Abstract
We evaluated longitudinal effects of alendronate on MRI-based trabecular bone structure parameters derived from dual thresholding and fuzzy clustering (BE-FCM) trabecular bone segmentation. Treatment effects were observed in the distal tibia after 24 months. The BE-FCM method increased correlations to HR-pQCT-based parameters.
Introduction
High-resolution magnetic resonance imaging (MRI) allows for non-invasive bone microarchitecture analysis. The goal of this study was to examine the potential of MRI-based trabecular bone structure parameters to monitor effects of alendronate in humans in vivo, and to compare the results to HR-pQCT and DXA measurements.
Materials and methods
Postmenopausal osteopenic women were divided into alendronate treatment and control groups, and imaged at baseline, 12 months, and 24 months (n=52 at baseline) using 3 T MRI, HR-pQCT, and DXA. Image acquisition sites included distal tibia (MRI and HR-pQCT), distal radius (MRI, DXA, and HR-pQCT), and the proximal femur (MRI and DXA). Two different regions of interest were evaluated. One contained the trabecular bone region within the entire MRI acquisition, and the second contained a subregion matched to the region contained in the HR-pQCT acquisition. The trabecular bone was segmented using two different methods; dual thresholding and BE-FCM. Trabecular bone structure parameters included bone volume fraction (BV/TV), number (Tb.N), spacing (Tb.Sp), and thickness (Tb.Th), along with seven geodesic topological analysis (GTA) parameters. Longitudinal changes and correlations to HR-pQCT and DXA measurements were evaluated.
Results
Apparent Tb.N and four GTA parameters showed treatment effects (p<0.05) in the distal tibia after 24 months in the entire MRI region using BE-FCM, as well as Tb.N using dual thresholding. No treatment effects after 24 months were observed in the HR-pQCT or in MRI analysis for the HR-pQCT-matched regions. Apparent BV/TV and Tb.N from BE-FCM had significantly higher correlations to HR-pQCT values compared to those derived from thresholding.
Conclusions
This study demonstrates the influence of computational methods and region of interest definitions on measurements of trabecular bone structure, and the feasibility of MRI-based quantification of longitudinal changes in bone microarchitecture due to bisphosphonate therapy. The results suggest that there may be a need to reevaluate the current standard HR-pQCT region definition for increased treatment sensitivity.
Keywords: Magnetic resonance imaging, Bone microarchitecture, Alendronate, Osteoporosis, Fuzzy clustering, DXA, HR-pQCT
Introduction
Osteoporosis is a skeletal disease characterized by low bone mass and deteriorated bone microarchitecture. It is associated with increased susceptibility to fracture and decreased bone strength [1]. Bisphosphonates are widely used in osteoporosis treatment due to their antiresorptive effects on bone. Alendronate is an extensively studied bisphosphonate which has been shown to increase bone mineral density (BMD) longitudinally [2–4], and to reduce fracture incidence [5]. While a vast majority of such studies measured BMD using dual-energy x-ray absorptiometry (DXA), there have been studies evaluating longitudinal effects of alendronate on postmenopausal osteoporosis using computed tomography [5], and high resolution peripheral quantitative computed tomography (HR-pQCT) [6,7].
In magnetic resonance imaging (MRI) of trabecular bone [8], bone marrow yields high signal intensity. Trabeculae are found adjacent to the marrow at locations with a lower signal intensity (Fig. 3). The relatively low spatial resolution achievable in vivo, is similar to or greater than the width of trabeculae. Therefore, trabecular bone parameters from MRI are typically annotated with the prefix apparent. Trabecular bone structure parameters analogous to those of bone histomorphometry in the literature include volume fraction (BV/TV), number (Tb.N), spacing (Tb.Sp), and thickness (Tb.Th) [9]. Geodesic topological analysis (GTA) is a new technique for trabecular bone structure quantification, focused on bone connectivity and structural anisotropy, that has shown promise in fracture discrimination [10]. The key processing step prior to trabecular structure parameter computations is trabecular bone segmentation. One approach to this step is a binarization of the trabecular region by a global dual threshold criterion [11]. However, this method is sensitive to partial volume effects and local intensity variations. A recent development designed to alleviate these problems is a partial membership segmentation by fuzzy clustering (BE-FCM) [12], where each pixel is assigned a value between 0 and 1 depending on local intensity and anisotropy.
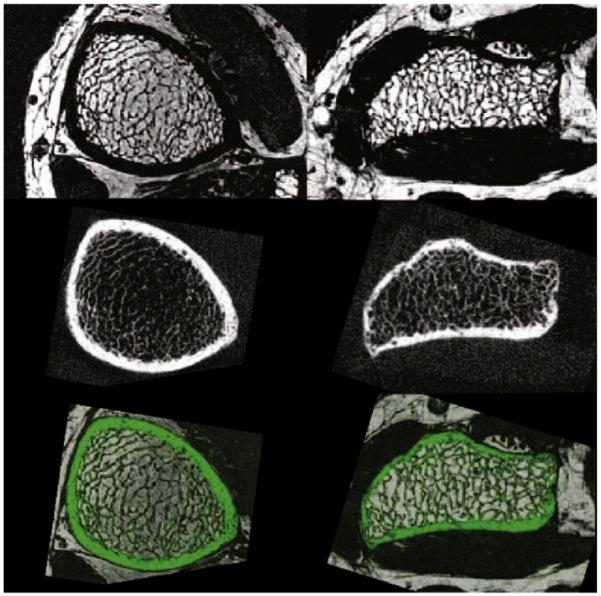
Fig. 3.
To the left: example images of the tibia, and to the right: example images of the radius. From top to bottom: a slice from an MR image, the corresponding slice from registered HR-pQCT image, and the registered HR-pQCT image (green) overlaid on the MR image.
Studies using MRI have demonstrated the potential to detect longitudinal changes in bone microarchitecture in vivo [13–16]. The ability of MRI-based trabecular bone analysis to detect changes in trabeculae in humans in vivo due to bisphosphonate treatment has not yet been well established. This study had three objectives: 1) to observe longitudinal changes in MRI-based trabecular bone structure parameters in postmenopausal osteopenic women treated with alendronate compared to a control group, and relate these changes to HR-pQCT and DXA measurements; 2) To compare the influence of trabecular bone segmentation using dual thresholding [11] vs. BE-FCM on the sensitivity and reliability of standard structural parameters; and 3) to evaluate two different region of interest definitions, one containing the trabecular bone region within the entire MRI acquisition, and the second containing a subregion matched to the region from the HR-pQCT acquisition.
