Abstract
Purpose
Vatalanib (PTK 787/ZK22584) is an oral polytyrosine kinase inhibitor with strong affinity for platelet-derived growth factor and vascular endothelial growth factor (VEGF) receptors. We conducted an open-label, phase II multicenter therapeutic trial investigating the efficacy and tolerability of vatalanib in patients with metastatic or advanced pancreatic cancer who failed first-line gemcitabine-based therapy.
Methods
Vatalanib treatment consisted of a twice daily oral dosing using a “ramp-up schedule,” beginning with 250 mg bid during week 1,500 mg bid during week 2, and 750 mg bid on week three and thereafter. the primary objective of this study was to evaluate the 6-month survival rate.
Results
Sixty-seven patients were enrolled. The median age was 64, and 66 %(N = 43) had only one prior regimen. Common grade 3/4 adverse events included hypertension (20 %; N = 13), fatigue (17 %; N = 11), abdominal pain (17 %; N = 11), and elevated alkaline phosphatase (15 %; N = 10). among the 65 evaluable patients, the 6-month survival rate was 29 % (95 % CI 18–41 %) and the median progression-free survival was 2 months. Fifteen patients survived 6 months or more. two patients had objective partial responses, and 28 % of patients had stable disease. Changes in biomarkers including soluble VEGF and vascular endothelial growth factor receptor did not correlate with response to drug.
Conclusion
Vatalanib was well tolerated as a second-line therapy and resulted in favorable 6-month survival rate in patients with metastatic pancreatic cancer, compared with historic controls.
Keywords: Vatalinib, Pancreatic adenocarcinoma, Tyrosine kinase inhibitor, Second-line treatment
Introduction
Pancreatic adenocarcinoma remains one of the deadliest cancers, with an estimated 43,920 new cases and 37,390 anticipated deaths in the United States 2013 [1]. Only a few patients are diagnosed early enough to be considered for a definitive surgery and those only 15–20 % live up to 5 years [2]. For patients with advanced and metastatic disease, the median survival remains between 6 and 11 months. the first active chemotherapy agent to be approved for meta static disease, gemcitabine, has a response rate of about 10 % and a median survival of 6.5 months. Numerous trials investigating combinations of gemcitabine with other chemotherapeutic agents failed to prove beneficial with respect to overall survival. Most recently, a combination of nab-paclitaxel and gemcitabine has been shown to be more active than gemcitabine alone, with median OS of 8.5 versus 6.7 months [3]. A multidrug combination of FOLFIRINOX has demonstrated considerable activity in a population of younger and ft patients (median OS of 11.2 months) but with some considerable toxicity [4]. In the second-line therapy setting, options are even more limited in terms of available and effective therapies [5].
Angiogenesis, the process by which new capillary blood vessels extend from established blood vessels creating additional vascular supply, is an essential feature of all solid tumors allowing growth beyond a limiting size by facilitating delivery of nutrients to the tumor and removal of waste products (reviewed in [6]). Pancreatic tumors express high levels of vascular endothelial growth factor (VEGF), VEGF receptors, and other angiogenic regulators. this aberrant expression may be due to the complex pancreatic cancer microenvironment which is highly hypoxic and characterized by extensive stromal proliferation, with >80 % of the tumor mass consisting of desmoplastic stroma composed of endothelial cells, fibroblasts, immune, and endocrine cells that secrete growth factors and cytokines [7–9].
Vatalanib, 4-chlorophenyl-(4-pyridin-4-ylmethyl-phthalazin-1-yl) amine succinate, is an orally active pan tyrosine kinase inhibitor with high receptor binding affinity for all known vascular endothelial growth factor receptor (VEGFR)- and platelet-derived growth factor receptor (PDGFR)-tyrosine kinases and also inhibits receptor for stem cell factor (c-Kit) and colony-stimulating factor 1 receptor (c-Fms) [10]. Several phase 1 and phase 2 studies have investigated the single agent and combined activity of vatalanib in a variety of solid tumors; however, no previous clinical studies have investigated vatalanib in pancreatic adenocarcinomas. In preclinical orthotopic pancreatic models, vatalanib has significant anti-tumor activity and is correlated with decreased microvessel density [11, 12]. Because vatalanib targets multiple targets implicated in pancreatic tumor survival and angiogenesis and shows promising preclinical activity, we hypothesized that it may have clinical benefit in pancreatic cancer.
