Abstract
The duper mutation in Syrian hamsters shortens the free-running period of locomotor activity (τDD) to about 23 h and results in a type 0 phase-response curve (PRC) to 15-min light pulses. To determine whether exaggerated phase shifts are specific to photic cues and/or restricted to subjective night, we subjected hamsters to novel wheel confinements and dark pulses during subjective day. Small phase shifts elicited by the nonphotic cue were comparable in mutant and wild-type (WT) hamsters, but dark pulses triggered larger shifts in dupers. To assess further the effects of the duper mutation on light-dark transitions, we transferred hamsters between constant light (LL) and constant dark (DD) or between DD and LL at various circadian phases. Duper hamsters displayed significantly larger phase shifts than WT hamsters when transferred from LL to DD during subjective day and from DD to LL during subjective night. The variability of phase shifts in response to all light/dark transitions was significantly greater in duper hamsters at all time points. In addition, most duper hamsters, but none of the WTs, displayed transient ultradian wheel-running patterns for 5 to 12 days when transferred from light to dark at CT 18. The χ2 periodogram and autocorrelation analyses indicate that these ultradian patterns differ from the disruption of rhythmicity by SCN lesions or exposure to constant bright light. We conclude that the duper mutation specifically amplifies phase shifts to photic cues and may destabilize coupling of circadian organization upon photic challenge due to weakened coupling among components of the circadian pacemaker. Mathematical modeling of the circadian pacemaker supports this hypothesis.
Keywords: novel wheel, dark pulse, light transitions, phase shift, phase-response curve, duper, hamster, ultradian, constant light, entrainment
Endogenous daily oscillations (circadian rhythms) not only coordinate behavior with the environment but also maintain internal temporal organization. In mammals, circadian rhythms are produced by transcriptional-translational feedback loops involving a set of core clock components that includes the Period, Cryptochrome, Clock, and Bmal1 genes and their protein products. Posttranslational processes, such as phosphorylation of clock proteins, also determine the period and entrained phase (Meng et al., 2008; Reischl and Kramer, 2011). The duper mutation in Syrian hamsters shortens the period of the free-running locomotor rhythm to approximately 23 h. Duper is the first recessive circadian mutation discovered in mammals, but its genetic basis is not yet known. It differs from the well-studied tau mutation in that it is not a sequence change in the coding region of csnk1e or csnk1d, which encode casein kinase 1ε or 1δ, respectively (Lowrey et al., 2000; Monecke et al., 2011).
Although the duper mutation has no effect on the precision or stability of activity rhythms in constant darkness (DD; Bittman, 2012), it markedly amplifies phase-shifting responses to 15-min light pulses (Krug et al., 2011). The effect of duper on responses to nonphotic zeitgebers is not yet known. Activity pulses of approximately 3 h during subjective day have been reported to induce phase advances of 1 to 3 h in Syrian hamsters, similar in magnitude to the effect of a light pulse during early subjective night (Mrosovsky et al., 1992; Antle and Mistlberger, 2000; Mistlberger et al., 2002; Mistlberger et al., 2003; Webb et al., 2014). Exploration of the influence of duper on nonphotic shifts may provide insight into its effects on circadian organization and reveal fundamental mechanisms of phase shifting. Light-induced phase shifts result from induction of Per1 and Per2 transcription upon glutamate release from retinohypothalamic terminals during the subjective night (Yamamoto et al., 2001; Yan and Silver 2002). In contrast, activity-induced phase shifts associated with novel running wheels are caused by suppression of Period gene expression in the SCN of wild-type (WT) hamsters during subjective day (Maywood et al., 1999). Furthermore, the magnitude of phase shifts in response to photic stimuli is dependent on photoperiod, whereas that of nonphotic phase shifts is not (Evans et al., 2004). The tau mutation has been reported to increase the amplitude of nonphotic responses (Mrosovsky, et al., 1992; Biello and Mrosovsky, 1996). To determine whether exaggerated phase shifts in duper hamsters occur only in response to photic cues or if the mutation also amplifies responses to nonphotic (activity-induced) cues, we examined the effect of 3-h novel wheel confinements.
The exaggerated effects of light pulses in duper hamsters are confined to the active zone of the PRC, raising the question of whether the effects of the duper mutation on phase lability are confined to subjective night. Dark pulses may be used to explore this question, as these stimuli can induce phase advances and small phase delays when given during subjective day (Boulos and Rusak, 1982; Canal and Piggins, 2006). We subjected hamsters to dark pulses to determine whether the effects of the duper mutation are phase specific as well as zeitgeber specific.
Photic pulses (light or dark) are compound stimuli of entry and exit of the new lighting condition. Duper may specifically alter the response to light onset or to light offset. Albers (1986) argued that responses to single transitions summate in order to produce the effect of pulses of light or dark. Whereas DD to LL transitions induce delays during subjective day and advances during subjective night, LL to DD transitions induce phase advances during subjective day and delays during subjective night (Albers, 1986). Thus we examined responses to LL to DD and DD to LL transitions in WT and duper mutant hamsters to determine whether the effects of the dark pulses depend on the phase of entry into dark, the reentry into light, or a combination of the two. Unexpected responses of duper hamsters to transitions from DD to LL led us to compare effects of the mutation to other treatments that compromise the stability of circadian rhythms and to explore the ability of current mathematical models to explain the duper phenotype on the basis of alterations in oscillator coupling.
MATERIALS AND METHODS
Animal Maintenance
Syrian hamsters (Mesocricetus auratus) were group housed in 14:10 light/dark cycle (LD) with ad libitum access to food and water until they reached adulthood (3–8 months). A total of 106 hamsters, including 36 WT (18 male, 18 female) and 70 dupers (44 males, 26 females), were used in these experiments. All hamsters were derived from stock obtained from Lakeview (LAK:LVG) and have been bred in our laboratory for multiple generations as previously described (Monecke et al., 2011). As adults, animals were housed individually with continuous access to a running wheel (17.5 cm diameter) as previously described (Krug et al., 2011). To minimize artifactual changes in phase and period, cages and water bottles were changed every 30 days during experiment 1 and every 10 to 20 days in experiments 2 and 3. Highly absorbent bedding (Bed-o-cob, Maumee, OH) was used throughout. Hamsters had the same running wheel throughout the experiment except during 3-h confinement periods in experiment 1, in which they were placed in a novel confinement wheel of the same size. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst and conform to all U.S. federal animal welfare requirements.
Experiment 1: 3-h Novel Wheel Confinements and Light Pulses
Animals were placed in DD, and running wheel activity patterns were recorded for 10 to 12 days. Actimetrics (Wilmette, IL) software was used to collect activity data in 10-min bins and to assess the quantity and phase of wheel revolutions as previously described (Krug et al., 2011). Hamsters were then placed in a novel confinement wheel for 3 h. A dim red light (<0.1 lux) was used to aid in transfers in and out of confinement wheels. All animals received at least 3 confinements beginning at CT 0, 4, and 8 in counterbalanced order (Figure 1). If an animal ran fewer than 3000 revolutions over the course of the 3-h interval, a confinement was repeated. Although some animals did not run more than 3000 revolutions after a second confinement at a given phase, no confinement was repeated more than once. Controls were performed at CT 4 by handling animals at the same times as the entry and exit of the confinement to mimic disruption, without placement in a novel wheel. To compare the effects of the nonphotic manipulation with the response to light pulses, each hamster received a 15-min light pulse (220 lux, white fluorescent light; Philips [Eindhoven, the Netherlands] 25 watt Hg, F32T8/ADV841/XLL) at CT 18.5 after the effects of the final confinement were assessed. This was followed by 10 to 12 days in DD and a final light pulse at CT 15.