Materials and methods
Subjects
Women between the ages of 45 and 65, with a mean age of 56 years (± 4 SD), were recruited for the study. Subjects had been postmenopausal for at least 1 but not more than 6 years, with a mean duration of postmenopause of 31 months. In addition, all subjects had a low BMD (T-score range − 1.1 to − 2.5) as determined by DXA at either the lumbar spine, the proximal femur trochanter, neck, or total proximal femur. Further subject characteristics can be seen in [6] Power calculations indicated a minimum group size of 23 subjects in order to detect a 5% treatment difference for apparent Tb.N at the radius over two years. Therefore, a total of 53 women were recruited and randomized 1:1; 26 in the treatment group (70 mg alendronate once weekly and 2800 IU of Vitamin D3 and Oscal daily) and 27 in the control group (2800 IU Vitamin D3 and Oscal daily). Exclusion criteria included fracture occurrence after the age of 50, history or evidence of metabolic bone disease other than postmenopausal bone loss, treatment within the previous year with any compound known to influence bone turnover, and estrogen usage within the past six months. Exclusion criteria due to the particular imaging techniques included weight greater than 250 lbs, presence of pacemakers, and claustrophobia. The study protocol was approved by the UCSF Committee of Human Research, and all patients gave written informed consent prior to participation. One subject was not correctly positioned for the HR-pQCT scan and was therefore not included in the study, resulting in a placebo group size of 26. A total of 18 subjects in the treatment group and 13 subjects in the placebo group completed the 24 month study for at least one imaging site, as can be seen in Fig. 1.
Fig. 1.
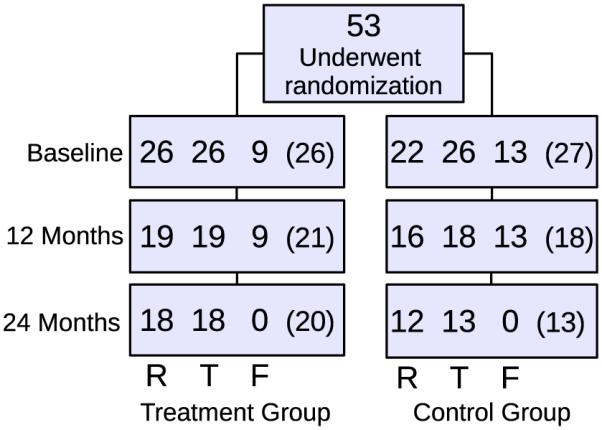
The number of subjects in the treatment and control groups at baseline, 12, and 24 months. The top three numbers represent the number of subjects that have completed distal radius (R), distal tibia (T), and proximal femur (F) MRI scans with sufficient image quality for further analysis, respectively. The number in parentheses represents the number of subjects scanned using HR-pQCT, while the lower number of completed MRI scans are due to incomplete scans because of patient discomfort, or inadequate image quality due to patient motion during the scan.
DXA measurements
Bone densitometry data were acquired using DXA at the proximal femur (trochanter), and distal radius (ultra-distal and total). At baseline, 42 subjects were scanned with a QDR 4500 (Hologic Inc., Bedford, Massachusetts, USA) and 11 subjects were scanned with a Lunar Prodigy (GE Healthcare, Piscataway, NJ, USA). Repeat measurements were obtained on the same scanners. BMD and T-scores were calculated at all sites. The standard NHANES III database was used to avoid discrepancies in the proximal femur. An example DXA measurement of the radius can be seen in Fig. 2.
Fig. 2.

To the left: an example DXA image of the radius with ultra-distal (UD) and total (largest rectangle) regions marked. To the right: the MRI view of the same radius with the MRI scan ROI (red) and the HR-pQCT-matched ROI (blue).
MRI acquisition
MR image acquisition was performed on a 3 T Signa scanner (General Electric Medical Systems, Waukesha, WI, USA) using a fully balanced steady-state free precession (bSSFP) sequence [17,18]. Images of the distal radius were obtained using a transmit/receive quadrature wrist coil (GE Medical). A 4-channel phased array coil (Nova Medical, Virginia, USA) was used to image the tibia and the proximal femur [19]. Due to exam time limitations, the proximal femur was only imaged if time and scheduling permitted. Distal tibia and radius scans were obtained over a 2.5 cm length. The bandwidth was ±122 Hz/pixel and the flip angle was 60° for all sites; additional scan parameters and spatial resolution are listed in Table 1. Example images of the tibia and distal radius can be seen in Fig. 3 and in Fig. 4 of the proximal femur.
Table 1.
Scan parameters and spatial resolution for the MRI acquisitions of the distal radius, distal tibia, and proximal femur.
| TR/TE (ms) | Scan time (min) | Resolution (μm3) | |
|---|---|---|---|
| Radius | 14.4–15.2/3.3–3.9 | 10 | 156×156×500 |
| Tibia | 16.8–17.8/6.5 | 15 | 156×156×500 |
| Femur | 11.7/4.6 | 10 | 234×234×1000 |
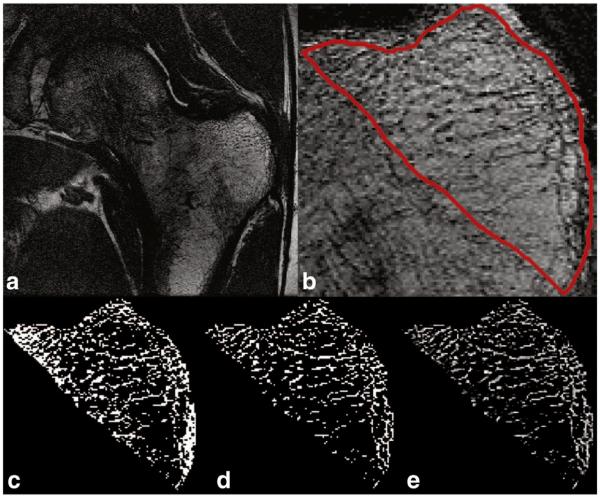
Fig. 4.
An example slice from a proximal femur MRI scan. a) the slice field of view. b) Image centered around the trochanter with the ROI delineated in red. c) Trabecular bone segmentation using dual thresholding. d) Trabecular bone segmentation using BE-FCM after thresholding. e) Trabecular bone segmentation using BE-FCM.
HR pQCT acquisition
HR-pQCT images were acquired using an in vivo scanner (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland), with an x-ray source with cone beam geometry (source potential 60 kVP, tube current 900 μA, and integration time 100 mms). A two-dimensional (2D) detector array is used to create a three-dimensional (3D) reconstruction. The distal radius and tibia were imaged over a 9mm slab (110 slices) with an isotropic nominal voxel size of 82 μm. The scan location began 9.5 mm proximal to the endplate in the radius, and 22.5 mm proximal to the endplate in the tibia. The effective dose of ionizing radiation was approximately 4.2 μSV per measurement.
