4 shows that the mean percentage of buccal mucosal cells in S-phase prior to administration of 3-AP was 0.02± 0.06 standard deviations (SD). Two hours after administration it was 1.9±4.4SD and was 2.4±2.3SD 4.0 hours after administration. This suggests that there is a significant increase in the pre-S phase percentage compared to the 4.0 hour S-phase percentage (p=0.04, paired, two tail t-test).
Table 4. Flow cytometric analysis for cell cycle arrest in buccal mucosal cells.
| Percent Cells in Diploid S-Phase (Mean±SD) | Percent Cells in Diploid G0/G1 (Mean±SD) | Percent Cells in Diploid G2/M (Mean±SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point | Pre-infusion 0.02±0.06 | 2h 1.9±4.4 | 4h 2.4±2.3 | Pre- infusion 99.1 ±2.3 | 2h 97.2±4.2 | 4h 96.9±2.5 | Pre- infusion 0.92±2.2 | 2h 0.91 ±1.5 | 4h 0.68±1.0 |
SD standard deviation
Discussion
No responses were seen with this regimen of 3-AP in predominantly GR advanced pancreatic cancer patients. Enrollment was suspended after 15 evaluable patients due to the inactivity of 3-AP and the rate of grade 3 and 4 toxicities, primarily hematologic, seen in our patients. We did not meet our primary endpoint in that only 21% of GR patients survived at least four months. Median OS (3.5 months) was low and similar historically to that of pancreatic cancer patients refractory to first-line chemotherapy [30].
To our knowledge this is the first published phase II evaluation of single-agent 3-AP in advanced pancreatic cancer patients. Mackensie and colleagues [34] recently reported a phase II study of 3-AP (105 mg/m2 as a 2-hour infusion days 1, 8 and 15 of a 28-day cycle) with gemcitabine in chemotherapy-naïve advanced pancreatic cancer patients. In these patients there was significant grade ≥ 3 hematologic toxicities and fatigue and no responses. While the Mackensie study did not meet its primary endpoints of RR and SD, median OS was 9 months, longer than in our study consistent with their use of gemcitabine and chemotherapy-naïve patients. Another phase II study [35] published only in abstract form also utilized a higher, less frequent dosing of 3-AP with chemotherapy-naïve patients and demonstrated a median OS of 8 months. While these trials were negative based on their primary endpoints, their survival data question whether the benefit of 3-AP for pancreatic cancer lies in its use as a first-line agent given less frequently or for a longer duration in combination with a second agent.
We performed correlative studies to explore mechanisms of action of 3-AP and relate them to clinical outcomes. First, we evaluated all patients for MDR polymorphisms. Because there were no responses, we searched for a correlation between MDR polymorphisms and toxicity. We found that the frequency of the T, or variant allele in C1236T and G2677T, was inversely associated with leukopenia (p< 0.0001). The decreased hematological toxicity in variant individuals, compared to wild-type individuals, may potentially be explained by a prolonged plasma half-life and increased drug exposure in those with wild-type MDR. This hypothesis needs to be confirmed with pharmacokinetic data and may be useful in future evaluations (of a more appropriate regimen) of 3-AP by identifying patients for whom 3-AP may be tolerable.
Second, we sought to correlate the level of expression of M2 mRNA and M2 protein with response. RR M2 expression was evaluated by AQUA® and compared to that in patients with non-pancreatic tumors on contemporaneous 3-AP studies at UW. RR M2 expression was low at baseline in our patients' pancreatic tumors relative to other solid tumors (p=0.03). This finding is hypothesis generating and suggests that one reason for 3-AP failures in the current study may be that there was an insufficient amount of target (RR M2). Additionally, it implies that this target may not play a critical role in the malignant potential of pancreatic cancer. However, poor tolerability of the study drug confounds this interpretation.
Third, as hypothesized, we demonstrated that 3-AP causes S-phase arrest in buccal mucosal cells. However, we did not test for this effect in our patients' corresponding tumor cells, where it may not have occurred. Alternatively, 3-AP may have caused S-phase arrest in these patients' pancreatic tumors, but doing so may not matter therapeutically in this setting.
