Abstract
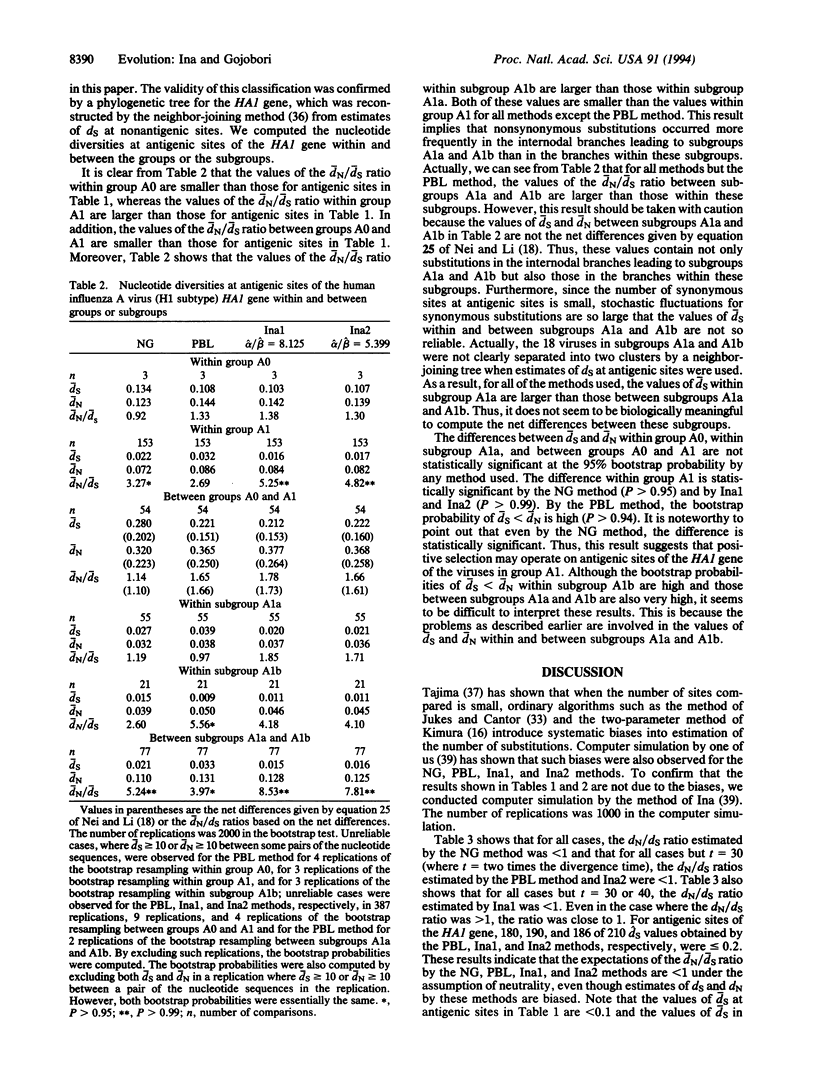
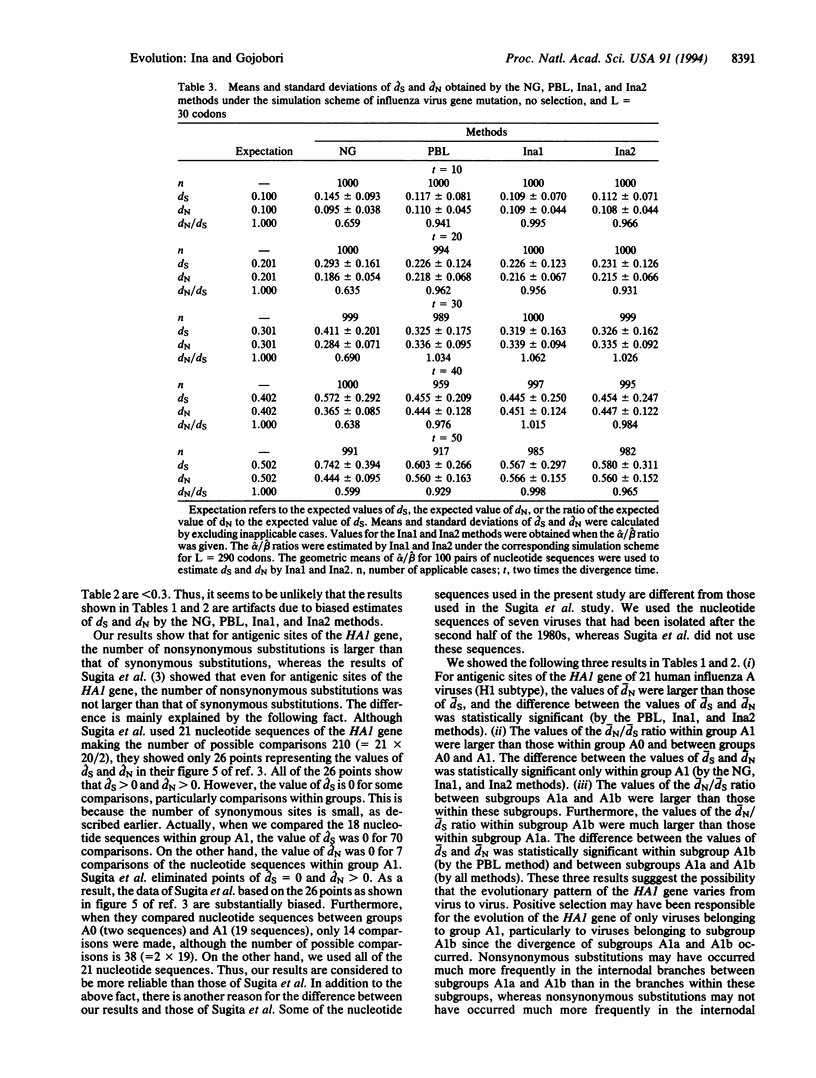
To examine whether positive selection operates on the hemagglutinin 1 (HA1) gene of human influenza A viruses (H1 subtype), 21 nucleotide sequences of the HA1 gene were statistically analyzed. The nucleotide sequences were divided into antigenic and nonantigenic sites. The nucleotide diversities for antigenic and nonantigenic sites of the HA1 gene were computed at synonymous and nonsynonymous sites separately. For nonantigenic sites, the nucleotide diversities were larger at synonymous sites than at nonsynonymous sites. This is consistent with the neutral theory of molecular evolution. For antigenic sites, however, the nucleotide diversities at nonsynonymous sites were larger than those at synonymous sites. These results suggest that positive selection operates on antigenic sites of the HA1 gene of human influenza A viruses (H1 subtype).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beklemishev A. B., Blinov V. M., Vasilenko S. K., Golovin S. Ia, Gutorov V. V. Sintez polnorazmernoi DNK-kopii gena gemaggliutinina virusa grippa A H1N1-podtipa, ee klonirovanie i opredelenie pervichnoi struktury. Bioorg Khim. 1984 Nov;10(11):1535–1543. [PubMed] [Google Scholar]
- Beklemishev A. B., Blinov V. M., Vasilenko S. K., Golovin S. Ia, Karginov V. A. Pervichnaia struktura polnorazmernoi DNK-kopii gena gemaggliutinina virusa grippa A/Kiev/59/79 (H1N1). Bioorg Khim. 1986 Mar;12(3):375–381. [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Concannon P., Cummings I. W., Salser W. A. Nucleotide sequence of the influenza virus A/USSR/90/77 hemagglutinin gene. J Virol. 1984 Jan;49(1):276–278. doi: 10.1128/jvi.49.1.276-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N. J., Black R. A., Kendal A. P. Pathways of evolution of influenza A (H1N1) viruses from 1977 to 1986 as determined by oligonucleotide mapping and sequencing studies. J Gen Virol. 1989 Feb;70(Pt 2):299–313. doi: 10.1099/0022-1317-70-2-299. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Leiter J. M., Li X. Q., Palese P. Positive Darwinian evolution in human influenza A viruses. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4270–4274. doi: 10.1073/pnas.88.