Region of interest definition
Regions of interest (ROIs) were defined at the endosteal surface of the cortical bone for both the MRI and HR-pQCT images. The MRI scans of the distal tibia and radius were analyzed using two different ROI definitions. One ROI contained the trabecular bone region within the entire MRI acquisition. For a better comparison between modalities the second ROI contained the trabecular bone region within the MRI acquisition which matched the region contained in the HR-pQCT acquisition (see Fig. 2).
To determine the common region analyzed in the HR-pQCT images within the three time points, a standard evaluation protocol provided by the HR-pQCT manufacturer was applied. This evaluation protocol is based on a 2D cross sectional area matching method. After a common region for the HR-pQCT images between time points was determined, the common region between the HR-pQCT images and the MR images was found using a 3D rigid registration technique [20]. At each time point HR-pQCT images were registered to corresponding MR images using a registration software package (Rview) based on a normalized mutual information measure [21]. The result of the 3D rigid registration, three Euler angles and a translation vector, was then applied to the HR-pQCT ROI to determine the proximal and distal slices off the MR analysis region for comparison to HR-pQCT results. This technique avoided errors associated with the interpolation of the original images while ensuring that the analysis regions for all three time points in both the HR-pQCT and MR images contained the same start and end anatomical locations.
MRI analysis
Trabecular bone segmentation
ROIs were manually outlined within the bone cortex in the slices defined as described in the Region of interest definition section for the tibia and distal radius. In the proximal femur, an ROI was manually outlined in the ten most central slices of the scans including trabecular bone contained between the epiphyseal line and the cortex of the greater trochanter. The trabecular bone within the defined ROIs was segmented using two different methods: binarization by a global dual threshold criterion [11], and partial membership segmentation by bone enhancement fuzzy C-means clustering (BE-FCM) [12]. The dual thresholding method requires a user to define regions consisting of cortical bone. A threshold is then determined to binarize the image based on cortical bone intensity and marrow intensity (defined as intensity histogram peak position plus half peak width) [22]. BE-FCM produces a partial membership segmentation fully automatically, in which, based on the local intensity and anisotropy, each voxel is assigned a value between 0 and 1 depending on the amount of bone it is estimated to contain. Examples of a binarization and a partial membership segmentation can be seen in Fig. 4.
Structural parameters
Apparent trabecular bone volume fraction (BV/TV) was calculated by voxel counting. Apparent trabecular number (Tb.N) was determined using standard two-dimensional (2D) histomorphometric methods, specifically the mean intercept length (MIL) technique based on the plate model [9]. Apparent trabecular thickness (Tb.Th) and spacing (Tb.Sp) were derived from these primary measures using plate model assumptions. These are referred to as standard structure parameters.
In addition, trabecular bone parameters obtained from geodesic topological analysis (GTA) were computed on binary trabecular bone images [10]. The BE-FCM trabecular bone segmentations were binarized by assigning all pixels with a bone membership >0.5 to trabecular bone, since this signifies that a majority of the pixel area was occupied by bone signal. The junctions of the binary images were found using skeletonization, and bone pixels were each assigned to their nearest junction in terms of geodesic distance. The GTA parameters use trabecular junctions to quantify scale, topology, and anisotropy, as specified below.
Volume of junction (Tb.VJ) corresponds to the bone volume a junction is supporting.
Convex hull of junction (Tb.CHJ) corresponds to the space encompassed by a junction and its set of bone voxels.
Junction spacing (Tb.JSp) is related to the minimum geodesic distance between junctions.
Distance to junction (Tb.DJ) is the maximum geodesic distance between a junction and its bone voxels.
Junction eccentricity (Tb.JE) measures the eccentricity of the bone voxels belonging to a junction.
Junction orientation (Tb.JO) measures the anisotropy of the bone voxels belonging to a junction.
Interjunction orientation (Tb.IJO) is a global measure of anisotropy of the closest connected junctions.
Compared to the standard trabecular bone structure parameters, GTA is focused on the bone connectivity and structural anisotropy. Healthy trabecular bone is expected to be connected, and thus have a larger number of junctions compared to bone expressing pathology. If there were fewer junctions in the trabecular structure, they would need to support more bone per junction. Hence Tb.VJ increases with a decrease in the number of bone junctions. In Fig. 5 this can be appreciated qualitatively; the subject in the top row has higher than average GTA parameters and appears to have fewer junctions than the subject with lower GTA parameters at the bottom. Similar reasoning can be applied to the other GTA parameters, Tb.CHJ, Tb.JSp, and Tb.DJ are expected to increase with a decrease in junctions. For a more detailed description of the GTA parameters we refer to the work of Carballido-Gamio et al. [10].
Fig. 5.
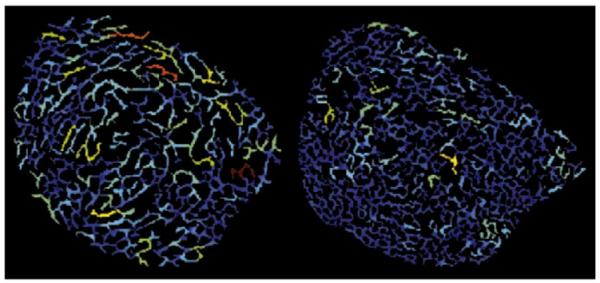
Example trabecular bone in a slice from an MRI image of a subject with higher GTA parameter values than average (left), and lower than average (right). Pixels belonging to the same junction have the same color. The color encodes the volume a junction is supporting, with red (blue) signifying high (low) apparent Tb.VJ.
HR-pQCT analysis
Trabecular bone segmentation
The HR-pQCT images were processed using the standard patient-style analysis offered by the manufacturer [23]. First, a semi-automated edge-finding algorithm generated a closed contour around the periosteal surface. Secondly, the bone cortex was masked and the trabecular region was identified using a fixed threshold of 16% of the global maximum image intensity after filtering with a Gaussian. The original image intensities inside the cortex were then convolved with a Laplace-Hamming filter, and trabecular bone was defined as intensities over 40% of the maximum intensity [24] (Fig. 6).
Fig. 6.

An example slice from a distal radius HR-pQCT scan. Left: the field of view of one slice with the ROI delineated in red. Right: trabecular bone segmentation using thresholding.
Structural parameters
The BV/TV calculation was based on volumetric BMD in the trabecular compartment and an assumed tissue density value of 1200 mg HA/cc. Tb.N was calculated in 3D using a mid-axis transformation to find trabecular ridges and the distance transform method [25]. Tb.Th and Tb.Sp were derived from BV/TV and Tb.N as described for the MRI analysis. GTA parameters were not calculated for HR-pQCT images since the current GTA software is limited to slice-by-slice analysis and might be suboptimal for the isotropic resolution of the HR-pQCT images.