One of the clinical challenges in the development of vatalanib has been optimization of the dosing schedule. Thomas et al. [13] reported their experience with twice a day (BID) dosing of vatalanib in patients with advanced solid tumors. The authors reported favorable toxicity profile with doses up to 750 mg BID daily. Interestingly, the BID dosing resulted in lower Cmax values but higher trough levels. The trough levels plateau was seen at and above the 500 mg BID dose level. There was evidence of early accumulation of the drug that leveled off after day 14, suggesting a role for ramp-up in dosing schedule. In the current study, we implemented a ramp-up over the first 2 weeks starting at 250 mg BID, up to 500 mg BID in the second week, and in patients experiencing no drug-related toxicity, up to 750 mg BID in the third of week and thereafter.
Soluble biomarkers in plasma and serum have been implicated as potential pharmacologic biomarkers for anti-angiogenic agents. results from two phase 1 trials in patients with colorectal cancer reported changes in plasma VEGF-A and bFGF correlated with one course of >1,000 mg dose of vatalanib, with only changes in plasma VEGF-a being significantly correlated with disease status [14]. In two phase 3 studies of vatalanib and chemotherapy in patients with colorectal cancer serum lactate dehydrogenase (LDH) appeared to correlate with longer progression-free survival (PFS), implicating LDH as a possible predictive biomarker of vatalanib [15, 16]. Prior clinical studies have also suggested that DCE-MRI may be a useful as imaging marker for monitoring response to vatalanib [14, 15, 17]. In the current study, we investigated soluble angiogenic factors, cytokines, serum LDH, and DCE-MRI as potential predictive markers.
Patients and methods
Eligibility criteria
Eligible patients were required to be 18 years of age or older with histologically or cytologically diagnosed pancreatic adenocarcinoma that was measurable or evaluable as determined by RECIST criteria, Zubrod performance status of 0–2, absolute neutrophil counts ≥1.5 × 109/L, platelets ≥100 × 109/L, hemoglobin (Hgb) ≥9 g/dl, serum creatinine ≤1.5 the upper limit of normal (ULN), serum bilirubin ≤1.5 ULN, aspartate aminotransferase (AST/SGOT)/alanine aminotransferase (ALT/SGPT) ≤3.0 × ULN (≤5 × ULN if liver metastases present), no proteinuria based on dipstick reading, or positive for protein on dipstick reading the total urinary protein ≤500 mg and measured creatinine clearance (CrCl) ≥50ml/min for a 24-h urine collection. All patients were required to have a life expectancy ≥12 weeks. Patients must have failed or progressed on prior gemcitabine-based therapy for advanced or metastatic disease (includes a combination of gemcitabine with another chemotherapy or biologic drug). Failure/progression was based on clinical assessment per the investigator's discretion. Patients deemed unable to tolerate gemcitabine-based therapy were also eligible. Written informed consent was obtained in compliance with federal and local regulatory guidelines. exclusion criteria included islet cell or neuroendocrine carcinomas of the pancreas, history of presence of central nervous system (CNS) disease (i.e., primary brain tumor, malignant seizures, CNS metastases or carcinomatous meningitis), and pleural effusion or ascites that causes respiratory compromise (≥CTCAE 3.0 grade 2 dyspnea). No other prior malignancy was allowed except for the following: (1) adequately treated basal cell or squamous cell skin cancer, (2) in situ cervical cancer, (3) adequately treated stage I or II cancers from which the patient is currently in complete remission and off any anticancer therapy for at least 2 years, or (4) any other cancer from which the patient was disease free for at least 5 years. No prior chemotherapy ≤21 days, prior biologic or immunotherapy ≤14 days, or prior full-field radiotherapy ≤28 days or limited field radiotherapy ≤14 days prior to registration was allowed. Patients must have recovered from all therapy-related toxicities. The site of previous radiotherapy should have evidence of progressive disease if this is the only site of disease. Major surgery (e.g., laparotomy) ≤28 or minor surgery ≤14 days prior to registration was not allowed. Minor surgery was defined as any surgery which did not require general anesthesia. Insertion of a vascular access device was not considered major or minor surgery in this regard. Patients were required to have recovered from all surgery-related toxicities. Patients who had received investigation drugs ≤28 days prior to registration, or prior therapy with anti-VEGF agents, were excluded. All study participants provided written informed consent.
Treatment plan
This was a multicenter phase II study conducted by the Pancreatic Cancer research team (PCRT) at eight clinical sites in the USA. All patients received vatalanib orally, twice daily. A cycle was defined as 28 days. A ramp-up dosing design was used in the first cycle, with patients receiving 250 mg bid for week one, 500 mg bid week 2, and 750 mg bid (full dose) week 3 and thereafter.
