Abstract
The virion host shutoff protein (Vhs) of herpes simplex virus type 1 induces destabilization of mRNA following infection. Our study of primary neurons from CD-1 mice demonstrates that vhs is functional in neurons but that more Vhs is required to mediate RNA degradation in neurons than in other susceptible cells.
The virion host shutoff protein (Vhs) of herpes simplex virus type 1 (HSV-1) is a tegument phosphoprotein which upon infection induces the destabilization of mRNA (2-4, 6, 8). vhs-deficient viruses grow normally in cell culture but show significant attenuation in growth in the nervous system and other tissues (9, 11, 12). It was previously reported that Vhs cannot induce RNA degradation in neurons, a finding that is incompatible with the hypothesis that Vhs activity is critical for neuropathogenesis (7). Data reported in this study show that Vhs activity occurs in neurons but requires a higher viral inoculum than it does in other cell types.
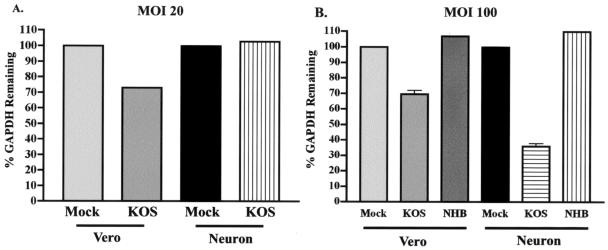
Vhs-mediated RNA degradation in neuron cultures was analyzed with the previously described RNA degradation assay (12). Primary superior cervical ganglion (SCG) cells were isolated from neonatal CD-1 mice according to procedures used previously for the isolation of neonatal rat SCG neurons (7). Briefly, 104 Vero cells or SCG neurons were cultured on collagen-coated 35-mm-diameter dishes, with one mouse pup yielding approximately 104 SCG neurons. Each sample in the Northern blot analysis required RNA extracted from eight plates of Vero cells or SCG neurons per virus type (7). Cells were mock infected or infected with wild-type virus (KOS) or a previously described vhs-null virus (UL41NHB) (12) at a multiplicity of infection (MOI) of 20 or 100. At 8 h postinfection, infected cells were treated with dissociation media (5), and total RNA was harvested with an RNeasy RNA preparation kit (QIAGEN, Valencia, Calif.) and analyzed by Northern blotting (12). Blots were probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA and for 28S rRNA as a loading control. Images were documented with a STORM phosphoimaging system (Amersham Biosciences, Piscataway, N.J.) and quantified with ImageQuant software. The value of each GAPDH measurement was normalized to the lowest 28S RNA value. The data were then expressed as the amount of GAPDH message remaining as a percentage of the amount remaining in mock-infected cells, which was set at a value of 100%. At MOIs of 20 and 100, infection with KOS resulted in the degradation of 25 to 35% of the GAPDH RNA in Vero cells (Fig. 1). As expected, the use of total RNA extracts, in contrast to the cytoplasmic extracts used previously (11), revealed more-modest levels of Vhs-mediated RNA degradation. This difference is found because Vhs mediates RNA degradation largely in the cytoplasm, and nuclear GAPDH RNA, while not immune to Vhs, is therefore protected from degradation due to its localization in the nucleus. The infection of SCG neurons with KOS at an MOI of 20 resulted in no RNA degradation, as found previously (9, 12). At an MOI of 100, however, KOS induced the degradation of 70% of GAPDH RNA. As expected, there was no RNA degradation in either Vero cells or SCG neurons infected with the vhs-null mutant UL41NHB at an MOI of 100, a result consistent with that of previous experiments with Vero cells infected at an MOI of 20 (12). A value slightly above 100% in the cells infected with UL41NHB at an MOI of 100 is an artifact of normalization. Vhs therefore mediates RNA degradation in neurons at high MOIs. The consistent amounts of RNA degradation found in Vero cells infected at MOIs of 20 and 100 may suggest a threshold level of Vhs activity in Vero cells, which is achieved at or below an MOI of 20. The increased amount of RNA degradation seen in neurons infected at an MOI of 100 may suggest that the unique anatomy of neurons, with a significantly larger cytoplasm, may require an increased concentration of Vhs to mediate RNA degradation.
FIG. 1.
RNA degradation assay by Northern blotting to measure Vhs activity. Vero cells or SCG neurons (104) were mock infected (Mock) or infected with KOS or UL41NHB (NHB) at an MOI of 20 (A) or 100 (B). Total RNA was harvested at 8 h postinfection, and a Northern blot analysis was performed. Northern blots were probed for GAPDH and then stripped and reprobed for 28S rRNA as a loading control. Images were quantified with ImageQuant software from Molecular Dynamics. Data are reported as the amount of GAPDH message remaining as a percentage of that in mock-infected cells. (A) Results from a single experiment; (B) combined data from two experiments.