Statistical analysis
A comparison between the groups and between the three time points for each of the parameters was made using a two-way analysis of variance with repeated measures over time (MANOVA). The statistical analysis was performed using the statistical software package JMP (SAS Institute, Cary, North Carolina, USA).
Pearson’s correlation coefficient was used to compare apparent BV/TV determined from MRI with BV/TV determined from HR-pQCT and BMD determined from DXA measurements. Comparison differences between correlations (comparing the correlation coefficient between variables j and h vs. correlation coefficient between j and k) were performed using Steiger’s statistics [26]. A statistical significance level of 5% was chosen, and a p-value between 5% and 10% was classified as a trend.
Results
Treatment evaluation of BMD from DXA
The difference between treatment and placebo groups in BMD measurements from DXA in the ultra-distal radius in a region matched to the HR-pQCT ROIs showed a trend towards treatment effects after 12 months (p=0.08) and 24 months (p=0.09). The BMD was maintained in the treatment group and decreased in the placebo group. The corresponding values for the total distal radius were significant after 12 months (p=0.02) and 24 months (p=0.006). For the proximal femur trochanter BMD measurements, there were no significant differences after 12 months but there was a significant difference between groups after 24 months (p=0.01). At 24 months, the BMD in the treatment group was increased, while being maintained in the placebo group throughout the study period. These results are summarized in Table 2.
Table 2.
Longitudinal results for BMD from DXA. Percent changes from baseline after 12 and 24 months for the ultra-distal radius (UD RAD), total distal radius (TOT RAD), and proximal femur trochanter are displayed (FEM), along with group difference for the treatment (T) and control (C) groups.
| 12 months | 24 months | |
|---|---|---|
| UD RAD T | −0.37† | −0.35† |
| UD RAD C | −2.42** ,† | −3.22** ,† |
| TOT RAD T | +0.17†† | +0.46†† |
| TOT RAD C | −1.67** ,†† | −2.20** ,†† |
| FEM T | +3.11** | +3.55†† |
| FEM C | +0.39 | +0.23†† |
Boldface signify statistical trend or significance;
*p<0.1 from baseline,
p<0.05 from baseline,
p<0.1 between treatment and control groups,
p<0.05 between treatment and control groups.
Treatment evaluation of trabecular bone structure parameters
BE-FCM-based parameters from MRI
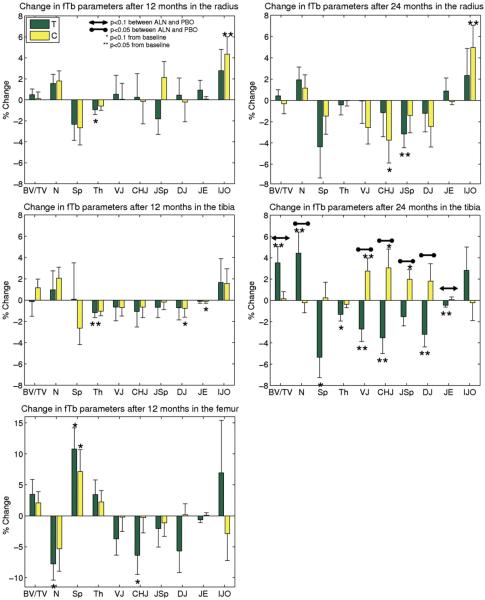
In standard trabecular bone structure parameters in the ROIs from the entire MRI acquisitions, there was a treatment effect in the distal tibia after 24 months for apparent Tb.N (p=0.03), and a trend towards a treatment effect for BV/TV (p=0.06). For these parameters, the values of the placebo group were maintained while in the treatment group the apparent BV/TV and Tb.N values increased compared to baseline (Fig. 7). Of the GTA parameters, apparent Tb.VJ (p=0.005), Tb.ChJ (p=0.009), Tb.JSp (p=0.01),and Tb.DJ (p=0.02) showed significant treatment effects after 24 months in the distal tibia. Tb.JE indicated some evidence of treatment (p=0.09). There was a decrease in the values of the treatment group, while the values of the placebo group were increased. No effects were evident in the distal radius or the proximal femur for any of the MRI parameters.
Fig. 7.
Longitudinal results of BE-FCM-based structure parameters from MRI using the entire MRI ROIs. Percent change (mean and SE) from baseline after 12 and 24 months for the distal radius, distal tibia, and proximal femur (after 12 months) are displayed, along with group difference for the treatment (T) and control (C) groups. Tb.JO was left out due to very large variations in the data.
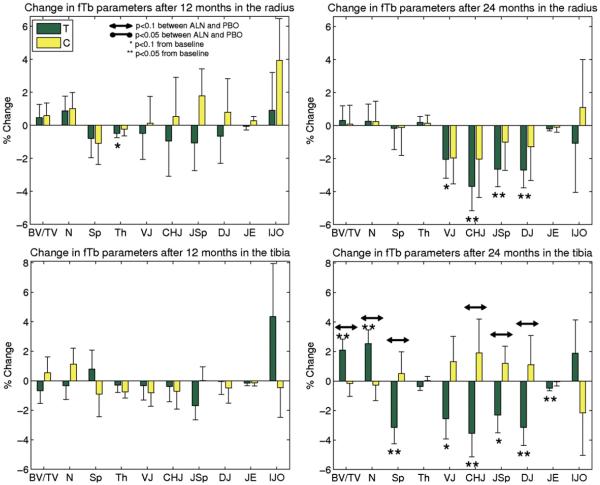
The corresponding results for the MRI analysis using HR-pQCT-matched ROIs can be seen in Fig. 8. Here, there were some trends towards treatment effects (p<0.1) similar to those obtained from the entire MRI region for some of the parameters.
Fig. 8.
Longitudinal results of BE-FCM-based structure parameters from MRI using HR-pQCT-matched ROIs. Percent change (mean and SE) from baseline after 12 and 24 months in the MRI data for the distal radius, and distal tibia, along with group differences for the treatment (T) and control (C) groups. Tb.JO was left out due to very large variations in the data.
Dual thresholding-based parameters from MRI
After 24 months, apparent Tb.N showed a treatment effect (p=0.04), and apparent Tb.VJ showed a trend towards a treatment effect (p<0.1) in the distal tibia. In the distal radius, there were no significant treatment effects after 24 months, although apparent Tb.JE showed an indication (p<0.1), and apparent Tb.JSp showed a treatment effect after 12 months (p=0.04). No effects were evident in the proximal femur. There were no evidence or indications of treatment effects in the HR-pQCT-matched ROIs.
Structure parameters from HR-pQCT
In the HR-pQCT data analysis only the volumetric BMD in the distal radius showed a difference between groups after 12 months (p=0.05), with no treatment effect after 24 months.
