Abstract
The major immediate-early (MIE) gene of human cytomegalovirus (HCMV) produces multiple mRNAs through differential splicing and polyadenylation. Reverse transcriptase PCR was used to characterize transcripts from exons 1, 2, 3, and 4 (immediate-early 1 [IE1]). The expected IE72 and IE19 mRNAs were detected, as well as two heretofore-uncharacterized transcripts designated IE17.5 and IE9. The IE72, IE19, and IE17.5 transcripts utilized the same 5′-splice site in exon 3. IE9 utilized a cryptic 5′-splice site within exon 3. The IE19, IE17.5, and IE9 transcripts all used different 3′-splice sites within exon 4. These spliced species occur in infected human foreskin fibroblast (HFF) cells, with accumulation kinetics similar to those of IE72 mRNA. IE19 and IE9 RNAs were much more abundant than IE17.5 RNA. Transfection of CV-1 cells with cDNAs resulted in IE19 and IE17.5 proteins detectable by antibodies to either N-terminal or C-terminal epitopes. No IE9 protein product has been detected. We have not been able to detect IE19, IE17.5, or IE9 proteins during infection of HFF, HEL, or U373MG cells. Failure to detect IE19 protein contrasts with a previous report (M. Shirakata, M. Terauchi, M. Ablikin, K. Imadome, K. Hirai, T. Aso, and Y. Yamanashi, J. Virol. 76:3158-3167, 2002) of IE19 protein expression in HCMV-infected HEL cells. Our analysis suggests that an N-terminal breakdown product of IE72 may be mistaken for IE19. Expression of IE19 or IE17.5 from its respective cDNA results in repression of viral gene expression in infected cells. We speculate that expression of these proteins during infection may be restricted to specific conditions or cell types.
The major immediate-early (MIE) gene of human cytomegalovirus (HCMV) is a complex region consisting of a promoter, five exons, and two poly(A) signals (Fig. 1). Alternative splicing and differential usage of the two polyadenylation signals give rise to at least five reported transcripts (23, 26). The two major transcripts encode the most studied MIE proteins (MIEPs), immediate-early 72 (IE72; IE1, IE1491aa, or ppUL123) and IE86 (IE2, IE2579aa, or ppUL122a). These proteins have been extensively studied for their effects on the transcriptional activities of viral and cellular promoters and their role in controlling the temporal expression of the HCMV genes (2, 6-10, 12, 14-17, 21, 22, 25, 27, 29). They have also been shown to regulate cellular processes such as cell cycle control, metabolism, and apoptosis (13, 28, 30).
FIG. 1.
The HCMV MIE gene encodes a number of alternatively spliced mRNA species. The schematic illustrates the MIE gene with its five major exons and two alternative polyadenylation (PA) signals. Exons 4 and 5 are traditionally considered the IE1 and IE2 regions, respectively. Previously identified transcripts from both the IE1 and IE2 regions are shown. The locations of primers used in RT-PCR analysis (primers 1, 2, and 3) are indicated with arrows.
In addition to the transcripts for IE72 and IE86, other MIE gene splice variants have been identified: IE19 (from the IE1 region) (20) and IE55 (IE2425aa or ppUL122b) (1) and IE18 from the IE2 region (11). IE19 has been reported to function in synergy with IE72 to transcriptionally coactivate the HsOrc1 promoter (20). The functions of IE55 and IE18 have not been extensively studied; however, available data suggest that IE55 can suppress transcriptional activation mediated by IE72 and IE86 (5, 12, 15, 25).
While some of the alternative MIE gene transcripts give rise to well-characterized proteins, the products of others have not been well studied (23, 26). In addition, it is quite likely that transcripts remain to be discovered, as few studies have been initiated using present technologies to carefully evaluate the variety of MIE transcripts.
In this study, we used reverse transcriptase PCR (RT-PCR) to identify splice variants from the IE1 region. In addition to IE72 and the recently reported IE19 transcript (20), we detected and characterized two other heretofore-uncharacterized splice variants. Interestingly, each of the IE1 variants arises from utilization of alternative splice sites, not exon skipping. Based on the molecular mass of their predicted translation products, we named the two transcripts IE17.5 and IE9. Nuclease protection analyses demonstrated that the IE17.5 and IE9 transcripts are expressed with immediate-early kinetics during HCMV infection, similar to those of IE72 and IE19. Despite the presence of IE19, IE17.5, and IE9 transcripts, we did not detect their protein products during HCMV infection in infected human foreskin fibroblast (HFF), HEL, and U373MG cells. Our observation that IE19 is not detectable in HCMV-infected cells differs from a previous study of this protein (20).
When cells were transfected with cDNAs encoding the IE1 transcripts, we readily detected protein expression of IE19 and IE17.5; however, we did not detect IE9. Expression of IE19 or IE17.5 in transfected cells was found to repress the MIE promoter and result in reduced levels of the MIEPs IE72 and IE86. In HCMV-infected U373MG cells transfected with IE19 cDNA, we also observed a reduction in the level of IE72 and IE86, as well as of a late viral protein, p28. These data suggest that IE19 and IE17.5 act as repressors of viral gene expression. Given these repressive effects and the inability to detect IE19 and IE17.5 during HCMV infection of permissive cells (HFF, HEL, and U373MG cells), we hypothesize that translation of these proteins may be limited to conditions or cell types which restrict the lytic cycle.
MATERIALS AND METHODS
Cell culture, transfections, and infections.