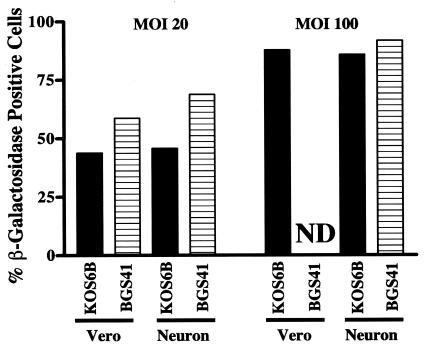
To address the hypothesis that the differential susceptibilities to Vhs of Vero cells and SCG neurons were due to differential levels of permissiveness to infection, cells were infected with either vhs-sufficient (KOS6B) (13) or vhs mutant (BGS-41) (12) viruses which express β-galactosidase. Vero cells and SCG neurons were infected at MOIs of 20 or 100 with either KOS6B or BGS-41 and stained for β-galactosidase at 12 h postinfection, a time point that allows optimum detection of viral gene expression prior to de novo virus production (Fig. 2). At an MOI of 20, 45 to 50% of Vero cells and SCG neurons infected with KOS and 55 to 70% of cells infected with BGS-41 were positive for β-galactosidase staining. Eighty-five to 90% of Vero cells and SCG neurons were β-galactosidase positive after infection at an MOI of 100 with KOS6B or BGS-41 (BGS-41 infection of Vero cells was not determined). SCG neurons and Vero cells are therefore equivalently infected at both MOIs, and the presence or absence of Vhs does not affect the ability of HSV-1 to infect either cell type.
FIG. 2.
Susceptibility of SCG neurons and Vero cells to infection by HSV-1. SCG neurons and Vero cells were plated at a density of 104 cells/well. The cells were infected with either KOS6B or BGS-41 at an MOI of 20 or 100. The cells were fixed, stained for β-galactosidase expression, and counted at 12 h postinfection. Data shown are representative of two experiments. ND, not detectable.
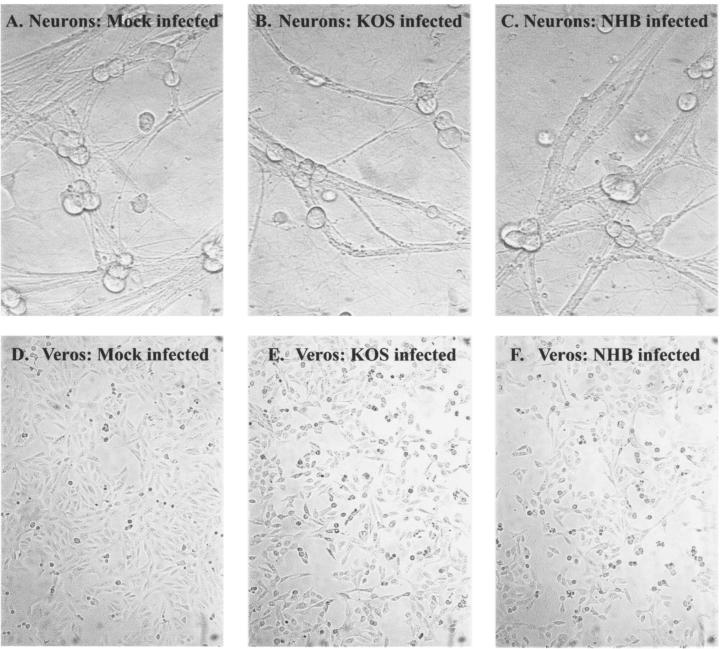
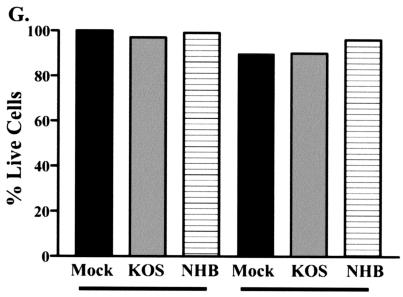
To address the hypothesis that the degradation of GAPDH in cells infected at an MOI of 100 resulted from virus-induced cell death rather than Vhs activity, Vero cells and SCG neurons were infected at an MOI of 100. Bright-field images of infected Vero cells and SCG neurons are shown in Fig. 3. These infected cultures were analyzed for viability with a Live/Dead viability-cytotoxicity kit (Molecular Probes, Eugene, Ore.). In this assay, live cells show green fluorescence due to uptake and conversion of Calcein-AM to calcein. Dead cells show red fluorescence due to the uptake and binding of ethidium homodimer (Eth-D1) to nucleic acids. In the methanol-treated dead-cell control, less than 1% of cells showed green fluorescence. In contrast, cells that were either mock infected or infected with KOS or UL41NHB showed 90% green fluorescence and less than 10% red fluorescence (Fig. 3). At 8 h postinfection (the time point at which RNA was harvested), therefore, both SCG neurons and Vero cells were viable, and the changes in GAPDH RNA levels can be attributed to Vhs activity, not cell death.
FIG. 3.