Figure 1.
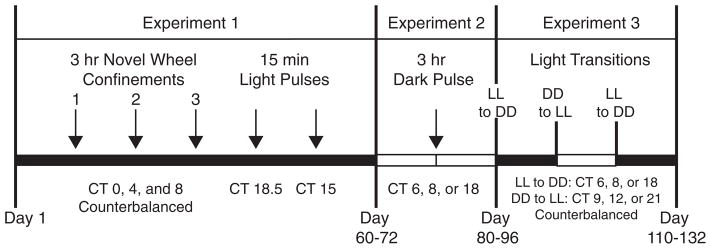
Timeline of experiments. Dark bars indicate constant darkness; open bars indicate constant light. The order of CT for novel wheel confinements and light transitions was counterbalanced. Animals were kept in a constant condition (light or dark) for 10 to 12 days between manipulations in all experiments. The 3 experiments generally lasted for a combined length of 110 to 132 days. If an animal repeated a novel wheel confinement due to a lack of activity, the total length of the experiment was extended to as much as 175 days. Novel wheel confinements using a modified Aschoff type II protocol were performed after this experimental timeline with different animals (see Suppl. Figure S1).
Upon finding that novel wheel confinement in DD produced small and inconsistent phase shifts, we performed additional experiments using modified Aschoff type II protocols that have been reported to produce larger effects (Webb et al., 2014). Fifteen additional animals (9 WT and 6 duper; 3 and 2 females, respectively) that had not been used in the other experiments were kept in a 14:10 LD cycle (120–150 lux, white fluorescent light) for 10 to 12 days while wheel-running patterns were recorded. At ZT 6 (6 hours before lights off), they were transferred to darkness with or without (control) a novel confinement wheel. After 3 h, the hamsters that had been confined to a novel wheel were released and their previous wheel was returned to them. After approximately 10 days of DD, the animals were returned to 14:10 LD for an additional 10 to 12 days. A second novel wheel confinement was performed with the modification that hamsters were exposed to 2 days of constant light (LL) before transfer to DD at ZT 6. All animals (excluding 2 females that were not part of the third manipulation) were subjected to all 3 novel wheel confinements in counterbalanced order. Cages were changed between manipulations at the time of return to the 14:10 LD cycle.
Experiment 2: 3-h Dark Pulses
After animals completed experiment 1, they were moved either to a 14:10 LD cycle for approximately 10 days or to dim LL. Additional duper females that had been housed in 14:10 LD but had not been included in experiment 1 were added to the study. After 10 to 12 days in dim LL (120–150 lux, white fluorescent light), a 3-h dark pulse was administered beginning in subjective day (CT 6 and 8) or subjective night (CT 18). At the end of the 3-h dark pulse, the animals were returned to LL for another 10 to 12 days so that phase shifts could be assessed.
Experiment 3: Light-Dark Transitions
Hamsters were moved from LL (of the same intensity as in experiment 2) to DD at CT 6, 8, or 18 and were maintained in darkness for approximately 10 days. Hamsters were then returned to dim LL at CT 9, 11, or 21 (to match the phase of the return to LL after a 3-h dark pulse in experiment 2). After 10 to 12 days in dim LL, they were returned to DD at CT 6, 8, or 18 for 10 to 12 days.
Statistical Analyses
Onset of activity was used as the phase marker to assess free-running period (assessed by linear regression; ClockLab software, Actimetrics). Phase shifts were calculated from the least squares fits plotted for 8 to 10 circadian cycles of steady-state wheel running before and after the manipulation (Bittman, 2012). Phase shifts of animals that displayed transients or ultradian behavior after LL to DD transitions at CT 18 were based on the steady state after they spontaneously regained consistent circadian rhythmicity.
All phase shifts are given in circadian hours (hours multiplied by 24 and divided by period of activity onsets preceding the manipulation). Variability of phase shifts was measured using tests for the concentration parameter (described below). The χ2 periodogram and autocorrelation were used to determine the period and strength of ultradian rhythms and were based on a minimum of 5 days of behavioral data. Novel wheel confinements and controls with prior exposure to LL or LD were analyzed using a repeated-measures analysis of variance (ANOVA) to compare manipulations within and between genotypes. When a significant difference was found, paired t tests were used to test for pairwise differences in means.
Circular Statistics
The resultant vector for a set of phases φk given in radians is , the circular mean is φ̄ = arg(r̄), and the circular standard deviation is . This value was multiplied by 12/π to convert phases from radians to circadian hours. To test whether the circular means of 2 or more groups are the same, we applied the Watson-Williams test (circular analog of the 1-factor ANOVA). The concentration parameter κ of a von Mises distribution (circular analog of the normal distribution) describes the spread of values: a higher value of κ corresponds to a narrower concentration about the circular mean (Fisher, 1993). To test whether 2 samples have the same concentration parameters, we applied a 2-sample test of equal concentration parameter with test statistic , where n1 and n2 are the sample sizes of the 2 groups.
Circular statistics were computed using MATLAB 2013a (MathWorks, Natick, MA) and CircStats Version 2012a (Berens, 2009).
RESULTS
Experiment 1: 3-h Novel Wheel Confinement and Light Pulses
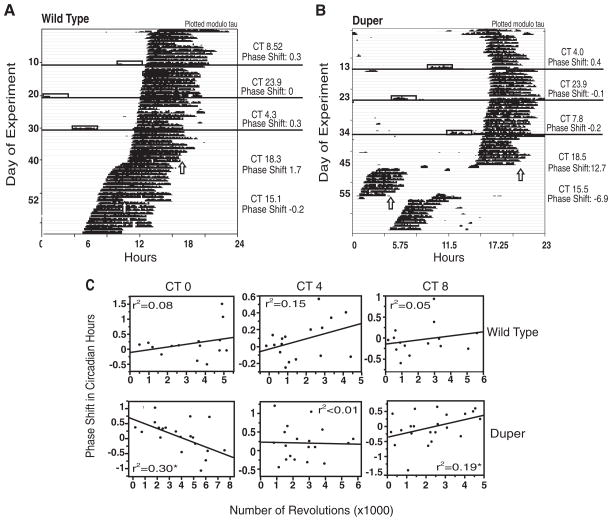
Confinement to a novel wheel in DD produced inconsistent phase shifts in both WT and duper hamsters at CT 0, 4, and 8 (all p > 0.3; Table 1 and Figure 2). At none of these phases was there a significant effect of genotype on the shift of phase of activity onset after confinement to a novel running wheel. At CT 4, phase shifts of duper but not WT hamsters were significantly larger than those of control animals of the same genotype that were handled but not placed in a novel wheel (p = 0.02 and p = 0.08, respectively; Table 1). All hamsters were of comparable age when tested, and there were no significant differences between males and females in phase-shift amplitude or the number of wheel revolutions (all p > 0.1). The number of wheel revolutions during the 3-h interval of confinement at all time points was similar between WT and duper hamsters for all trials as well as for trials in which animals ran more than 3000 revolutions (Suppl. Table S2). In neither genotype were phase shifts of hamsters that ran more than 3000 revolutions during the 3-h confinement significantly greater than the average of the entire group, and there was no genotype effect in these more active hamsters upon phase shifts elicited by confinement at CT 0, 4, or 8 (all p > 0.2; Table 1). Duper hamsters showed a small but statistically significant negative correlation between the number of revolutions and magnitude of phase advances at CT 0 (r2 = 0.30, slope = −0.001716, p = 0.01) and positive correlation at CT 8 (r2 = 0.19, slope = 0.00024, p = 0.04; Figure 2C). There were no other significant correlations between number of revolutions during the 3-h wheel confinement and phase shifts.