Dosing modifications
The established full dose was 1,500 mg/day (after ramp-up phase), with two dose de-escalation levels: 1,000 and 750 mg/day. In case of grade 3 or higher study drug-related toxicity, the treatment was discontinued for 1 week. The treatment was then resumed at the lower dose level (–1, 1000 mg/day) provided toxicity improved to grade 2 or lower. If the patient experienced another episode of grade 3 or higher non-hematological toxicity, the dose was further decreased another dose level (–2, 750 mg/day). Further dose reductions were not allowed.
Safety assessment
Adverse events were graded according to the NCI CTCAE3.0. Pre-treatment assessment included: physical examination, chemistries, and complete blood counts, urine dipstick, and imaging study within 28 days from treatment start. Repeat physical examination, toxicity checks, and laboratory evaluations were done weekly during the 4-week ramp-up period an every 28 days thereafter. Restaging computerized tomography (CT) of chest/abdomen and pelvis was performed every 8 weeks.
Efficacy assessments
Radiographic assessment (CT scan with contrast) of response was carried out at baseline and every 8 weeks (=2 cycles) for patients continuing on study. RECIST 1.1 criteria were used for response determination [18].
Soluble protein assessment
Blood was collected at baseline and following the first cycle of treatment (28 days) in heparin-coated tubes and immediately spun at 3000×g for 15 min to isolate the plasma layer. Plasma was stored at −80 °C until time of analysis. Patient plasma was assayed for: VEGF-A, VEGF-C, VEGF-R1, VEGF-R2, platelet-derived growth factor beta heterodimer, (PDGF-BB), osteopontin (OPN), angiopoietin 2 (ANG-2), interleukin-6 (Il-6), and soluble interleukin six receptor (sIL-6R) (Searchlight, Pierce Biotechnology Multiplex ELISA and IL-6 and sIL-6R using R&D Systems). Serum LDH levels were analyzed in the local commercial clinical laboratories.
Dynamic contrast-enhanced MRI
DCE-MRI was performed on a 1.5-T MRI scanner (GE) using an eight channel phased array coil (GE). DCE-MRI data were collected from subjects who repeated a “breathe-in, breathe-out, and hold” pattern, with the imaging occurring during each held-expiration period. Prior to the dynamic portion of the scanning, four pre-contrast three-dimensional gradient recalled echo (3D-GRE) images were obtained at flip angles of 15, 23, 30, and 60° so that a pre-contrast t1 map could be calculated in each case. Parameters for the 3D-gre imaging were, 12 slices reconstructed to a matrix size of 256 × 256, slice thickness = 5 mm, TR = 5.0 ms, TE = 2.1 ms, and α = 30°. after 1–2 pre-contrast images had been acquired as a baseline and to ensure adequate image quality, gadolinium contrast (Omniscan®, GE Healthcare) was injected at a dose of 0.1 mmol/kg at a rate of 4 ml/s using a power injector (Mallinckrodt Cincinnati, OH), followed with a 20-ml saline flush injected at 4 ml/s. Pixel-by-pixel pharmacokinetic analysis of DCE-MRI data was performed using a two-compartment model [19]. Prior to pharmacokinetic analysis, sequential elastic registration was performed on the DCE-MRI images to reduce misregistration arising from inconsistent breathholding, as described previously [20]. Three physiologically relevant parameters were calculated for each pixel, the volume transfer constant (Ktrans), volume fraction of the extravascular extracellular space (ve), and plasma volume fraction (vp), by fitting image data to the following equation [19]:
where Cp(t) is the arterial input function (AIF) calculated from a region-of-interest (ROI) placed over the descending aorta, and Ct(t) is the volume-averaged concentration of the contrast agent in a pixel. Ct(t) was calculated assuming a linear dependence of signal enhancement on the concentration of contrast agent, from the known in vitro longitudinal relaxivity of the contrast agent. The images were captured at baseline (within 7 days from the first dose) and 24 h following first dose.
Statistics
The primary outcome measured was 6-month survival rate. Other objectives included PFS, toxicity, and response rate. Exploratory analyses included changes in circulating angiogenic factors, serum LDH, and DCE-MRI. Pre- and post-therapy serum LDH baseline levels were correlated with PFS using log rank test and compared with response categories using the Wilcoxon rank sum test. The cutoff for high/low serum LDH levels was 190 U/L. Soluble plasma protein markers were analyzed using descriptive statistics including calculation of the average, standard deviation, and mean for each protein at each time point. To investigate the correlation between proteins at baseline values, the Spearman correlation was used. To investigate the association between survival and changes in soluble protein expression at baseline and end of cycle one, the Cox proportional hazard model was used.