Life-extended HFF cells, U373MG human glioblastoma cells, and human embryonic lung cells (HEL-299) were propagated and maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, Glutamax, and antibiotics. CV-1 cells were propagated and maintained as described above, except that 5% fetal calf serum was used. For transfections, CV-1 and U373MG cells were seeded in six-well plates at 60 to 70% confluency. Transfections were performed with cDNA plasmids (described below) by the FuGene method (Roche). For viral infections, life-extended HFF, U373MG, and HEL-299 cells were infected with HCMV (Towne strain; multiplicity of infection = 2.5).
RNA isolation, primers, and RT-PCR.
Total RNA was isolated from infected cells at various times postinfection with the SV Total RNA kit (Promega). RT-PCR was performed using the one-step RT-PCR kit (Qiagen). Oligonucleotide primers used were as follows: primer 1, 5′-CCGGGACCGATCCAGCCTCCGCG-3′, annealing to exon 1; primer 2, 5′-AGTTTACTGGTCAGCCTTGC-3′, annealing to the end of exon 4; and primer 3, 5′-CCTTGATTCTATGCCGCACCA-3′, annealing to the beginning of exon 4 (Fig. 1). Reverse transcriptase reactions were performed at 51°C for 32 min. Reactions were stopped by heating the reaction mixtures to 95°C for 15 min. To amplify the cDNA, samples were denatured at 94°C for 30 min and annealed at 55°C for 40 s and extension was done at 72°C for 5 min. Cycles were repeated 35 times using a Mastercycler (Eppendorf). RT-PCR products were subjected to agarose gel electrophoresis followed by ethidium bromide staining.
Cloning of cDNAs and plasmids.
cDNAs of IE72, IE19, IE17.5, and IE9 were cloned into the pCR3.1 expression vector (Invitrogen) and into pGem4 (Promega) for functional analysis, sequencing, and production of probes for RNase protection. To generate the IE19(−) mutant, a mutation in the IE19 splice acceptor site in exon 4 was inserted into a plasmid containing a genomic fragment encoding IE72 (i.e., containing exons 1 through 4) by use of the Quick Change kit for site-directed mutagenesis (Stratagene). The IE19 5′-splice site in exon 4 was changed from CCCAGAGTCCC to CCCAAAAAACC. A plasmid containing a Flag-tagged version of IE19 was the gift of Yuji Yamanashi (20).
RNase protection analysis.
To detect the presence of mRNAs in HCMV-infected HFF cells, total RNA was analyzed by RNase protection assay. 32P-labeled antisense probes for IE19, IE17.5, and IE9 cDNAs were made by in vitro transcription with corresponding pGem plasmids and appropriate RNA polymerases. Total RNA from mock-infected and HCMV-infected cells was added to labeled RNA probe for 4 min at 95°C and then annealed at 50°C overnight in a citrate-based hybridization buffer (Ambion). The reaction mixtures were then treated for 30 min at 37°C with RNase T1/A to digest the single-stranded RNA. The remaining RNA was precipitated and separated on a denaturing 5% polyacrylamide gel. Gels were dried and autoradiographed.
Immunoprecipitations and Western blot analyses.
Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer. Monoclonal antibody (MAb) 810 (Chemicon) was used at a dilution of 1:1,000 to immunoprecipitate the immediate-early proteins. Immunoprecipitates were collected using protein G beads (Gibco-BRL). Beads were washed in RIPA buffer, and proteins were eluted and separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (9 or 11% polyacrylamide gels, as indicated). For Western analyses, proteins were transferred to nitrocellulose membranes which were blocked by incubation for 1 h at room temperature in 5% nonfat milk diluted in phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBS-T). Anti-exon 2/3 polyclonal antibody (1:2,000 dilution), which recognizes the first 85 amino acids of the MIEPs encoded in exons 2 and 3 (28), and anti-exon 4 antibody (gift of William Britt), which recognizes the C-terminal end of IE72 (1:500 dilution), were used to detect immediate-early proteins. Anti-p28 (1:1,000 dilution; Advanced Biotechnologies) was used to detect the late protein p28. The membranes were washed three times in PBS-T and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G for 45 min at room temperature, followed by three more washes in PBS-T. Secondary antibody was detected by ECL (Amersham).
Immunofluorescence microscopy.
Expression plasmids containing cDNAs for IE72, IE19, IE17.5, and IE9 were individually transfected into CV-1 cells, grown on glass coverslips, according to the Fugene-6 protocol, as recommended by the manufacturer (Roche). Cells were fixed with 2% formaldehyde in PBS with 0.2% Triton X-100 for 15 min at room temperature and then washed with PBS and incubated with chilled acetone for 10 min at 4°C. Cells were washed again and incubated in PBS-Tween-5% bovine serum albumin for 30 min at room temperature for blocking. Cells were incubated with primary antibody MAb 810 (1:4,000; diluted in PBS containing 5% bovine serum albumin) at room temperature for 1 h. Cells were washed three times with PBS and incubated with fluorescein isothiocyanate-conjugated anti-mouse antibody (1:200 dilution) for 45 min. After three washes in PBS, the coverslips were mounted on slides with Vectashield (Vector Laboratories Inc.). Stained cells were examined under a Nikon Eclipse E600 fluorescence microscope. Images were captured at an ×40 magnification by use of the appropriate filter to visualize green or red fluorescence.
Luciferase reporter assay.
With use of the Fugene-6 system (Roche) cells were cotransfected with a luciferase reporter plasmid driven by the MIE promoter and cDNA-containing plasmids encoding IE72, IE86, IE19, or IE17.5. Cells were harvested 48 h posttransfection. A luciferase reporter kit (Promega) was used to perform the reporter assay according to the manufacturer's protocol. Ten micrograms of protein was used per reaction, and luciferase activities were determined using a Perkin-Elmer luminometer.
Nucleotide sequence accession numbers.
