Abstract
Simian virus 40 (SV40) large T antigen (T Ag) interacts with the tumor suppressor p53 and the transcriptional coactivators CBP and p300. Binding of these cellular proteins in a ternary complex has been implicated in T Ag-mediated transformation. It has been suggested that the ability of CBP/p300 to modulate p53 function underlies p53's regulation of cell proliferation and tumorigenesis. In this study, we provide further evidence that CBP activity may be mediated through its synergistic action with p53. We demonstrate that SV40 T Ag is acetylated in vivo in a p53-dependent manner and T Ag acetylation is largely mediated by CBP. The acetylation of T Ag is dependent on its interaction with p53 and on p53's interaction with CBP. We have mapped the site of acetylation on T Ag to the C-terminal lysine residue 697. This acetylation site is conserved between the T antigens of the human polyomaviruses JC and BK, which are also known to interact with p53. We show that both JC and BK T antigens are also acetylated at corresponding sites in vivo. While other proteins are known to be acetylated by CBP/p300, none are known to depend on p53 for acetylation. T Ag acetylation may provide a regulatory mechanism for T Ag binding to a cellular factor or play a role in another aspect of T Ag function.
Simian virus 40 (SV40) is the most well-characterized member of the polyomavirus family. Its ability to immortalize and transform primary cell cultures and form tumors in animals provides a useful model system for studying oncogenesis and growth regulation at the molecular level. SV40 large tumor antigen (T Ag) is a 708-amino-acid multifunctional viral oncoprotein that plays an important role in productive infection and virus-induced cell transformation. T Ag is required for viral replication in permissive monkey kidney cells and is also essential for SV40-mediated transformation. When expressed alone, T Ag is sufficient to immortalize and transform a variety of cell types, including primary mouse embryo fibroblasts (MEFs). For example, T Ag expression confers on MEFs the ability to grow in low serum and to grow in an anchorage-independent manner and cooperates to induce tumor formation in nude mice (33). The ability of T Ag to transform cells depends on its interaction with and regulation of several key cellular regulatory proteins. The principal targets of T Ag are the retinoblastoma family of proteins (including pRb, p107, and p130) and the tumor suppressor p53. The LXCXE motif of T Ag, residues 103 to 107, mediates binding of T Ag to the pRb family proteins. T Ag residues 1 to 82, the J domain, cooperate with the LXCXE motif to inactivate pRb family proteins and may contribute to additional transforming activity (2). In addition, T Ag can be posttranslationally modified by phosphorylation, glycosylation, acylation, ADP ribosylation, and adenylation. Different forms of T Ag may be responsible for its various biological activities and interactions with host proteins (35).
Two regions in the carboxyl terminus of T Ag have been shown to mediate p53 binding: amino acids 351 to 450 and 533 to 626. However, a mutant T Ag with residues 434 to 444 deleted has been shown to be completely defective for p53 binding (22). Interestingly, the regions of T Ag that are required for binding to p53 have also been shown to be necessary for T Ag association with the transcriptional transactivators CBP and p300 and the related p400 (3, 13, 25). Deletion of residues 501 to 550 of T Ag results in loss of binding to both p300 and p53. T Ag forms a ternary complex with p53 and CBP/p300 (25). It is not known if T Ag binding to CBP/p300 is direct or if the interaction occurs indirectly through p53.
Several other viral oncoproteins also target the CBP/p300 and p53 tumor suppressor proteins. Adenovirus (Ad) E1A and polyomavirus large T Ag bind to CBP/p300, while Ad E1b (55 kDa) binds to p53. While polyomavirus large T Ag does not bind to p53, it has been shown to interact with p300, and this interaction may be essential for transformation and viral replication (12, 39). Interaction of Ad E1A with CBP/p300 is also known to be required for Ad-induced cellular transformation (40). The transforming ability of all of these viral oncoproteins is dependent on their binding to and inactivation of p53 or binding to CBP/p300.
CBP and p300 share several distinct motifs characteristic of transcriptional coactivator proteins and have numerous overlapping activities. CBP and p300 interact with a variety of transcription factors that are involved in a wide range of complex biological activities including cell differentiation and proliferation, development, and transformation (10). One important transcription factor that interacts with CBP/p300 is the p53 tumor suppressor protein (16, 24). The interaction of CBP/p300 with p53 results in the synergistic activation of transcription of target genes (16). Furthermore, CBP/p300 serves as a transcriptional adaptor for p53 and modulates p53's G1 cell cycle checkpoint activation function (24). This suggests that CBP/p300 may target p53 in order to carry out its cellular functions. Many of p53's functions, including the ability to arrest cells, may be dependent on CBP/p300 (24). Since binding of CBP/p300 is implicated in the transformation functions of viral oncoproteins, including E1A and SV40 T Ag, disruption or modulation of the interaction of CBP/p300 with p53 may be part of the mechanism contributing to viral oncogenesis (24, 25).
Another aspect of CBP/p300 function that viral oncoproteins seek to disrupt is its histone acetyltransferase (HAT) activity. Both CBP and p300 possess HAT activity and use this chromatin modification as a mechanism to regulate gene expression (29). In addition to histones, CBP and p300 have been shown to acetylate many other viral and cellular nonhistone proteins, including p53 and Ad E1A (28, 44). Acetylation of proteins on specific lysine residues can affect a variety of biological processes, including protein stability and localization as well as protein-protein and protein-DNA interactions (8). Upon binding to CBP/p300, Ad E1A is acetylated at specific residues on its C terminus by CBP/p300 as well as p300/CBP-associated factor (PCAF) (28, 44). When E1A becomes acetylated, it can no longer bind the transcriptional corepressor C-terminal binding protein (CtBP). It is believed that the disruption of this interaction leads to the increased transforming potential of E1A (44). E1A may also alter CBP/p300 HAT activity by specifically increasing or decreasing HAT activity at some promoters or redirecting HAT activity to other promoters (1, 9, 17).
