Abstract
Tropical wetlands are not included in Earth system models, despite being an important source of methane (CH4) and contributing a large fraction of carbon dioxide (CO2) emissions from land use, land use change, and forestry in the tropics. This review identifies a remarkable lack of data on the carbon balance and gas fluxes from undisturbed tropical wetlands, which limits the ability of global change models to make accurate predictions about future climate. We show that the available data on in situ carbon gas fluxes in undisturbed forested tropical wetlands indicate marked spatial and temporal variability in CO2 and CH4 emissions, with exceptionally large fluxes in Southeast Asia and the Neotropics. By upscaling short-term measurements, we calculate that approximately 90 ± 77 Tg CH4 year−1 and 4540 ± 1480 Tg CO2 year−1 are released from tropical wetlands globally. CH4 fluxes are greater from mineral than organic soils, whereas CO2 fluxes do not differ between soil types. The high CO2 and CH4 emissions are mirrored by high rates of net primary productivity and litter decay. Net ecosystem productivity was estimated to be greater in peat-forming wetlands than on mineral soils, but the available data are insufficient to construct reliable carbon balances or estimate gas fluxes at regional scales. We conclude that there is an urgent need for systematic data on carbon dynamics in tropical wetlands to provide a robust understanding of how they differ from well-studied northern wetlands and allow incorporation of tropical wetlands into global climate change models.
Keywords: carbon dioxide, decomposition, methane, net primary productivity, tropical, wetland
1. Introduction
Tropical wetlands play an important role in the global carbon (C) cycle [Page et al., 2011]. Currently, they are under considerable pressure from agriculture [Houghton, 2012] resulting in substantially increased carbon dioxide (CO2) emissions from these ecosystems. For example, 1–3% of annual fossil fuel emissions or 355–855 Mt C year−1 in Indonesia alone [Hooijer et al., 2010] are estimated to originate from tropical peatlands. Undisturbed tropical wetlands emit between 85 and 184 Tg of methane (CH4) each year, accounting for two thirds of global emissions from wetlands [e.g., Richey et al., 2002; Jauhiainen et al., 2005; Hooijer et al., 2006; Nahlik and Mitsch, 2011; Melton et al., 2013].
The dominant wetland ecosystems in the tropics are forested peatlands, swamps, and floodplains (Table1) [Aselmann and Crutzen, 1989]. Of these, only peatlands accumulate substantial C deposits (between 0.5 and 11 m deep) [Phillips et al., 1997; Page et al., 1999; Shimada et al., 2001; Hope et al., 2005; Page et al., 2011; Lähteenoja et al., 2012]. However, controls on the formation of deep peats in the tropics are not well understood. As expected from their capacity for C accumulation, tropical peatlands comprise a significant proportion of terrestrial C: an estimated 89 Gt C or 19% of the C stored in peatlands worldwide [Page et al., 2011]. Accumulation of C in tropical peatlands is under threat from land use and climate change, which can transform tropical wetlands into C sources [Furukawa et al., 2005; Laiho, 2006; Meehl et al., 2007; Hooijer et al., 2010].
Table 1.
Description of Wetland Typesa
| Wetland Type | Description | Area (km2) |
|---|---|---|
| Swamps | Forested freshwater wetlands on waterlogged or inundated soils where little or no peat accumulation takes place. For this review we have limited data to forested system. | 230,000 |
| Peatlands | Peat producing wetlands in moist climates where organic materials have accumulated over long periods. | 441,000 |
| Floodplains | Periodically follower areas along rivers or lakes showing considerable variation in vegetation cover. In the Amazon flood plain two separate systems are defined Varzea forests which are feb by muddy rivers and Igapo forests located in blackwater and clearwater tributaries | 715,000 |
For this review we have limited data to forested systems.
There are considerable uncertainties regarding the spatial extent of tropical wetlands (Figure1). Observational data suggest that tropical wetland areas range between 2.8 and 6.0 × 106 km2, while models predict a much larger range (1.3–38.8 × 106 km2) [Melton et al., 2013]. Uncertainties regarding the relative distribution of tropical wetland types are even larger; areal estimates of different wetland types are presented in Table1 [Aselmann and Crutzen, 1989; Page et al., 2011]. Given the contrasting environmental conditions associated with these different wetland types (e.g., peat accumulation and nutrient-poor conditions in peatlands and seasonal variation in the degree of inundation in floodplain systems), tropical wetlands are not only expected to differ in C accumulation as peat but also their release of CO2 and CH4.
Figure 1.
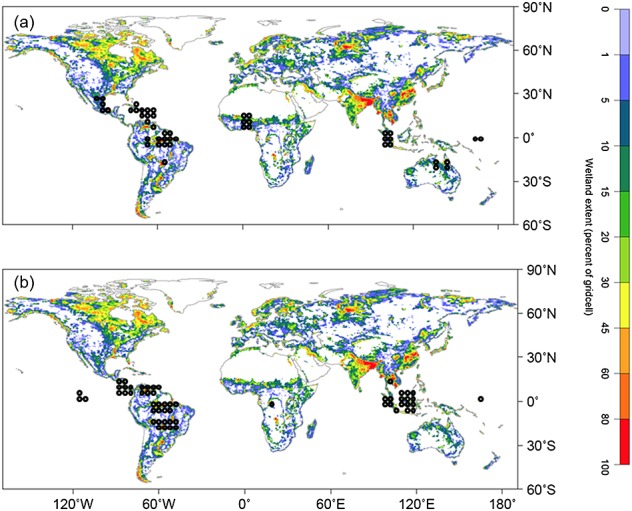
The wetland map is based on remotely sensed inundation data and GIEMS refers to the Global Inundation Extent from Multi-Satellites; the GIEMS inundation data set is plotted as the mean annual maximum value across between 1993 and 2004 [Melton et al., 2013]. (a) The spatial distribution of NPP data sets (data in Table2) and (b) greenhouse gas flux data sets (data in Table4).
The rate of increase in CH4 concentration in the atmosphere has varied during the past three decades reassuming its increase after 2006 to ca. 6 Tg CH4 y−1 [Kirschke et al., 2013]; with tropical wetlands playing a major role in the renewed increase of atmospheric CH4 [IPCC, 2013]. The magnitude of this increase has been observed to differ depending on whether the estimate is based on a top-down (atmospheric inversion models) or a bottom-up (process-based models; adding up independently estimated flux components) analytical approach. Higher estimates have been reported using the bottom-up approach, where the estimates of fluxes from natural wetlands carry an uncertainty of at least 50% [Kirschke et al., 2013]. The uncertainty in the bottom-up approach of the CH4 emissions from wetlands is mainly due to the lack of a reliable estimate of the global extent of wetlands [Melton et al., 2013] and to the scarcity of wetland CH4 flux measurements [Riley et al., 2011].
Our rudimentary understanding of CH4 emissions in the tropics is underlined by the discrepancy between emissions of CH4 from the surface of wetlands and the high concentrations of this gas in the tropical atmosphere [Melack et al., 2004; Miller et al., 2007; Bergamaschi et al., 2009; Bloom et al., 2010]; with CH4 emissions from top-down and bottom-up approaches differing the most in tropical South America [Kirschke et al., 2013]. Addressing this knowledge gap is of particular importance as models predict a global increase in CH4 emissions of 77%, due largely to increased emissions from existing tropical wetlands in response to increasing temperatures [Shindell et al., 2004]. The model used by Shindell et al. [2004] calculates CH4 emissions based on relationships between temperature, water table depth, and net primary productivity (NPP). Some progress has been made in testing these relationships [Walter and Heimann, 2000], but data are limited, particularly regarding NPP and temperature responses; such issues must be considered in greater detail [Farmer et al., 2012].
Several existing wetland modeling tools may be suitable for application to tropical peatlands and some might be useful in Earth system models [Farmer et al., 2012]. However, the inclusion of tropical wetlands in such models is hampered by a lack of suitable data to validate them. Current models of global CH4 emissions [Bridgham et al., 2013; Melton et al., 2013] use estimates of tropical CH4 fluxes from a small number of review papers [e.g., Matthews and Fung, 1987; Aselmann and Crutzen, 1989; Bartlett and Harriss, 1993] that estimated CH4 emissions from a limited number of measurements. It is therefore not surprising that outputs from wetland models that estimate current CH4 emissions from tropical areas vary widely, with values between 85 ± 7 and 184 ± 11 Tg CH4 year−1 [Melton et al., 2013]. Without appropriate data on C dynamics from undisturbed tropical wetlands, it will be difficult to predict how degradation of these systems will impact on global climate. Key input data needed to model C dynamics in tropical wetlands are aboveground and belowground net primary productivity (NPP), litter input and decay, and information on soil properties, including nutrient status, and hydrology [Farmer et al., 2012]. Good quality CO2 and CH4 flux data, i.e., data accounting for temporal and spatial variability in fluxes are also needed to evaluate model predictions and close the gap between top down and bottom up modeling approaches [Farmer et al., 2012].
Compared to the more intensively studied boreal and temperate peatlands, tropical peatlands are poorly understood with respect to the controls on decomposition and C storage; the C sink strength of tropical peatlands therefore remains poorly quantified [Dommain et al., 2011]. However, tropical wetlands have common characteristics, such as high mean annual temperature with little seasonal variation, high rainfall, generally high hydraulic conductivity at the surface in the case of peatlands, and the presence of overstorey rainforest providing the main input of organic matter [Page et al., 1999; Sjögersten et al., 2010; Lähteenoja and Page, 2011; Wright et al., 2011]. Carbon accumulation in ecosystems is determined by the balance between inputs and output. In high-latitude wetlands, the main control of C accumulation is slow decomposition of recalcitrant litter inputs, often Sphagnum spp., in cold wet soils [Clymo, 1984], whereas the situation in the tropics is less well understood. In contrast to cold regions, temperature is unlikely to be a major factor in limiting decomposition. The recalcitrance of litter inputs is less constrained as they are produced from different plant tissue types and plant species. Chimner and Ewel [2005] suggested that relatively slow root decomposition may be instrumental in the formation of tropical peat, implying that root production rate is important in determining C balance. However, the relationship between NPP and long-term C storage within tropical wetlands has not been explored.
We calculated current C balances for a wide range of tropical wetlands by compiling data for long-term net C accumulation rates and CO2 and CH4 emissions from flooded tropical wetlands/peatlands. It was anticipated that C accumulation rates would be greater in tropical than in temperate and boreal peatlands, but that CO2 and CH4 emissions would be high due to the substantial inputs of fresh litter and stable high temperatures. The hypothesis that C accumulation in tropical peatlands is driven by slow decomposition rather than high NPP was tested by comparing decomposition rates and NPP with tropical wetlands that do not accumulate peat.