GenBank accession numbers for mRNA sequences were as follows: IE19, AY436380; IE17.5, AY445660; IE9, AY445661.
RESULTS
Identification of splice variants with IE1-specific primers.
Total RNA was prepared from HCMV-infected HFF cells at various times after infection (up to 72 h). To detect RNAs produced from the IE1 region, RNA was subjected to RT-PCR with primers which annealed to the 5′ end of exon 1 (primer 1) and 3′ end of exon 4 (primer 2) (Fig. 1). The amplified products were separated on a 1.2% agarose gel and stained with ethidium bromide (Fig. 2). Three amplification products of approximately 1.7, 1.0, and 0.65 kb were observed by 4 h postinfection (hpi). Each band was excised from the gel and cloned into the eukaryotic expression plasmid pCR3.1. Nucleotide sequencing revealed that the 1.7-kb cDNA matched the expected cDNA encoding full-length IE72. The 1.0-kb cDNA matched the sequence of a cellular RNA binding protein and was not further considered. When the resulting colonies from the 0.65-kb cDNA were screened by digestion with EcoRI, three distinct clones were detected having inserts of approximately 0.65, 0.60, and 0.55 kb in length (Fig. 2, inset). The 0.65-kb cDNA sequence matched the recently described IE19 transcript (20) (Fig. 3 and 4). The 0.6-kb cDNA resembled that of IE19; however, it lacked 36 nucleotides at the 5′ end of exon 4 (Fig. 3). Since the predicted molecular mass of this protein product is 17.5 kDa (Fig. 4), we refer to it as IE17.5. The 0.55-kb cDNA sequence lacked 16 nucleotides at the 3′ end of exon 3, as well as 40 nucleotides at the 5′ end of exon 4 (Fig. 3). As indicated in Fig. 4, the use of an alternative splice donor site in exon 3 and splice acceptor site in exon 4 results in a frameshift in the open reading frame of this gene. In this alternative open reading frame, translation is terminated after only two codons in exon 4 (Fig. 4). The predicted molecular mass of this protein product is 9 kDa, so we refer to it as IE9.
FIG. 2.
Identification of previously uncharacterized splice variants from the HCMV IE1 region by RT-PCR. RT-PCR was performed using total RNA isolated from HCMV-infected HFFs harvested at the times indicated. Primers 1 and 2 were used (Fig. 1). The RT-PCR products were separated on a 1.2% agarose gel and stained with ethidium bromide. Band sizes (kilobases) are shown on the right. The 0.65-kb band was cloned into the eukaryotic expression vector pCR3.1, and clones were screened by digestion with EcoRI. Three independent clones were identified having inserts of 0.65, 0.60, and 0.55 kb in length (inset).
FIG. 3.
Alignment of nucleotide sequences of each alternatively spliced transcript. The start and stop codons within each transcript are marked in bold and underlined. The dashed lines represent deletions relative to the IE19 mRNA.
FIG. 4.
Amino acid alignment of protein products predicted for each alternatively spliced transcript. Vertical lines represent exon demarcation. Dashed lines represent deletions relative to IE19. The two amino acids translated in an alternative reading frame in IE9 are underlined.
Comparison of the 5′- and 3′-splice sites of each alternatively spliced transcript (Fig. 5A) shows that, while none of the sites conform precisely to mammalian consensus splice donor and acceptor elements, all agree with the GU-AG rule, with the exception of IE9 (GU-AU). In addition, each of the newly identified transcripts has a polypyrimidine track (PPT) which precedes its splice acceptor in exon 4.
FIG. 5.
IE19 and IE17.5 utilize consensus AU/AG splice sites while IE9 does not. (A) Sequences surrounding the 3′ end of exon 3 and the 5′ end of exon 4 for each transcript are shown, exons in uppercase and introns in lowercase. Sequences of the 5′- and 3′-splice sites are underlined and shaded. The polypyrimidine track (PPT) preceding each 3′-splice site is indicated. (B) Towne strain sequences containing and surrounding the 3′-splice sites (3′-SS) in exon 4 and the 5′-splice sites (5′-SS) in exon 3 were aligned and compared with the sequences of the Ad169 strain and four clinical HCMV isolates (Toledo, fix, phoebe, and tr). Intron-exon junctions are indicated by a slash. Nucleotide differences are shaded.
We also compared the sequences containing and surrounding the 5′- and 3′-splice sites of the Towne strain with those of another laboratory strain, Ad169, and with four clinical isolates (Toledo, fix, phoebe, and tr). Figure 5B shows that these regions are highly conserved. Only two base changes are noted: (i) a G (Towne, Ad169, and Toledo)-to-A (fix, phoebe, and tr) change upstream of the IE9 5′-splice site, which is not predicted to affect this site's utilization, and (ii) an A (Towne and Ad169)-to-G (Toledo, fix, phoebe, and tr) change between the 3′-splice sites for the IE17.5 and IE9 mRNAs. This difference is not predicted to alter the utilization of the IE17.5 3′-splice site. Since the IE9 3′-splice site is not conventional, it is not possible to predict what the change would do to its utilization; however, in a normal splice site a G in this position would not be expected to alter utilization. Thus, we conclude that all of the viruses examined could perform the splicing necessary to make the IE19, IE17.5, and IE9 transcripts.
Exon 2 and 3 skipping was not detected.