Like Ad E1A, SV40 T Ag has been shown to interact with the transcriptional coactivators CBP and p300; however it is unknown whether this interaction is direct or mediated through T Ag binding to p53 and p53 binding in turn to CBP/p300 (3, 13, 25). T Ag binding and stabilization of p53 may enhance the formation of stable CBP/p300-p53-T Ag complexes, thereby modulating the function of each of these cellular proteins. The inactivation of p53 transactivation function is closely related to T Ag transformation ability and suggests that interfering with some aspect of CBP/p300 activity might also be crucial for oncogenesis. The functional consequence of T Ag's interaction with CBP/p300 is not well understood although it has been suggested that T Ag increases and modulates CBP HAT activity (38).
It is possible that, like E1A, T Ag can also serve as a substrate for acetylation by CBP/p300. Here, we show that SV40 T Ag is acetylated in vivo in a manner that is dependent on its binding to p53. In addition, we provide evidence to suggest that CBP specifically contributes to T Ag acetylation. We have mapped the site of T Ag acetylation to a conserved lysine residue in the C terminus that is shared with the T antigens of the human polyomaviruses JC and BK and demonstrate that both JC and BK T antigens are also acetylated at this site in vivo. Acetylation of SV40 T Ag could provide a regulatory mechanism for any of T Ag's functions, including viral replication and transformation.
MATERIALS AND METHODS
Cells.
The human osteosarcoma U2OS, mouse fibroblast NIH 3T3, and African green monkey kidney COS-1 cell lines were obtained from the American Type Culture Collection. HCT116 and p53-null derivatives were supplied by B. Vogelstein (7). Wild-type (wt) and p53−/− mouse embryo fibroblasts (MEFs) were generated from 14.5-day-old littermate embryos of a p53+/− pregnant female mouse (Jackson Laboratory) as described previously (43). p300−/− and CBP−/− MEFs have been previously described (23). PCAF+/+ and PCAF−/− MEFs were obtained from K. Ozato (41). Knockout and wt MEFs stably expressing T Ag were generated by retroviral transfer of T Ag using the pBabe-Puro shuttle vector or by calcium phosphate cotransfection of pSG5 T Ag along with pEpuro in a 5:1 ratio. The cells were selected in puromycin-containing medium (1 μg/ml). All cells were cultured in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% Fetal Clone-I serum (HyClone), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. U2OS, NIH 3T3, and HCT116 cells were transfected with Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's protocol. A total of 4 μg of plasmid DNA was transfected per 100-mm-diameter dish.
TSA treatment.
Two hours prior to cell lysis, medium was replaced with fresh medium containing 100 ng of trichostatin A (TSA)/ml, a histone deacetylase (HDAC) inhibitor (Sigma) (42). Cells were cultured at 37°C until the time of harvest, and TSA was added to the lysis buffer at the same concentration.
Plasmids.
The SV40 large T Ag cDNA expression plasmids pSG5-T and pSG5-del434-444 have been previously described (11, 43). The K697R, K698R, and double K697,698R point mutant versions of pSG5-T were generated by PCR mutagenesis using the Stratagene QuikChange kit with appropriate primers according to the manufacturer's protocol. The human p53 expression plasmids pCMV wt p53, pCMV p53 22,23, and pCMV 6KR have been previously described (14, 16, 26, 32). T Ag truncations were constructed by cloning T Ag residues 83 to 627, 83 to 708, 250 to 627, and 250 to 708 in frame with an N-terminal influenza virus hemagglutinin (HA) epitope (YPYDVPDYA) tag and the SV40 nuclear localization signal (SPKKKRKVED) in the pcDNA3 expression vector (Invitrogen). pcDNA3 JCT was provided by K. Khalili, and pcDNA3 BKT was provided by M. Imperiale.
Antibodies.
The following antibodies were used for immunoprecipitation and/or Western blot analysis: anti-T Ag mouse monoclonal antibodies PAb 901 (provided by S. S. Tevethia) and 419 (18), anti-p53 PAb 240 and DO-1 (NeoMarkers), anti-HA HA-11 (Covance) and 12CA5, and anti-acetylated lysine mouse monoclonal and rabbit polyclonal antibodies (Upstate Biotechnology and Cell Signaling Technology). T Ag acetylation site-specific rabbit polyclonal antibodies (Ac-K697) were generated by Bethyl Laboratories using the peptide RGFTCFAc-KKPPTPPPEPET, chemically acetylated at position 697 for immunization. Cross-linking of anti-T Ag antibodies to protein A-Sepharose beads was carried out with dimethyl pimelimidate.
Immunoprecipitations.
Cells were washed twice in phosphate-buffered saline and lysed in 500 μl of EBC-150 buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% Nonidet P-40) supplemented with protease inhibitor set I (Calbiochem) per 100-mm dish. Extracts were then cleared by centrifugation at 14,000 × g for 10 min, and protein concentrations were determined by Bradford assay (Bio-Rad). One milligram or 500 μg of cell lysates was incubated with the relevant antibodies and protein A-Sepharose beads (or antibodies cross-linked to protein A-Sepharose beads) in a total volume of 500 μl of EBC-150 for at least 2 h at 4°C. Immune complexes were collected, washed three times with NET-N (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40), and boiled in sodium dodecyl sulfate-containing buffer.
Western blots.
Immunoprecipitated proteins and/or whole-cell lysates (50 or 100 μg) were separated in sodium dodecyl sulfate-6 or 7.5% polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5% milk in TBS-T (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20) for at least 1 h at room temperature prior to incubation with the appropriate antibody in TBS-T for 2 h at room temperature. Detection of proteins was performed with the appropriate horseradish peroxidase-conjugated secondary goat antibody (Pierce) at a 1:2,500 dilution in TBS-T containing 0.5% goat serum. Immunoblots were developed by enhanced chemiluminescence (Pierce) according to the manufacturer's protocol.
RESULTS
T Ag is acetylated in vivo.