2. Methods
2.1. Data Collation
The Web of Knowledge and Google Scholar were used to collate information on CO2 and CH4 fluxes, peat depth, NPP, and C accumulation from the relevant published literature using the following search terms: Tropical, Amazon, Pantanal, Africa, Southeast Asia, peatlands, wetlands, methane, peat, carbon dioxide, biomass, litter, NPP, and root. Based on the references obtained, all relevant original research pertaining to forested tropical wetland areas was used to identify additional references. We consider only freshwater wetlands.
To assess litter decomposition rates, a data set of decay constants (k) was compiled for different litter types from in situ decomposition in tropical and subtropical wetlands, with high k values corresponding to more rapid decay. Half times (half time = ln(2)/k) were calculated for different tissue types.
2.2. Data Processing and Analysis
We used two approaches to estimate NPP, (i) by summing C inputs and (ii) by using a conversion between litter production and total NPP. To construct a C balance for wetlands on organic and mineral soil, using the first approach, plant production was estimated by summing leaf litterfall, reproductive litterfall (flowers, fruit, and seed), branch litterfall, other litter (e.g., chaff), wood increment, and fine root production. No data were found for coarse woody debris or coarse root production. Published data for litter production were generally presented as mass of material, for conversion to C inputs a 50% C content was assumed [Wright et al., 2013]. We assumed that data for some of the litter pools needed for estimating NPP this way would be limited. Therefore, we used our second approach for estimating NPP. This was based on a linear relationship between NPPtotal and NPPcanopy reported for lowland rainforest [Malhi et al., 2011], and we chose this approach since data availability for canopy litter production in tropical wetlands was the most regularly measured component of the C inputs. The relationship was used to estimate NPP based on the assumption that NPPtotal = 2.27(NPPcanopy). NPPcanopy was calculated as leaf litter + reproductive litterfall + branch litterfall + other litter again assuming a C content of 50% to convert litterfall to C inputs. Net ecosystem production (NEP) was calculated by subtracting total C losses (in the form of average gaseous losses as CO2 and CH4 and aquatic losses as dissolved organic carbon (DOC) across all sites from which data were available) from the substrate from NPPtotal.
Calculations of NEP were separated between the organic and mineral soil components, and estimates of heterotrophic respiration were based on upscaling of short-term in situ ground surface flux measurements to the annual scale to enable comparison with litter inputs. The measurements of surface CO2 flux combine both autotrophic and heterotrophic respiration; as measurements were largely collected during the daytime period, this may have introduced bias within the data. Furthermore, collection of flux data during different seasons may also have influenced the balance between C inputs and output (inputs were based on litterfall data normally collected over an annual cycle). Potential data limitations are highlighted in the discussion.
Tests for significant differences in CO2 and CH4 fluxes and NPPtotal between tropical wetland types (e.g., peat forming versus wetlands on mineral soil) and geographical regions were conducted using an unbalanced analysis of variance (ANOVA). CO2 and CH4 flux data were square root and log transformed, respectively, to meet the normality assumption of ANOVA. All statistical analysis was carried out using GENSTAT version 15. To assess the impacts of data gaps in the C balance, we carried out a sensitivity analysis calculating potential errors associated with particular data gaps relative to the total C inputs using existing studies from either tropical wetlands or tropical lowland rainforest system.
3. Carbon Accumulation
Carbon accumulates in both mineral and peat-forming tropical wetlands and a wide range of peat accumulation rates have been reported for tropical peatlands; for example, Chimner and Ewel [2005] estimated accumulation on the island of Kosrae in Micronesia to be 300 g C m−2 yr−1, at the higher end of the range reported for the tropics. In Kalimantan, mean accumulation rates were estimated to be 31–77 g C m−2 yr−1 [Dommain et al., 2011] and 94 g C m−2 yr−1 [Moore et al., 2013], while comparable values of 39–85 g C m−2 yr−1 have been reported for Peruvian Amazon peatlands [Lähteenoja et al., 2009] and 43–55 g C m−2 yr−1 in Panamanian peatlands (J. Hoyos, unpublished data, 2014). Furthermore, peat accumulation rates appear to be greater in coastal lowland peatlands than in inland peatlands [Dommain et al., 2011]. Hirano et al. [2009] reported that net ecosystem C production (NEP) in a drained peatland forest in Kalimantan ranged from 296 to 594 g C m−2 yr−1, at the upper end of range of long-term C accumulation rates.
Carbon accumulation is also substantial in depositional sedimentary flood plain systems. Moreira-Turcq et al. [2004] suggested a rate of 100 g C m−2 yr−1 for the varzea of the Amazon, while Devol et al. [1984] suggested a rate of 44 g C m−2 yr−1 based on depositional systems connected to the Amazon for only 6 months of the year. In Lake Rawa Danau, West Java, Indonesia, sedimentary deposition of organic C was lower at 11.75 g C m−2 yr−1. Flux data are lacking for C inputs into the Bengal delta plain, even though this region may represent an important store given the high outflow of sediments with C contents ranging between 0.05 and 1.4% [Datta et al., 1999].
Carbon accumulation rates in boreal and temperate peatlands are generally lower than in the tropics, although substantial variation occurs depending on peatland type, with values as high as 132–198 g C m−2 yr−1 being recorded for bogs in the USA [Craft et al., 2008]. However, lower peat accretion rates are also common; for example, rates close to 21 g C m−2 yr−1 were reported in Scotland [Anderson, 2002] and Canada [Roulet et al., 2007]. Accumulation rates in boreal peatlands are generally lower than in temperate and tropical peatlands. For example, accumulation rates in boreal peatlands in Canada range between 6 and 22 g C m−2 yr−1 [Robinson and Moore, 1999; Turunen and Turunen, 2003; Sannel and Kuhry, 2009], while accumulation rates in Finland were between 15 and 35 g C m−2 yr−1 [Turunen et al., 2002; Ukonmaanaho et al., 2006]. In summary, C accumulation rates are, with a few exceptions, greatest in the tropics and decrease with latitude.
The high long-term C accumulation in tropical peatlands may be driven by their high mean NPP, with aboveground biomass production of 1000–1300 g C m−2 yr−1 [Nebel et al., 2001] and NPP of 1100 g C m−2 yr−1 [Chimner and Ewel, 2005]. Our calculations of NPPtotal (Table2) and existing data from Nebel et al. [2001] and Chimner and Ewel [2005] suggest that C inputs from NPP are generally high in tropical wetlands, although there is considerable variability among wetland types. Maximum values for NPP based on litterfall data were 1929 g C m−2 yr−1 in a forested wetland in Puerto Rico [Frangi and Lugo, 1985], while the lowest recorded value was 430 g C m−2 yr−1 in a floodplain forest in Australia [Payntner, 2005]. NPPtotal was significantly greater in tropical wetlands on organic soils (mean ± SE: 1206 ± 93 g C m−2 yr−1) than on mineral soils (mean ± SE: 880 ± 77 g C m−2 yr−1) (F1,49 = 7.15; P = 0.01; Table2). These high rates of productivity generally yield large C stocks, but pool sizes are poorly quantified (Table3).
Table 2.
Net Primary Productivity Based on Litterfall Data in a Range of Forested Tropical Wetlands
| Region, Country | Forest Type, Site Name | Soil Type | NPPtotala (g C m−2 yr−1) | Reference |
|---|---|---|---|---|
| Puerto Rico | Pterocarpus officinalis forest | Organic | 1277 | Easse and Aide [1999] |
| Luquillo, Puerto Rico | Flood plain palm forest | Organic | 616 | Frangi and Lugo [1998b] |
| Puerto Rico | Prestoea montana forest | Organic | 1929 | Frangi and Lugo [1985] |
| Veracruz, Mexico | Forested wetlands, Apompal | Organic | 1056 | Mata et al. [2012] |
| Veracruz, Mexico | Forested wetlands, Mancha | Organic | 1101 | Mata et al. [2012] |
| Veracruz, Mexico | Forested wetlands, Chica | Organic | 1691 | Mata et al. [2012] |
| Veracruz, Mexico | Forested wetlands, Cienaga | Mineral | 1566 | Mata et al. [2012] |
| Veracruz, Mexico | Forested wetlands, Salado | Organic | 1419 | Mata et al. [2012] |
| Puerto Rico | Pterocarpus officinalis forest, Mayaguez | Organic | 1600 | Alvarez-Lopez [1990] |
| Puerto Rico | Pterocarpus officinalis forest, Patillas | Organic | 1351 | Alvarez-Lopez [1990] |
| Puerto Rico | Pterocarpus officinalis forest, Dorado | Mineral | 987 | Alvarez-Lopez [1990] |
| Guadeloupe | Pterocarpus officinalis swamp forest | Organic | 1476 | Miegot and Imbert [2012] |
| Guadeloupe | Pterocarpus officinalis swamp forest | Organic | 1606 | Miegot and Imbert [2012] |
| Guadeloupe | Pterocarpus officinalis swamp forest | Organic | 1189 | Miegot and Imbert [2012] |
| Panama | Riverine forest | Mineral | 1318 | Golley et al. [1975] |
| Peru | Flood plain forest, high restinga | Mineral | 796 | Nebel et al. [2001] |
| Peru | Flood plain forest, low restinga | Mineral | 810 | Nebel et al. [2001] |
| Peru | Flood plain forest, Tahuampa | Mineral | 787 | Nebel et al. [2001] |
| Orinoco Llanos, Venezuela | Palm swamp forest, flood-prone | Organic | 560 | San-José et al. [2010] |
| Orinoco Llanos, Venezuela | Palm swamp forest, flood plain | Organic | 2438 | San-José et al. [2010] |
| Brazil | Swamp forest | Mineral | 647 | Terror et al. [2011] |
| Pantanal, Brazil | Flooded forest | Mineral | 1021 | Haase [1999] |
| Manaus, Brazil | Swamp forest, Igapo | Organic | 772 | Adis et al. [1979] |
| Manaus, Brazil | Flood plain forest | Mineral | 726 | Franken et al. [1979] |
| Manaus, Brazil | Swamp forest | Organic | 760 | Franken et al. [1979] |
| Para, Brazil | Swamp forest | Organic | 976 | Klinge [1978] |
| Para, Brazil | Flood plain forest | Mineral | 193 | Klinge [1978] |
| Para, Brazil | Swamp forest | Organic | 874 | Silva and Lobo [1982] |
| Para, Brazil | Flood plain forest | Mineral | 976 | Silva and Lobo [1982] |
| Para, Brazil | Flood plain forest | Mineral | 1566 | Cattanio et al. [2004] |
| Amazonia | Floodplain forest, varzea, 40 year old | Mineral | 1190 | Naiman [2005] |
| Amazonia | Floodplain forest, varzea, 80 year old | Mineral | 1680 | Naiman [2005] |
| Australia | Flood plain forest Mimosa pigra | Mineral | 430 | Payntner [2005] |
| Australia | Flood plain forest, Melaleuca spp.—Mangrove, northeastern Queensland | Mineral | 470 | Duke [1982] |
| Australia | Melaleuca spp. forest, Magela flood plain | Mineral | 350 | Finlayson et al. [1993] |
| Australia | Melaleuca spp forest, Magela flood plain | Mineral | 750 | Finlayson [1988] |
| Ivory coast | Water logged forest, VG | Mineral | 919 | Devineau [1976] |
| Ivory coast | Riverine forest, TR6 | Mineral | 783 | Devineau [1976] |
| Ivory coast | Riverine forest, gallery, MS | Mineral | 965 | Devineau [1976] |
| Ivory coast | Riverine forest, gallery, TR4 | Mineral | 704 | Devineau [1976] |
| Ivory coast | Riverine forest, gallery, BD | Mineral | 874 | Devineau [1976] |
| Ivory coast | Riverine forest, gallery, TR2 | Mineral | 602 | Devineau [1976] |
| Malaysia, Tasek Bera | Riverine forest, Eugenia swamp | Organic | 1039 | Furtado et al. [1980] |
| Sumatra, Indonesia | Peat swamp forest, PS3 | Organic | 1351 | Brady [1997] |
| Sumatra, Indonesia | Peat swamp forest, SE6 | Organic | 829 | Brady [1997] |
| Sumatra, Indonesia | Peat swamp forest, PI6 | Organic | 783 | Brady [1997] |
| Sumatra, Indonesia | Peat swamp forest, PI9 | Organic | 624 | Brady [1997] |
| Sumatra, Indonesia | Peat swamp forest, PI12 | Organic | 624 | Brady [1997] |
| Yela, Micronesia | Peat swamp forest | Organic | 1689 | Chimner and Ewel [2005] |
| Yewak, Micronesia | Peat swamp forest | Organic | 1716 | Chimner and Ewel [2005] |
NPPtotal is based on conversion of NPPcanopy using NPPtotal = 2.27*NPPcanopy [Malhi et al., 2011], where total NPP was not reported.