One mechanism by which splice variants of the IE1 region could be produced is by skipping (i.e., splicing out) of exons 2 and/or 3 (Fig. 1). Exon skipping was not detected in any of the amplification products described above. To examine the possibility of exon skipping more directly, we performed RT-PCR analysis on total RNA isolated from HCMV-infected cells using a pair of primers which anneal to exon 1 (primer 1, used above) and the 5′ end of the IE72 exon 4 (primer 3) (Fig. 1). The amplified products were separated on a 1.5% agarose gel and stained with ethidium bromide. We observed a single amplification product of approximately 0.4 kb in length, the expected size for a transcript containing exons 1, 2, and 3; sequencing of the subcloned fragment confirmed this (data not shown). Thus, all of our RT-PCR analyses suggest that the MIE transcripts that include exon 4 (or part of it, due to alternative splicing) also utilize exons 1 and 2 and all or part of exon 3.
IE19, IE17.5, and IE9 transcripts are present in infected cells.
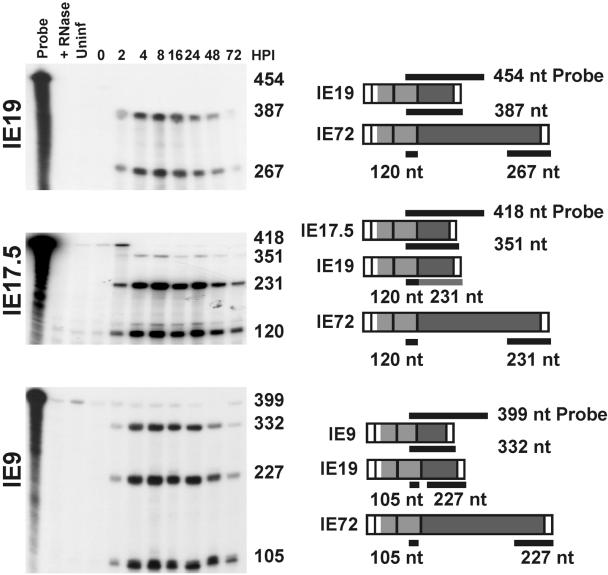
The fact that IE19, IE17.5, and IE9 transcripts are so similar in size makes them difficult to separate on an agarose gel. When we performed Northern blot analyses using a probe from the IE1 region to detect these transcripts in infected cells, we observed a broad and diffuse band matching the approximate size of these transcripts, but the band did not resolve into distinct species (data not shown). Therefore, to confirm the presence of IE19, IE17.5, and IE9 transcripts in infected cells, we analyzed total RNA isolated from HCMV-infected cells using nuclease protection. 32P-labeled antisense RNA probes were prepared by in vitro transcription with T7 polymerase and cDNA templates of IE19, IE17.5, and IE9 (each cloned into the pGem4 vector and then linearized with HpaI). Figure 6 shows diagrams of the probe for each RNA species as well as the probe fragments which are expected to be protected after hybridization to mRNAs and subsequent nuclease digestion (Fig. 6). In each analysis, we detected the expected protected fragment for each transcript, as well as other protected fragments resulting from hybridization of the probe to other spliced species (Fig. 6, left). For example, in the analysis of IE9, when a 399-nucleotide probe (spanning from the T7 promoter to the HpaI site in exon 3) was hybridized to IE9 RNA, a 332-nucleotide fragment was protected. In addition, the IE9 probe hybridized to other transcripts from the IE1 region, IE72 and IE19, resulting in protected fragments of 227 and 105 nucleotides, respectively.
FIG. 6.
Detection of IE19, IE17.5, and IE9 transcripts in HCMV-infected cells. Nuclease protection assays were performed using total RNA isolated from HCMV-infected HFF cells. Antisense RNA probes were generated specifically for IE19, IE17.5, and IE9. A schematic illustrating the design of each probe and the expected protected fragments is shown to the right of each figure. The size of each protected RNA fragment (in nucleotides [nt]) is marked to the right of each gel.
Nuclease protection analysis for IE19, IE17.5, and IE9 revealed that each transcript accumulates with similar kinetics. Like IE72, IE19, IE17.5, and IE9 are first detected at 2 hpi, reach maximum levels between 4 and 24 hpi, and decline thereafter, indicating that each of these genes is expressed with characteristic immediate-early kinetics. The bands corresponding to IE19 and IE9 RNAs are relatively intense, similar to that of IE72. In contrast, IE17.5 appears to be less abundant relative to the other IE1 variants.
Gene products of the IE19 and IE17.5 cDNAs are produced in transfected CV-1 cells.
To determine whether protein products were produced from IE19, IE17.5, and IE9, the cDNAs were inserted into a eukaryotic expression vector and transfected into CV-1 cells. In addition, an IE72 expression plasmid was included as a control. Cell lysates were prepared in RIPA buffer and subjected to Western blot analysis with use of a polyclonal antibody which recognizes the first 85 amino acids encoded by exons 2 and 3 (anti-exon 2/3); these 85 amino acids are common to all known MIEPs. Figure 7A shows expression of the expected 72- and 39-kDa proteins (20) encoded by the IE72 and IE19 cDNAs, respectively. In addition, a 30-kDa protein was produced by the IE17.5 cDNA. It has previously been observed that the IE19 gene product migrates with a higher-than-expected molecular weight (20); the IE17.5 protein, being very similar to IE19, also appears to migrate anomalously. A protein product of the IE9 cDNA was not detected.
FIG. 7.
Expression of IE19 and IE17.5 from cDNAs. (A) CV-1 cells were transfected with the cDNAs indicated. Transfected cells were harvested in RIPA buffer, separated on an SDS-12% polyacrylamide gel, transferred to nitrocellulose, and probed using an antibody specific for exon 2/3. (B) CV-1 cells were transfected with cDNAs encoding IE17.5, IE9, or a plasmid expressing only exons 2 and 3. Lysates were analyzed by Western blotting as described for panel A.