Table 1.
Phase shifts in response to novel wheel confinements and light pulses.
| Manipulation | Measurement | All Trials
|
|
|---|---|---|---|
| WT | Duper | ||
| Novel confinement wheels in DD | |||
| CT 0 | Phase shift (n) | 0.2 ± 0.4 (15) | 0.04 ± 0.6 (21) |
| CT 4 | Phase shift (n) | 0.1 ± 0.4 (18) | 0.3 ± 0.4 (19)* |
| CT 8 | Phase shift (n) | 0.03 ± 0.3 (15) | 0.3 ± 0.6 (24) |
| Revolutions (all confinements) | 2551 ± 211(48)* | 2583 ± 209 (64)* | |
| Control (DD) | |||
| CT 4 | Phase shift (n) | −0.03 ± 0.08 (5) | −0.003 ± 0.10 (8) |
| Revolutions | 5 ± 2 | 10 ± 5 | |
| Light pulses | |||
| CT 18.5 | Phase shift (n) | 2.6 ± 0.9 (15)* | −11.6 ± 2.6 (16)*† |
| CT 15 | Phase shift (n) | −1.3 ± 1.1 (15) | −5.6 ± 1.1 (16)† |
| Modified Aschoff type II novel wheel confinements at ZT 6 | |||
| LD to DD | Phase shift (n) | 0.54 ± 0.25 (9) | 1.87 ± 0.98 (6)† |
| Revolutions | 522 ± 123 | 1047 ± 364 | |
| LD to DD Control | Phase shift (n) | 0.58 ± 0.40 (9) | 2.07 ± 0.83 (6)† |
| Revolutions | 3 ± 2 | 183 ± 76* | |
| LL to DD | Phase shift (n) | 1.95 ± 0.78 (9)* | See text. |
| Revolutions | 733 ± 165 | See text. | |
All phase shifts are presented as circular mean ± circular SD given in circadian hours (number of animals). Number of revolutions for all experiments is provided as mean ± SEM. Significant differences (p < 0.05) between experimental and control groups within genotype and between genotypes are indicated (* and †, respectively). Where values are not provided for dupers in the modified Aschoff type II novel wheel confinement experiment, the sample size is too small for statistical analysis; see text for details. WT, wild type.
Figure 2.
Duper and wild-type (WT) hamsters show small and comparable phase shifts in response to novel wheel confinements. Representative actograms of responses of (A) WT and (B) duper hamsters to novel wheel confinement at CT 0, 4, and 8 (open boxes) and to light pulses (vertical arrows) at CT 15 and CT 18.5 are shown. Actograms are plotted modulo τDD for each genotype. (C) Linear regression of phase shifts in circadian time on the number of revolutions during the novel wheel confinement at CT 0, 4, and 8. Significant correlations (*p < 0.03) were found at CT 0 and CT 8 in duper hamsters. The number of revolutions during confinement was similar in WT and duper hamsters.
Further experiments used modified Aschoff type II protocols that have been reported to elicit larger and more consistent nonphotic shifts (Webb et al., 2014). The 3-h confinement to a novel wheel coincident with transfer from 14:10 LD to DD at ZT 6 induced phase shifts similar to those of nonconfined controls in both WT and duper hamsters (p = 0.84 and p = 0.51, respectively; Table 1). Dupers showed significantly larger phase shifts than WT hamsters in both conditions (repeated-measures ANOVA: p = 0.0007; paired t test: novel wheel p < 0.014; control p < 0.006; Table 1 and Suppl. Figure S1). The number of wheel revolutions during the 3-h interval of confinement was similar between WT and duper hamsters (Table 1). In control trials, dupers showed more activity than WT hamsters (p < 0.03; Table 1). There was no correlation between phase-shift amplitude and number of wheel revolutions within either genotype.
Exposure to 2 days of LL prior to confinement and transfer to DD at ZT 6 amplified phase shifts of WTs compared with those observed after wheel confinement in LD cycles (repeated-measures ANOVA between all manipulations: p < 0.001; paired t test: LL to DD, 1.95 ± 0.27 h vs. LD to DD, 0.54 ± 0.09 h, p < 0.001). Wheel confinement of WT hamsters in LL also increased the size of the shift compared with nonconfined controls (0.46 ± 0.19 h, p = 0.002). WT hamsters showed no correlation between phase-shift amplitude and the amount of activity (p > 0.1). The 4 duper hamsters subjected to such transfers showed highly variable shifts (0.78, 0.88, 2.29, and −9.5 h). The duper hamster exhibiting a 9.5-h phase delay ran more than other hamsters (5557 revolutions). However, other dupers that ran more than 3000 revolutions during confinement had phase shifts comparable to animals that were not active.
When given 15-min light pulses after the completion of assessment of the effects of novel wheel confinements in DD, duper hamsters showed larger phase shifts at CT 18.5 and CT 15 than did WT animals (Figure 2A,B and Table 1). At both phases, the circular mean phase shifts of the duper hamsters were significantly greater than those of the WT (Watson-Williams test: CT 15, F = 98.7, p < 0.001; CT 18.5, F = 152, p < 0.001).
Experiment 2: 3-h Dark Pulses
The 3-h dark pulses at CT 6, 8, and 18 elicited variable phase shifts. Duper hamsters tended to show larger phase shifts than did WT during subjective day (CT 6 and 8; Figure 3A,B), but genotype had no significant effect on circular mean (Watson-Williams test, F = 1.35, p = 0.26; Figure 4). Phase shifts in response to dark pulses at CT 6 and 8 were significantly more variable in duper than in WT hamsters (2-sample test of equal concentration parameter, F = 10.9, p < 0.001). The number of revolutions during the dark pulse was variable, did not differ significantly between WT and duper hamsters (983 ± 475 vs. 186 ± 156 revolutions, respectively, p > 0.14), and showed no correlation with the amplitude of the phase shift at any time point (CT 6: WT r2 = 0.32, duper r2 = 0.09; CT 8: r2 = 0.18, r2 = 0.03; CT 18: r2 = 0.07, r2 = 0.14, all p > 0.18).