To investigate the possibility that SV40 large T Ag was acetylated, we performed immunoprecipitations of T Ag from MEFs stably expressing wt T Ag and NIH 3T3 cells as a negative control. We then compared these sets of immunoprecipitations after Western blotting with an antibody generated against acetylated lysine residues (Fig. 1A). When immunoprecipitated, T Ag reacts with this acetyl-lysine antibody, indicating that it is indeed acetylated. We repeated this experiment with four different acetyl-lysine antibodies, two polyclonal and two monoclonal antibodies from two different companies, and each time we could see that T Ag is acetylated (data not shown).
FIG. 1.
SV40 T Ag is acetylated in vivo. (A) Immunoprecipitations for T Ag from MEFs stably expressing T Ag or vector only were Western blotted with an antibody generated against acetylated lysine residues (bottom, Ac-Lys) or a T Ag antibody (top). (B) TSA treatment enhances T Ag acetylation. MEFs stably expressing T Ag or COS-1 cells were treated (+) or not treated (−) with 100 ng of TSA/ml for 2 h prior to lysis. T Ag was then immunoprecipitated with protein A-Sepharose beads cross-linked to a T Ag antibody and then Western blotted with an antibody generated against acetylated lysine residues.
We then treated cell lines that stably express wt T Ag with TSA, a chemical inhibitor of HDACs, to see if T Ag acetylation could be enhanced (42). MEF T cells and COS-7 cells were treated with 100 ng of TSA/ml for 2 h prior to lysis. T Ag was then immunoprecipitated with a T Ag antibody cross-linked to protein A-Sepharose beads and Western blotted with an acetyl-lysine antibody (Fig. 1B). T Ag acetylation increased in both cell lines with TSA treatment. Acetylation of p53 bound to T Ag also increased with TSA treatment, as has been previously shown (6), providing an internal positive control for the effectiveness of TSA in our system.
T Ag acetylation is dependent on binding to p53.
Since it has been suggested that T Ag binds to CBP/p300 through p53, if CBP and p300 are responsible for T Ag acetylation, it is possible that p53 would also be required in order for T Ag to be acetylated. To test this idea, we compared levels of acetylation of T Ag in the presence and absence of p53. First, we generated stable cell lines from a matched set of p53+/+ and p53−/− MEFs by stably expressing wt T Ag. We then compared levels of acetylation of T Ag in p53+/+ and p53−/− MEFs by immunoprecipitation and Western blotting (Fig. 2A). T Ag was immunoprecipitated from these cells and Western blotted with an acetyl-lysine antibody. In p53+/+ MEFs stably expressing T Ag, acetylation of T Ag and p53 was visible, as we had previously seen. However, when we compared acetylation of T Ag in wt MEFs with that in p53−/− MEFs, we saw a dramatic reduction in T Ag acetylation (Fig. 2A, bottom). While the expression of T Ag in the p53+/+ MEFs was relatively equivalent to that in p53−/− MEFs, T Ag acetylation was undetectable in p53−/− MEFs. Western blots of lysates from these cells show T Ag and p53 expression (Fig. 2A, top and middle). p53 levels in the vector-only wt MEFs were very low, and only when T Ag was introduced to stabilize p53 was it then detectable by Western blotting at this exposure (Fig. 2A, middle, first two lanes).
FIG. 2.
T Ag acetylation is dependent on p53. (A) T Ag acetylation is reduced in p53−/− MEFs. Whole-cell lysates from p53+/+ or p53−/− MEFs stably expressing T Ag or vector only were Western blotted for T Ag (top) and p53 (middle). T Ag was then immunoprecipitated from these cells and blotted with antibody generated against acetylated lysine residues (bottom). (B) Reintroduction of wt and 6KR mutant but not 22,23 mutant p53 restores T Ag acetylation in p53−/− MEFs. p53−/− MEFs were transfected with wt human p53, the CBP/p300 binding-deficient 22,23 mutant p53, and the acetylation site 6KR mutant p53. T Ag was immunoprecipitated from these cells and compared to T Ag immunoprecipitated from p53+/+ MEFs that stably express T Ag. Western blotting was performed for T Ag (top), p53 (middle), and Ac-Lys (bottom).
To further determine the role of p53 in T Ag acetylation, we performed a reintroduction experiment with various forms of p53, both wt and mutant, using p53−/− MEFs to see how T Ag acetylation was affected. Since T Ag was acetylated in MEFs with wt p53 but is not acetylated in cells lacking p53, reintroduction of wt p53 to p53−/− MEFs should restore T Ag acetylation. In addition to wt p53, we also tested two mutant p53s. First, we tested the 22,23 mutant p53 that was previously characterized as having a double point mutation in the transactivation domain and that has been shown to be deficient in binding to CBP (16). This mutant protein is useful in helping to determine not only if p53 regulates T Ag acetylation but also if CBP/p300 binding plays a role in acetylation of T Ag. Second, we tested the 6KR mutant p53, which contains six lysine-to-arginine point mutations at known sites of acetylation (14, 32). Since CBP/p300 is known to acetylate p53 (15, 20, 27, 34), it is possible that the acetylation of T Ag is dependent on acetylation of p53.
We transiently transfected p53−/− MEFs stably expressing T Ag with human wt p53 and human 22,23 and 6KR mutant p53 constructs and compared T Ag acetylation from these cells with that from p53+/+ MEFs stably expressing T Ag. T Ag was immunoprecipitated from all of the cells and Western blotted for T Ag, p53, and acetyl-lysine (Fig. 2B). Interestingly, while all of the p53 proteins could bind to T Ag, only the wt and 6KR mutant p53s restored T Ag acetylation. Both T Ag and p53 bound to T Ag were acetylated in p53+/+ MEFs (lane 1), as we have shown. T Ag expressed in p53−/− cells was not acetylated (lane 2), but when a wt human p53 was reintroduced into these cells, T Ag as well as p53 acetylation was restored (lane 3). However, a mutant p53 that is deficient in CBP binding, and that therefore was not acetylated itself, cannot promote T Ag acetylation (lane 4), suggesting a role for CBP/p300 in T Ag acetylation. The 6KR acetylation site mutant p53 effectively promoted T Ag acetylation although it was not acetylated itself (lane 5). This indicates that acetylation of T Ag and acetylation of p53 are distinct and independent events. While T Ag expression was slightly greater in the p53+/+ MEFs, relative levels of T Ag in the other cells were roughly equivalent. Differences in gel migration of the various forms of p53 are due to differences in species as well as mutations.