Given the poor clinical activity and intolerability of this dosing schedule of 3-AP in our patients and in others with solid tumors [36–38] we do not feel that further clinical development of this regimen of 3-AP in pancreatic cancer is warranted. 3-AP, however, may be useful as an anticancer agent in combination with other treatment modalities, such as radiation [39], or in the treatment of advanced hematologic malignancies [40, 41].
Acknowledgments
This study was supported by NCI grant N01 CM-62205; NCI Translational Research Initiative Subcontract 24XS090; grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health (NIH); and NIH grant T32 CA009614 Physician Scientist Training in Cancer Medicine (Dr. Attia).
Contributor Information
Steven Attia, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
Jill Kolesar, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
Michelle R. Mahoney, Mayo Clinic Cancer Center, Rochester, MN, USA
Henry C. Pitot, Mayo Clinic Cancer Center, Rochester, MN, USA
Daniel Laheru, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD, USA.
James Heun, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
Wei Huang, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
Jens Eickhoff, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
Charles Erlichman, Mayo Clinic Cancer Center, Rochester, MN, USA.
Kyle D. Holen, Email: kdh@medicine.wisc.edu, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, 600 Highland Avenue, K4/528, Madison, WI 53792, USA.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 4.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 5.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Richards D, Ramanathan RK, van Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D, Arning M, Kindler HL. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 7.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, Zaniboni A, Ducreux M, Aitini E, Taieb J, Faroux R, Lepere C, de Gramont A. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 9.Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzyme Regul. 1999;39:3–12. doi: 10.1016/s0065-2571(98)00017-x. [DOI] [PubMed] [Google Scholar]
- 10.Cory JG, Cory AH, Rappa G, Lorico A, Liu MC, Lin TS, Sartorelli AC. Inhibitors of ribonucleotide reductase. Comparative effects of amino- and hydroxy-substituted pyridine-2-carboxaldehyde thiosemicarbazones. Biochem Pharmacol. 1994;48:335–344. doi: 10.1016/0006-2952(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 11.Murren J, Modiano M, Clairmont C, Lambert P, Savaraj N, Doyle T, Sznol M. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9:4092–4100. [PubMed] [Google Scholar]
- 12.Feun L, Modiano M, Lee K, Mao J, Marini A, Savaraj N, Plezia P, Almassian B, Colacino E, Fischer J, MacDonald S. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother Pharmacol. 2002;50:223–229. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 13.Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemi-carbazone, administered by 96-hour intravenous continuous infusion. J Clin Oncol. 2004;22:1553–1563. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- 14.Fromm MF. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv Drug Deliv Rev. 2002;54:1295–1310. doi: 10.1016/s0169-409x(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappa G, Lorico A, Liu MC, Kruh GD, Cory AH, Cory JG, Sartorelli AC. Overexpression of the multidrug resistance genes mdr1, mdr3, and mrp in L1210 leukemia cells resistant to inhibitors of ribonucleotide reductase. Biochem Pharmacol. 1997;54:649–655. doi: 10.1016/s0006-2952(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 17.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 19.Rittberg DA, Wright JA. Relationships between sensitivity to hydroxyurea and 4-methyl-5-amino-1-formylisoquinoline thiosemicarbazone (MAIO) and ribonucleotide reductase RNR2 mRNA levels in strains of Saccharomyces cerevisiae. Biochem Cell Biol. 1989;67:352–357. doi: 10.1139/o89-055. [DOI] [PubMed] [Google Scholar]
- 20.Wright JA, Cory JG. Alterations in the components of ribonucleotide reductase in hydroxyurea-resistant hamster cells. Biosci Rep. 1983;3:741–748. doi: 10.1007/BF01120985. [DOI] [PubMed] [Google Scholar]
- 21.Yen Y, Grill SP, Dutschman GE, Chang CN, Zhou BS, Cheng YC. Characterization of a hydroxyurea-resistant human KB cell line with supersensitivity to 6-thioguanine. Cancer Res. 1994;54:3686–3691. [PubMed] [Google Scholar]