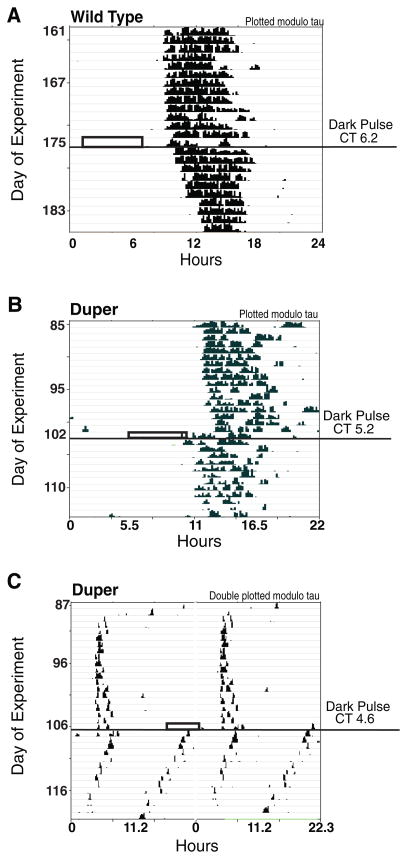
Figure 3.
The 3-h dark pulses during LL led to variable phase shifts and splitting in duper but not wild-type (WT) hamsters. Representative examples of responses of (A) WT and (B) duper hamsters to a 3-h dark pulse at approximately CT 6 are shown. Boxes indicate time of dark pulse, and circadian time of onset is indicated to the right of the actogram. Note that actograms are plotted modulo τLL. (C) Actogram of a duper hamster that split immediately after the dark pulse.
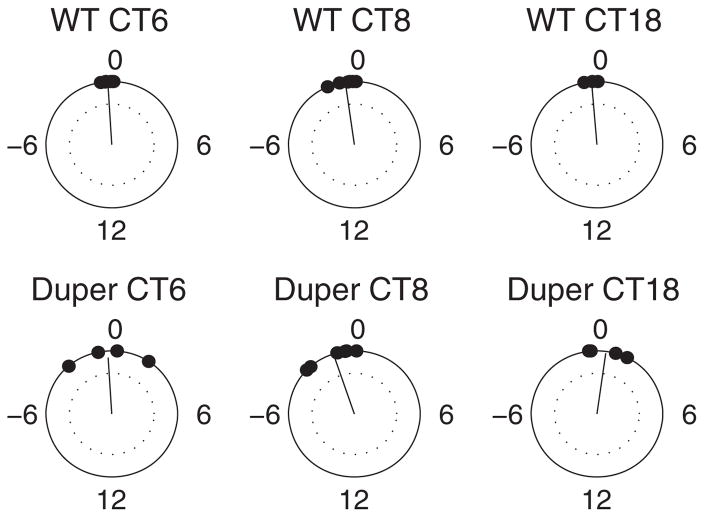
Figure 4.
Rayleigh plot of phase shifts in response to a 3-h dark pulse at CT 6, 8, and 18. The resultant vector’s direction indicates the circular mean of the phase shifts; the dotted circle indicates the α = 0.05 significance line for the Rayleigh test for nonuniformity (i.e., if the resultant’s endpoint is inside the dotted circle, the phases are not statistically different from the uniform distribution). Variability of phase shift was significantly greater in dupers than in WT hamsters at CT 6 to 8.
Splitting or arrhythmicity occurred after 3-h dark pulses in 8 of 21 duper hamsters and 0 of 16 WT hamsters. Two duper mutants split immediately after the dark pulse and 3 within 12 days after the dark pulse (Figure 3C), while 3 became arrhythmic immediately following a dark pulse.
Experiment 3: Light-Dark Transitions
Both LL to DD and DD to LL transitions resulted in significantly larger phase shifts in duper than in WT hamsters at particular circadian phases. Both types of light-dark transitions induced phase shifts that were significantly more variable in dupers than in WT hamsters (Figure 5).
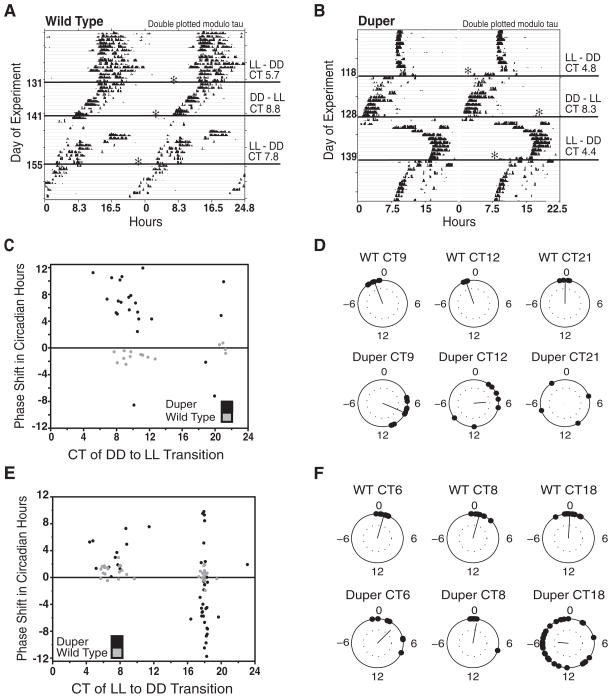
Figure 5.
Duper hamsters show larger phase shifts than wild-type (WT) hamsters in response to light and dark transitions. Representative responses of (A) WT and (B) duper hamsters to LL to DD and DD to LL transitions. The asterisk (*) marks time of transition. Actograms are double plotted modulo τ. (C) DD to LL transitions induced significantly greater phase shifts in duper than in WT hamsters at CT 9 and 12. Seven of 34 duper and 2 of 20 WT hamsters were excluded from analysis because they split in response to the DD to LL transition. (D) Variability of phase shifts was significantly greater in duper hamsters at all time points. (E) LL to DD transitions at CT 18, but not during subjective day, induced significantly larger phase shifts in duper (black points) than in WT hamsters (gray symbols; p< 0.001). (F) Phase shifts in subjective night (CT 18) were significantly greater than in subjective day (CT 6 and 8) in both duper and WT hamsters. Variability of phase shifts was significantly greater in duper hamsters at all time points (p < 0.001). Dotted circles in E and F are as in Figure 4.
DD to LL transitions during late subjective day (CT 9) and early subjective night (CT 12) induced significantly larger phase shifts in duper mutants than in WT hamsters (Watson-Williams test: F = 99, p < 0.001, at CT 9; F = 10.3, p = 0.009 at CT 12; Figure 5C,D). Duper hamsters were also more variable (2-sample test of equal concentration parameter: F = 6.5, p = 0.02 at CT 9; F = 204, p < 0.001 at CT 12). WT hamsters showed phase delays of 1.3 ± 0.8 and 1.2 ± 0.3 circadian hours at CT 9 and 12, respectively (circular mean ± circular SD). In contrast, duper hamsters exhibited phase advances ranging from 5 to 11 h at CT 9. At CT 12, both large advances (ranging from 3–7 h) and delays (8–12 h) occurred in the mutants (Figure 5C,D).
Phase shifts of WT hamsters transferred from DD to LL in late subjective night (CT 21) were uniformly less than 1 h (mean ± SEM, 0.03 ± 0.6). Dupers showed great variability in resetting (−10.6 ± 5.4 circadian hours), with both phase advances and delays occurring and a significantly different concentration parameter than WT (2-sample test of equal concentration parameter, F = 79.0, p < 0.001; Figure 5C,D). Change in τ after DD to LL transitions did not differ between WT and duper hamsters at any phase (WT vs. duper at CT 9: 0.21 ± 0.05 h vs. −0.04 ± 0.23 h; at CT 12: 0.32 ± 0.12 h vs. 0.02 ± 0.23 h; at CT 21: 0.15 ± 0.05 h vs. −0.37 ± 0.44 h, all p > 0.14) or between phases within phenotype (WT, all p > 0.2; duper, all p > 0.4).