We next wanted to confirm our finding that T Ag acetylation is reduced in the absence of p53 in human cells that lack p53. HCT116 p53+/+ and p53−/− cells are a matched set of human carcinoma cell lines where p53 has been removed by homologous recombination (7). We transiently transfected each of these cell lines with a wt T Ag construct and compared levels of acetylation of T Ag in the presence and absence of p53 (Fig. 3A). When T Ag was immunoprecipitated and Western blotted with an acetyl-lysine antibody, T Ag acetylation in p53−/− cells was dramatically reduced compared to that in p53+/+ cells (Fig. 3A, bottom). This confirmed that T Ag acetylation was reduced in the absence of p53 in both mouse and human cells. Western blots of lysates for T Ag and p53 show that levels of expression of T Ag were relatively equivalent in both cell types and that p53 was expressed only in p53+/+ cells, as expected (Fig. 3A, top and middle, respectively). p53 expression was slightly higher upon introduction of T Ag (lane 2).
FIG. 3.
T Ag acetylation requires binding to p53 in both human and mouse cells. (A) T Ag is acetylated in p53+/+ but not p53−/− human cells. HCT116 cells were transfected with vector only or T Ag and Western blotted for T Ag (top) and p53 (middle). T Ag immunoprecipitations were blotted with an Ac-Lys antibody (bottom). (B) A p53-binding mutant T Ag is not acetylated. NIH 3T3 cells were transfected with wt T Ag or the p53-binding-deficient del434-444 mutant T Ag. T Ag was immunoprecipitated from these cells and compared to T Ag immunoprecipitated from wt MEFs that stably express wt T Ag. Western blotting was done for T Ag (top), p53 (middle), and Ac-Lys (bottom).
To determine if the presence of p53 was sufficient to promote T Ag acetylation, or if T Ag binding to p53 was required, we wanted to see if a p53 binding mutant T Ag was acetylated. The del434-444 mutant T Ag contains a 10-amino-acid deletion in its p53 binding domain and is completely deficient in p53 binding (22). We transiently transfected NIH 3T3 cells with wt T Ag and del434-444 and compared them to wt MEFs stably expressing wt T Ag. T Ag was immunoprecipitated and Western blotting was done for T Ag, p53, and acetyl-lysine (Fig. 3B). The del434-444 mutant T Ag was not acetylated compared to wt T Ag, supporting the role for p53 binding to achieve acetylation of T Ag. Expression of the del434-444 mutant T Ag was slightly lower than that of wt T Ag, probably due to increased instability, and it did not bind to p53 (Fig. 3B, top and middle, respectively).
CBP plays a role in T Ag acetylation.
Since T Ag is known to bind to the HATs CBP and p300, we sought to determine if either of these proteins contributed to T Ag acetylation. To look at the effect of these proteins individually, we generated stable cell lines expressing wt T Ag in CBP−/− and p300−/− MEFs. MEFs were stably transfected with a plasmid expressing wt T Ag along with pEpuro in a 5:1 ratio. After selection, T Ag acetylation was analyzed by immunoprecipitation and Western blotting as described in Materials and Methods and compared to that for wt MEFs expressing wt T Ag that were generated at the same time as the knockout cell lines. Western blots of lysates from these MEFs showed that wt MEFs have both CBP and p300 while the respective knockouts were missing either CBP or p300 (Fig. 4A, top two panels). When T Ag was immunoprecipitated, it was found that T Ag levels were approximately equivalent in all cell lines (Fig. 4A, third panel). However, when the T Ag immunoprecipitations were Western blotted with an acetyl-lysine antibody, T Ag acetylation in CBP−/− MEFs was reduced 15-fold compared to that for wt MEFs (Fig. 4A, bottom panel). Furthermore, T Ag acetylation in the p300−/− MEFs was roughly unchanged compared to that for wt MEFs. This experiment was repeated several times with different clones of both CBP and p300 knockout MEFs, with T Ag stably introduced by three different methods, and each time we saw a significant reduction of T Ag acetylation in CBP−/− MEFs, but not p300−/− MEFs, compared to wt MEFs (data not shown).
FIG. 4.
CBP specifically contributes to T Ag acetylation. (A) T Ag acetylation is reduced in CBP−/− but not p300−/− MEFs. T Ag was stably introduced into wt, p300−/−, and CBP−/− MEFs. Lysates were Western blotted for p300 (top) and CBP (second from top). T Ag was immunoprecipitated from these cells and blotted for T Ag (second from bottom) and Ac-Lys (bottom). (B) Levels of T Ag acetylation in PCAF+/+ and PCAF−/− MEFs are comparable. T Ag was immunoprecipitated from PCAF+/+ and PCAF−/− MEFs stably expressing T Ag and Western blotted for T Ag (top) or Ac-Lys (bottom).
To determine if the residual acetylation of T Ag seen in CBP−/− MEFs was due to another cellular HAT, we tested to see if PCAF could contribute to T Ag acetylation. While T Ag is not known to associate with PCAF, since T Ag has been shown to bind to CBP/p300 and PCAF also binds to CBP/p300 and has HAT activity, it is possible that T Ag is brought into a complex with PCAF and acetylated by it. To test this, we obtained a matched set of PCAF+/+ and PCAF−/− MEFs and stably introduced wt T Ag. T Ag was immunoprecipitated and Western blotted for T Ag and acetyl-lysine (Fig. 4B). While relatively equivalent levels of T Ag were immunoprecipitated, the acetyl-lysine blot showed no significant difference in T Ag acetylation between PCAF+/+ and PCAF−/− MEFs.