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojobori T., Moriyama E. N., Kimura M. Molecular clock of viral evolution, and the neutral theory. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10015–10018. doi: 10.1073/pnas.87.24.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida H., Toh H., Kikuno R., Miyata T. Evolution of influenza virus genes. Mol Biol Evol. 1985 Jul;2(4):289–303. doi: 10.1093/oxfordjournals.molbev.a040352. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Davis A. R., Nayak D. P. Complete sequence analysis shows that the hemagglutinins of the H0 and H2 subtypes of human influenza virus are closely related. Virology. 1981 May;111(1):113–124. doi: 10.1016/0042-6822(81)90658-9. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968 Feb 17;217(5129):624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ohta T. On some principles governing molecular evolution. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2848–2852. doi: 10.1073/pnas.71.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Preponderance of synonymous changes as evidence for the neutral theory of molecular evolution. Nature. 1977 May 19;267(5608):275–276. doi: 10.1038/267275a0. [DOI] [PubMed] [Google Scholar]
- Li W. H. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993 Jan;36(1):96–99. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985 Mar;2(2):150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T. Molecular evolution of mRNA: a method for estimating evolutionary rates of synonymous and amino acid substitutions from homologous nucleotide sequences and its application. J Mol Evol. 1980 Sep;16(1):23–36. doi: 10.1007/BF01732067. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Nakajima K., Kendal A. P. Identification of the binding sites to monoclonal antibodies on A/USSR/90/77 (H1N1) hemagglutinin and their involvement in antigenic drift in H1N1 influenza viruses. Virology. 1983 Nov;131(1):116–127. doi: 10.1016/0042-6822(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Nei M., Jin L. Variances of the average numbers of nucleotide substitutions within and between populations. Mol Biol Evol. 1989 May;6(3):290–300. doi: 10.1093/oxfordjournals.molbev.a040547. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamilo P., Bianchi N. O. Evolution of the Zfx and Zfy genes: rates and interdependence between the genes. Mol Biol Evol. 1993 Mar;10(2):271–281. doi: 10.1093/oxfordjournals.molbev.a040003. [DOI] [PubMed] [Google Scholar]