Data from 1980.
Table 3.
Fluxes and Pools of C in Tropical Wetlands on Organic Peat Soil and Mineral Soils; Values are Mean (Standard Deviation; n), n/d Refers to No Data, References in Addition to Those in Table1 as Listed Belowa
| Organic |
Mineral |
|||
|---|---|---|---|---|
| Fluxes (g C m−2 yr−1) | ||||
| Reproductive litter | 71.7 | (62.6; 17) | 73.6 | (44.8; 10) |
| Leaves | 333.3 | (95.7; 17) | 281.2 | (86.1; 17) |
| Fine woody litter | 104.9 | (51.2; 16) | 90.5 | (34.1; 9) |
| Coarse wood | 155.0 | (183.8; 2) | n/d | |
| Live wood increment | 379.8 | (71.7; 2) | 547.9 | (323.4; 6) |
| Other litter | 28.6 | (14.0; 12) | 29.0 | (2.0; 2) |
| Fine root production | 112.1 | (140.3; 7) | n/d | |
| CO2 efflux | −875.1 | (481.3; 17) | −901.4 | (728.0;18) |
| CH4 efflux | −40.1 | (66.1; 15) | −54.0 | (52.1; 29) |
| DOCb | −75.5 | (17; 2) | −120 | (n/d; 1) |
| Pools (kg C m−2) | ||||
| Leaves | n/d | 0.6 | (n/d; 1) | |
| Wood | 12.4 | (4.5; 3) | 17.1 | (8.2; 4) |
| Forest floor litter | 1.2 | (0.9; 8) | 0.3 | (0.1; 3) |
| Downed logs | 0.8 | (n/d; 2) | n/d | |
| Fine roots | 1.9 | (2.2; 13) | 2.4 | (1.7; 5) |
A further important aspect of C inputs to tropical wetlands is a more rapid root turnover rate (70% yr−1) than in equivalent temperate and boreal systems (55 and 45% yr−1, respectively) [Gill and Jackson, 2000; Chimner and Ewel, 2005]. This observation suggests that C inputs from root turnover might contribute significantly to the high C accumulation rates in tropical wetlands, but data for root production are scarce (Table3).
4. Carbon Dioxide and Methane Fluxes From Tropical Swamps
Depending on prevailing environmental conditions, primarily the oxygen content and redox potential of the peat, microbial degradation of organic material in wetlands can induce the release of predominantly CO2 or simultaneous release of both CO2 and CH4. Measurements of daily, monthly, and seasonal variation in gas fluxes show that specific wetlands can switch between production of mainly CO2 and a greater contribution of CH4 [Hadi et al., 2005; Jauhiainen et al., 2005; Melling et al., 2005a, 2005b; Wright et al., 2013]. Only a few studies have addressed temporal variability in gas fluxes in tropical peatlands, although strong seasonal variation in CH4 fluxes has been reported in floodplain wetlands in the Amazon [e.g., Devol et al., 1988; Bartlett et al., 1990]. Gas fluxes can also vary strongly among vegetation types, which in turn are linked to nutrient status [Wright et al., 2013]. Given the diversity of forest types present on tropical wetland soils, this provides a substantial degree of variability. Information on fluxes is almost entirely lacking for many geographical regions; for example, we identified only two papers on CO2 emissions and one on CH4 emissions from African wetlands. No data were found for gas fluxes from peatlands in the Amazon basin despite their vast spatial extent (150,000 km2) [Lähteenoja et al., 2009], although detailed data exist from the floodplains in the region [Bartlett et al., 1988, 1990; Crill et al., 1988; Devol et al., 1988, 1990].
4.1. Carbon Dioxide
Fluxes of CO2 from forested tropical wetlands vary greatly, with reported values ranging between 30 and 4055 mg m−2 h−1 (Table4). The lowest values were reported for a palm swamp in Venezuela [Bracho and San José, 1990], while values were greatest for a forested peatland in Kalimantan, Indonesia [Melling et al., 2005a]. The majority of available data on CO2 fluxes from forested tropical wetlands are from Southeast Asian peatlands, but these tend to be disturbed by human activity, making it difficult to assess regional variation in CO2 losses from tropical peatlands. We found no significant differences in CO2 efflux among geographical regions (P >0.05; Figure2b), although data are absent or very limited for some regions, including both Africa and the Amazon basin, which limits the strength of any conclusions. CO2 emission rates tended to be greater in tropical peatlands (Table4) than in temperate and boreal systems [Silvola et al., 1996; Clair et al., 2002; Bubier et al., 2003; Crow and Wieder, 2005; Makiranta et al., 2009], although fluxes within specific tropical regions were highly variable and affected by local conditions. Interestingly, the greater range of CO2 emissions from flooded forested tropical peatlands [e.g., Hadi et al., 2005; Melling et al., 2005b] were within the same range (i.e., approximately 1000 mg CO2 m−2 h−1) as those found for tropical peatlands with substantially lowered water tables (up to 1 m below the peat surface) [Couwenberg et al., 2010]. Upscaling the CO2 fluxes to pantropical wetland areas suggests a release of approximately 4540 ± 1480 Tg CO2 year−1 (mean ± standard deviation (SD)). This calculation is based on the simplistic assumption that that the CO2 flux from mineral soil (Figure2a) is related to the area covered by swamps and floodplains (Table1), and the flux from organic soil (Figure2a) was related to the area covered by peatlands. Substantial additional uncertainty around this mean will arise from current poor understanding of tropical wetland area [Melton et al., 2013; Lähteenoja et al., 2009]. Despite the general accumulation of organic matter in tropical peatlands, there was no significant difference in CO2 fluxes between tropical wetlands on organic and mineral soils (P >0.05; Figure1a). Furthermore, there was no systematic variation in CO2 efflux among wetland types (P >0.05; Figure2c).
Table 4.