The site of acetylation on T Ag maps to the carboxyl terminus.
Since T Ag contains 63 lysine residues located throughout the protein, the number of potential acetylation sites was too great to map the site of acetylation by individually mutating each lysine residue. To determine the site(s) of acetylation, several fragments of T Ag lacking various domains of T Ag were constructed in order to narrow down the region of interest. Since we had already demonstrated that p53 binding is required for T Ag acetylation, it was necessary to include the p53 binding domain on all of the fragments. Figure 5A illustrates the amino acids included in the four truncations of T Ag that we used in our experiments: 83 to 627, 83 to 708, 250 to 627, and 250 to 708. All fragments were constructed with an N-terminal HA tag and transiently transfected in HCT116 p53+/+ cells. The fragments were immunoprecipitated with an anti-HA antibody, and Western blotting was done for HA, p53, and acetyl-lysine (Fig. 5B). Levels of expression of all of the T Ag fragments were comparable, and all of the truncations bound to p53 (Fig. 5B, top and bottom left, respectively). Fragments containing amino acids 83 to 627 and 250 to 627 were not acetylated, while their counterparts 83 to 708 and 250 to 708, that contain the entire C terminus of T Ag, were acetylated (Fig. 5B, right). This suggests that the site of acetylation on T Ag is located on a lysine residue between 627 and 708.
FIG. 5.
The site of SV40 T Ag acetylation maps to the carboxy terminus. (A) Schematic representation of domains encompassed by various T Ag truncations. (B) The C terminus of T Ag (627 to 708) is required for T Ag acetylation. U2OS cells were transfected with various T Ag truncations as shown in panel A. HA immunoprecipitations were Western blotted (WB) with an HA antibody (top left) to show expression, p53 (bottom left), and Ac-Lys (right). (C) Lysine 697 is a potential site of T Ag acetylation. wt T and K697R, K698R, and K697,698R mutant T antigens were transfected in U2OS cells. T Ag was immunoprecipitated and then Western blotted for T Ag (top), p53 (middle), or Ac-Lys (bottom).
There are only four lysine residues on T Ag between amino acids 627 and 708, located at positions 645, 652, 697, and 698. Since acetylation of lysine residues tends to occur more frequently on lysines that are next to each other or that are tightly clustered together (19), we first sought to individually mutate lysines 697 and 698. Point mutations of each lysine to an arginine residue (in order to conserve the positive charge) were generated, and a double point mutation of both lysines to arginines was also generated. We then transfected these mutant T antigens along with wt T Ag into HCT116 cells to look for differences in acetylation. All T antigens were immunoprecipitated with a C-terminal T Ag antibody cross-linked to protein A-Sepharose beads and Western blotted for T Ag, p53, and acetylated lysines (Fig. 5C). Interestingly, while wt T Ag and a mutant T Ag with single point mutation at lysine 698 (K698R) were equally acetylated, mutation of lysine 697 to arginine either individually (K697R) or simultaneously with lysine 698 (K697,698R) completely abolished T Ag acetylation. This indicated that lysine 697 was the potential site of T Ag acetylation. In addition, p53 acetylation was unchanged regardless of the status of T Ag acetylation, further suggesting that the acetylation of p53 and that of T Ag were separable and independent of one another. Levels of T Ag expression were relatively equal for all constructs, and the mutant T antigens retained binding to p53 (Fig. 5C, top two panels).
T Ag is acetylated on lysine 697.
To definitively prove that lysine 697 was the site of acetylation on T Ag, we generated an acetylation site-specific antibody against a T Ag peptide containing an acetylation modification on the residue corresponding to lysine 697. We then tested the reactivity of this antibody (Ac-K697) in a Western blot against lysates from various MEFs stably expressing T Ag that we have used in previous experiments, along with NIH 3T3 cells that do not express T Ag as a negative control (Fig. 6A). wt T Ag was recognized by the acetylation site-specific antibody, proving that lysine 697 was an acetylation site on T Ag. Furthermore, only the K698R mutant T Ag and not the K697R or K697,698R mutant T Ag reacted with this antibody, showing that it was specific for lysine 697. In addition, wt T Ag stably expressed in p53−/− MEFs was not recognized by the acetylation-site-specific antibody, confirming our previous findings that T Ag acetylation required p53.
FIG. 6.
SV40 T Ag is acetylated on lysine residue 697. (A) An acetylation site-specific antibody generated against K697 was used to Western blot MEFs stably expressing wt T Ag or various acetylation site mutant T antigens, as well as NIH 3T3 cells and p53−/− MEFs that stably express wt T Ag. (B) The Ac-K697 antibody is effectively competed with acetylated K697 peptide in a dose-dependent manner but not with unacetylated K697 peptide. Lysates from MEFs stably expressing wt T Ag were Western blotted with the Ac-K697 antibody preincubated with either 0, 1, 2, or 5 μg of acetylated K697 peptide or 5 μg of unacetylated K697 peptide as indicated. The bottom blot shows equivalent levels of T Ag in all lanes.
To further demonstrate the specificity of our new Ac-K697 T Ag site-specific antibody, we performed a peptide competition experiment. The Ac-K697 antibody was preincubated with increasing amounts of the acetylated peptide that was used to generate the antibody or a corresponding maximum amount of unacetylated peptide. These antibodies were then used in Western blots on lysates from MEFs stably expressing wt T Ag (Fig. 6B). The acetylated peptide effectively competed the Ac-K697 antibody in a dose-dependent manner, while the unacetylated peptide was unable to compete the Ac-K697 antibody even at the highest concentration tested. This indicated that the Ac-K697 antibody was specific for acetylation on lysine 697 (Fig. 6B, top). T Ag was equally loaded in all lanes (bottom).
JC and BK large T Ags are also acetylated on the conserved lysine corresponding to residue 697 of SV40 T Ag.