LL to DD transitions induced similar phase shifts at CT 6 and 8 in duper and WT hamsters (circular mean F = 0.98, p = 0.33, Watson-Williams test), but the variability was much greater in duper hamsters (2-sample test of equal concentration parameter, F = 11.6, p < 0.001; Figure 5E,F). Phase shifts in response to LL to DD transitions at CT 18 were significantly larger in duper than in WT hamsters (Watson-Williams test, F = 26.9, p < 0.001), and duper hamsters again exhibited greater variability (2-sample test of equal concentration parameter, F = 23.5, p < 0.001; Figure 5E,F). Duper and WT hamsters did not differ in change of τ after LL to DD transitions at any phase (CT 6: WT −0.25 ± 0.09 h vs. duper: −0.14 ± 0.11 h; CT 8: −0.20 ± 0.07 h vs. −0.35 ± 0.29 h; CT 18: −0.20 ± 0.05 h vs. 0.10 ± 0.16 h, all p > 0.3), nor did the phase of transition have an effect on the change of τ within either genotype (WT, all p > 0.6; duper, all p > 0.13).
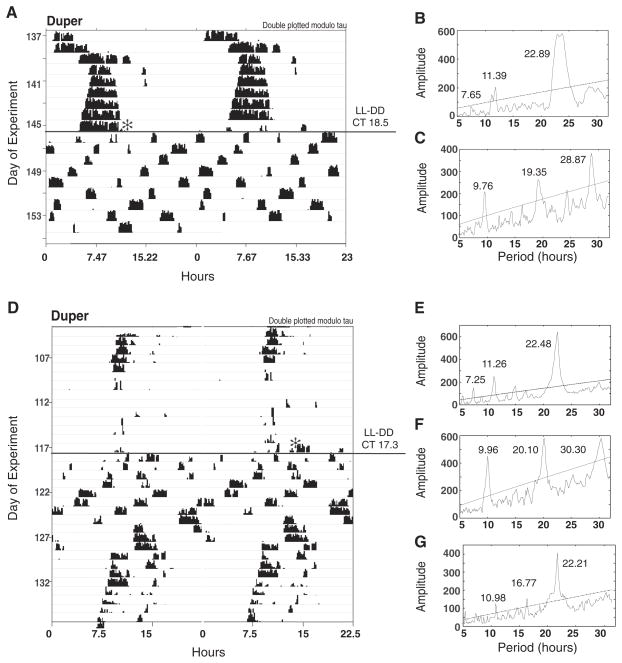
LL to DD transitions at CT 18 induced ultradian patterns of wheel-running behavior in 29 of 43 duper and 0 of 21 WT hamsters. This did not occur when the dupers were shifted to DD at other phases. Ultradian locomotor behavior lasted for approximately 6 to 12 days following the transition to DD (Figure 6). The period of the ultradian rhythms averaged 7.47 ± 1.67 h (range, 5–10 h). The period of the ultradian rhythm was not correlated with the number of days of ultradian activity (r2 = 0.04), the amplitude of the phase shift (r2 = 0.02), or the change in period after rhythmicity was regained (r2 = 0.03; all p > 0.4). When kept in DD, all duper hamsters spontaneously regained stable free-running patterns (Figure 6D). There was no significant change in the free-running period before (τLL) versus after (τDD) the interval of ultradian activity, regardless of whether the χ2 periodogram or autocorrelation was used to analyze these effects (Suppl. Figures S3 and S4).
Figure 6.
LL to DD transition at CT 18 leads to ultradian locomotor rhythms in some duper hamsters. Actograms are plotted modulo τLL. (A, D) Two examples of duper hamsters in which circadian locomotor patterns were transiently disrupted upon transfer from LL to DD during mid-subjective night. Twenty-nine of 43 duper and 0 of 21 WT hamsters displayed ultradian locomotor behavior for at least 5 days following the shift to DD. This did not occur when dupers were shifted from LL to DD at other phases. (B, E) The χ2 periodogram analysis of locomotor patterns in LL before the transition; (C, F) analysis of ultradian behavior during its duration after the LL to DD transition; (G) analysis of locomotor patterns after the spontaneous resumption of circadian rhythmicity in DD.
Mathematical Modeling
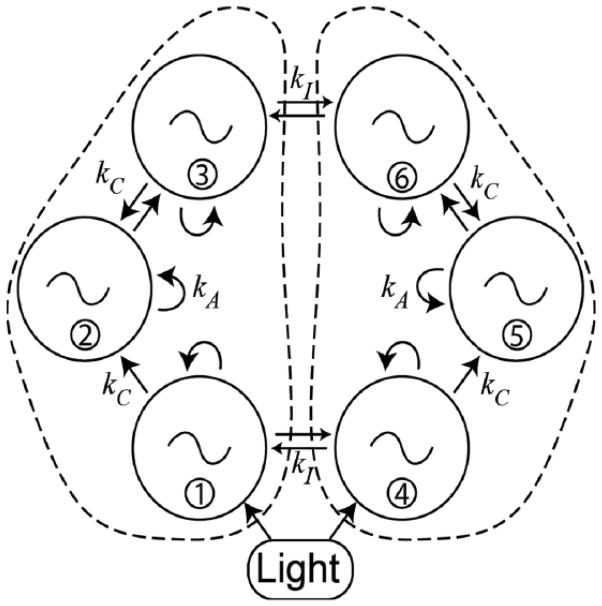
To examine whether reduced coupling can account for the altered resetting and other duper properties, we developed a mathematical model to simulate the duper mutation. We sought to determine whether a reduction of coupling strength could reproduce the essential features of the duper behavioral phenotype: shortened period, reduced amplitude (suggested by the gene expression analysis in Krug et al., 2011), type 0 photic PRC, increased range of entrainment (Bittman, 2014), and large, variable shifts following LL to DD transitions at CT 18, sometimes with transient loss of circadian rhythms. To this end, we employed a relatively simple model (Figure 7) consisting of coupled Goodwin oscillators adapted from Gonze et al. (2005). Details of the model, including parameter values, are given in the supplementary online material.
Figure 7.
Diagram of a simple model of the SCN using 6 Goodwin oscillators. Each oscillator represents a regional cluster of SCN neurons (2 light-responsive core clusters and 4 shell clusters), coupled as indicated by the arrows and organized into left and right lobes. The model is adapted from Gonze et al. (2005).