Carbon Dioxide (CO2) and Methane (CH4) Fluxes From Tropical Wetlands Showing the Mean Fluxesa and (Ranges) if Available
| Location | Type | Soil Type | CO2 Efflux (mg m−2 h−1) |
CH4 Efflux (mg m−2 h−1) |
Reference |
|---|---|---|---|---|---|
| Kalimantan, Indonesia | Forested peatland | Organic | na | 1.1 ± 0.61 | Inubushi et al. [1998] |
| Kalimantan, Indonesia | Secondary forest | Organic | 501 ± 180 (146–843) | 0.18 ± 0.06 (0–1) | Inubushi et al. [2003] |
| Kalimantan, Indonesia | Forested peatland | Organic | 317–950 | na | Hirano et al. [2009] |
| Kalimantan, Indonesia | Secondary forest | Organic | 513 | 0.19 | Hadi et al. [2001] |
| Kalimantan, Indonesia | Secondary forest | Organic | 395 (183–4055) | 0.50 (0–3.33) | Hadi et al. [2005] |
| Kalimantan, Indonesia | Forested peatland | Organic | 399 ± 36 (50–550) | 0.16 ± 0.65 (−0.1–0.35) | Jauhiainen et al. [2005] |
| Kalimantan, Indonesia | Forested peatland | Organic | 563 (79–1580) | na | Sundari et al. (2012) |
| Sumatra, Indonesia | Forested peatland | Organic | 380 ± 55 | 0.89 ± 0.48 | Furukawa et al. [2005] |
| Sumatra, Indonesia | Forested peatland | Organic | 278 ± 16 | 1.21 ± 1.36 | Furukawa et al. [2005] |
| Sumatra, Indonesia | Forested peatland | Organic | 376 ± 107 | 0.77 ± 0.27 | Furukawa et al. [2005] |
| Malaysia | Forested peatland | Organic | 905 (366–1953) | na | Melling et al. [2005a] |
| Malaysia | Forested peatland | Organic | na | 0.0029 (−0.006–0.011) | Melling et al. [2005b] |
| Malaysia | Forested peatland | Organic | 444 | Murayama and Bakar [1996] | |
| Thailand | Forest peatland | Organic | na | 1.12 ± 2.7 (0.19–12.6) | Ueda et al. [2000] |
| Micronesia | Forested peatland | Organic | 396 ± 36 (340–402) | na | Chimner [2004] |
| Mauim, Hawaii | Montane peatland | Organic | 285 ± 75 | Chimner [2004] | |
| Bocas del Toro, Panama | Forested peatland | Organic | 212 (11–1694) | 23 (−5.35–143) | Wright et al. [2011] |
| Bocas del Toro, Panama | Forested peatland | Organic | 238 (62–801) | 17 (−3.53–98.3) | Wright et al. [2011] |
| Bocas del Toro, Panama | Open peatland | Organic | 259 (7–950) | 31 (−6.40–7.88) | Wright et al. [2011] |
| Colon, Panama | Forested peatland | Organic | na | 14.4 (0–48) | Keller [1990] |
| Kalimantan, Indonesia | Forested peatland | Organic | na | Pangala et al. [2013] | |
| Ka'au, Hawaii | Montane swamp | Organic | 127 ± 47 | na | Chimner [2004] |
| Orinoco Llanos, Venezuela | Palm swamp | Organic | 30 (17–54) | na | Bracho and San José [1990] |
| Sumatra, Indonesia | Forested floodplain | Mineral | 410 ± 35 | na | Ali et al. [2006] |
| Sumatra, Indonesia | Forested floodplain | Mineral | 884 ± 212 | na | Ali et al. [2006] |
| Ka'au crater, Hawaii | Forested floodplain | Mineral | na | 5.25 ± 0.42 (2.08–14.17) | Grand and Gaidos [2010] |
| La Selva, Costa Rica | Flooded forest | Mineral | na | 23.3 ± 14.6 | Nahlik and Mitsch [2011] |
| La Selva, Costa Rica | Flooded forest | Mineral | na | 40.4 ± 13.1 | Nahlik and Mitsch [2011] |
| Earth wetlands, Costa Rica | Secondary forest | Mineral | na | 5.7 ± 1.4 | Nahlik and Mitsch [2011] |
| Earth wetlands, Costa Rica | Secondary forest | Mineral | na | 4.5 ± 0.78 | Nahlik and Mitsch [2011] |
| Orinoco, Venezuela | Forested floodplain | Mineral | na | 4.6 | Smith et al. [2000] |
| Orinoco, Venezuela | Forested floodplain | Mineral | na | 10.7 (0–78) | Smith and Lewis [1992] |
| Orinoco, Venezuela | Forested floodplain | Mineral | na | 12.8 (0.125–95.3) | Smith and Lewis [1992] |
| Orinoco, Venezuela | Forested floodplain | Mineral | na | 7.27 (0–68.7) | Smith and Lewis [1992] |
| Orinoco, Venezuela | Forested floodplain | Mineral | na | 10.3 (0–114) | Smith and Lewis [1992] |
| Amazon river, Brazil | Forested floodplain | Mineral | na | 4.6 (0.24-31.7) | Devol et al. [1988] |
| Amazon river, Brazil | Forested floodplain | Mineral | na | 1.88 (0–8.33) | Wassmann et al. [1992] |
| Amazon river, Brazil | Forested floodplain | Mineral | na | 2.29 ± 0.54 (0.014–47.3) | Devol et al. [1990] |
| Amazon river, Brazil | Forested floodplain | Mineral | na | 8 ± 1.12 | Bartlett et al. [1988] |
| Amazon river, Brazil | Forested floodplain | Mineral | na | 5.25 ± 0.83 | Bartlett et al. [1990] |
| Amazon river, Brazil | Forested floodplain | Mineral | 237 | 0.1 | Richey et al. [1988] |
| Amazon river, Brazil | Forested floodplain | Mineral | 36 | 7.5 | Richey et al. [1988] |
| Itu, Negro river, Brazil | Forested interfluvial wetland | Mineral | 375 | 1.9 | Belger et al. [2011] |
| Araca, Negro river, Brazil | Forested interfluvial wetland | Mineral | 583 | 2.5 | Belger et al. [2011] |
| Pantanal, Brazil | Floodplain | Mineral | na | 5.9 ± 13.1 (0.042–91.1) | Marani and Alvala [2007] |
| Pantanal, Brazil | Floodplain | Mineral | 554 | 5.8 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 444 | 2.9 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 507 | 2.9 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 317 | 8.6 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 364 | 8.6 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 428 | 11.5272 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 586 | 11.5 | Hamilton et al. [1995] |
| Pantanal, Brazil | Floodplain | Mineral | 1062 | 17.3 | Hamilton et al. [1995] |
| Congo river basin, Congo | Flooded forest | Mineral | na | 4.41 | Tathy et al. [1992] |
Error is standard deviation. As the fluxes reported here are from studies extending over different time periods, they should be used for indicative purposes to illustrate the range of fluxes in tropical wetlands. The forested tropical wetlands shown in the table were not managed. Positive fluxes represent a release of CO2 or CH4 from the peat, and negative CH4 fluxes indicate CH4 oxidation in the peat. na, not available.
Figure 2.
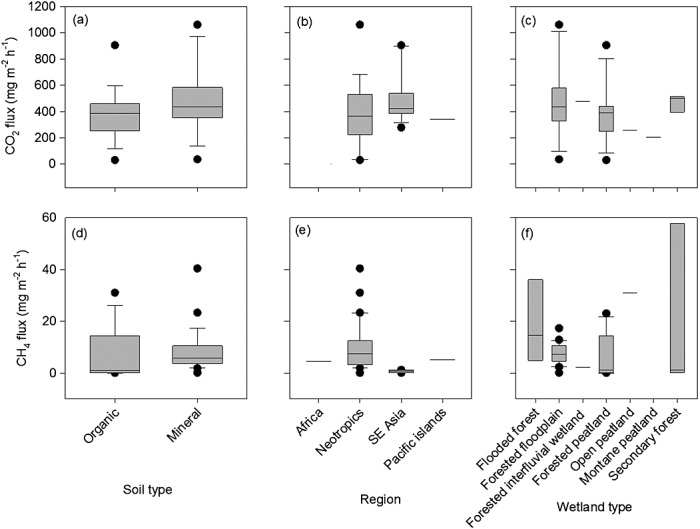
Box plots comparing (a–c) CO2 and (d–f) CH4 fluxes from different: (Figures2a and 2d) soil types, (Figures2b and 2e) regions, and (Figures2c and 2f) wetland types. The box plots show the lowest and highest observations and the lowest, median, and upper quartiles as well as values which may be considered as outliers. The statistics describing these results are reported in the text.
4.2. Methane
Estimated fluxes of CH4 from peatlands are typically several orders of magnitude lower than those for CO2 (Table4). Indeed, CH4 emissions are undetectable in some peatlands and uptake from the atmosphere might occur instead. Reported CH4 fluxes vary among wetland types (F5,42 = 6.77, P <0.001), ranging from −0.1 to 40 mg CH4 m−2 h−1; the highest values were recorded across a range of wetland systems (Figure2f), including forested peatland and floodplain ecosystems [Keller, 1990; Devol et al., 1998, 1990; Nahlik and Mitsch, 2011; Wright et al., 2011]. CH4 fluxes in Southeast Asian forested peatlands were typically lower (<2 mg CH4 m−2 h−1), while the highest, albeit variable, fluxes were reported for the Neotropics (F3,42 = 12.88; P <0.001; Figure2e). For example, fluxes from peatlands in Panama ranged between −5.35 and 143 mg CH4 m−2 h−1 (Table4 [Wright et al., 2011]), highlighting the potential for very high CH4 fluxes and marked temporal variability. The highest average CH4 emissions were from wetlands on mineral soils (F1,42 = 6.97, P <0.05), with mean fluxes of 8.22 and 6.10 mg CH4 m−2 h−1 in mineral and organic soils, respectively (Figure2d). The high emissions found in tropical wetlands have also been observed in subtropical wetland systems. A maximum emission of 19 mg CH4 m−2 h−1 was found in a subtropical forested floodplain in Australia [Boon et al., 1997], which is comparable to fluxes in swamp forests in the Everglades, USA, [Bartlett and Harriss, 1993] and 77 mg CH4 m−2 h−1 from forested floodplains in South Africa [Otter and Scholes, 2000]. In contrast, maximum CH4 fluxes from flooded temperate and boreal peatlands are lower, ranging between 10 and 14 mg CH4 m−2 h−1 [Couwenberg et al., 2010, and references therein]. Indeed, when comparing the estimated CH4 fluxes from tropical wetland to CH4 fluxes to higher-latitude wetland (e.g., subarctic and boreal; mean fluxes 4.7 and 3.0 mg CH4 m−2 h−1, respectively) and other types of wetlands (e.g., bog and fens; mean fluxes 4.0 and 3.9 mg CH4 m−2 h−1, respectively), mean tropical CH4 fluxes are higher [Turetsky et al., 2014].
Simple upscaling of short-term measurements to the pantropics suggests that approximately 91.6 ± 77 Tg CH4 year−1 (mean ± SD) is released from tropical wetlands, assuming that the CH4 flux from mineral soil (Figure2d) is related to the area covered by swamps and floodplains (Table1), and the flux from organic soil (Figure2d) was related to the area covered by peatlands. Our estimates of CH4 emissions from the peat surface of tropical wetlands are within the lower range of fluxes predicted by models [Melton et al., 2013]. In this context, it important to acknowledge the importance of tree stems and canopies for CH4 release [Pangala et al., 2013]. This pathway was not included in our calculations, which are therefore likely to underestimate actual fluxes. It will be important to include stem fluxes in future CH4 budgets. Additionally, tropical rivers represent an important source of CH4 to the atmosphere with recent estimates of CH4 emissions from rivers in the Amazon basin amounting to 0.40 to 0.58 Tg C year−1 which should be considered in the context of tropical CH4 emissions [Sawakuchi et al., 2014].
The much lower emissions of CH4 relative to CO2 suggest that only a small component of net C losses result from CH4 release. However, given its greater global warming potential compared to CO2 [Meehl et al., 2007], CH4 emissions at the upper end of the reported emissions range from tropical wetlands are still important from the perspective of radiative forcing.