Results
Patient population
Patient characteristics are detailed in table 1. The first patient was enrolled on December 2, 2005, and the accrual was completed on January 16, 2008 (67 patients enrolled, 65 received study drug). Median age at registration was 64 years, and patients had a median of one prior treatment regimen (range one to four). Of the 65 evaluable patients, 92 % had distant metastatic disease with the remainder having locally advanced disease. Thirty patients (46 %) had Zubrod performance status of 0, 32 patients (49 %) had performance status of 1, and three patients had performance status of two. Sixty-five patients began treatment, and 62 patients had discontinued treatment at the time of the final analysis, due to progression (N = 35), toxicity/side effects/complications (N = 18), refusing further treatment (N = 6), death (N = 1), or other reasons (N = 2). Two patients were taken off study without receiving any protocol treatment and are considered not analyzable for survival, response, or adverse events. Fifty-five patients had died at the time of analysis, including the two patients that never received study treatment. Median treatment duration was 6.5 weeks (range 0.1–39 weeks).
Table 1. Patient characteristics.
| Age | % | Disease status at registration | % | ||
|---|---|---|---|---|---|
| Median | 64 | Locally advanced | 5 | 8 | |
| Minimum | 39 | Distant metastatic | 60 | 92 | |
| Maximum | 83 | ||||
| Zubrod performance status | Number of prior regimens | ||||
| 0 | 30 | 46 | 1 | 43 | 66 |
| 1 | 32 | 49 | 2 | 19 | 29 |
| 2 | 3 | 5 | 3 | 2 | 3 |
| 4 | 1 | 2 | |||
Safety and toxicity
The most common adverse events (all grades) included fatigue, nausea, hypertension, abdominal pain, and liver function test elevations. Significant adverse events (≥grade 3) occurring in at least 10 % of patients are outlined in table 2. All treated patients were evaluated for adverse events. grade 2 toxicity attributable to study agent was experienced by 32 % patient, 43 % had grade 3, and 12 % had grade 4 maximum toxicity. Hypertension and fatigue were the most common severe (grade 3 or higher) toxicities that were attributable to study treatment, occurring in 20 and 17 %, respectively, of treated patients. Forty-nine different serious adverse events (SAE) were recorded, representing a total of 76 adverse events in 34 unique patients. Twenty-three of these 76 adverse events were determined to be possibly or probably related to treatment, with the remaining 53 SAEs unlikely or unrelated to treatment. None of the 14 deaths that occurred during or within 30 days of discontinuation of study treatment were attributed to study drug. Nine were related to disease progression, one each to lung infection and multi-organ failure, and three to other causes.
Table 2. Significant adverse events (≥grade 3) occurring in >10 % of patients.
| Adverse event | N | % |
|---|---|---|
| Hypertension | 13 | 20 |
| Fatigue | 11 | 17 |
| Abdominal pain | 11 | 17 |
| Elevated alkaline phosphatase | 10 | 15 |
| Elevated AST | 9 | 14 |
| Nausea | 7 | 11 |
| Dehydration | 7 | 11 |
| Hyponatremia | 7 | 11 |
Efficacy
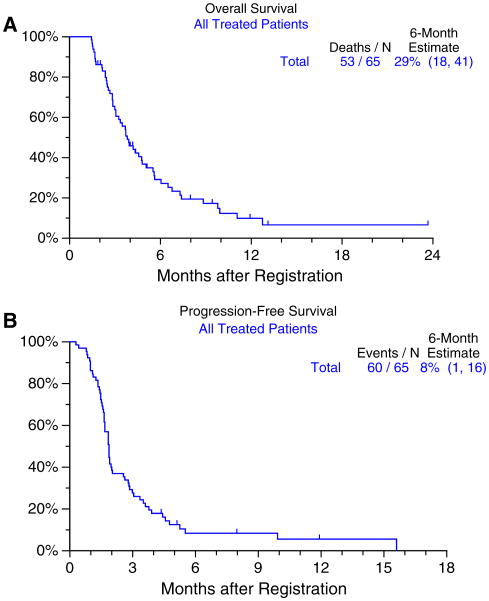
Treatment efficacy data are summarized in table 3. The primary outcome of this study was the 6-month survival rate. Among the 65 treated patients, the 6-month survival rate was 29 % (95 % CI 18–41 %) (Fig. 1a). There were 15 patients who survived 6 months or more. Progression-free survival at 6 months was 8 % (Fig. 1b).