To further investigate the lack of the IE9 product, CV-1 cells were transfected with expression plasmids containing the cDNAs for IE17.5 and IE9 and a cDNA which expresses solely the 85-amino-acid peptide encoded in exons 2 and 3 (Fig. 7B). The polyclonal anti-exon 2/3 antibody again detected the IE17.5 gene product as well as the first 85 amino acids (exon 2/3) but did not detect a gene product from the IE9 cDNA. As shown in Fig. 4, the predicted IE9 protein contains 80 of the 85 amino acids encoded by exon 2/3, the five missing amino acids being replaced by two new ones. It is possible that this difference may make the IE9 gene product unrecognizable by available antibodies. Alternatively, the IE9 mRNA may be untranslatable, or the protein may be very unstable.
Cellular localization of IE1 splice variants.
To determine the cellular localization of IE19 and IE17.5, CV-1 cells were transfected with cDNA expression plasmids and proteins were detected by immunofluorescence microscopy with a MAb which recognizes the common N-terminal region of the MIEPs (MAb 810) and a secondary antibody conjugated to fluorescein isothiocyanate (see Materials and Methods). IE19 and IE17.5, like IE72, showed very similar nuclear staining and very little, if any, cytoplasmic staining; no immunofluorescence was detected in cells transfected with IE9 cDNA (data not shown).
Transcriptional repression of the MIE promoter by IE19 and IE17.5.
IE86 and IE72 are known to affect the activity of the MIE promoter: IE72 is reported to activate the promoter, while IE86 represses it (25). To determine what effect IE19 and IE17.5 have on the MIE promoter, we cotransfected CV-1 cells with a luciferase reporter plasmid containing the MIE promoter and a plasmid expressing cDNAs for either IE19, IE17.5, IE72, IE86, or a combination of these. The results are shown in Table 1. Fold activation is expressed relative to the activity of the MIEP promoter transfected with a vector control plasmid. Consistent with earlier findings (25), we observed a five- to sixfold activation of the MIE promoter by IE72 and a 50% repression by IE86. However, unlike IE72, the IE1 region variants, IE19 and IE17.5, each repressed the MIE promoter by 50 to 60%. When IE19 or IE17.5 was expressed with IE72, each significantly repressed IE72's ability to activate the MIE promoter. When IE19 or IE17.5 was expressed along with the IE86-expressing plasmid, we observed additional repression of the MIE promoter. We conclude that the IE1 variants IE19 and IE17.5 have MIE promoter repression functions similar to those of the IE2 protein IE86.
TABLE 1.
Effect of MIEPs on MIE promoter activitya
| MIEP expressed | Fold activity of MIE promoter |
|---|---|
| Control plasmid | 1.0 |
| IE19 | 0.8 ± 0.02 |
| IE17.5 | 0.8 ± 0.02 |
| IE72 | 5.3 ± 0.04 |
| IE72 + IE19 | 2.2 ± 0.04 |
| IE72 + IE17.5 | 2.5 ± 0.05 |
| IE86 | 0.7 ± 0.04 |
| IE86 + IE19 | 0.4 ± 0.01 |
| IE86 + IE17.5 | 0.4 ± 0.01 |
CV-1 cells were transfected using the Fugene method. One microgram of MIE promoter-luciferase reporter plasmid and 0.5 μg of MIEP-expressing plasmids were used. The final DNA concentration was 2 μg; differences were made up with vector control plasmid.
IE19 and IE17.5 expressed from transfected plasmids repress IE72 and IE86 protein expression from a plasmid utilizing the MIE promoter.
To confirm that IE19 and IE17.5 repress MIE promoter activity, we cotransfected CV-1 cells with expression plasmids containing the cDNA for IE72 or IE86 driven by the MIE promoter, along with expression plasmids for either IE19 or IE17.5. After 48 h, the transfected cells were lysed in RIPA buffer and the proteins were subjected to Western blot analysis with MAb 810. The first four lanes of Fig. 8A show the levels of IE19, IE17.5, IE72, and IE86 alone. The last four lanes show the effect of IE19 and IE17.5 on expression levels of IE72 and IE86. In the presence of either IE19 or IE17.5, there was a 60 to 75% reduction in levels of IE72 and IE86, suggesting that both IE19 and IE17.5 repress the MIE promoter. A similar experiment using the IE9-expressing plasmid showed no effect on the levels of IE72 or IE86 (data not shown) in agreement with the observation that the IE9 plasmid expresses no detectable protein product.
FIG. 8.
Repression of IE72 and IE86 by IE19 and IE17.5. (A) CV-1 cells were transfected or cotransfected with the cDNAs indicated. Proteins were separated on an SDS-10% polyacrylamide gel and transferred to nitrocellulose. A polyclonal antibody specific for exons 2 and/or 3 was used to detect immediate-early proteins. (B) U373MG cells were transfected with a Flag-tagged IE19 cDNA and then infected with HCMV after 24 h. Cells were harvested 24 hpi. Lysates were analyzed as described above. The top panel shows a Western blot probed with the exon 2/3 antibody, and the bottom panel shows a Western blot probed with an antibody against the late protein p28. Protein bands are identified at right.
IE19 expressed from a transfected plasmid represses viral protein synthesis during infection.
To examine the effect of the IE1 region variants on expression of IE72 and IE86 in HCMV-infected cells, we transfected U373MG cells with a plasmid expressing a Flag-tagged version of IE19 (pME19F; gift of Yuji Yamanashi) (20) or a control plasmid. After 24 h, the cells were infected with HCMV. Cells were harvested 24 hpi and subjected to Western analysis with the anti-exon 2/3 antibody to detect all the MIEPs. Figure 8B shows that, in the presence of IE19 expressed from the plasmid, there is a marked decrease in the levels of IE72 and IE86 expressed during viral infection.