5. Balance Between Carbon Inputs and Outputs
The high C effluxes presented above clearly suggest that most of the substantial quantity of C entering wetland systems eventually decomposes and does not contribute to accumulation of C in soil. This is also illustrated by the high litter decay constants (k) and short half times (mean 1.6 year) for in situ litter decomposition in tropical and subtropical wetlands (Figure3). Carbon accumulation in tropical wetlands is therefore attributable to the relatively small residual fraction compared to the much larger inputs (litter and root exudates) and outputs (heterotrophic respiration and DOC leaching) of C. This ultimately results in high CO2 and CH4 emissions from wetlands (Figure2 and Table4), in which environmental conditions are important in determining the proportions released as CO2 and CH4. For example, drainage of peatland for agriculture result enhances heterotrophic respiration and large CO2 losses from SE Asian peatlands (172 Tg C yr−1 [Hooijer et al., 2006]) amounting to 12% of C losses arising from deforestation and degradation on the tropics (1.4 Pg year−1 [Houghton, 2012]). In addition, the compilation of CH4 emissions suggests that the low CH4 emissions from wetlands in Southeast Asia reported by Couwenberg et al. [2010] are not representative of tropical wetlands globally (Figure2 and Table4). It is clear that various natural tropical wetland systems, including peatlands, are potentially significant sources of both CH4 and CO2 emissions.
Figure 3.
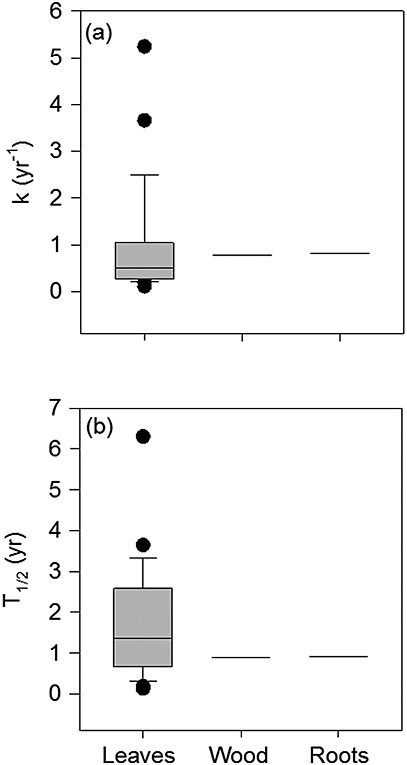
Box plots showing (a) litter decay constants for different tissue types reported in the literature and (b) calculated half times for different tissue types. Data are from in situ decomposition in tropical or subtropical wetlands. Given the small sample size for wood and roots (n = 2), only the median values are shown in the graph. The box plots show the lowest and highest observations and the lower, median, and upper quartiles, as well as observations which may be considered as outliers. The statistics describing these results are reported in the text. (Values are from Furtado et al. [1980], Irmler and Furch [1980], Frangi and Lugo [1985], Brady [1997], Rejmankova [2001], Del Valle-Arango [2003], Gamage and Asaeda [2005], Chimner and Ewel [2005], Troxler and Childers [2009], and Yule and Gomez [2009]).
Although k values for leaf litter decay were high, they differed among tree species and tissue types: the highest and lowest values reported for leaf tissue are, respectively, 5.64 and 0.11 year−1 (Figure3). The corresponding values for wood and roots are within the same range as for leaf tissue, but only one study appears to have examined in situ wood and root decay in tropical wetlands [Chimner and Ewel, 2005]. Decay constants >1 for some leaf litter types illustrate that some components of the litter input are likely to decompose fully, contributing to the substantial CO2 and CH4 efflux from tropical wetlands. Based on the existing limited data for different tissue types, it is currently impossible to ascertain whether specific tissue types degrade more slowly than others. However, the low decay constants for leaf litter reported in some studies (Figure3) clearly indicate that leaf materials, as well as wood and roots, contribute to peat formation. As wood and roots were important components for plant biomass production (approximately 50 and 10%, respectively [Chimner and Ewel, 2005]), information on their decay rates is needed to establish the relative contribution of tissue types to peat formation.
Based on the compilation of litter production and C loss data (Tables2 and 3), C balances were constructed for two types of tropical wetlands: those that are peat-forming, and those occurring on mineral soils (Figure4). Carbon inputs estimated as NPPtotal (Table2) and from the different litter fractions (Table3) provided comparable results for organic soils (1206 and 1185 g C m−2 yr−1 for NPPtotal and NPPcombined, respectively). As the data set for NPPtotal was based on a larger number of studies, we used this to calculate NEP. Mean C losses from the soil in the form of respiration (autotrophic and heterotrophic losses and CO2 and CH4 fluxes combined) and DOC losses for organic soils were lower for organic soils (991 g C m−2 yr−1) than for mineral soils (1075 g C m−2 yr−1). In contrast, NPPtotal was greater in wetlands with organic soils (1206 g C m−2 yr−1) than for those with mineral soils (880 g C m−2 yr−1). This resulted in NEP of 215 and −195 g C m−2 yr−1 for organic and mineral soils, respectively. The estimated NEP is within the range of the long-term C accumulation in tropical peatlands, which ranged between 30 and 300 g C m−2 yr−1 (see above), but is lower than reported by Hirano et al. [2007], who recorded NEP values of 310, 380, and 600 g C m−2 yr−1 in three consecutive years in a drained peat swamp forest and a papyrus swamp in Uganda (approximately 1000 g C m−2 yr−1 [Saunders et al., 2012]).
Figure 4.
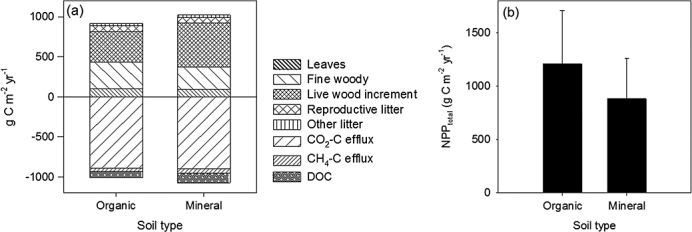
(a) Comparison of mean C inputs and outputs in tropical wetlands on organic and mineral soil, respectively. Note that the number of observations used for the means is highly variable (cf. Table3). There are also some important gaps in the comparison of the C balance between wetlands on organic and mineral soils, namely root (fine and coarse) production and coarse woody litter fall due to lack of data: (b) estimated C inputs (NPPtotal) from fine litterfall data sets (data in Table3) separated between wetlands on organic and mineral soil.
These balances suggest that positive NEP values are reflected by peat accumulation. However, the negative NEP for wetlands on mineral soils clearly indicates that the data must be used with caution; indeed, reliable estimates of NEP cannot be calculated from actual litter production due to the severe limitations in the available database. More specifically, we found only seven studies of fine root production, all on peat soils, and none containing data on coarse root production; these components of the C cycle are therefore not included in Figure4. This is a major concern, given their potentially large contribution to the overall C budget. Based on Chimner and Ewel [2005], fine root production amounted to approximately 11% of total plant production in a tropical peatland forest, while Malhi et al. [2011] estimated that coarse root production contributed approximately 7% to total plant production in tropical rainforest on mineral soil. Similarly, very few references report data for woody growth, which might represent a large flux of C in tropical wetlands (Table3). Data from Chimner and Ewel [2005] suggest that this might introduce an error of 25–30% in estimates of plant production. Omission of belowground and wood increment data from calculations of C balance may therefore lead to underestimations of C inputs of approximately 40–50%.
Similar problems exist with organic C data for fluvial soils. Ting-Hsuan et al. [2012] present data for overall regional trends of C export from tropical rivers suggesting that fluvial C losses from tropical rivers are 8.3 g C m−2 yr−1 with fluxes being estimated to be 2.2, 11.0, and 20.4 g C m−2 yr−1 for Africa, America, and Asia, respectively. Estimates of carbon exports of 8.5 g C m−2 yr−1 from the Amazon were presented by Richey et al. [1988]. However, these studies do not isolate the contribution from wetlands. Data from Moore et al. [2013], including TOC losses of 63 and 97 g C m−2 yr−1 from intact and disturbed peat swamp forests, respectively, in Kalimantan, suggest a potentially notable contribution of fluvial C losses from NEP calculations for peatland systems in Southeast Asia (approximately 10% increased C losses compared to the above calculations of gaseous losses and 22% compared with local accumulation rates). However, any available TOC or DOC data are integrated over large areas [Richey et al., 2002; Moore et al., 2011, 2013], in contrast to the measurement of litter production and C gas release. Furthermore, high variability of temporal fluvial C in relation to flood and rain events [e.g., Bass et al., 2011], combined with a low number of high-resolution temporal studies, also contribute to the limitations of aquatic C estimates. Given the limited available data, DOC fluxes appear to be of the same order of magnitude as CH4 fluxes about an order of magnitude smaller than CO2 losses (Figure4 and Table3). Although variation in the reported DOC flux data was substantial between organic and mineral soils systems (60%), the limitations of the available data mean that it is not possible to test whether this is a systematic difference.
Data availability was better for fine litterfall from the canopy, which was used in to calculate NPPtotal. However, the relationship between NPPtotal and NPPcanopy established for lowland rainforests may not be applicable to forested wetlands and may also differ between ombrotrophic and minerotrophic wetlands. Indeed, covariation between nutrient availability, forest composition, and peat depth/organic chemistry [Phillips et al., 1997; Sjögersten et al., 2010] suggests that nutrient availability may provide a strong control of C cycling in tropical wetlands. Care is therefore needed when interpreting these data.
Bearing in mind the data limitation noted above, NPPtotal appeared to be greater in tropical peatlands than in systems that were not accumulating peat (F1,48 = 7.15: P = 0.01: Figure4b). Data for litterfall and C effluxes were often not available for the same wetland systems, making it difficult to make valid comparisons of C inputs and outputs. Furthermore, the time frame for soil respiration measurements was highly variable, and there were neither long-term data sets on soil CO2 efflux nor diurnal variation with respect to plant-mediated gas transport [Pangala et al., 2013]. As a result, comparison of C inputs, which tend to be estimated on an annual basis, and the temporally discrete point measurements of CO2 emissions are unbalanced, which is likely to introduce a large error in the estimated NEP.
To assess the C budget of tropical wetlands fully, there is also an urgent need to separate autotrophic and heterotrophic respiration. Based on studies of an Acacia plantation on peat soil, Jauhiainen et al. [2012] concluded that up to 80% of the CO2 efflux from tropical peatlands might originate from root respiration, while work in well-drained tropical forests suggests that root respiration could account for 25–50% of the total soil CO2 efflux [Nottingham et al., 2010].