Table 3. Response summary.
| Best response on treatment | N | Percent |
|---|---|---|
| Partial response | 2 | 3.1 |
| Stable disease | 18 | 27.7 |
| Increasing disease | 34 | 52.3 |
| Symptomatic deterioration | 4 | 6.2 |
| Not determinable | 7 | 10.8 |
Fig. 1. Overall and progression-free survival in all patients treated with vatalanib (n = 65).
Correlative studies
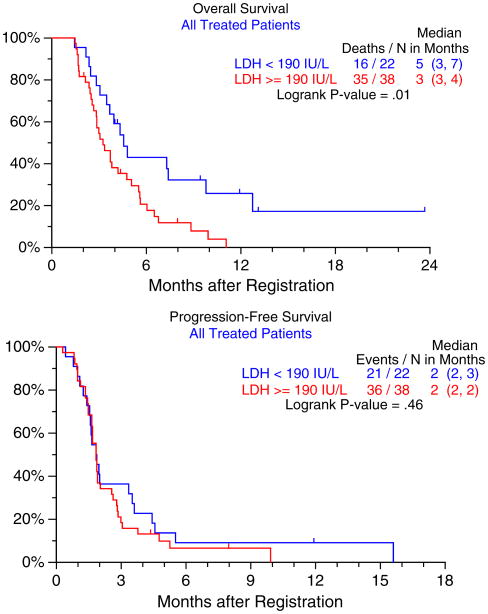
No significant difference in the baseline serum LDH levels was observed for different response categories (PR or stable vs. progressive disease, Wilcoxon rank sum, p = 0.75). There was a significant correlation observed between baseline LDH levels and 6-month survival (p = 0.01), but not PFS (p = 0.46) (Fig. 2).
Fig. 2. Overall and progression-free survival in patients stratified by high and low serum LDH level.
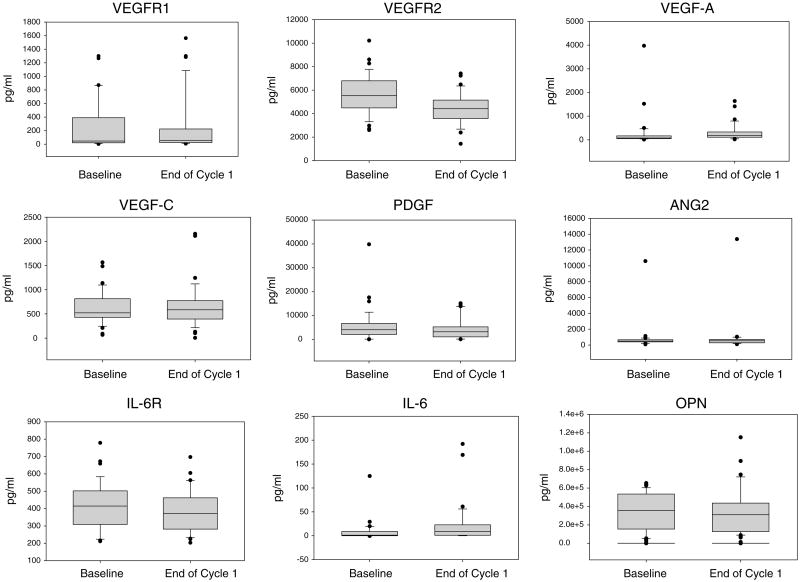
Soluble angiogenic and cytokine levels were analyzed in a total of 37 paired samples (Fig. 3). Because many of these proteins are regulated by overlapping signaling pathways, we looked for correlations between protein expression at baseline. Correlations were observed between VEGF-C and VEGF-R1 (p = 0.0496); VEGF-C and VEGF-A (p = 0.0092); PDGF-BB and ANG-2 (p = 0.0–003); IL-6 and ANG-2 (p = 0.0218); and IL-6 sR and OPN (p = 0.005). Only baseline IL-6 was predictive for survival, with lower IL-6 values associated with longer survival (p = 0.0227). The changes in protein levels post-treatment were not predictive of survival.
Fig. 3.