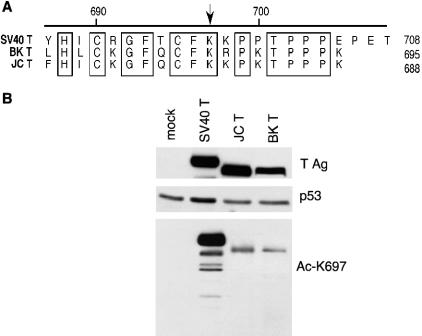
The human polyomaviruses JC and BK both express large T antigens that have many regions of sequence homology with SV40 T Ag and, although less well studied, are thought to function similarly to SV40 T Ag (30). We were interested in seeing whether or not the acetylation site lysine 697 of SV40 T Ag was in a conserved region with JC and BK T antigens. Figure 7A shows a diagram of the residues conserved among the C-terminal domains of SV40, JC, and BK T antigens. The acetylation site lysine 697 on SV40 T Ag is conserved in both BK and JC T antigens, corresponding to lysines 687 and 680, respectively.
FIG. 7.
The acetylation site of SV40 large T Ag is conserved with JC and BK large T antigens. (A) Alignment of C-terminal domains of SV40, BK, and JC large T antigens. Boxes, conserved residues; arrow, position of the conserved lysine residue corresponding to the acetylation site K697 on SV40 large T Ag. (B) BK and JC T antigens are acetylated on a conserved lysine residue. U2OS cells were transfected with SV40, BK, or JC T antigens and Western blotted for T Ag (top) and p53 (middle). Immunoprecipitations for T Ag were Western blotted with a site-specific acetylation antibody corresponding to the conserved lysine at position 697 on SV40 T Ag.
Since the acetylation site on SV40 T Ag was located on a lysine that is conserved in BK and JC T antigens, we were interested to know whether BK and JC T antigens were also acetylated on this residue. Due to the high degree of homology at the C termini of all three T antigens, it was possible that our SV40 T Ag acetylation-site-specific antibody Ac-K697 could also recognize the corresponding regions of BK and JC T antigens. To test this, we transiently transfected SV40, JC, and BK T Ag constructs in U2OS cells, immunoprecipitated the T Ag proteins, and Western blotted with the Ac-K697 SV40 acetylation-site-specific antibody (Fig. 7B). JC and BK T antigens did, in fact, appear to be acetylated at a conserved lysine residue corresponding to lysine 697 on SV40 T Ag. While all three T antigens were expressed well, the SV40 T Ag had a stronger reactivity with the Ac-K697 antibody than the JC and BK T antigens, which were comparable to one another. p53 expression was consistent among transfected cells.
DISCUSSION
The posttranslational modification of histone and nonhistone proteins by acetylation is an important regulatory process that controls gene transcription. Due to its importance in governing cellular activities, many viruses seek to interfere with the acetylation pathway in order to regulate cellular and viral gene expression or gain some functional advantage. Some viruses encode proteins that can interact with cellular HATs and modulate their activity. One consequence of this interaction is that in some cases the viral protein itself can serve as a substrate for acetylation. For example, human immunodeficiency virus type 1 (HIV-1) Tat and Ad E1A can both be acetylated by cellular HATs. The acetylation of these viral proteins has various effects on different aspects of their function. For HIV-1 Tat, it can be acetylated by three different HATs on three specific lysine residues. Acetylation at each lysine results in a new functional property of Tat that is critical to the HIV-1 life cycle (8). Like several other viral proteins, SV40 T Ag interacts with the cellular HATs CBP and p300 and is thought to affect their acetyltransferase activity (38).
In this study, we demonstrate that SV40 T Ag is acetylated in vivo in a manner that depends on T Ag's ability to bind to p53. The dependence of T Ag acetylation on p53 binding suggests that one purpose of T Ag's binding to and stabilizing p53 is so that it can bind to CBP/p300 and get acetylated. Since the 22,23 mutant p53 that is deficient in binding to CBP cannot support T Ag acetylation, this provides evidence that CBP/p300 contributes to T Ag acetylation. This observation supports the model that p53 serves as a bridging protein, allowing T Ag and CBP/p300 to interact indirectly, as has been previously suggested (25). Interestingly, T Ag acetylation does not depend on p53 acetylation, although it does depend on T Ag binding to p53 and p53 binding to CBP/p300 (Fig. 2B and 5C). While p53 itself is also acetylated by CBP/p300, it appears that p53 acetylation and T Ag acetylation are independent events and therefore probably serve distinct functions. This also suggests that p53 bound to T Ag need not be functionally active in order to promote binding to CBP/p300 and therefore T Ag acetylation. p53 may simply serve as a binding scaffold allowing T Ag access to the HAT activity of CBP/p300.
While most studies have illustrated the functional redundancy between p300 and CBP, some evidence suggests that p300 and CBP may have distinct roles in vivo. For example, there is a differential requirement for the PHD fingers (in the C/H2 region) of p300 and CBP in their acetyltransferase activities. While CBP requires an intact PHD finger motif to acetylate histones and p53, p300 does not (5, 21). This suggests that structural differences between p300 and CBP may contribute to the specialized functions of the proteins. In this study, CBP appears to serve as the major contributor to T Ag acetylation (Fig. 4). However, even in the absence of CBP, T Ag acetylation is not completely abolished, as is the case in the absence of p53. The apparent decrease in T Ag acetylation in CBP −/− MEFs may reflect the preferred acetylation of T Ag specifically by CBP, with a small contribution by p300 or the upregulation of p300 activity in the absence of CBP. It is also possible that PCAF contributes to a small percentage of T Ag acetylation (<10%) that is not detectable through the elimination of PCAF alone (Fig. 4B).
Simultaneous elimination of both CBP and p300, together or in combination with PCAF, would allow us to address this question and see if T Ag acetylation can be completely eliminated to a level comparable to that in p53−/− MEFs. However, since CBP and p300 are both essential for cell growth and development, a double knockout of CBP and p300 is technically difficult due to its lethality in cells. A transient knockdown of p300 activity in CBP−/− MEFs, or vice versa, by RNA interference or small-molecule inhibitors may provide another approach to address this question. A similar approach could be used to more precisely determine the contribution, if any, of PCAF to T Ag acetylation.