Comparison of our tentative C budgets for tropical wetlands with tropical forest on well-drained soils [Malhi et al., 2011] shows that NPPtotal from peat forming wetlands is comparable to lowland rainforest, but that NPPtotal from wetlands on mineral soils are lower. Decomposition rates in the wetland systems were generally lower (approximately 900 and 1350 g C m−2 yr−1 for wetlands and lowland forests, respectively). Together with the higher NPPtotal in wetlands on organic peat soils, this suggests that C accumulation in tropical peatlands is driven by a combination of lower decomposition rates and higher NPP.
6. Conclusions
Our metaanalysis suggests that greenhouse gas fluxes from tropical wetlands are high, with CH4 emissions being highest from mineral soils, although data quality is variable, with substantial data gaps for some regions (Figure1). NEP was greater in peat-forming wetlands than on mineral soils, but missing data for key components of the C balance again add significant uncertainty to our estimates of NEP.
The high CH4 emissions, particularly in the Neotropics, might partially explain the high atmospheric CH4 concentrations reported for tropical regions [Mikaloff Fletcher et al., 2004a, 2004b; Meirink et al., 2008]. The growing body of recent data for CO2 and CH4 fluxes from a range of tropical wetlands should be utilized in global wetland models, setting a challenge for the modeling community. However, our ability to assess the role of tropical wetlands in the global C cycle is limited by severe gaps in current understanding of net C inputs (with very limited data on root inputs and woody growth) and outputs (data are largely lacking on DOC losses and separation of autotrophic and heterotrophic respiration), presenting field researchers with an equally important challenge. Without such data, we cannot assess how these ecosystems influence global climate and how their role in the global C cycle may be impacted by future change in land use and climate [Melton et al., 2013].
Acknowledgments
The project was supported by a University of Nottingham New Investigator grant. Emma Wright received a PhD scholarship from the UK Biotechnology and Biological Sciences Research Council. Jorge Hoyos received funding for a PhD scholarship from CONACYT, Mexico. The data used for this literature review are available in the published literature.
Key Points
Released from tropical wetlands are 90 Tg CH4 year−1 and 3860 Tg CO2 year−1
CH4 emissions were greatest from mineral soils
NEP appears greater in peat-forming wetlands than on mineral soil
References
- Adis J, Furch K. Irmler U. Litter production of Central-Amazonian blackwater inundation forest. Trop. Ecol. 1979;20:236–245. [Google Scholar]
- Ali M, Taylor D. Inubushi K. Effects of environmental variations on CO2 efflux from a tropical peatland in eastern Sumatra. Wetlands. 2006;26:612–618. [Google Scholar]
- Alvarez-Lopez JA. Ecology of Pterocarpus officinalis forested wetlands in Puerto Rico. In: Brown S, editor; Lugo AE, Brinson M, editors. Ecosystems of the World, 15 Forested Wetlands. Netherlands: Elsevier; 1990. pp. 251–265. [Google Scholar]
- Anderson DE. Carbon accumulation and C/N ratios of peat bogs in North West Scotland. Scott. Geogr. J. 2002;118:323–341. [Google Scholar]
- Aselmann I. Crutzen PJ. Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J. Atmos. Chem. 1989;8:307–358. [Google Scholar]
- Bartlett KB. Harriss RC. Review and assessment of methane emissions from wetlands. Chemosphere. 1993;26:261–320. [Google Scholar]
- Bartlett KB, Crill PM, Sebacher DI, Harriss RC, Wilson JO. Melack JM. Methane flux from the central Amazonian floodplain. J. Geophys. Res. 1988;93:1574–1582. [Google Scholar]
- Bartlett KB, Crill PM, Bonassi JA, Richey JE. Harriss RC. Methane flux from Central Amazonian floodplains: Emission during rising water. J. Geophys. Res. 1990;95:16,733–16,788. [Google Scholar]
- Bass AM, Bird MI, Liddell MJ. Nelson PN. Fluvial dynamics of dissolved and particulate organic carbon during periodic discharge events in a steep tropical rainforest catchment. Limnol. Oceanogr. 2011;56:2282–2292. [Google Scholar]
- Belger L, Forsberg BR. Melack JM. Carbon dioxide and methane emissions from interfluvial wetlands in the upper Negro River basin, Brazil. Biogeochemistry. 2011;105:171–183. [Google Scholar]
- Bergamaschi P. Inverse modeling of global and regional CH4 emissions using SCIAMACHY satellite retrievals. J. Geophys. Res. 2009 et al. 114, D22301, doi: 10.1029/2009JD012287. [Google Scholar]
- Bloom AA, Palmer PI, Fraser A, Reay DS. Frankenberg C. Large-scale controls of methanogenesis inferred from methane and gravity spaceborne data. Science. 2010;327:322–325. doi: 10.1126/science.1175176. [DOI] [PubMed] [Google Scholar]
- Boon PI, Mitchell A. Lee K. Effects of wetting and drying on methane emissions from ephemeral floodplain wetlands in southeastern Australia. Hydrobiologia. 1997;357:73–87. [Google Scholar]
- Bracho R. San José J. Energy fluxes in a morichal (swamp palm community) at the Orinoco Llanos, Venzuela: Microclimate, water vapour and CO2 exchange. Photosynthetica. 1990;24:468–494. [Google Scholar]
- Brady MA. 1997. Canada The Univ. of British Columbia Organic material dynamics of coastal peat deposits in Sumatra, Indonesia, PhD thesis.
- Bridgham SD, Cadillo-Quiroz H, Keller JK. Zhung Q. Methane emissions from wetlands: Biogeochemical, microbial and modelling perspectives from local to global scales. Global Change Biol. 2013;19:1325–1346. doi: 10.1111/gcb.12131. [DOI] [PubMed] [Google Scholar]
- Bubier JL, Bhatia G, Moore TR, Roulet NT. Lafleur PM. Spatial and temporal variability in growing-season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems. 2003;6:353–367. [Google Scholar]
- Cattanio JH, Anderson AB, Rombold JS. Nepstad DC. Phenology, litterfall, growth, and root biomass in a tidal floodplain forest in the Amazon estuary. Rev. Bras. Bot. 2004;27:703–712. [Google Scholar]
- Chimner RA. Soil respiration rates of tropical peatlands in Micronesia and Hawaii. Wetlands. 2004;24:51–56. [Google Scholar]
- Chimner RA. Ewel KC. A tropical freshwater wetland: II. Production, decomposition and peat formation. Wetlands Ecol. Manage. 2005;13:671–684. [Google Scholar]
- Clair TA, Arp P, Moore TR, Dalva M. Meng FR. Gaseous carbon dioxide and methane, as well as dissolved organic carbon losses from a small temperate wetland under a changing climate. Environ. Pollut. 2002;116:S143–S148. doi: 10.1016/s0269-7491(01)00267-6. [DOI] [PubMed] [Google Scholar]
- Clymo RS. The limits to peat bog growth. Philos. Trans. R. Soc. London, Ser. B. 1984;303:605–654. [Google Scholar]
- Couwenberg J, Dommain R. Joosten H. Greenhouse gas fluxes from tropical peatlands in south-east Asia. Global Change Biol. 2010;16:1715–1732. [Google Scholar]
- Craft C, Washburn C. Parker A. Wastewater Treatment, Plant Dynamics and Management in Constructed and Natural Wetlands. New York: Springer Science + Business Media BV; 2008. Latitudinal trends in organic carbon accumulation in temperate freshwater peatlands; pp. 23–31. [Google Scholar]
- Crill PM, Bartlett KB, Harrisss RC, Melack JM, MacIntyre S, Lesack L. Smith-Morrill L. Tropospheric methane from an Amazonian floodplain lake. J. Geophys. Res. 1988;93:1564–1570. and, doi: 10.1029/JD093iD02p01564. [Google Scholar]
- Crow SE. Wieder RK. Sources of CO2 emission from a northern peatland: Root respiration, exudation, and decomposition. Ecology. 2005;86:1825–1834. [Google Scholar]
- Datta DK, Gupta LP. Subramian V. Distribution of C, N and P in the sediments of the Ganges ± Brahmaputra ± Meghna river system in the Bengal Basin. Org. Geochem. 1999;30:75–82. [Google Scholar]
- Del Valle-Arango JI. Descomposición de la hojarasca fina en bosques pantanosos del pacífico colombiano. Interciencia. 2003;28:148–153. [Google Scholar]
- Devineau JL. Données preliminaries sur la litière e al chute feuilles dans quelque forestières semi-décidues de la moyenne Côte d'Ivore. Oecol. Plant. 1976;11:375–395. [Google Scholar]
- Devol AH, Zaret TM. Forsberg BR. Sedimentary organic matter diagenesis and its relation to the carbon budget of tropical Amazon floodplain lakes. Verh. Int. Verein. Theor. Angew. Limnol. 1984;22:1299–1304. [Google Scholar]
- Devol AH, Richey JE, Clark WA. King SL. Methane emissions to the troposphere from the Amazon floodplain. J. Geophys. Res. 1988;93:1583–1492. and, doi: 10.1029/JD093iD02p01583. [Google Scholar]
- Devol AH, Richey JE, Forsberg BR. Martinelli LA. Seasonal dynamics in methane emissions from the Amazon River floodplain to the troposphere. J. Geophys. Res. 1990;95:16,417–16,426. and, doi: 10.1029/JD095iD10p16417. [Google Scholar]
- Dommain R, Couwenberg J. Joosten H. Development and carbon sequestration of tropical peat domes in south-east Asia: Links to post-glacial sea-level changes and Holocene climate variability. Quat. Sci. Rev. 2011;30:999–1010. [Google Scholar]
- Duke NC. 1982. Mangrove litterfall data from Hinchbrook Island, north-eastern Australia, Australian Institute of Marine Science Data Report Coastal Studies Series, Townsville, Australia.