Soluble protein expression in patient plasma at baseline and end of cycle 1 was measured for 37 paired samples. Protein levels were measured using ELISA based assays and expression normalized to plasma volume
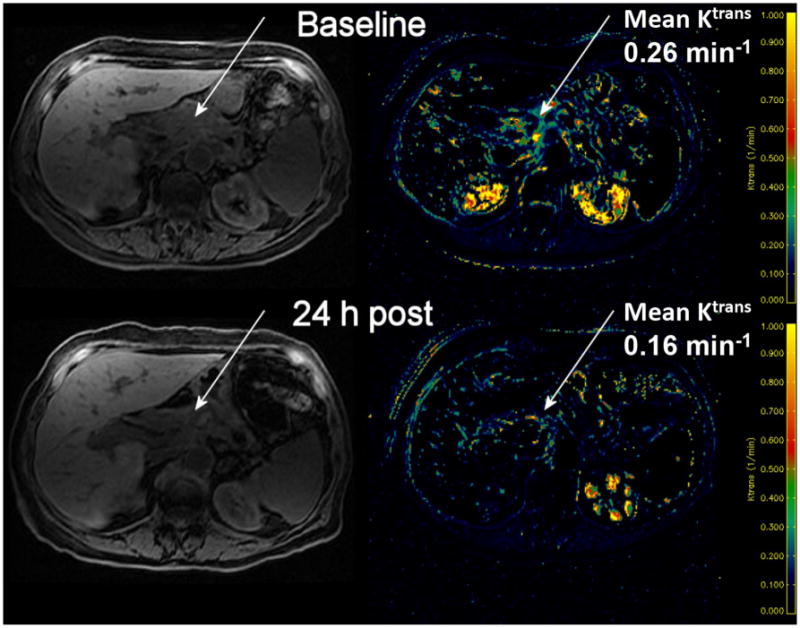
DCE-MRI studies were conducted in three patients, all of whom were on 250 mg twice daily dosing at the time of analysis. First, the baseline mean Ktrans value was established for each patient prior to treatment, and the DCE-MRI study was repeated 24 h after the first drug dose. While in two patients there were no significant changes post-treatment, in the third patient there was a decrease in the mean Ktrans value 24 h after the first dose of vatalanib (Fig. 4).
Fig. 4. DCE-MRI changes in a patient before and 24-h after the first dose of vatalanib.
Discussion
Single-agent gemcitabine has been the mainstay of systemic treatment for advanced pancreatic adenocarcinoma for more than a decade. The addition of erlotinib to gemcitabine in the first-line setting added a statistical, but clinically modest overall survival benefit in unselected patients compared to gemcitabine alone [21]. After many negative combination trials, two recent front-line combination regimens have shown significant improvement in overall survival over single-agent gemcitabine in randomized phase 3 trials and may be regarded as standard of care for appropriate patients [3, 4].
Although most clinicians offer second-line chemotherapy to patients with preserved performance status who progress on front-line therapy, so far there is a paucity of level one evidence for efficacy of systemic therapy after prior chemotherapy failure in this disease. A recent review of the literature identified a trend for survival benefit for patients receiving treatment with systemic therapy compared to best supportive care (BSC) [22]. Although randomized data are lacking, the summary of the available trials appears to favor a combination of platinum with either fluoropyrimidines or gemcitabine [22]. However, only a fraction of patients progressing on first-line treatment are able to receive subsequent therapy, and thus, the results of small phase 1 and 2 trials need to be evaluated with caution.
One of the major problems in drug development for advanced pancreatic adenocarcinoma has been the difficulty to accrue sufficient patient numbers to draw meaningful conclusions. This need clearly has to be addressed through multi-institutional efforts, as recently reviewed in the Journal of Clinical Oncology [23]. The feasibility of large multi-institutional trials in the USA was recently demonstrated in the MPAC trial (Metastatic Pancreatic adenocarcinoma Clinical trial), which enrolled over 800 patients with untreated metastatic pancreatic carcinoma, and established the superiority of nab-paclitaxel plus gemcitabine over gemcitabine alone in this setting [3]. Similarly, large randomized trials are lacking thus far in second-line setting, and very few phase three studies have been reported. the German CONKO Study group published the results of a phase 3 trial comparing the combination of oxaliplatin, 5FU, and leucovorin (“OFF” regimen) with BSC; the trial was closed after 46 patients due to poor accrual, but reported 9.9 month OS in GMM-OFF arm [24, 25].
The current study was undertaken based on convincing preclinical data on the efficacy of multi-kinase target inhibition (mainly of the VEGF pathway) in pancreatic adenocarcinoma models [11, 12]. the “ramp-up” dosing schedule was selected to optimize trough drug levels, based on previous phase 1 experience [13]. The observed 6-month OS rate of 29 % (the primary endpoint) is consistent with historical single-agent efficacy in the second-line setting for pancreatic adenocarcinomas: a summary of 29 previous trials reported on average a PFS and OS of 1.6 and 5.3 months, respectively [22]. Doublet platinum containing regimens have slightly higher PFS and OS of 4 and 6 months, respectively, but multi-agent chemotherapy studies are more toxic and the modestly better results are likely influenced by selection bias, different definitions for progression, etc. [22]. Also, it is noteworthy that the majority of the previous trials have been single- and oligo-institutional studies with relatively few patients on each trial [22], which might further explain the differences observed between our trial and historical data [26]. The main adverse events observed, hypertension and fatigue, are well described with VEGF-R targeting small molecules and generally manageable [27].