Comparison of MRI structure parameters and DXA measurements
A comparison between apparent BV/TV from the proximal femur trochanter and the DXA measurements of BMD at the same site revealed a correlation which was not significant when using dual thresholding segmentation for all measurements throughout the study period (R=0.12, p<0.4). Using BE-FCM, the correlation to DXA measurements was somewhat improved (R=0.32, p=0.04). The correlation was also improved for the distal radius; from (R=0.22, p=0.02) using thresholding to (R=0.28, p=0.003) using BE-FCM. The improvements were not significant using Steiger’s statistic (Tables 3 and 4).
Table 3.
Longitudinal results of BE-FCM-based trabecular structure parameters for the ROI in the entire MRI acquisition. Percent changes from baseline after 12 and 24 months for the distal radius, distal tibia, and proximal femur (after 12 months) are displayed, along with group difference for the treatment (T) and control (C) groups. Tb.JO was left out due to very large variations in the data.
| Radius 12 | Radius 24 | Tibia 12 | Tibia 24 | Femur 12 | |
|---|---|---|---|---|---|
| fBV/TV T | +0.48 | +0.43 | −0.13 | 3.53** ,† | +3.45 |
| fBV/TV C | +0.10 | −0.32 | +1.16 | 0.14† | +2.08 |
| fTb.N T | +1.55 | +1.94 | +0.97 | 4.43** ,†† | − 7.74* |
| fTb.N C | +1.79 | +1.14 | +2.07 | −0.22†† | −5.31 |
| fTb.Sp T | −2.33 | −4.36 | +0.08 | −5.35* | +10.80* |
| fTb.Sp C | −2.67 | −1.46 | −2.63 | +0.23 | +7.13* |
| fTb.Th T | −0.94* | −0.43 | −1.18** | −1.32* | +3.41 |
| fTb.Th C | −0.58 | −0.04 | −1.04 | −0.36 | +2.24 |
| fTb.VJ T | +0.52 | −0.06 | −0.65 | −2.69** ,†† | −3.73 |
| fTb.VJ C | +0.04 | −2.54 | −0.69 | 2.75** ,†† | −0.20 |
| fTb.ChJ T | +0.24 | −1.14 | −1.06 | −3.52** ,†† | −6.37* |
| fTb.ChJ C | −0.15 | −3.74* | −0.65 | 3.06* ,†† | −0.30 |
| fTb.JSp T | −1.82 | −3.16** | −0.67 | −1.54†† | −2.05 |
| fTb.JSp C | +2.12 | −1.41 | −0.17 | 1.96* ,†† | −1.11 |
| fTb.DJ T | +0.44 | −1.22 | −0.71 | −3.20** ,†† | −5.68 |
| fTb.DJ C | −0.22 | −2.44 | −0.77* | 1.79†† | +0.17 |
| fTb.JE T | +0.91 | +0.88 | −0.11 | −0.46** ,† | −0.66 |
| fTb.JE C | +0.06 | −0.11 | −0.11* | +0.07† | +0.11 |
| fTb.IJO T | +2.77 | +2.33 | +1.65 | +2.82 | +6.93 |
| fTb.IJO C | +4.36** | +4.98** | +1.55 | −0.21 | −2.85 |
Boldface signify statistical trend or significance;
p<0.1 from baseline,
p<0.05 from baseline,
p<0.1 between treatment and control groups,
p<0.05 between treatment and control groups.
Table 4.
Longitudinal results of BE-FCM-based structure parameters from MRI using HR-pQCT-matched ROIs. Percent change from baseline after 12 and 24 months in the MRI data for the distal radius, and distal tibia, along with group differences for the treatment (T) and control (C) groups. Tb.JO was left out due to very large variations in the data.
| Radius 12 | Radius 24 | Tibia 12 | Tibia 24 | |
|---|---|---|---|---|
| fBV/TV T | +0.74 | +0.31 | −0.68 | +2.09** ,† |
| fBV/TV C | +0.58 | +0.09 | +0.54 | −0.15† |
| fTb.N T | +0.88 | +0.27 | −0.33 | +2.54** ,† |
| fTb.N C | +1.00 | +0.24 | +1.12 | −0.27† |
| fTb.Sp T | −0.79 | −0.17 | +0.79 | −3.14** ,† |
| fTb.Sp C | −1.09 | −0.12 | −0.91 | +0.51† |
| fTb.Th T | −0.49* | +0.20 | −0.29 | −0.37 |
| fTb.Th C | −0.24 | +0.14 | −0.76 | +0.04 |
| fTb.VJ T | −0.50 | −2.04* | −0.34 | −2.55* |
| fTb.VJ C | +0.12 | −1.96 | −0.81 | +1.31 |
| fTb.ChJ T | −0.95 | −3.69** | −0.38 | −3.55** ,† |
| fTb.ChJ C | +0.53 | −2.03 | −0.71 | +1.91† |
| fTb.JSp T | −1.08 | −2.65** | −1.69 | −2.30* ,† |
| fTb.JSp C | +1.79 | −1.01 | +0.00 | +1.19† |
| fTb.DJ T | −0.67 | −2.69** | −0.05 | −3.16** ,† |
| fTb.DJ C | +0.79 | −1.28 | −0.48 | +1.09† |
| fTb.JE T | −0.06 | −0.19 | −0.16 | −0.49** |
| fTb.JE C | +0.27 | −0.10 | −0.14 | −0.05 |
| fTb.IJO T | +0.92 | −1.07 | +4.35 | +1.88 |
| fTb.IJO C | +3.94 | +1.09 | −0.47 | −2.15 |
Boldface signify statistical trend or significance;
p<0.1 from baseline,
p<0.05 from baseline,
p<0.1 between treatment and control groups,
††p<0.05 between treatment and control groups.
Comparison of MRI-based and HR-pQCT-based structure parameters
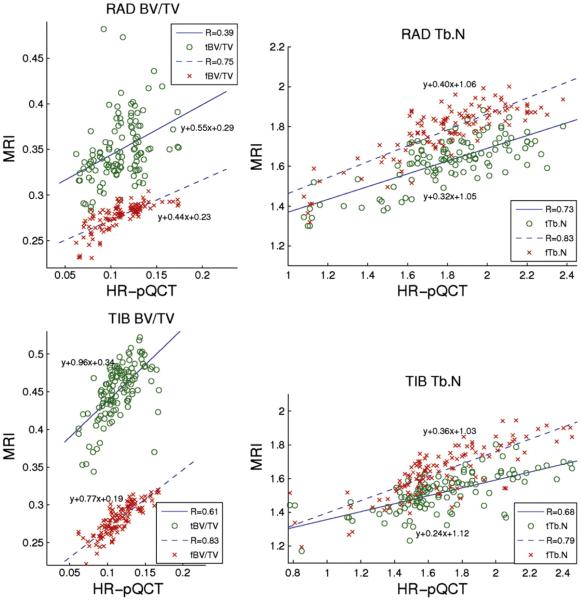
Correlations between trabecular bone structural parameters from HR-pQCT and MRI computed using both dual thresholding and BE-FCM trabecular bone segmentation and data from all three time points can be seen in Fig. 9 and Table 5. All apparent structural parameters from MRI are highly correlated to HR-pQCT structural parameters, with the exception of apparent BV/TV from thresholding and the apparent Tb.Th estimates for which correlations are less significant.