We have mapped the site of acetylation on T Ag to the carboxyl-terminal lysine at position 697. From our experiments mutating the single lysine 697 to arginine, we were able to eliminate the acetylation of T Ag. This difference in acetylation between the mutant T antigens is not due to changes in protein stability or cellular localization, as acetylation mutant T antigens had a half-life indistinguishable from that of wt T Ag and all forms of T Ag localized to the nucleus (data not shown). We confirmed that lysine 697 is a site of T Ag acetylation by generation of a site-specific antibody. We cannot rule out the possibility, although it is unlikely from our studies, that other sites of acetylation exist on T Ag and that mutation of the site at K697 somehow alters T Ag to prevent it from being acetylated at other sites. If this is the case, each acetylation site on T Ag could result in a distinct effect on various aspects of T Ag function.
The conservation of the acetylation site lysine in SV40 T Ag among human polyomaviruses JC and BK large T antigens suggests that T Ag acetylation may serve a common purpose that is important to all of these viruses. While the Ac-K697 site-specific antibody strongly recognized SV40 T Ag, JC and BK T antigens reacted somewhat more weakly. This could be due to the slight sequence variation between SV40, JC, and BK T antigens in the region that the antibody recognizes or the decreased ability of JC and BK T antigens to bind p53 compared to that of SV40 T Ag (Fig. 7A) (4). Interestingly, mouse polyomavirus large T Ag has also been reported to be acetylated, and it is thought that this modification contributes to the activation of replication at the polyomavirus origin (39). While the sites of polyomavirus T Ag acetylation were not mapped, polyomavirus T Ag shows no homology to the SV40 T Ag C-terminal domain. In addition, polyomavirus T Ag is acetylated by PCAF rather than by CBP/p300 (12). These differences, in addition to the fact that polyomavirus T Ag binds to CBP/p300 directly and does not bind to p53, suggest not only a different mechanism but also a distinct functional role for the acetylation of SV40 and polyomavirus T antigens.
The acetylation of viral proteins has been shown to affect many aspects of protein function, including cellular localization, protein stability, and protein-protein as well as protein-DNA interactions. There are numerous possible effects of T Ag acetylation on any of its functions. For example, the site of acetylation maps to the previously characterized carboxyl-terminal host range/Ad helper function (hr/hf) domain of SV40 T Ag (31, 36, 37). This host range phenotype, in addition to many other functions of T Ag, could be regulated by T Ag acetylation by either promotion or disruption of the association of cellular host factors with the C terminus of T Ag. T Ag acetylation could result in a functional advantage that is mediated by its interaction with cellular proteins.
One example of acetylation regulating the binding of cellular factors to a viral oncoprotein is found in adenovirus E1A. E1A is specifically acetylated on lysine 239, located in its C-terminal regulatory domain, by PCAF, GCN5, and CBP/p300. This region also interacts with the transcriptional corepressor CtBP, but acetylation of lysine 239 inhibits this interaction. This could help to increase the transforming potential of E1A by releasing the repressing function of CtBP. Similarly, acetylation of SV40 T Ag could regulate its interaction with some cellular corepressor or coactivator proteins. While we have found that the majority of T Ag (>60%) appears to be acetylated in vivo (data not shown), two distinct populations of T Ag, acetylated and unacetylated, may serve different purposes. Acetylation could affect T Ag's relationship with any of its known binding partners or it could be responsible for promoting or interfering with T Ag binding to a novel cellular factor. Further studies with our acetylation site mutant T Ags may be able to shed some light on this possibility by allowing us to compare proteins bound to acetylated and unacetylated forms of T Ag. In this way, T Ag acetylation may indirectly contribute to the transforming function or other functions of T Ag. Alternatively, the acetylation of T Ag may reflect its ability to modulate the function of the cellular proteins that it comes into contact with. T Ag clearly alters the HAT activity of CBP/p300 since it is able to redirect the acetyltransferase activity onto itself. In this way, T Ag may sequester CBP/p300's HAT activity by displacing it from its cellular functional complex and directing it to serve the virus's purpose. The conservation of T Ag acetylation among polyomaviruses likely indicates a common functional advantage of this modification of viral proteins.
Acknowledgments
We thank K. Ozato for the PCAF+/+ and PCAF−/− MEFs and B. Vogelstein for the p53+/+ and p53−/− HCT116 cells. We thank Jocelyn Kasper for pBabe-Puro T Ag.
This work was supported in part by Public Health Service grant RO1CA063113.