The correlative studies in our trial examined the role of LDH, and a number of serum cytokines implicated in the VEGF pathway, as potential predictive markers for vatalanib. While serum LDH levels did not correlate with PFS, implicating LDH is not predictive of response to vatalanib, we did observe a significant correlation between LDH and survival at 6 months, confirming the prognostic value of this marker, which has been also reported in other solid tumors [28, 29]. Higher LDH levels were associated with longer PFS in exploratory analyses of a previous trial of vatalanib in colorectal carcinoma [16]. However, the CONFIRM-1 trial combined vatalanib with a chemotherapy backbone (FOLFOX4), the association with PFS was retrospective, the drug combination was assessed in the first-line setting, included patients with a different malignancy (i.e., colorectal carcinoma), using a once daily dosing of 1,250 mg of vatalanib, and included a total of 1,168 patients, thus potentially explaining the different results compared to our study [16].
In a subset of 37 patients with available serum samples for angiogenic factor analysis, only pre-treatment IL-6 levels correlated with survival, while none of the factors measured pre- or post-treatment predicted for response to vatalanib. Higher levels of IL-6, a pleiotropic cytokine with pro-inflammatory properties, have been associated in patients with pancreatic adenocarcinoma with worse outcomes and correlated with poor performance status and weight loss in a previous study [30]. Thus, our study confirms the previously described role of IL-6 and LDH as potential prognostic markers in pancreatic adenocarcinoma. However, none of the serum angiogenic factors measured in our patients had predictive value. It is also likely that our study was not adequately powered to detect potentially small changes in serum markers tested.
With the emergence of ever more anti-angiogenic therapies [27], the search for useful markers of response and resistance to such therapies has been a very active area of research, and the current challenges in identifying and validating appropriate candidates have been discussed elsewhere [31].
While the majority of clinical benefit seen with vatalanib was due to disease stabilization, we did observe objective tumor responses in two patients. In addition, one of three tumors with available DCE-MRI data showed an early decrease in the mean Ktrans value, suggesting intratumoral perfusion changes in patients as early as 24 h after the first vatalanib dose. These clinical and radiographic observations implicate a possibility of real clinical benefit in a subset of patients. Recent paper that reviews the outcomes of pancreatic cancer gene sequencing underscores the heterogeneity of molecular pathway disruption in pancreatic cancers [32]. Perhaps, it is not surprising then that two large phase 3 trials evaluating addition of bevacizumab to gemcitabine-based chemotherapy in patients with metastatic pancreatic cancer failed to reach their efficacy endpoints as study populations were not enriched for patients sensitive to anti-angiogenic therapy [33, 34]. This approach recently identified a small subset (about 8 %) of urothelial carcinomas which harbor TSC1 (tuberous sclerosis complex 1) mutations and thus are susceptible to the mTOR inhibitor everolimus [35]. Interestingly, a recent paper by Reni et al. [36] reports clinical activity of another oral multi-kinase inhibitor, sunitinib, when given as a maintenance therapy in patients with metastatic pancreatic cancer after first-line therapy. This, together with observed clinical activity of vatalanib in our trial may warrant further investigations of vatalanib in the maintenance setting and/or in a biomarker-defined subset of pancreatic cancer patients.
Acknowledgments
The authors are thankful to Ms. Amy Stoll from TGEN/PCRT, Drs. Denise Roe and Haiyan Cui from Biometry Core at the University of Arizona Cancer Center, and Ms. Tamara Burk head for the help with trial coordination and manuscript preparation. This work was supported in part by P50 CA95060 (E. Gerner), CA017094, and P30 CA023074 from the national Cancer Institute to University of Arizona Cancer Center (TD and AFB), for correlative science. Additional support for imaging studies was provided by GE Healthcare. Supported by Investigator Initiated Grant (from Novartis) to T.D.
Footnotes
Presented in abstract form at the 2009 ASCO annual Meeting in Orlando Florida.
Conflict of interest: None.
Contributor Information
T. Dragovich, Email: tomislav.dragovich@bannerhealth.com, Banner MD Anderson Cancer Center, 1900 N. Higley Road, Gilbert, AZ 85234, USA; The University of Arizona Cancer Center, Tucson, AZ, USA.