Our model of the circadian pacemaker consists of 6 regional oscillators, mimicking the simple multioscillator model network postulated by Yamaguchi et al. (2013), except that we include both left and right lobes. Coupling between these oscillators is indicated in Figure 7: kA denotes the autofeedback of each region to itself (representing communication among neurons within each regional cluster), kC denotes coupling between regions in the same lobe, and kI denotes interlobe communication. The autofeedback increases both the amplitude and period of each oscillator and is required for generating self-sustained oscillations. The WT parameters were chosen to reproduce a typical WT period, responses to light pulses, and ability to entrain to 24-h LD cycles. In particular, the model with the WT parameter values yields a free-running period of 23.8 h in DD, a lower range of entrainment of T22, and exhibits a weak resetting curve with all phase shifts less than 2 h in response to a bright 1-h photic pulse. Phase shifts in response to LL to DD transitions at all phases are modest (0–2 h), and oscillators always remain well synchronized. In the absence of coupling, oscillators are self-sustained for the WT parameters.
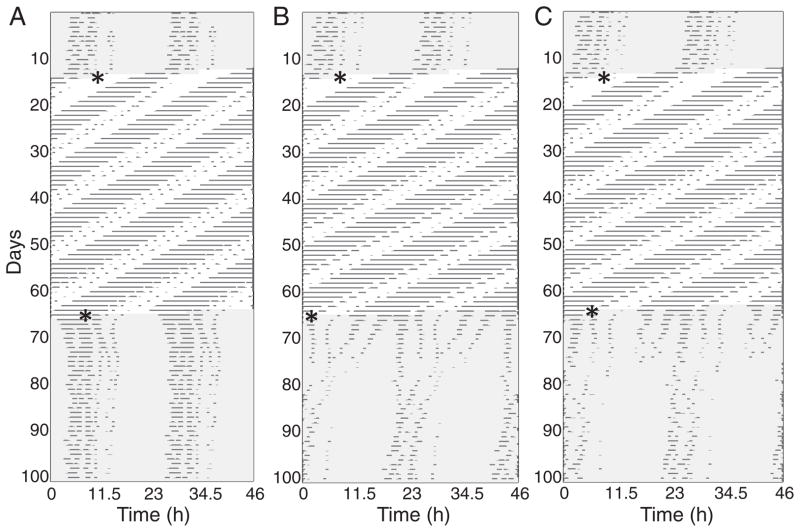
To simulate mutant behavior, we reduced the parameters controlling strength of coupling, among the components of the model to 63% of its WT value. All other parameter values, including light parameters, are the same as in the WT parameter set. The reduction in the coupling strength parameter results in a free-running period of 22.9 h, significantly reduced amplitude in all components, an extended range of entrainment that goes down to T18.5, and a strong resetting curve with 12-h phase shifts near CT 18. The response to LL to DD transitions at CT 18 is more dramatic, with severe disruptions of the circadian rhythm sometimes occurring. Slight changes to the period of a light-responsive oscillator result in large changes to the response to LL to DD transitions at CT 18, possibly explaining the large variability observed experimentally in Figure 5. See Figure 8 for examples of simulated actograms showing DD to LL and LL to DD transitions.
Figure 8.
Actograms of simulations of the duper mutant with DD to LL and LL to DD transitions at CT 18 (indicated by *). (A) Simulation using original parameter set with s4 = 1.055 (which controls the period of oscillator 4), in which oscillators remain synchronized. The system advances by 5 h in response to the DD to LL transition and delays by 4.5 h in response to the LL to DD transition. (B) Simulation of system with s4 = 1.058 (resulting in slightly shortened period), in which oscillators become disassociated in response to the LL to DD transition but resynchronize after several weeks. The system experiences a large, nearly anti–phase shift following the LL to DD transition. (C) System with s4 = 1.059, in which left and right sets of oscillators split for a week following the LL to DD transition, then exhibit weak rhythms for a week before spontaneously regaining a coherent free-running circadian rhythm. Actograms are constructed from the simulations by adding Gaussian noise at 10% of the maximum amplitude for each oscillator, then smoothing with a discrete wavelet transform to isolate the circadian component for each oscillator in the system. Activity occurs when any oscillator is near its peak value. Circadian time CT 12 corresponds to activity onset in the simulated actograms.
Although the model does not capture all details of hamster activity, it shows that the major features of the duper phenotype can be reproduced qualitatively as a result of reduced coupling within the circadian pacemaker.
DISCUSSION
As part of an ongoing effort to analyze the duper mutation, we have sought a deeper understanding of its behavioral phenotype. We used novel wheel confinements, dark pulses, and light/dark transitions to determine whether large phase shifts of duper mutants are specific to certain zeitgebers or restricted to particular circadian phases.
Our results provide no evidence that the duper mutation amplifies circadian responses to a nonphotic cue. This suggests that the mutation selectively alters responses to photic zeitgebers and/or its effects are restricted to subjective night. Unlike WT hamsters, duper mutants that were confined to novel wheels in DD showed significantly greater phase shifts at CT 4 than did handled controls. Nevertheless, the effect was small even in the duper hamsters. Genotype did not influence levels of activity over the 3 h spent in the novel wheel. Furthermore, restriction of our analysis to animals that ran more than 3000 revolutions over the course of the confinement revealed no effect of genotype. Despite the fact that our animals were comparatively active, the phase shifts that we observed were much smaller than those elicited by light in WT, let alone duper mutant hamsters. Given that the tau mutation can shift the phase of peak sensitivity to nonphotic zeitgebers (Mrosovsky et al., 1992; Biello and Mrosovsky, 1996) it is possible that we might find a greater response of duper hamsters at other circadian times, but the variability and small amplitude of the effects of the novel wheel in both genotypes at 3 different phases during the subjective day make this doubtful.
Although the phase shifts we observed in WT hamsters placed in novel wheels in DD were smaller than some that have been reported previously, the effects of this procedure have been highly variable in other laboratories (Reebs and Mrosovsky, 1989b; Mrosovsky et al., 1992; Bobrzynska and Mrosovsky, 1998; Duncan et al., 2014). Mrosovsky et al. (1992) reported that novel wheel confinement elicited only small phase shifts at CT 0 and 8. At CT 4, they obtained variable results: although shifts as great as 2 to 4 h occurred in many of the hamsters, some animals exhibited little or no phase shift even when they ran a substantial amount (Mrosovsky et al., 1992). Previous studies reported even more varied results with a 2-h pulse at CT 6, with most animals displaying phase shifts of less than an hour, similar to controls (Reebs and Mrosovsky, 1989a). Due to the variable results, Reebs and Mrosovsky (1989a) repeated the experiment with varying durations of novel wheel confinement (1, 3, and 5 h). They found that 3-h and 5-h confinements showed significantly larger phase shifts (2–3 h) than the 1-h confinements (0–1 h) but still noted that about 25% of the animals in longer confinements did not shift.
Other investigations of nonphotic zeitgebers have used modified Aschoff type II protocols, and some laboratories have found larger effects using such procedures (Bobrzynska and Mrosovsky, 1998; for review, see Webb et al., 2014). Thus, we followed our initial studies comparing novel wheel confinement in DD with additional experiments using Aschoff type II designs. Our findings were comparable to those of others who have found highly variable results and lack of an effect of the novel wheel using comparable procedures (Evans et al., 2004; Duncan et al., 2014). Although duper hamsters did show larger phase shifts when exposed to a light/dark cycle prior to novel wheel confinement at ZT 6 (compared to DD before confinement), this is likely a confound of the light stimulus given that there was no difference between control and experimental conditions. Although we found that WT hamsters had significantly larger phase shifts when exposed to LL for 2 days prior to confinement than when the novel wheel was introduced at ZT 6 of a 14:10 schedule or at CT 4 or 8 after 10 days in DD, this procedure also confounded the light with the nonphotic stimulus, and hamsters to whom a wheel was provided at the time of LD to DD transfer at ZT 6 showed no greater phase shifts than did controls. The profound differences between duper and WT hamsters in responses to transfer between LL and DD (experiments 2 and 3) greatly complicate interpretation of Aschoff type II designs in experiments on nonphotic phase shifting. Furthermore, the short period of duper hamsters results in a more positive phase angle and greater masking in LD cycles (Krug et al., 2011). This complicates assessment of the circadian phase at which the nonphotic stimulus is provided and makes comparison of duper and WT responses still more difficult.