Viability assay of SCG neurons and Vero cells infected at an MOI of 100. SCG neurons and Vero cells were plated at a density of 104 cells/well. The cells were then either mock infected (Mock) or infected with KOS or UL41NHB (NHB) at an MOI of 100. At 8 h postinfection, cells were incubated in the presence of Calcein-AM and ethidium homodimer and counted by fluorescence microscopy. A methanol-incubated dead-cell control showed 100% killing (data not shown). (A to C) Bright-field images of SCG neurons mock infected or infected with KOS or UL41NHB at 8 h postinfection; (D to F) Vero cells mock infected or infected with KOS or UL41NHB at 8 h postinfection; (G) graphical representation of the cytotoxicity data. Data shown are representative of two independent experiments.
This work demonstrates that Vhs activity can occur in SCG neurons. Our results are consistent with previous data showing that Vhs activity does not occur in SCG neurons infected at an MOI of 20 (7), but Vhs activity clearly does occur in SCG neurons infected at higher MOIs. The differences in Vhs activity in Vero cells and SCG neurons were not due to differential levels of permissiveness or to decreased cell viabilities. Species origin and cell type (primary versus continuous) also cannot explain the differences in Vhs activity between Vero cells and SCG neurons, since Vero cells and mouse embryo fibroblasts have been shown to have equivalent susceptibilities to Vhs (10). The higher MOI required for Vhs activity in neurons suggests that perhaps as much as 10-fold more Vhs is required to mediate RNA degradation in neurons than in Vero cells. Nichol et al. showed that nerve growth factor-differentiated PC12 cells have increased resistance to Vhs compared to that of undifferentiated cells (7). This differential resistance does not discriminate between differentiation to a unique neuronal anatomy or the possible expression of a specific Vhs inhibitor but suggests that nerve growth factor differentiation may reduce susceptibility to Vhs.
During HSV-1 infection, it is unknown how much Vhs is expressed in neurons, but vhs-deficient viruses clearly show significant attenuation in growth in the nervous system. This finding is consistent with the idea that Vhs is important for the growth and spread of HSV (9, 12). The observation that a high MOI is required for Vhs activity in SCG neurons, contrasted with the important role that Vhs plays in neurovirulence, presents an obvious paradox. A number of possible explanations exist. First, Vhs may be a multifunctional protein, suggesting that functions in addition to mRNA degradation may be important to neurovirulence. Second, the impact of Vhs-mediated RNA degradation at nonneuronal sites such as the cornea or skin may significantly affect the ability of HSV-1 to seed the nervous system and therefore indirectly affect neurovirulence. Third, it is also clear that cultured neurons do not recapitulate the complex interactions of neurons with other cells in the nervous system. It is therefore possible that Vhs activity in support cells within the nervous system may significantly modify the biology of the neuron and may have a major impact on damage within the nervous system. In light of the finding that Vhs activity can occur in neurons, studies of latency and pathogenesis should therefore account for this possibility. Additionally, many gene therapy applications require a bolus of HSV to be stereotactically injected into specific sites in the nervous system (1). In this circumstance, Vhs activity could result in decreased levels of transgene expression, and our findings underscore a possible advantage of using vhs-deficient vectors in such applications.
Acknowledgments
We thank Eugene Johnson and Patricia Lampe for the dissection and culture of the SCG neurons.
This work was supported by NIH grants to David A. Leib (EY10707) and the Department of Ophthalmology and Visual Sciences, Washington University School of Medicine (P30 EY02687). Support from Research to Prevent Blindness to the department and a Lew Wasserman Scholarship to David A. Leib are gratefully acknowledged.
REFERENCES
- 1.Burton, E. A., D. J. Fink, and J. C. Glorioso. 2002. Gene delivery using herpes simplex virus vectors. DNA Cell Biol. 21:915-936. [DOI] [PubMed] [Google Scholar]
- 2.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 75:10272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenwick, M. L., and R. D. Everett. 1990. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 71:2961-2967. [DOI] [PubMed] [Google Scholar]
- 4.Fenwick, M. L., and M. McMenamin. 1984. Synthesis of alpha (immediate-early) proteins in Vero cells infected with pseudorabies virus. J. Gen. Virol. 65:1449-1456. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, P. G., and R. P. Lisak. 1980. Astrocytes and oligodendrocytes in dissociated cell culture of adult rat optic nerve. Neurosci. Lett. 16:229-233. [DOI] [PubMed] [Google Scholar]
- 6.Lu, P., F. E. Jones, H. A. Saffran, and J. R. Smiley. 2001. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 75:1172-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1994. Infection of sympathetic and sensory neurones with herpes simplex virus does not elicit a shut-off of cellular protein synthesis: implications for viral latency and herpes vectors. Neurobiol. Dis. 1:83-94. [DOI] [PubMed] [Google Scholar]
- 8.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73:467-470. [DOI] [PubMed] [Google Scholar]
- 9.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith, T. J., R. H. Silverman, and D. A. Leib. 2003. RNase L activity does not contribute to host RNA degradation induced by herpes simplex virus infection. J. Gen. Virol. 84:925-928. [DOI] [PubMed] [Google Scholar]
- 11.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 70:5665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers, B. C., and D. A. Leib. 2002. Herpes simplex virus type 1 origins of DNA replication play no role in the regulation of flanking promoters. J. Virol. 76:7020-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]