To determine whether the IE19-induced decrease in the MIEPs affected later viral gene expression, the transfer was subsequently probed with a MAb specific for the late protein p28 (Fig. 8B, lower panel). The level of p28 expressed during infection was significantly reduced in IE19-transfected cells.
The reduction of IE72, IE86, and p28 by IE19 is underestimated in these experiments since, although all of the cells are infected, only about 30% of the cells are transfected. Thus, inhibition of protein expression in the infected cells which are also transfected may be very dramatic. The data suggest that the expression of IE19 can significantly impede viral infection.
IE19 and IE17.5 are not expressed in HCMV-infected HFF, HEL, and U373MG cells.
As shown in Fig. 8B, IE19 was not detected in HCMV-infected cells transfected with the control plasmid. MAb 810 should detect virally produced IE19. To directly examine the expression of IE19 and IE17.5 in HCMV-infected cells, we infected three permissive cell lines: HFF, HEL-299, and U373 cells. The infected cells were harvested at 0 (mock), 6, 24, and 72 h after infection,. As positive controls showing IE19 and IE17.5 migration, lysates of CV-1 cells transfected with IE19 or IE17.5 cDNA expression plasmids were analyzed in parallel. Fifteen micrograms of each cell lysate was immunoprecipitated with MAb 810. The precipitates were washed and subjected to Western analysis with a MAb which recognizes an epitope in the C terminus of exon 4 (p63-27) (18). This region of epitope 4 is contained in IE72, IE19, and IE17.5. The results in Fig. 9 show that the exon 4-specific antibody recognized IE19 and IE17.5 in transfected CV-1 cells. However, only IE72 and sumoylated IE72 (sIE72) were detected in HCMV-infected HFF, HEL-299, and U373MG cells. We have observed similar results when [35S]methionine-labeled proteins from HCMV-infected cells were immunoprecipitated (data not shown).
FIG. 9.
IE19 and IE17.5 proteins are not detectable during HCMV infection. HFF, HEL, and U373MG cells were infected with HCMV and harvested at the indicated times. Lysates were prepared in RIPA buffer, and IE proteins were immunoprecipitated using MAb 810. CV-1 cells were transfected with cDNAs encoding IE19 and IE17.5 as a positive control for the migration of these two proteins. Immunoprecipitates were separated by SDS-10% polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with an antibody specific for exon 4. Proteins are indicated at the right; sIE72 refers to the sumoylated form of IE72.
Mutagenesis of the IE19 3′-splice site in exon 4.
Although IE19 expression has been reported in HCMV-infected HEL-299 cells harvested at 24 hpi (20), we have been unable to reproduce these results (Fig. 9). To address this discrepancy, we generated an IE19 splice mutant in WT IE72, a plasmid containing a portion of the HCMV genome encoding IE72 exons 1 through 4 and capable of producing the IE19 splice (Fig. 10A). The mutant IE19(−) has the predicted IE19 splice acceptor site in exon 4 mutated as shown in Fig. 10A (see also Materials and Methods); it is incapable of generating the IE19 splice. The mutation was designed to minimize amino acid changes in IE72. The nuclease protection data in Fig. 10B confirm that the mutation eliminated the IE19 splice. Specifically, the 387-nucleotide protected fragment diagnostic for IE19 mRNA (see diagram in Fig. 6) is not present in RNA extracted from CV-1 cells transfected with the IE19(−) plasmid; however, it is present in the RNA from the cells transfected with WT IE72.
FIG. 10.
Mutagenesis of the IE19 splice acceptor site in exon 4 eliminates IE19 transcript and protein. (A) IE19(−), a genomic IE1 plasmid (WT IE72) containing a mutation in the IE19 splice acceptor site in exon 4, was generated by site-directed mutagenesis (site of mutation indicated with an asterisk in panel C; see text for details). (B) CV-1 cells were transfected with the plasmids indicated. Total RNA was analyzed by RNase protection assay to confirm that the IE19(−) mutation eliminated production of the IE19 transcript; details of the probe and bands are shown in Fig. 6. Numbers at right are lengths in nucleotides. (C) Western analysis were performed using lysates from transfected and HCMV-infected cells (as indicated) with antibodies specific for exon 4 (top) or exons 2 and 3 (bottom).
Expression of IE19 was examined in CV-1 cells transfected with WT IE72 (the wild-type genomic construct [Fig. 10A]), IE19(−) (the mutant genomic construct [Fig. 10A]), and the expression plasmid containing the IE19 cDNA, as well as in HCMV-infected HFF cells. Extracts were subjected to Western analysis (Fig. 10C) and probed with two antibodies: anti-exon 2/3 and anti-exon 4 (both described above). IE19 produced from the IE19 cDNA is detected by both antibodies. In cells transfected with plasmids containing the genomic constructs IE19(−) and WT IE72 or in cells infected with HCMV, the exon 2/3 antibody recognized a band (marked with an asterisk) that migrates slightly slower than IE19 expressed from the cDNA; this band was not detected by the exon 4 antibody.
Two lines of evidence suggest that the higher-mobility band recognized by the exon 2/3 antibody does not correspond to IE19: (i) the band is present in the IE19(−) sample where the IE19 splice cannot occur and (ii) the band is not detected by the exon 4 antibody, which does recognize the IE19 cDNA product. While the precise identity of this higher-mobility protein band is not yet clear, we suspect that it is an N-terminal degradation product of IE72, since it is present in all samples containing IE72 and is recognized exclusively by anti-exon 2/3. This band is also enhanced when the cells are harvested in SDS-containing sample buffer which contains insufficient levels of protease inhibitors, further suggesting that it is a breakdown product of IE72 (data not shown). It is interesting that we did not detect IE19 protein in the transfection with the genomic WT IE72 (Fig. 10C) plasmid even though the IE19 splice occurs (Fig. 10B). Thus, we cannot verify the previous report (20) of IE19 synthesis in HCMV-infected cells, or from genomic MIE gene fragments, even though the IE19 splice can be made.