- Rajakumar A., Swierkosz E. M., Schulze I. T. Sequence of an influenza virus hemagglutinin determined directly from a clinical sample. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4154–4158. doi: 10.1073/pnas.87.11.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond F. L., Caton A. J., Cox N. J., Kendal A. P., Brownlee G. G. Antigenicity and evolution amongst recent influenza viruses of H1N1 subtype. Nucleic Acids Res. 1983 Oct 25;11(20):7191–7203. doi: 10.1093/nar/11.20.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. Sequence analysis of the haemagglutinin of A/Taiwan/1/86, a new variant of human influenza A(H1N1) virus. J Gen Virol. 1987 Apr;68(Pt 4):1205–1208. doi: 10.1099/0022-1317-68-4-1205. [DOI] [PubMed] [Google Scholar]
- Rocha E., Cox N. J., Black R. A., Harmon M. W., Harrison C. J., Kendal A. P. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J Virol. 1991 May;65(5):2340–2350. doi: 10.1128/jvi.65.5.2340-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. Polymorphism and evolution of influenza A virus genes. Mol Biol Evol. 1986 Jan;3(1):57–74. doi: 10.1093/oxfordjournals.molbev.a040381. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sugita S., Yoshioka Y., Itamura S., Kanegae Y., Oguchi K., Gojobori T., Nerome K., Oya A. Molecular evolution of hemagglutinin genes of H1N1 swine and human influenza A viruses. J Mol Evol. 1991 Jan;32(1):16–23. doi: 10.1007/BF02099924. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kato H., Naeve C. W., Webster R. G. Single-amino-acid substitution in an antigenic site of influenza virus hemagglutinin can alter the specificity of binding to cell membrane-associated gangliosides. J Virol. 1989 Oct;63(10):4298–4302. doi: 10.1128/jvi.63.10.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Unbiased estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol. 1993 May;10(3):677–688. doi: 10.1093/oxfordjournals.molbev.a040031. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Brownlee G. G. Nucleotide sequence of the haemagglutinin gene of a human influenza virus H1 subtype. Nature. 1981 Jul 2;292(5818):72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]