Fig. 9.
Correlations between structure parameters from MR and HR-pQCT computed using dual thresholding (t) and BE-FCM (f) trabecular bone segmentation. All correlations were statistically significant (p<0.0001).
Table 5.
Correlations between structure parameters from MR and HR-pQCT. Correlation coefficients (R) are listed. Correlations of structure parameters obtained using dual thresholding (prefixed t) and fuzzy clustering (prefixed f) trabecular bone segmentation are listed.
| Tibia (R) | Radius (R) | |
|---|---|---|
| tBV/TV | 0.61 | 0.39 |
| fBV/TV | 0.83*** | 0.75*** |
| tTb.N | 0.68 | 0.73 |
| fTb.N | 0.79* | 0.83** |
| tTb.Sp | 0.77 | 0.67 |
| fTb.Sp | 0.78 | 0.88*** |
| tTb.Th | 0.43 | 0.32 |
| fTb.Th | 0.53 | 0.15* |
Stars signify differences between correlations using Steiger's statistic;
p<0.05,
p<0.001,
p<0.0001.
Discussion
Study summary
In this study, we evaluated longitudinal effects of alendronate on trabecular bone structure parameters from MRI scans of the distal radius, distal tibia, and proximal femur. Significant treatment effects were found in the distal tibia for apparent Tb.N, Tb.VJ, Tb.ChJ, Tb.JSp, and Tb.DJ after 24 months using BE-FCM trabecular bone segmentation over the 2.5 cm length of the MRI acquisition. The dual threshold-based analysis showed similar trends, with a significant treatment effect for apparent Tb.N in the distal tibia after 24 months. The HR-pQCT analysis using the standard patient-style ROI over a 9 mm length showed no treatment effects after 24 months. Similarly, the MRI analysis using HR-pQCT-matched ROIs showed no treatment effects. Treatment effects were less evident in the structural parameters based on dual thresholding trabecular bone segmentation, and they were not as well correlated to HR-pQCT parameters as structural parameters from BE-FCM segmentation.
The benefits of BE-FCM compared to dual thresholding can be appreciated visually in Fig. 4; in areas with a low average intensity the dual thresholding overestimates the amount of bone. Many pixels contain both bone and marrow, and BE-FCM accounts for this type of partial volume effects by assigning a partial membership value, instead of a binary, to each pixel.
Related MRI based studies
Chesnut et al. [16] showed significant improvement in standard trabecular bone parameters in postmenopausal osteoporotic women treated with calcitonin vs. a control group. Zhang et al. [14] showed longitudinal changes in trabecular bone elastic moduli and standard structural parameters after two years in hypogonadal men treated with testosterone. In a similar study, Wehrli et al. [15] examined the effects of estrogen supplementation in postmenopausal women after one year using μMRI. The treatment group maintained more plate-like bone microarchitecture and apparent BV/TV was significantly different between treatment and control groups. Takahashi et al. [13] demonstrated an increase in BV/TV and trabecular connectivity after eight weeks of bisphosphonate (risedronate) treatment in an in vivo animal model study using 16 rabbits exposed to glucocorticoids. To our knowledge, effects of alendronate using MRI in humans in vivo have previously not been documented.
Treatment effects
In the work of Seeman et al. [7], effects of alendronate were evaluated using HR-pQCT. Trabecular volumetric BMD was shown to be higher in alendronate subjects compared to placebo in the distal tibia but not in the radius after 12 months. In our study, treatment effects were found in the distal tibia only. This may be related to the difference in weight-bearing required by the two sites, where the distal tibia is considered a high weight-bearing site and the radius is considered a non-weight-bearing site. Although no evidence have been found that alendronate would add to the effects of weight-bearing exercise [27], longitudinal differences in BMD between weight-bearing and non-weight-bearing bones have been detected after total hip arthroplasty [28]. Also, there is evidence that variation in BMD over time can be explained by weight and thus load on weight-bearing bones [29].
No changes were detected in the proximal femur, which is likely due to a smaller sample size, combined with lower spatial resolution compared to other imaging sites. This site was added to the MRI analysis as a technique development component and an exploratory endpoint. Given the high fracture occurrence at this site, the development of higher quality MR images of the proximal femur is of great interest to the medical community [19].
Given the antiresorptive effect of bisphosphonates, one would expect maintained values in the treatment group and a decrease in the control group longitudinally. However, some studies show conflicting evidence. Rizzoli et al. [30] found no treatment effects of alendronate after 12 months using HR-pQCT. However they did find an increase in Tb.N for alendronate subjects, which they attributed to possible changes in attenuation characteristics related to secondary mineralization. A related study showed a significant increase in trabecular volumetric BMD from HR-pQCT after 24 months of alendronate treatment in the same cohort as our study [6]. An increase in BMD may be related to improvements in trabecular structure parameters [31]. The number of MRI studies is limited; however Takahashi et al. reported an increase both in BV/TV and in trabecular connectivity in their MRI study. They suggested that bisphosphonate treatment leads to a rod-to-plate conversion of the bone microstructure. Our study showed an increase in apparent BV/TV, and a decrease in the GTA parameters with alendronate treatment, where the latter corresponds to higher trabecular connectivity. Further research is necessary to establish how the improvements in trabecular bone structure parameters from MRI relate to effects of bisphosphonates, and how accurately such mechanisms can be quantified given the limited spatial resolution of the MRI data.
The attrition rate was higher than expected, resulting in study group sizes smaller than the targeted number. It appeared related to recently reported potential safety concerns with long term bisphosphonate use [32]. However a recent study did not support the association between bisphosphonates and atypical fractures [33], and the relation between bisphosphonates and atrial fibrillation is yet unclear [34]. The aim of this study was not to establish the suitability of alendronate as a therapeutic treatment of osteoporosis, but rather to assess how documented bone changes due to alendronate treatment would relate to changes in trabecular bone structure parameters from in vivo MRI data.