DISCUSSION
The HCMV MIE gene (Fig. 1) is often thought of as the gene encoding the two most studied MIEPs, IE72 (IE1) and IE86 (IE2). However, its complex structure provides the potential for producing numerous mRNAs via alternative splicing, exon skipping, and the utilization of two different polyadenylation signals. Indeed, several splice variants have been noted previously, as discussed in the introduction. We chose to use RT-PCR to characterize transcripts from the IE1 region, involving splicing among exons 1, 2, 3, and 4 (Fig. 1). Besides the expected transcripts for IE72 and IE19 (20), we also detected two previously uncharacterized transcripts which we refer to as IE17.5 and IE9, denoting the size of their predicted translation products. The IE72, IE19, and IE17.5 transcripts all utilize the same 5′-splice site in exon 3, while IE9 utilizes a cryptic 5′-splice site within exon 3. The IE19, IE17.5, and IE9 transcripts all use a different alternative 3′-splice site within exon 4. Interestingly, we have yet to detect evidence of skipping of exon 2 or 3, an occurrence which would have the potential for expanding the diversity of proteins encoded by the MIE gene.
Our analyses show that the IE19, IE17.5, and IE9 splicing patterns occur in infected HFF cells, resulting in RNAs which accumulate over a 72-h time course with kinetics similar to that of IE72. The IE19 and IE9 RNAs were comparable in quantity to IE72; however, the IE17.5 transcript was in much lower abundance in these cells. Sequence comparison with several clinical strains of HCMV indicates that the various 3′- and 5′-splice sites are conserved; thus, all the strains are capable of the splicing necessary to make the IE9, IE17.5, and IE19 transcripts.
IE19 and IE17.5 cDNAs encode proteins which can be synthesized after transfection; however, no protein product from the IE9 cDNA has been detected. One of the antibodies that we use to detect these proteins recognizes epitopes within the first 85 amino acids encoded in exons 2 and 3. In IE9, the last five of these amino acids are replaced with two new amino acids; thus, it is possible that the epitope has been disrupted and is not recognized by the antibody. The other antibody that we use detects an epitope in the C terminus (exon 4) which is present in IE72, IE19, and IE17.5 but not in IE9. Therefore, it is possible that there are no available antibodies to detect IE9. For this reason we cannot conclude whether an IE9 protein product is made during transfection or infection.
Although either antibody can detect IE17.5 and IE19 in extracts from CV-1 cells transfected with the cDNAs, we have not been able to detect these proteins in infected HFF, HEL, or U373MG cells. We have detected the mRNAs, and they are present in the cytoplasm (data not shown); thus, transport from the nucleus does not seem to be a problem. Precedence for low expression of MIEP variants comes from IE55 (Fig. 1), which is expressed at such low levels in HCMV-infected cells that detection requires cycloheximide treatment and removal (1). We have performed similar cycloheximide experiments aimed at detecting IE19, IE17.5, and IE9 but have found none (data not shown). The inability to detect IE19 protein in infected HEL cells was surprising, given that Shirakata et al. (20) have reported the presence of IE19 protein in HCMV-infected HEL cells at 24 hpi. Instead of authentic IE19, we consistently detect a protein from infected cells with a slightly lower electrophoretic mobility than that of cDNA-produced IE19. In Fig. 10B, we used differential antibody detection to establish that this protein was not IE19 but most likely an N-terminal breakdown product of IE72. The difference in mobility between this breakdown product and cDNA-produced IE19 is small and noted only when samples are compared side by side on the same gel; thus, without the differential antibody analysis, the breakdown product could be mistaken for IE19.
We also failed to detect IE19 and IE17.5 in transfections using a plasmid containing the genomic WT IE72 region from the MIE gene (Fig. 10), even though the RNA with the IE19 splice was detected by nuclease protection. Thus, in all cases we have detected expression of IE19 only from the cDNA and never from the genomic context. In the genomic context the homologous MIE promoter and the homologous IE1 polyadenylation signal are used. In the cDNA context the homologous MIE promoter is used, but splicing is eliminated and polyadenylation is provided by the bovine growth hormone polyadenylation signal. These differences may be significant since it is well known that transcription, splicing, and polyadenylation are coupled (3, 4). It is possible that polyadenylation in the genomic context is inefficient when coupled to IE19 or IE17.5 splicing; specifically, the poly(A) tail produced may be too short for efficient association with ribosomes (19). That such a mechanism may occur is suggested by previous studies (24) in which polysome-associated MIE transcripts were examined. It was determined that the IE72 RNA was present on polysomes in higher proportions than other IE RNAs were. Thus, preferential association of different MIE transcripts with the ribosome may occur by mechanisms which can rely on poly(A) tail length.
Why would the virus make these transcripts which are so inefficiently expressed? The answer may lie in their effect on HCMV gene expression. Both IE19 and IE17.5, produced from cDNAs, can significantly reduce the synthesis of the MIEPs IE72 and IE86, and this results in the reduction of later viral gene expression. Hence, these proteins may be expressed only in specific cells; for example, it has been demonstrated that the IE2 region-derived IE18 mRNA is limited to monocyte-derived macrophages (1). In addition, these proteins may become expressed only under conditions where the viral lytic cycle is slowed or inhibited, for example leading to latency.