REFERENCES
- 1.Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 2.Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. [DOI] [PubMed] [Google Scholar]
- 3.Avantaggiati, M. L., M. Carbone, A. Graessmann, Y. Nakatani, B. Howard, and A. S. Levine. 1996. The SV40 large T antigen and adenovirus E1a oncoproteins interact with distinct isoforms of the transcriptional co-activator, p300. EMBO J. 15:2236-2248. [PMC free article] [PubMed] [Google Scholar]
- 4.Bollag, B., W. F. Chuke, and R. J. Frisque. 1989. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J. Virol. 63:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordoli, L., S. Husser, U. Luthi, M. Netsch, H. Osmani, and R. Eckner. 2001. Functional analysis of the p300 acetyltransferase domain: the PHD finger of p300 but not of CBP is dispensable for enzymatic activity. Nucleic Acids Res. 29:4462-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, C. L., and W. Gu. 2003. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 15:164-171. [DOI] [PubMed] [Google Scholar]
- 7.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 8.Caron, C., E. Col, and S. Khochbin. 2003. The viral control of cellular acetylation signaling. Bioessays 25:58-65. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarti, D., V. Ogryzko, H. Y. Kao, A. Nash, H. Chen, Y. Nakatani, and R. M. Evans. 1999. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell 96:393-403. [DOI] [PubMed] [Google Scholar]
- 10.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 11.Chao, H. H. A., A. M. Buchmann, and J. A. DeCaprio. 2000. Loss of p19ARF eliminates the requirement for the pRB-binding motif in simian virus 40 large T antigen-mediated transformation. Mol. Cell. Biol. 20:7624-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho, S., Y. Tian, and T. L. Benjamin. 2001. Binding of p300/CBP co-activators by polyoma large T antigen. J. Biol. Chem. 276:33533-33539. [DOI] [PubMed] [Google Scholar]
- 13.Eckner, R., J. W. Ludlow, N. L. Lill, E. Oldread, Z. Arany, N. Modjtahedi, J. A. DeCaprio, D. M. Livingston, and J. A. Morgan. 1996. Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol. 16:3454-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, J., L. Nie, D. Wiederschain, and Z. M. Yuan. 2001. Identification of p53 sequence elements that are required for MDM2-mediated nuclear export. Mol. Cell. Biol. 21:8533-8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 16.Gu, W., X. L. Shi, and R. G. Roeder. 1997. Synergistic activation of transcription by CBP and p53. Nature 387:819-823. [DOI] [PubMed] [Google Scholar]
- 17.Hamamori, Y., V. Sartorelli, V. Ogryzko, P. L. Puri, H. Y. Wu, J. Y. Wang, Y. Nakatani, and L. Kedes. 1999. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein twist and adenoviral oncoprotein E1A. Cell 96:405-413. [DOI] [PubMed] [Google Scholar]
- 18.Harlow, E., L. V. Crawford, D. C. Pim, and N. M. Williamson. 1981. Monoclonal antibodies specific for simian virus 40 tumor antigens. J. Virol. 39:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, S., Y. Qiu, Y. Shi, Z. Xu, and S. J. Brandt. 2000. P/CAF-mediated acetylation regulates the function of the basic helix-loop-helix transcription factor TAL1/SCL. EMBO J. 19:6792-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, A., C. H. Lai, X. Zhao, S. Saito, M. H. Hamilton, E. Appella, and T. P. Yao. 2001. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20:1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalkhoven, E., H. Teunissen, A. Houweling, C. P. Verrijzer, and A. Zantema. 2002. The PHD type zinc finger is an integral part of the CBP acetyltransferase domain. Mol. Cell. Biol. 22:1961-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kierstead, T. D., and M. J. Tevethia. 1993. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J. Virol. 67:1817-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung, A. L., V. I. Rebel, R. T. Bronson, L. E. Ch'ng, C. A. Sieff, D. M. Livingston, and T. P. Yao. 2000. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14:272-277. [PMC free article] [PubMed] [Google Scholar]
- 24.Lill, N. L., S. R. Grossman, D. Ginsberg, J. DeCaprio, and D. M. Livingston. 1997. Binding and modulation of p53 by p300/CBP coactivators. Nature 387:823-827. [DOI] [PubMed] [Google Scholar]
- 25.Lill, N. L., M. J. Tevethia, R. Eckner, D. M. Livingston, and N. Modjtahedi. 1997. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J. Virol. 71:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, J., J. Chen, B. Elenbaas, and A. J. Levine. 1994. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8:1235-1246. [DOI] [PubMed] [Google Scholar]
- 27.Liu, L., D. M. Scolnick, R. C. Trievel, H. B. Zhang, R. Marmorstein, T. D. Halazonetis, and S. L. Berger. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison, D. L., P. Yaciuk, R. P. Kwok, and J. R. Lundblad. 2002. Acetylation of the adenovirus-transforming protein E1A determines nuclear localization by disrupting association with importin-alpha. J. Biol. Chem. 277:38755-38763. [DOI] [PubMed] [Google Scholar]
- 29.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 30.Pipas, J. M. 1992. Common and unique features of T antigens encoded by the polyomavirus group. J. Virol. 66:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipas, J. M. 1985. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J. Virol. 54:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez, M. S., J. M. Desterro, S. Lain, D. P. Lane, and R. T. Hay. 2000. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol. Cell. Biol. 20:8458-8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saenz-Robles, M. T., C. S. Sullivan, and J. M. Pipas. 2001. Transforming functions of simian virus 40. Oncogene 20:7899-7907. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12:2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider, J., and E. Fanning. 1988. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J. Virol. 62:1598-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence, S. L., and J. M. Pipas. 1994. Simian virus 40 large T antigen host range domain functions in virion assembly. J. Virol. 68:4227-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tornow, J., and C. N. Cole. 1983. Nonviable mutants of simian virus 40 with deletions near the 3′ end of gene A define a function for large T antigen required after onset of viral DNA replication. J. Virol. 47:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valls, E., X. de la Cruz, and M. A. Martinez-Balbas. 2003. The SV40 T antigen modulates CBP histone acetyltransferase activity. Nucleic Acids Res. 31:3114-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie, A. Y., V. P. Bermudez, and W. R. Folk. 2002. Stimulation of DNA replication from the polyomavirus origin by PCAF and GCN5 acetyltransferases: acetylation of large T antigen. Mol. Cell. Biol. 22:7907-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaciuk, P., M. C. Carter, J. M. Pipas, and E. Moran. 1991. Simian virus 40 large-T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol. Cell. Biol. 11:2116-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi, T., J. Yamauchi, T. Kuwata, T. Tamura, T. Yamashita, N. Bae, H. Westphal, K. Ozato, and Y. Nakatani. 2000. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl. Acad. Sci. USA 97:11303-11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida, M., M. Kijima, M. Akita, and T. Beppu. 1990. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265:17174-17179. [PubMed] [Google Scholar]
- 43.Zalvide, J., and J. A. DeCaprio. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol. Cell. Biol. 15:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Q., H. Yao, N. Vo, and R. H. Goodman. 2000. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl. Acad. Sci. USA 97:14323-14328. [DOI] [PMC free article] [PubMed] [Google Scholar]