D. Laheru, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA
F. Dayyani, Banner MD Anderson Cancer Center, 1900 N. Higley Road, Gilbert, AZ 85234, USA
V. Bolejack, Cancer research and Biostatistics, Seattle, WA, USA
L. Smith, South Texas Oncology Hematology, San Antonio, TX, USA
J. Seng, Virginia Piper Cancer Institute, Minneapolis, MN, USA
H. Burris, Sarah Cannon Cancer Center, Nashville, TN, USA
P. Rosen, Tower Cancer research Foundation, Beverly Hills, CA, USA
M. Hidalgo, Spanish national Cancer research Centre (CNIO), Madrid, Spain
P. Ritch, Medical College of Wisconsin, Milwaukee, WI, USA
A. F. Baker, The University of Arizona Cancer Center, Tucson, AZ, USA
N. Raghunand, The University of Arizona Cancer Center, Tucson, AZ, USA
J. Crowley, Cancer research and Biostatistics, Seattle, WA, USA
D. D. Von Hoff, Virginia G. Piper Cancer Center at Scottsdale Healthcare, Scottsdale, AZ, USA
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Lillemoe KD, Castillo CF. Pancreatic surgery for adenocarcinoma. Curr Opin Gastroenterol. 2012;28(5):488–493. doi: 10.1097/MOG.0b013e3283567f2c. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Campen CJ, Dragovich T, Baker AF. Management strategies in pancreatic cancer. Am J Health Syst Pharm. 2011;68(7):573–584. doi: 10.2146/ajhp100254. [DOI] [PubMed] [Google Scholar]
- 6.Abdelrahim M, et al. Angiogenesis: an update and potential drug approaches (review) Int J Oncol. 2010;36(1):5–18. [PubMed] [Google Scholar]
- 7.Kleeff J, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 8.Whipple C, Korc M. Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks arch Surg. 2008;393(6):901–910. doi: 10.1007/s00423-008-0280-z. [DOI] [PubMed] [Google Scholar]
- 9.Garcea G, et al. Hypoxia and angiogenesis in pancreatic cancer. ANZ J Surg. 2006;76(9):830–842. doi: 10.1111/j.1445-2197.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 10.Bold G, et al. New anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesis. J Med Chem. 2000;43(12):2310–2323. doi: 10.1021/jm9909443. [DOI] [PubMed] [Google Scholar]
- 11.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62(7):1996–2003. [PubMed] [Google Scholar]
- 12.Solorzano CC, et al. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biother Radiopharm. 2001;16(5):359–370. doi: 10.1089/108497801753354267. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AL, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23(18):4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Drevs J, et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol. 2005;16(4):558–565. doi: 10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 15.Drevs J, et al. A phase IA, open-label, dose-escalating study of PTK787/ZK 222584 administered orally on a continuous dosing schedule in patients with advanced cancer. Anticancer res. 2010;30(6):2335–2339. [PubMed] [Google Scholar]
- 16.Hecht JR, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29(15):1997–2003. doi: 10.1200/JCO.2010.29.4496. [DOI] [PubMed] [Google Scholar]
- 17.Morgan B, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21(21):3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz LH, et al. evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45(2):261–267. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7(1):91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 20.Rajaraman S, et al. Automated registration of sequential breath-hold dynamic contrast-enhanced MR images: a comparison of three techniques. Magn Reson Imaging. 2011;29(5):668–682. doi: 10.1016/j.mri.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the national Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 22.Rahma OE, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol. 2013;24(8):1972–1979. doi: 10.1093/annonc/mdt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoos WA, et al. Pancreatic cancer clinical trials and accrual in the United States. J Clin Oncol. 2013;31(27):3432–3438. doi: 10.1200/JCO.2013.49.4823. [DOI] [PubMed] [Google Scholar]
- 24.Pelzer U, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47(11):1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Saif MW. New developments in the treatment of pancreatic cancer. Highlights from the “44th ASCO annual Meeting”. Chicago, IL, USA. May 30–June 3, 2008. JOP. 2008;9(4):391–397. [PubMed] [Google Scholar]
- 26.Bafeta A, et al. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: metaepidemiological study. BMJ. 2012;344:e813. doi: 10.1136/bmj.e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morabito A, et al. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11(7):753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30(27):3402–3407. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 29.Weide B, et al. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer. 2012;107(3):422–428. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebrahimi B, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101(12):2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kindler HL, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28(22):3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Cutsem E, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27(13):2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 35.Iyer G, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reni M, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer. 2013;49(17):3609–3615. doi: 10.1016/j.ejca.2013.06.041. [DOI] [PubMed] [Google Scholar]