- Easse AM. Aide TM. Patterns of litter production across a salinity gradient in a Pterocarpus officinalis tropical wetland. Plant. Ecol. 1999;145:307–315. [Google Scholar]
- Farmer J, Matthews R, Smith JU, Smith P. Singh BK. Assessing existing peatland models for their applicability for modelling greenhouse gas emissions from tropical peat soils. Curr. Opin. Environ. Sustainability. 2012;3:339–349. [Google Scholar]
- Finlayson CM. Productivity and nutrient dynamics of seasonally inundated floodplains in the Northern Territory Floodplains Res. In: Wade-Marshall D, Loveday P, editors. Northern Australia: Progress and Prospects. Vol. 2. Canberra, Australia: ANU Press; 1988. pp. 58–83. edited by, vol. [Google Scholar]
- Finlayson CM, Cowie ID. Bailey BJ. Biomass and litter dynamics in a Melaleuca forest on a seasonally inundated floodplain in tropical, northern Australia. Wetlands Ecol. Manage. 1993;2:177–188. [Google Scholar]
- Frangi JL. Lugo AE. Ecosystem dynamics of a subtropical floodplain forest. Ecol. Monogr. 1985;55:351–369. [Google Scholar]
- Frangi JL. Lugo AE. A flood plain palm forest in the Luquillo Mountains of Puerto Rico five years after hurricane Hugo. Biotropica. 1998;30:339–348. [Google Scholar]
- Franken M, Irmler U. Klinge H. Litterfall in inundation, riverine and terra firme forest of Central Amazonia. Trop. Ecol. 1979;20:225–235. [Google Scholar]
- Furtado JI, Verghese S, Liew KS. Lee TH. Litter production in a freshwater swamp forest Tasek Bera, Malasia. In: Furtado JI, editor; Tropical Ecology and Development. Proceedings of the Vth International Symposium of Tropical Ecology. Kuala Lumpur, Malaysia: International Society of Tropical Ecology; 1980. pp. 815–822. edited by, and. [Google Scholar]
- Furukawa Y, Inubishi K, Ali M, Itang AM. Tsuruta H. Effect of changing groundwater levels caused by land-use changes on greenhouse gas fluxes from tropical peat lands. Nutr. Cycling Agroecosyst. 2005;71:81–91. [Google Scholar]
- Gamage NPD. Asaeda T. Decomposition and mineralization of Eichhornia crassipes litter under aerobic conditions with and without bacteria. Hydrobiologia. 2005;541:13–27. [Google Scholar]
- Gill RA. Jackson RB. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000;147:13–31. [Google Scholar]
- Golley FB, McGinnis JT, Clements RG, Child GI. Duever MJ. Mineral Cycling in a Tropical Moist Forest Ecosystem. Univ. Georgia; 1975. [Google Scholar]
- Grand M. Gaidos E. Methane emission from a tropical wetland in Ka'au Crater, O'ahu, Hawaii. Pac. Sci. 2010;64:57–72. [Google Scholar]
- Haase R. Litterfall and nutrient return in seasonally flooded and non-flooded forest of the Pantanal, Mato Grosso, Brazil. For. Ecol. Manage. 1999;117:129–147. [Google Scholar]
- Hadi A, Haridi M. Inubushi K. Effect of land-use change on the microbial population and emission of greenhouse gasses. Micro. Environ. 2001;16:79–86. [Google Scholar]
- Hadi A, Inubushi K, Furukawa Y, Purnomo E, Rasmadi M. Tsuruta H. Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia. Nutr. Cycling Agroecosyst. 2005;71:73–80. [Google Scholar]
- Hamilton SK, Sippel SJ. Melack JM. Oxygen depletion and carbon dioxide and methane production in waters of the Pantanal wetland of Brazil. Biogeochemistry. 1995;30:115–141. [Google Scholar]
- Hirano T, Segah H, Harada T, Limin S, June T, Hirata R. Osaki M. Carbon dioxide balance of a tropical peat swamp forest in Kalimantan, Indonesia. Global Change Biol. 2007;13:412–425. [Google Scholar]
- Hirano T, Jauhiainen J, Inoue T. Takahashi H. Controls on the carbon balance of tropical peatlands. Ecosystems. 2009;12:873–887. [Google Scholar]
- Hooijer A, Silvius M, Wosten H. Page S. 2006. 2Delft Hydraulics Report Q3943, 36 p and PEAT–CO2Assessment of CO emissions from drained peatlands in SE Asia.
- Hooijer A, Page S, Canadell JG, Silvius M, Kwadijk J, Wosten H. Jaukiainen J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences. 2010;7:1505–1514. [Google Scholar]
- Hope G, Chokkalingam U. Anwar S. The stratigraphy and fire history of the Kutai Peatlands, Kalimantan, Indonesia. Quat. Res. 2005;64:407–417. [Google Scholar]
- Houghton RA. Carbon emissions and the drivers of deforestation and forest degradation in the tropics. Curr. Opin. Environ. Sustainability. 2012;4:1–7. [Google Scholar]
- Inubushi K, Hadi A, Okazaki M. Yonebayashi K. Effect of converting wetland forest to sago palm plantations on methane gas flux and organic carbon dynamics in tropical peat soil. Hydrol. Process. 1998;12:2073–2080. [Google Scholar]
- Inubushi K, Furukawa Y, Hadi A, Purnomo E. Tsuruta H. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere. 2003;52:603–608. doi: 10.1016/S0045-6535(03)00242-X. [DOI] [PubMed] [Google Scholar]
- Stocker TF, editor. IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, U. K., and New York: Cambridge Univ. Press; 2013. edited by et al. [Google Scholar]
- Irmler U. Furch K. Weight, energy, and nutrient changes during the decomposition of leaves in the emersions phase of Central-Amazonian inundation forests. Pedobiologia. 1980;20:18–130. [Google Scholar]
- Jauhiainen J, Takahashi H, Heikkinen JEP, Martikainen PJ. Vasanders H. Carbon fluxes from a tropical peat swamp forest floor. Global Change Biol. 2005;11:1788–1797. [Google Scholar]
- Jauhiainen J, Hooijer A. Page SE. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra Indonesia. Biogeosciences. 2012;8:8270–9302. [Google Scholar]
- Keller M. 1990. Princeton, N. J Princeton Univ Biological sources and sinks of methane in tropical habitats and tropical atmospheric chemistry, Cooperative thesis 126.
- Kirschke S. Three decades of global methane sources and sinks. Nat. Geosci. 2013;6:813–823. [Google Scholar]
- Klinge H. Litter production in tropical ecosystems. Malay. Nat. J. 1978;30:415–422. [Google Scholar]
- Lähteenoja O. Page S. High diversity of tropical peatland ecosystem types in the Pastaza-Maranon basin, Peruvian Amazonia. J. Geophys. Res. 2011;116 and, G02025, doi: 10.1029/2010JG001508. [Google Scholar]
- Lähteenoja O, Ruokolainen K, Schulman L. Oinonen M. Amazonian peatlands: An ignored C sink and potential source. Global Change Biol. 2009;15:2311–2320. [Google Scholar]
- Lähteenoja O, Reátegui YR, Räsänen M, Torres DDC, Oinonen M. Page S. The large Amazonian peatland carbon sink in the subsiding Pastaza-Marañón foreland basin, Peru. Global Change Biol. 2012;18:164–178. [Google Scholar]
- Laiho R. Decomposition in peatlands: Reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biol. Biochem. 2006;38:2011–2024. [Google Scholar]
- Makiranta P, Laiho R, Fritze H, Hytonen J, Laine J. Minkkinen K. Indirect regulation of heterotrophic peat soil respiration by water level via microbial community structure and temperature sensitivity. Soil Biol. Biochem. 2009;41:695–703. [Google Scholar]
- Malhi Y, Doughty C. Galbraith D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc., B. 2011;366:3225–3245. doi: 10.1098/rstb.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marani L. Alvala PC. Methane emissions from lakes and floodplains in Pantanal, Brazil. Atmos. Environ. 2007;41:1627–1633. [Google Scholar]
- Mata DI, Moreno-Casasola P. Madero-Vega C. Litterfall of tropical forested wetlands of Veracruz in the coastal floodplains of the Gulf of Mexico. Aquat. Bot. 2012;98:1–11. [Google Scholar]
- Matthews E. Fung I. Methane emissions from natural wetlands: Global distribution, area and environmental characteristics of sources. Global Biogeochem. Cycles. 1987;1:61–86. [Google Scholar]
- Meehl GA. Global climate projections. In: Solomon S, editor. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, U. K., and New York: Cambridge Univ. Press; 2007. edited by et al.,, et al. ( [Google Scholar]
- Meirink JF, Bergamaschi P. Krol MC. Four-dimensional variational data assimilation for inverse modelling of atmospheric methane emissions: Analysis of SCIAMACHY observations. J. Geophys. Res. 2008;11 and, D17301, doi: 10.1029/2007JD009740. [Google Scholar]
- Melack JM, Hess LL, Gastil M, Forsberg BR, Hamilton SK, Lima IBT. Novo ELM. Regionalization of methane emissions in the Amazon Basin with microwave remote sensing. Global Change Biol. 2004;10:530–544. [Google Scholar]
- Melling L, Hatano R. Goh KJ. Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol. Biochem. 2005a;37:1445–1453. [Google Scholar]
- Melling L, Hatano R. Goh KJ. Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus Ser. B-Chem. Phys. Met. 2005b;57:1–11. [Google Scholar]
- Melton JR. Present state of global wetland extent and wetland methane modelling: Conclusion from a model inter-comparison project (WETCHIMP) Biogeosciences. 2013;10:753–788. et al. doi: 10.5194/bg-10-753-2013. [Google Scholar]
- Miegot J. Imbert D. Phenology and production of litter in a Pterocarpus officinalis (Jacq.) swamp forest of Guadeloupe (Lesser Antilles) Aquat. Bot. 2012;101:18–27. [Google Scholar]
- Mikaloff Fletcher SE, Tans PP, Bruhwiler LM, Miller JB. Heimann M. CH4 sources estimated from atmospheric observations of CH4 and its 13C/12C isotopic ratios: 1. Inverse modeling of source processes. Global Biogeochem. Cycles. 2004a;18 and, GB4004, doi: 10.1029/2004GB002224. [Google Scholar]
- Mikaloff Fletcher SE, Tans PP, Bruhwiler LM, Miller JB. Heimann M. CH4 sources estimated from atmospheric observations of CH4 and its 13C/12C isotopic ratios: 2. Inverse modeling of CH4 fluxes from geographical regions. Global Biogeochem. Cycles. 2004b;18 and, GB4005, doi: 10.1029/2004GB002224. [Google Scholar]