Comparison between apparent BV/TV from MRI and BMD DXA
Values obtained using BE-FCM showed higher correlations compared to those from dual thresholding, hence BE-FCM may capture more trabecular bone information of the two techniques. The correlations are in the lower range, which is in accordance with the literature. Kazakia et al. [23] found no significant correlation between MRI and DXA for the radius, and a small correlation for the tibia (R2 = 0.13, p<0.04). Link et al. [35] found no significant correlation between MRI and DXA in the femoral trochanter (R=0.20). The relatively low correlations are related to the differences between the measurement techniques. DXA measurements are made from 2D projections of the bone, include both trabecular and cortical bone, and measure the bone mineralization, whereas the MRI measurements include trabecular bone only, and measure bone volume fraction. The low correlations suggest that the MRI-based parameters are detecting changes that are different from what can be found using DXA. The sensitivity to treatment effects using DXA suggests that a combination of cortical and trabecular measurements may be superior for quantification of bone quality in relation to osteoporosis. Imaging methods like HR-pQCT and MRI provide the opportunity to do so in a spatially resolved manner, and finding a suitably weighted combination of trabecular and cortical measurements for improved fracture prediction presents an interesting future research opportunity.
Comparison between MRI and HR-pQCT
Structural parameters obtained using BE-FCM were better correlated to corresponding HR-pQCT parameters compared to parameters obtained by dual thresholding. Relatively low correlations were obtained for apparent Tb.Th and Tb.Sp, presumably due to the low spatial resolution of the MRI data. The in-plane resolution in MRI was in the same order of magnitude as the average thickness of trabeculae. These parameters did not indicate any treatment effects longitudinally. The higher correlations for parameters determined using BE-FCM are likely due to a reduced sensitivity to partial volume effects, and a lower variability since the method does not rely on a fixed cortical bone intensity reference. With the spatial resolution currently achievable in in vivo MRI, it might be advantageous to focus on trabecular structure parameters that are robust to such limitations, such as fuzzy or probabilistic techniques. Saha and Wehrli [36] employed a fuzzy technique for trabecular bone analysis by developing a fuzzy distance transform for measurements of trabecular bone thickness. The method could provide more reliable thickness measurements in MRI data with limited spatial resolution. The technique is not to be confused with BE-FCM, which provides a fuzzy segmentation of the trabecular bone. However both approaches demonstrate how fuzzy techniques can alleviate partial volume effects introduced by the limited spatial resolution.
The two different imaging modalities introduce widely different artifacts into the images, making neither the MRI nor the HR-pQCT data in this study eligible as a gold standard [23]. However the HR-pQCT images with their higher resolution and less complex bone segmentation due to the better discrimination between bone and surrounding tissues can provide some standard of reference for the MRI postprocessing. The comparison was made using the HR-pQCT-matched ROI definition which, in agreement with the HR-pQCT analysis, showed no treatment effects.
MRI-based trabecular bone structure parameters showed significant treatment effects using the entire MRI acquisition ROIs, but not for the HR-pQCT-matched ROIs. There were no treatment effects after 24 months in the HR-pQCT trabecular parameters, and for the BMD from DXA there were significant treatment effects in the total radius but not in the ultra-distal radius, where the latter is more closely corresponding to the HR-pQCT ROI. These results suggest that either a larger ROI than the current patient-style HR-pQCT scan prescription may be necessary for detecting significant changes in bone micro-architecture, or that the location of the standard patient-style HR-pQCT region may not be optimal for detecting longitudinal changes. Rizzoli et al. used an ROI with a length of 9 mm at a distance of 22.5 mm proximally to the distal joint limit, where they found no significant treatment effects with alendronate. In order to evaluate the importance of ROI definition, we revisited the longitudinal analysis of apparent Tb.N using BE-FCM, which showed a significant difference (p<0.05) between treatment and placebo groups after 24 months for the entire MRI ROI but not for the HR-pQCT-matched ROI. Analyzing the entire MRI ROI except for the slices in the HR-pQCT-matched ROI (approximately a length of 1.8 mm of the tibia), Tb.N showed a similar difference between groups (p<0.05) compared to the entire MRI ROI. We also divided the MRI ROIs into slabs with the average HR-pQCT-matched ROI thickness (7.5 mm), centered at 33 mm, 30.5 mm, 27 mm (current standard), and 24.5 mm proximal to the endplate. The resulting analysis showed a significant difference between groups only for the slab centered 30.5 mm proximal to the endplate. This preliminary analysis suggests that the standard HR-pQCT ROI definition may be sufficient in length, but may benefit from a reevaluation in order to determine a more sensitive region. This region is possibly located further from the endplate in the proximal direction compared to the current standard patient-style protocol.
Conclusions
In conclusion, this study demonstrates the feasibility of detecting longitudinal response to alendronate treatment in osteopenic women in vivo, using MRI-based trabecular bone structure parameters. Analysis methods that account for partial volume effects can improve the sensitivity to changes in bone microarchitecture from MRI, and geodesic topological analysis may be able to provide measurable changes in trabecular connectivity. A further improvement of image quality of the MRI and HR-pQCT techniques is desirable as the limited spatial resolution and relative high noise in these images may at least partly explain the failure to detect longitudinal changes of bone structure in the forearm. Although further studies with larger cohorts are necessary, these results suggest that these methods may in the future contribute to new insights into effects of osteoporosis and related therapies without the use of ionizing radiation.
Acknowledgments
The authors would like to thank David Newitt, Benedict Hyun, Thelma Munoz, Jingyi Yu, Nicole Cheng, Melissa Guan, and Ayako Suzuki for contributions in subject recruitment, image acquisition, and database management. This work was supported by grants from Merck & Co., Inc., and NIH RO1 AG17762.