- 22.Zhou BS, Hsu NY, Pan BC, Doroshow JH, Yen Y. Overexpression of ribonucleotide reductase in transfected human KB cells increases their resistance to hydroxyurea: M2 but not M1 is sufficient to increase resistance to hydroxyurea in transfected cells. Cancer Res. 1995;55:1328–1333. [PubMed] [Google Scholar]
- 23.Maurer-Schultze B, Siebert M, Bassukas ID. An in vivo study on the synchronizing effect of hydroxyurea. Exp Cell Res. 1988;174:230–243. doi: 10.1016/0014-4827(88)90157-7. [DOI] [PubMed] [Google Scholar]
- 24.Foltz LM, Dalal BI, Wadsworth LD, Broady R, Chi K, Eisenhauer E, Kobayashi K, Kollmannsburger C. Recognition and management of methemoglobinemia and hemolysis in a G6PD-deficient patient on experimental anticancer drug Triapine. Am J Hematol. 2006;81:210–211. doi: 10.1002/ajh.20547. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Boom R, Sol CJ, Heijtink R, Wertheim-van Dillen PM, van der Noordaa J. Rapid purification of hepatitis B virus DNA from serum. J Clin Microbiol. 1991;29:1804–1811. doi: 10.1128/jcm.29.9.1804-1811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 28.Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–5494. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- 29.Rubin MA, Zerkowski MP, Camp RL, Kuefer R, Hofer MD, Chinnaiyan AM, Rimm DL. Quantitative determination of expression of the prostate cancer protein alpha-methylacyl-CoA racemase using automated quantitative analysis (AQUA): a novel paradigm for automated and continuous biomarker measurements. Am J Pathol. 2004;164:831–840. doi: 10.1016/s0002-9440(10)63171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA, 3rd, Green MR, Tarassoff PG, Brown TD, Casper ES, Storniolo AM, Von Hoff DD. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–353. doi: 10.1093/oxfordjournals.annonc.a010600. [DOI] [PubMed] [Google Scholar]
- 31.Sargent DJ, Chan V, Goldberg RM. A three-outcome design for phase II clinical trials. Control Clin Trials. 2001;22:117–125. doi: 10.1016/s0197-2456(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EMP. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Cox D. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–202. [Google Scholar]
- 34.Mackenzie MJ, Saltman D, Hirte H, Low J, Johnson C, Pond G, Moore MJ. A Phase II study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) and gemcitabine in advanced pancreatic carcinoma. A trial of the Princess Margaret hospital Phase II consortium. Invest New Drugs. 2007;25:553–558. doi: 10.1007/s10637-007-9066-3. [DOI] [PubMed] [Google Scholar]
- 35.Greeno E, Kindler HL, Peeters M, Trowbridge RC, Chong G, Valle JW, Johnson B, Allain EL, Burris HA. A phase II study of triapine in combination with gemcitabine (G) in patients (pts) with unresectable or metastatic pancreatic cancer (PC). Abstract. J Clin Oncol, 2006 ASCO Annual Meeting Proceedings Part I. 2006;24:4123. [Google Scholar]
- 36.Knox JJ, Hotte SJ, Kollmannsberger C, Winquist E, Fisher B, Eisenhauer EA. Phase II study of Triapine in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161) Invest New Drugs. 2007;25:471–477. doi: 10.1007/s10637-007-9044-9. [DOI] [PubMed] [Google Scholar]
- 37.Atieh DMM, Shriberg L, Brafman L, Szno M, Vahdat L. A phase II trial of 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone (3-AP) in patients with metastatic breast cancer. Abstract. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2004;22:864. [Google Scholar]
- 38.Ma B, Goh BC, Tan EH, Lam KC, Soo R, Leong SS, Wang LZ, Mo F, Chan AT, Zee B, Mok T. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine((R))) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2007 doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 39.Barker CA, Burgan WE, Carter DJ, Cerna D, Gius D, Hollings-head MG, Camphausen K, Tofilon PJ. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) Clin Cancer Res. 2006;12:2912–2918. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 40.Gojo I, Tidwell ML, Greer J, Takebe N, Seiter K, Pochron MF, Johnson B, Sznol M, Karp JE. Phase I and pharmacokinetic study of Triapine, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk Res. 2007;31:1165–1173. doi: 10.1016/j.leukres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Karp JE, Giles FJ, Gojo I, Morris L, Greer J, Johnson B, Thein M, Sznol M, Low J. A Phase I study of the novel ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine((R))) in combination with the nucleoside analog fludarabine for patients with refractory acute leukemias and aggressive myeloproliferative disorders. Leuk Res. 2008;32:71–77. doi: 10.1016/j.leukres.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]