Dark pulses provide another tool to explore the effects of the duper mutation during subjective day. In previous studies using WT Syrian hamsters, a 2- to 3-h dark pulse initiated at CT 6 to 8 resulted in an average phase advance of 2.5 h, with a range of 0 to 9 h (Boulos and Rusak, 1982; Canal and Piggins, 2006). Although we found the response of duper hamsters to be variable, none of our hamsters showed shifts larger than 4 h. Previous studies have shown that activity during the dark pulses contributes to the amplitude of the phase shift (Reebs et al., 1989). However, we found no significant difference between duper and WT hamsters in running activity during the 3 h of darkness. Furthermore, the number of revolutions during the 3-h dark pulse was not correlated with the amplitude of phase shifts. These observations further support the hypothesis that the effect of the duper mutation is specific to photic stimuli.
The light pulses typically used to provide insight into entrainment are complex stimuli. They include not only the interval of exposure to light or dark at a specific phase of a free run in otherwise constant lighting conditions but also acute transfers into or out of light and darkness. We used light to dark (LL to DD) and dark to light (DD to LL) transitions to isolate these events and to investigate further the effects of exposure to light and darkness at various circadian phases. The lability of phase in response to both DD to LL and LL to DD transitions was uniformly greater in duper than in WT hamsters. Consistent with previous studies (Albers, 1986; Aschoff, 1994), WT animals displayed phase shifts of 2 h or less at all phases tested, regardless of whether the transition was from light to darkness or vice versa. In contrast, duper hamsters experienced very large phase shifts, and within groups of mutants experiencing transitions at the same phase, we observed some delays and some advances. It is possible that shifts in response to a DD to LL transition during subjective day (during the dead zone of the PRC for light pulses) are not immediate but are triggered by an effect of light after the start of subjective night. However, dupers experienced larger phase shifts in response to a DD to LL transition at CT 9 or 12 than they did to a 15-min light pulse at CT 12 (Krug et al., 2011).
Although the duper mutation does not affect the stability or precision of free-running circadian rhythms in DD (Bittman, 2012, 2014), several of our observations of responses to photic manipulations provide evidence of lability and can be used to gain insight into circadian organization. First, we found that more than half of the duper hamsters transferred from LL to DD at CT 18 experienced a striking loss of circadian rhythmicity. In Siberian hamsters, a 5-h delay of an LD cycle with lights off at CT 17 can induce arrhythmicity or lead to an inability to reentrain (Ruby et al., 1996). This state seems to reflect a compromise of pacemaker function (Grone et al., 2011). Thus, it seems possible that the duper mutation may disrupt the SCN function or block its output or that the integration of cellular rhythms may be altered in a way similar to that seen in constant bright light. To evaluate these possibilities, we used autocorrelation analysis to compare these records of duper hamsters showing ultradian rhythms after this transition with those of SCN-lesioned tau mutant hamsters in DD (Bittman and Monecke, unpublished data) or of dupers in constant bright light (~300 lux; Bittman, 2014). SCN-lesioned tau hamsters displayed weak circadian rhythms or were arrhythmic but did not show ultradian wheel-running activity. Duper hamsters split or became arrhythmic when maintained in constant light but did not exhibit ultradian rhythms of locomotor behavior. We conclude that ultradian wheel-running patterns of duper hamsters when transferred from light to dark at CT 18 are a novel transition state rather than a suppression of SCN function.
A second indication of an effect of the duper mutation on the lability of the circadian system is our observation that a quarter of dupers split either immediately or within 10 days after a 3-h dark pulse (Figure 3C). Splitting implies decoupling of circadian components, such as the morning and evening oscillators (Pittendrigh and Daan, 1976; Daan and Berde, 1978). Boulos and Rusak (1982) observed occasional splitting several days after administration of dark pulses to WT hamsters, but immediate splits have not been observed previously. The immediacy and frequency of splitting in the dupers, which we did not observe in any of the WTs, suggests that the duper mutation may weaken coupling of pacemaker components.
A third indication that duper destabilizes the circadian system is the high variability of phase shifts in response to dark pulses at CT 6 and 8 and at all LL to DD and DD to LL transitions. An effect of duper to reduce oscillator amplitude (Krug et al., 2011) may also explain increased variability in phase shifts. Exaggerated responses of core clock components downstream from the perturbation of Per1 may contribute to or account for variability and lability of resetting.
Taken together, our results provide insight into the coupling strength and stability of the duper circadian system. We used simulations based on a mathematical model to test whether the behavior of duper mutants can be mimicked by manipulation of a coupling parameter. We found that reduction of coupling strength qualitatively captures the essential features of the duper behavioral phenotype: shortened period, reduced amplitude, type 0 photic PRC, increased range of entrainment, and large, variable shifts following LL to DD transitions at CT 18, sometimes with transient loss of circadian rhythms. While a simplified model cannot capture all details of hamster activity, the modeling does reproduce the features of interest in the duper phenotype through an overall decrease in coupling strength. Destabilization of the circadian pacemaker in dupers may be a consequence of reduced communication among the constituent components.
Although the genetic basis of the duper mutation is as yet unknown, our observations provide a basis on which to generate testable hypotheses. We cannot rule out the possibility that the duper mutation affects cell-autonomous properties, perhaps by altering expression of core clock genes. Nevertheless, our modeling suggests that a reduction of coupling between SCN neurons can parsimoniously explain the wide range of altered circadian dynamics observed in the duper mutant.
The retinorecipient core of the SCN is most directly affected by photic cues and thus may be the locus of changes that alter circadian function in duper mutant hamsters. Manipulation of VIP/VPAC2r (Aton et al., 2005; Hughes and Piggins, 2008), GABA (Freeman et al., 2013), vasopressin receptors (Yamaguchi et al., 2013), BMAL1 (Ko et al., 2010), or CLOCK (Vitaterna et al., 2006; Shimomura et al., 2013) reduces oscillator amplitude or cellular coupling. The decreased amplitude that is often a consequence of weakened coupling can drive some of the changes in dynamics, including type 0 PRCs. In various clock mutants, reduced amplitude of oscillations of gene expression has been observed to result in enhanced phase resetting. This may be explained theoretically using simple amplitude models (Lakin-Thomas et al., 1991; Vitaterna et al., 2006). Furthermore, coupling can directly affect circadian period. For instance, increased VIP activity in the SCN lengthens period (Aton et al., 2005; Pantazopoulos et al., 2010; Lucassen et al., 2012), and this finding can be reproduced by manipulating a VIP-like coupling mechanism in a model (Gonze et al., 2005).