References
- [1].Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, de Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, Melton L, Oden A, Papapoulos S, Pols H, Rizzoli R, Silman A, Tenenhouse A. A new approach to the development of assessment guidelines for osteoporosis (on behalf of the Internatrional Osteoporosis Foundation and the National Osteoporosis Foundation, USA) Osteoporos Int. 2002;13:527–36. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- [2].Bone HG, Hosking D, Devogelaer J-P, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2003;350(12):1189–99. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- [3].Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs R, Dequeker J, Favus M, Seeman E, Recker RR, Capizzi T, Santora AC, Lombardi A, Shah R, Hirsch LJ, Karpf DB. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333(22):1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- [4].Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- [5].Black DM, an dD SRC, Karpf B, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, an RM, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- [6].Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treament in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25(12):2558–71. doi: 10.1002/jbmr.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25(8):1886–94. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging. 2007;25:390–409. doi: 10.1002/jmri.20807. [DOI] [PubMed] [Google Scholar]
- [9].Parfitt A, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, Ott S, Recker R. Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- [10].Carballido-Gamio J, Krug R, Huber MB, Hyun B, Eckstein F, Majumdar S, Link T. Geodesic topological analysis of trabecular bone microarchitecture from high-spatial resolution magnetic resonance images. Magn Reson Med. 2009;61:448–56. doi: 10.1002/mrm.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Majumdar S, Genant HK, Grampp S, Newitt DC, Truong V-H, Lin JC, Mathur A. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res. 1997;12(1):111–8. doi: 10.1359/jbmr.1997.12.1.111. [DOI] [PubMed] [Google Scholar]
- [12].Folkesson J, Carballido-Gamio J, Eckstein F, Link T, Majumdar S. Local bone enhancement fuzzy clustering for segmentation of MR trabecular bone images. Med Phys. 2010;37(1):295–302. doi: 10.1118/1.3264615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi M, Saha PK, Wehrli FW. Skeletal effects of short-term exposure to dexamethasone and response to risedronate treatment studied in vivo in rabbits by magnetic resonance micro-imaging and spectroscopy. J Bone Miner Metab. 2006;24:467–75. doi: 10.1007/s00774-006-0712-1. [DOI] [PubMed] [Google Scholar]
- [14].Zhang XH, Liu XS, Vasilic B, Wehrli FW, Benito M, Rajapakse CS, Snyder PJ, Guo XE. In vivo μMRI-based finite element and morphological analyses of tibial trabecular bone in eugonadal and hypogonadal men before and after testosterone treatment. J Bone Miner Res. 2008;23(9):1426–34. doi: 10.1359/JBMR.080405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wehrli FW, Ladinsky GA, Jones C, Benito M, Magland J, Vasilic B, Popescu AM, Zemel B, Cucchiara AJ, Wright AC, Song HK, Saha PK, Peachey H, Snyder PJ. In vivo magnetic resonance detects rapid remodeling changes in the topology of the trabecular bone network after menopause and the protective effect of estradiol. J Bone Miner Res. 2008;23(9):1426–34. doi: 10.1359/JBMR.080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chesnut CH, Majumdar S, Newitt DC, Shields A, Pelt JV, Laschansky E, Azria M, Kriegman A, Eriksen MOEF, Mindeholm L. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the quest study. J Bone Miner Res. 2005;20(9):1548–61. doi: 10.1359/JBMR.050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bangerter N, Hargreaves B, Vasanawala S, Pauly J, Gold G, Nishimura D. Analysis of multiple-acquisition SSFP. Magn Reson Med. 2004;51(5):1038–47. doi: 10.1002/mrm.20052. [DOI] [PubMed] [Google Scholar]
- [18].Banerjee S, Han E, Krug R, Newitt D, Majumdar S. Application of refocused steady-state free-precession methods at 1.5 and 3 T to in vivo high-resolution MRI of trabecular bone, simulations and experiments. Magn Reson Imaging. 2005;21(6):818–25. doi: 10.1002/jmri.20348. [DOI] [PubMed] [Google Scholar]
- [19].Krug R, Banerjee S, Han ET, Newitt DC, Link TM, Majumdar S. Feasibility of in vivo structural analysis of high-resolution magnetic resonance images of the proximal femur. Osteoporos Int. 2005;16:1307–14. doi: 10.1007/s00198-005-1907-3. [DOI] [PubMed] [Google Scholar]
- [20].Goldenstein J, Kazakia G, Majumdar S. In-vivo evaluation of the presence of bone marrow in cortical porosity in postmenopausal osteopenic women. Ann Biomed Eng. 2010;38(2):235–46. doi: 10.1007/s10439-009-9850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Studholme C, Hill D, Hawkes D. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit. 1999;32:71–86. [Google Scholar]
- [22].Newitt DC, van Rietbergen B, Majumdar S. Processing and analysis of in vivo high-resolution MR images of trabecular bone for longitudinal studies: reproducibility of structural measures and micro-finite element analysis derived mechanical properties. Osteoporos Int. 2002;13:278–87. doi: 10.1007/s001980200027. [DOI] [PubMed] [Google Scholar]
- [23].Kazakia G, Hyun B, Burghardt A, Newitt D, de Papp A, Link T, Majumdar S. In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res. 2008;23(4):463–74. doi: 10.1359/jbmr.071116. [DOI] [PubMed] [Google Scholar]
- [24].Laib A, Ruegsegger P. Comparison of structure extraction methods for in vivo trabecular bone measurements. Comput Med Imaging Graph. 1999;23(2):69–74. doi: 10.1016/s0895-6111(98)00071-8. [DOI] [PubMed] [Google Scholar]
- [25].Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- [26].Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87(2):245–51. [Google Scholar]
- [27].Uusi-Rasi K, Kannus P, Cheng S, Sievanen H, Pasanen M, Heinonen A, Nenonen A, Halleeen J, Fuerst T, Genant H, Vuori I. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: a randomized controlled trial. Bone. 2003;33(1):132–43. doi: 10.1016/s8756-3282(03)00082-6. [DOI] [PubMed] [Google Scholar]
- [28].Hirano Y, Hagino H, Nakamura K, Katagiri H, Okano T, Kishimoto H, Morimoto K, Teshima R, Yamamoto K. Longitudinal change in periprosthetic, peripheral, and axial bone mineral density after total hip arthroplasty. Mod Rheumatol. 2001;11(3):217–21. doi: 10.3109/s101650170007. [DOI] [PubMed] [Google Scholar]
- [29].Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–73. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- [30].Rizzoli R, Laroche M, Krieg M-A, Frieling I, Thomas T, Delmas P, Felsenberg D. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int. 2010;30:1341–8. doi: 10.1007/s00296-010-1542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen P, Miller PD, Recker R, Resch H, Rana A, Pavo I, Sipos AA. Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. J Bone Miner Res. 2007;22(8):1173–80. doi: 10.1359/jbmr.070413. [DOI] [PubMed] [Google Scholar]
- [32].Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–65. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- [33].Black DM, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. The New England Journal of Medicine. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- [34].Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356(18):1895–6. doi: 10.1056/NEJMc076132. [DOI] [PubMed] [Google Scholar]
- [35].Link TM, Vieth V, Langenberg R, Meier N, Lotter A, Newitt D, Majumdar S. Structure analysis of high resolution magnetic resonance imaging of the proximal femur: in vitro correlation with biomechanical strength and BMD. Calcif Tissue Int. 2003;72:156–65. doi: 10.1007/s00223-001-2132-5. [DOI] [PubMed] [Google Scholar]
- [36].Saha PK, Wehrli FW. Measurement of trabecular bone thickness in the limited resolution regime of in vivo MRI by fuzzy distance transform. IEEE Trans Med Imaging. 2004;23(1):53–62. doi: 10.1109/TMI.2003.819925. [DOI] [PubMed] [Google Scholar]