Acknowledgments
We thank William Britt and Yuji Yamanishi for providing valuable reagents, Inma Barrasa and Eain Murphy for sequence comparison and alignment, Sherri Adams for comments on the manuscript, and the Alwine laboratory members for helpful suggestions and support. Cheers to all.
This work was supported by Public Health Service grants CA28379 and GM45773 awarded to J.C.A. by the National Cancer Institute and the National Institute of General Medical Sciences, respectively.
REFERENCES
- 1.Baracchini, E., E. Glezer, K. Fish, R. M. Stenberg, J. A. Nelson, and P. Ghazal. 1992. An isoform variant of the cytomegalovirus immediate-early auto repressor functions as a transcriptional activator. Virology 188:518-529. [DOI] [PubMed] [Google Scholar]
- 2.Caswell, R., L. Bryant, and J. Sinclair. 1996. Human cytomegalovirus immediate-early 2 (IE2) protein can transactivate the human hsp70 promoter by alleviation of Dr1-mediated repression. J. Virol. 70:4028-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke, C., and J. C. Alwine. 2002. Characterization of specific protein-RNA complexes associated with the coupling of polyadenylation and last-intron removal. Mol. Cell. Biol. 22:4579-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooke, C., H. Hans, and J. C. Alwine. 1999. Utilization of splicing elements and polyadenylation signal elements in the coupling of polyadenylation and last-intron removal. Mol. Cell. Biol. 19:4971-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghazal, P., J. Young, E. Giulietti, C. DeMattei, J. Garcia, R. Gaynor, R. M. Stenberg, and J. A. Nelson. 1991. A discrete cis element in the human immunodeficiency virus long terminal repeat mediates synergistic trans activation by cytomegalovirus immediate-early proteins. J. Virol. 65:6735-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemeier, C., S. Walker, R. Caswell, T. Kouzarides, and J. Sinclair. 1992. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J. Virol. 66:4452-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagemeier, C., S. M. Walker, P. J. G. Sissons, and J. H. Sinclair. 1992. The 72K IE1 and 80K IE2 proteins of human cytomegalovirus independently trans-activate the c-fos, c-myc and hsp70 promoters via basal promoter elements. J. Gen. Virol. 73:2385-2393. [DOI] [PubMed] [Google Scholar]
- 8.Hermiston, T. W., C. L. Malone, P. R. Witte, and M. F. Stinski. 1987. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J. Virol. 61:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, L., C. L. Malone, and M. F. Stinski. 1994. A human cytomegalovirus early promoter with upstream negative and positive cis-acting elements: IE2 negates the effect of the negative element, and NF-Y binds to the positive element. J. Virol. 68:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerry, J. A., M. A. Priddy, and R. M. Stenberg. 1994. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J. Virol. 68:4167-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerry, J. A., A. Sehgal, S. W. Barlow, V. J. Cavanaugh, K. Fish, J. A. Nelson, and R. M. Stenberg. 1995. Isolation and characterization of a low-abundance splice variant from the human cytomegalovirus major immediate-early gene region. J. Virol. 69:3868-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klucher, K. M., M. Sommer, J. T. Kadonaga, and D. H. Spector. 1993. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol. Cell. Biol. 13:1238-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukac, D. M., and J. C. Alwine. 1999. Effects of human cytomegalovirus major immediate-early proteins in controlling the cell cycle and inhibiting apoptosis: studies with ts13 cells. J. Virol. 73:2825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukac, D. M., N. Y. Harel, N. Tanese, and J. C. Alwine. 1997. TAF-like functions of human cytomegalovirus immediate-early proteins. J. Virol. 71:7227-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malone, C. L., D. H. Vesole, and M. F. Stinski. 1990. Transactivation of a human cytomegalovirus early promoter by gene products from the immediate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J. Virol. 64:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monick, M. M., L. J. Geist, M. F. Stinski, and G. W. Hunninghake. 1992. The immediate early genes of human cytomegalovirus upregulate expression of the cellular genes myc and fos. Am. J. Respir. Cell Mol. Biol. 7:251-256. [DOI] [PubMed] [Google Scholar]
- 18.Plachter, B., W. Britt, R. Vornhagen, T. Stamminger, and G. Jahn. 1993. Analysis of proteins encoded by IE regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 193:642-652. [DOI] [PubMed] [Google Scholar]
- 19.Salles, F. J., W. G. Richards, and S. Strickland. 1999. Assaying the polyadenylation state of mRNAs. Methods 17:38-45. [DOI] [PubMed] [Google Scholar]
- 20.Shirakata, M., M. Terauchi, M. Ablikin, K. Imadome, K. Hirai, T. Aso, and Y. Yamanashi. 2002. Novel immediate-early protein IE19 of human cytomegalovirus activates the origin recognition complex I promoter in a cooperative manner with IE72. J. Virol. 76:3158-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staprans, S. I., D. K. Rabert, and D. H. Spector. 1988. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J. Virol. 62:3463-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the α gene product TRS1 in addition to IE1 and IE2. J. Virol. 66:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenberg, R. M. 1996. The human cytomegalovirus major immediate-early gene. Intervirology 39:343-349. [DOI] [PubMed] [Google Scholar]
- 24.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal. 1990. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J. Virol. 64:1556-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenberg, R. M., P. R. Witte, and M. F. Stinski. 1985. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J. Virol. 56:665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo, Y. D., C.-J. Chiou, K. S. Choi, Y. Yi, S. Michelson, S. Kim, G. S. Hayward, and S.-J. Kim. 1996. The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor β1 gene through an Egr-1 binding site. J. Virol. 70:7062-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurochko, A. D., T. F. Kowalik, S.-M. Huong, and E.-S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]