We propose that the duper mutation affects a signaling pathway critical to normal coupling among neurons in the SCN. Further genetic studies to identify the location of the duper mutation, and thus elucidate the molecular clock mechanism, will test this prediction.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Mental Health grant RO1-MH070019, the National Institute of Child Health and Human Development R21-HD078863, and generous funding from the Department of Biology and the College of Natural Sciences of the University of Massachusetts Amherst. The authors thank Ian Webb for his advice on novel wheel confinements.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material is available on the journal’s website at http://jbr.sagepub.com/supplemental.
References
- Albers HE. Response of hamster circadian system to transitions between light and darkness. Am J Physiol Regul Integr Comp Physiol. 1986;250:R708–R711. doi: 10.1152/ajpregu.1986.250.4.R708. [DOI] [PubMed] [Google Scholar]
- Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. On the aging of circadian systems. In: Hiroshige T, Honma K, editors. Evolution of Circadian Clock. Sapporo: Hokkaido University Press; 1994. pp. 23–44. [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: A MATLAB toolbox for circular statistics. J Stat Software. 2009;31:1–21. [Google Scholar]
- Biello SM, Mrosovsky N. Phase response curves to neuropeptide Y in wild type and tau mutant hamsters. J Biol Rhythms. 1996;11:27–34. doi: 10.1177/074873049601100103. [DOI] [PubMed] [Google Scholar]
- Bittman EL. Does the precision of a biological clock depend upon its period? Effects of the duper and tau mutations in Syrian hamsters. PLoS One. 2012;7:e36119. doi: 10.1371/journal.pone.0036119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL. Effects of the duper mutation on responses to light: Parametric and non-parametric responses, range of entrainment, and masking. J Biol Rhythms. 2014;29:97–109. doi: 10.1177/0748730413520399. [DOI] [PubMed] [Google Scholar]
- Bobrzynska K, Mrosovsky N. Phase shifting by novelty-induced running: Activity dose-response curves at difference circadian times. J Comp Physiol. 1998;182:251–258. doi: 10.1007/s003590050175. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rusak B. Circadian phase response curves for dark pulses in the hamster. J Comp Physiol. 1982;146:411–417. [Google Scholar]
- Canal MM, Piggins HD. Resetting of the hamster circadian system by dark pulses. Am J Physiol Regul Integr Comp Physiol. 2006;290:R785–R792. doi: 10.1152/ajpregu.00548.2005. [DOI] [PubMed] [Google Scholar]
- Daan S, Berde C. Two coupled oscillators: simulations of the circadian pacemaker in mammalian activity rhythms. J Theoretical Biol. 1978;70:297–313. doi: 10.1016/0022-5193(78)90378-8. [DOI] [PubMed] [Google Scholar]
- Duncan M, Franklin KM, Peng X, Yun C, Legan SJ. Circadian rhythm disruption by a novel running wheel: Roles of exercise and arousal in blockade of the luteinizing hormone surge. Physiol Behav. 2014;131:7–16. doi: 10.1016/j.physbeh.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;286:R539–R546. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of spherical data. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78:799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonze G, Bernard S, Waltermann C, Kramer A, Herzel H. Spontaneous synchronization of coupled circadian oscillators. Biophys J. 2005;89:120–129. doi: 10.1529/biophysj.104.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Chang D, Bourgin P, Cao V, Fernald RD, Heller HC, Ruby NF. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- Hughes ATL, Piggins HD. Behavioral responses of Vipr2−/−mice to light. J Biol Rhythms. 2008;23:211–219. doi: 10.1177/0748730408316290. [DOI] [PubMed] [Google Scholar]
- Ko CH, Yamada YR, Welsh DK, Buhr ED, Liu AC, Zhang EE, Ralph SA, Forger DB, Takahashi JS. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 2010;8:e1000513. doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug S, Brewer JM, Bois AS, Bittman EL. Effects of the Duper mutation on circadian responses to light. J Biol Rhythms. 2011;26:293–304. doi: 10.1177/0748730411411570. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S, Coté GG. Amplitude model for the effects of mutations and temperature on period and phase resetting of the Neurospora circadian oscillator. J Biol Rhythms. 1991;6:281–297. doi: 10.1177/074873049100600401. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–491. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, van Diepen HC, Houben T, Michel S, Colwell CS, Meijer JH. Role of vasoactive intestinal peptide in seasonal encoding by the suprachiasmatic nucleus clock. Eur J Neuro. 2012;35:1466–1474. doi: 10.1111/j.1460-9568.2012.08054.x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian Period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci U S A. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NRJ, Piggins HD, et al. Setting clock speed in mammals: The CK1ε tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Webb IC, Jones M, Weinberg J, Pollock MS. Circadian clock resetting by arousal in Syrian hamsters: The role of stress and activity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R917–R925. doi: 10.1152/ajpregu.00222.2003. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: Dark pulses revisited. J Biol Rhythms. 2002;17:227–237. doi: 10.1177/07430402017003006. [DOI] [PubMed] [Google Scholar]
- Monecke S, Brewer JM, Krug S, Bittman EL. Duper: A mutation that shortens hamster circadian period. J Biol Rhythms. 2011;26:283–292. doi: 10.1177/0748730411411569. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Salmon PA, Menaker M, Ralph MR. Nonphotic phase shifting in hamster clock mutants. J Biol Rhythms. 1992;7:41–49. doi: 10.1177/074873049200700104. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Dolatshad H, Davis FC. Chronic stimulation of the hypothalamic vasoactive intestinal peptide receptor lengthens circadian period in mice and hamsters. Am J Physiol Regul Integr Comp Physiol. 2010;299:R379–R385. doi: 10.1152/ajpregu.00176.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- Reebs SG, Lavery RJ, Mrosovsky N. Running activity mediates the phase-advancing effects of dark pulses on hamster circadian rhythms. J Comp Physiol A. 1989;165:809–816. doi: 10.1007/BF00610879. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: Entrainment and phase response curve. J Biol Rhythms. 1989a;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N. Large phase-shifts of circadian rhythms caused by induced running in a re-entrainment paradigm: the role of pulse duration and light. J Comp Physiol A. 1989b;165:819–825. doi: 10.1007/BF00610880. [DOI] [PubMed] [Google Scholar]
- Reischl S, Kramer A. Kinases and phosphatases in the mammalian circadian clock. FEBS Lett. 2011;585:1393–1399. doi: 10.1016/j.febslet.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Saran ATUL, Kang T, Franken P, Heller HC. Siberian hamsters free run or become arrhythmic after a phase delay of the photocycle. Am J Physiol Regul Integr Comp Physiol. 1996;40:R881–R890. doi: 10.1152/ajpregu.1996.271.4.R881. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Kumar V, Koike N, Kim TK, Chong J, Buhr E, Whiteley AR, Low SS, Omura C, Fenner D, et al. Usf1, a suppressor of the circadian Clock mutant, reveals nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. eLife. 2013;2:e00426. doi: 10.7554/eLife.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduced circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Antle MC, Mistlberger RE. Regulation of circadian rhythms in mammals by behavioral arousal. Behav Neurosci. 2014;128:304–325. doi: 10.1037/a0035885. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: Brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin JM, Yamazaki F, Mizuguchi N, Zhang J, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.