- Miller JB, Gatti LV, d'Amelio MTS, Crotwell AM, Dlugokencky EJ, Bakwin P, Artaxo P. Tans PP. Airborne measurements indicate large methane emissions from the eastern Amazon basin. Geophys. Res. Lett. 2007;34 and, L10809, doi: 10.1029/2006GL029213. [Google Scholar]
- Moore S, Gauci V, Evans CD. Page SE. Fluvial organic carbon losses from a Bornean blackwater river. Biogeosciences. 2011;8:901–909. [Google Scholar]
- Moore S, Evans CD, Page SE, Garnett MH, Jones TG, Freeman C, Hooijer A, Wiltshire AJ, Limin SH. Gauci V. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature. 2013;493:660–663. doi: 10.1038/nature11818. [DOI] [PubMed] [Google Scholar]
- Moreira-Turcq P, Jouanneau JM, Turcq B, Seyler P, Weber O. Guyot JL. Carbon sedimentation at Lago Grande de Curuai, a floodplain lake in the low Amazon region: Insights into sedimentation rates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004;214:27–40. [Google Scholar]
- Murayama S. Bakar ZA. Decomposition of tropical peat soils, 2. Estimation of in situ decomposition by measurement of CO2 flux. Jpn. Agric. Res. Q. 1996;30:153–158. [Google Scholar]
- Nahlik AM. Mitsch WJ. Methane emissions from tropical freshwater wetlands located in different climatic zones of Costa Rica. Global Change Biol. 2011;17:1321–1334. [Google Scholar]
- Naiman RJ. Riparia: Ecology, Conservation, and Management of Streamside Communities. Mass: Academic Press; 2005. p. 140. [Google Scholar]
- Nebel G, Dragsted J. Vega AS. Litterfall, biomass and net primary production in flood plain forests in the Peruvian Amazon. For. Ecol. Manage. 2001;150:93–102. [Google Scholar]
- Nottingham AT, Turner BL, Winter K, van der Heijden MGA. Tanner EVJ. Arbuscular mycorrhizal mycelial respiration in a moist tropical forest. New Phytol. 2010;186:957–967. doi: 10.1111/j.1469-8137.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- Otter LB. Scholes MC. Methane sources and sinks in a periodically flooded South African savanna. Global Biogeochem. Cycles. 2000;14:97–111. [Google Scholar]
- Page SE, Rieley JO, Shotyk ØW. Weiss D. Interdependence of peat and vegetation in a tropical peat swamp forest. Philos. Trans. R. Soc., B. 1999;354:1885–1897. doi: 10.1098/rstb.1999.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SE, Rieley JO. Banks CJ. Global and regional importance of the tropical peatland carbon pool. Global Change Biol. 2011;17:798–818. [Google Scholar]
- Pangala SR, Moore S, Hornibrook ERC. Gauci V. Trees are major conduits for methane egress from tropical forested wetlands. New Phytol. 2013;197:524–531. doi: 10.1111/nph.12031. [DOI] [PubMed] [Google Scholar]
- Payntner Q. Evaluating the impact of a biological control agent Carmenta mimosa on the woody wetland weed Mimosa pigra in Australia. J. Appl. Ecol. 2005;42:1054–1062. [Google Scholar]
- Phillips S, Rouse GE. Bustin RM. Vegetation zones and diagnostic pollen profiles of a coastal peat swamp, Bocas del Toro, Panama. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997;128:301–338. [Google Scholar]
- Rejmankova E. Effect of experimental phosphorus enrichment on oligotrophic tropical marshes in Belize, Central America. Plant Soil. 2001;236:33–53. [Google Scholar]
- Richey JE, Devol AH, Wofy SC, Victoria R. Riberio MN. Biogenic gases and the oxidation and reduction of carbon in Amazon River and floodplain waters. Limnol. Oceanogr. 1988;33:551–561. [Google Scholar]
- Richey JE, Melack JM, Aufdenkampe AK, Ballester VM. Hess LL. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature. 2002;416:617–620. doi: 10.1038/416617a. [DOI] [PubMed] [Google Scholar]
- Riley WJ, Subin ZM, Lawrence DM, Swenson SC, Torn MS, Meng L, Mahowald NM. Hess P. Barriers to predicting changes in global terrestrial methane fluxes: Analyses using CLM4Me, a methane biogeochemistry model integrated in CESM. Biogeosciences. 2011;8:1925–1953. and, doi: 10.5194/bg-8-1925-2011. [Google Scholar]
- Robinson SD. Moore TR. Carbon and peat accumulation over the past 1200 years in a landscape with discontinuous permafrost, northwestern Canada. Global Biogeochem. Cycles. 1999;13:591–601. [Google Scholar]
- Roulet NT, Lafleur PM, Richard PJH, Moore TR, Humphreys ER. Bubier J. Contemporary carbon balance and late Holocene carbon accumulation in a northern peatland. Global Change Biol. 2007;13:397–411. [Google Scholar]
- San-José J, Montes R, Angel Mazorra M, Aguirre Ruiz E. Matute N. Patterns and carbon accumulation in the inland water-land palm ecotone (morichal) across the Orinoco lowlands, South America. Plant Ecol. 2010;206:361–374. [Google Scholar]
- Sannel ABK. Kuhry P. Holocene peat growth and decay dynamics in sub-arctic peat plateaus, west-central Canada. Boreas. 2009;38:13–24. [Google Scholar]
- Saunders MJ, Kansiime F. Jones MB. Agricultural encroachment: Implications for carbon sequestration in tropical African wetlands. Global Change Biol. 2012;18:1312–1321. [Google Scholar]
- Sawakuchi HO, Bastviken D, Sawakuchi AO, Krusche AV, Ballester MVR. Richey JE. Methane emissions from Amazonian Rivers and their contribution to the global methane budget. Global Change Biol. 2014;9:2829–2840. doi: 10.1111/gcb.12646. [DOI] [PubMed] [Google Scholar]
- Shimada S, Takahashi H, Haraguchi A. Kaneko M. The carbon content characteristics of tropical peats in Central Kalimantan, Indonesia: Estimating their spatial variability in density. Biogeochemistry. 2001;53(3):249–267. [Google Scholar]
- Shindell DT, Walter BP. Faluvegi G. Impacts of climate change on methane emissions from wetlands. Geophys. Res. Lett. 2004;31 and, L21202, doi: 10.1029/2004GL021009. [Google Scholar]
- Silva MFF. Lobo MGA. Nota sobre decomposição äo de materia orgânica em floresta de terra firme, várzea e igapó. Bol. Mus. Para. Emilio Goeldi (S Bot) 1982;1:111–158. [Google Scholar]
- Silvola J, Alm J, Ahlholm J, Nykanen H. Martikainen PJ. CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J. Ecol. 1996;84:219–228. [Google Scholar]
- Sjögersten S, Cheesman AW, Lopez O. Turner BL. Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry. 2010;104:147–163. [Google Scholar]
- Smith LK. Lewis WM. 1992. and Methane emissions from the Orinoco River floodplain, Venezuela. ASLO 92, Aquatic Science Meeting, Santa Fe, NM, February 9–14.
- Smith LK, Lewis WM, Chanton JP, Cronin G. Hamilton SK. Methane emissions from the Orinoco River floodplain, Venezuela. Biogeochemistry. 2000;51:113–140. [Google Scholar]
- Sundari S, Hirano T, Yamada H. Kusin LS. Effect of ground water level on soil respiration in tropical peat swamp forests. J. Agric. Meteorol. 2012;68:121–134. [Google Scholar]
- Tathy JP, Cros B, De Imas RA, Marenco A, Servant J. Labal M. Methane emissions from flooded forest in Central Africa. J. Geophys. Res. 1992;97:6159–6168. and, doi: 10.1029/90JD02555. [Google Scholar]
- Terror VL, de Sousa HC. Rodrigues Kozovit A. Produção, decomposição e qualidade nutricional da serapilheira foliar em uma fl oresta paludosa de altitude. Acta. Bot. Bras. 2011;25:113–121. [Google Scholar]
- Ting-Hsuan H, Yu-Han F, Pei-Yi P. Chen-Tung AC. Fluvial carbon fluxes in tropical rivers. Curr. Opin. Environ. Sustainability. 2012;4:162–169. [Google Scholar]
- Troxler T. Childers DL. Litter decomposition promotes differential feedbacks in an oligotrophic southern Everglades wetland. Plant Ecol. 2009;200:69–82. [Google Scholar]
- Turetsky MR. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Global Change Biol. 2014;20:2183–2197. doi: 10.1111/gcb.12580. et al. doi: 10.1111/gcb.12580. [DOI] [PubMed] [Google Scholar]
- Turunen C. Turunen J. Development history and carbon accumulation of a slope bog in oceanic British Columbia, Canada. Holocene. 2003;13:225–238. [Google Scholar]
- Turunen J, Tomppo E, Tolonen K. Reinikainen A. Estimating carbon accumulation rates of undrained mires in Finland—Application to boreal and subarctic regions. Holocene. 2002;12:69–80. [Google Scholar]
- Ueda S, Go CSU, Yoshioka T, Yoshida N, Wada E, Miyajima T, Sugimoto A, Boontanon N, Vijarnsorn P. Boonprakub S. Dynamics of dissolved O2, CO2, CH4 and N2O in a tropical coastal swamp in southern Thailand. Biogeochemistry. 2000;49:191–215. [Google Scholar]
- Ukonmaanaho L, Nieminen TM, Rausch N, Cheburkin A, Le Roux G. Shotyk W. Recent organic matter accumulation in relation to some climatic factors in ombrotrophic peat bogs near heavy metal emission sources in Finland. Global Planet. Change. 2006;53:259–268. [Google Scholar]
- Walter BP. Heimann M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Global Biogeochem. Cycles. 2000;14:745–765. and, doi: 10.1029/1999GB001204. [Google Scholar]
- Wassmann R, Thein UG, Whiticar MJ, Rennenbergh H, Seiler W. Junk WJ. Methane emissions from the Amazon floodplain characterization of production and transport. Global Biogeochem. Cycles. 1992;6:3–13. [Google Scholar]
- Wright EL, Black C, Turner BL. Sjögersten S. Environmental controls of temporal and spatial variability in CO2 and CH4 fluxes in a neotropical peatland. Global Change Biol. 2013;19:3775–3789. doi: 10.1111/gcb.12330. [DOI] [PubMed] [Google Scholar]
- Wright E, Black CR, Cheesman AW, Drage T, Large D, Turner BL. Sjögersten S. Contribution of subsurface peat to CO2 and CH4 fluxes in a neotropical peatland. Global Change Biol. 2011;17:2867–2881. [Google Scholar]
- Yule CM. Gomez LN. Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetlands Ecol. Manage. 2009;17:231–241. [Google Scholar]


