Abstract
Infection with West Nile virus (WNV) causes fatal encephalitis more frequently in immunocompromised humans than in those with a healthy immune system. Although a complete understanding of this increased risk remains unclear, experiments with mice have begun to define how different components of the adaptive and innate immune response function to limit infection. Previously, we demonstrated that components of humoral immunity, particularly immunoglobulin M (IgM) and IgG, have critical roles in preventing dissemination of WNV infection to the central nervous system. In this study, we addressed the function of CD8+ T cells in controlling WNV infection. Mice that lacked CD8+ T cells or classical class Ia major histocompatibility complex (MHC) antigens had higher central nervous system viral burdens and increased mortality rates after infection with a low-passage-number WNV isolate. In contrast, an absence of CD8+ T cells had no effect on the qualitative or quantitative antibody response and did not alter the kinetics or magnitude of viremia. In the subset of CD8+-T-cell-deficient mice that survived initial WNV challenge, infectious virus was recovered from central nervous system compartments for several weeks. Primary or memory CD8+ T cells that were generated in vivo efficiently killed target cells that displayed WNV antigens in a class I MHC-restricted manner. Collectively, our experiments suggest that, while specific antibody is responsible for terminating viremia, CD8+ T cells have an important function in clearing infection from tissues and preventing viral persistence.
West Nile virus (WNV) is a single-stranded positive-polarity RNA virus and the etiologic agent of West Nile encephalitis. WNV is maintained in a natural cycle between mosquitoes and birds but also infects humans, horses, and other vertebrates. It is endemic in parts of Africa, Europe, the Middle East, and Asia (20), and outbreaks are occurring annually in North America. Humans develop a febrile illness, with a subset of cases progressing to a meningitis or encephalitis syndrome (20). Currently, no specific therapy or vaccine has been approved for human use.
Host factors influence the expression of WNV disease in humans. Those with impaired immune systems are at greatest risk for severe neurological disease (2, 20, 66). Similarly, in animals, the integrity of the immune system correlates with resistance to WNV infection (14, 15, 69). Through the use of animal models of WNV infection, the immunologic basis for protection is beginning to be understood (10). T and B lymphocytes protect against WNV infection: SCID and RAG1 mice (T and B cell deficient) (9, 17) and B-cell-deficient mice uniformly succumb to WNV infection (9). Macrophages also have important functions, as their depletion increases the neuroinvasiveness of attenuated WNV strains (3).
Humoral immunity is an essential component of the immune response to WNV and other flaviviruses, as neutralizing antibodies limit dissemination of infection. Passive transfer of polyclonal or monoclonal immunoglobulin G (IgG) prior to infection protects mice against lethal flavivirus challenge (4, 9, 16, 18, 19, 22, 26, 48, 60-62). The importance of antibodies in protection against WNV infection has been highlighted by recent studies of immunodeficient mice. Mice that lack the ability to produce either anti-WNV IgM (11) or anti-WNV IgG (9) developed lethal encephalitis after infection with WNV; high levels of virus and viral RNA were detected both peripherally and in the central nervous system (CNS).
T lymphocytes are believed to contribute to the eradication of WNV from infected cells (7, 10). Antigen-restricted cytotoxic T lymphocytes (CTL) kill, proliferate, and release inflammatory cytokines after exposure to flavivirus-infected cells (12, 23, 30-34, 44, 52, 65). While T cells are believed to be protective in vivo, their precise role in the control of and recovery from infection by WNV and other encephalitic flaviviruses remains to be elucidated. Athymic nude mice that lack T cells have increased susceptibility to infection with Japanese encephalitis virus (35), and adoptive transfer of virus-specific CTL protected mice against lethal challenge with Japanese encephalitis virus (52). Moreover, gamma interferon (IFN-γ)-producing γδ T cells are also essential for the control of WNV infection (67). Nevertheless, because of their potential to kill infected neurons, the function of CD8+ T cells in protection against WNV infection has remained controversial. For example, a recent study suggested that CD8+ T cells may participate in both the recovery and the immunopathological phases of WNV infection; depending on the intravenous inoculating dose (103 or 108 PFU), the absence of CD8+ T cells had a detrimental or beneficial effect on mortality, respectively (68). Finally, an independent study with the closely related Murray Valley encephalitis virus demonstrated that a lack of either perforin or Fas ligand, two molecules that mediate CTL effector activity, protected mice against encephalitis and mortality (41). Thus, it remains unclear under what circumstances CD8+ T cells protect against disseminated infection or contribute to the pathogenesis of WNV-related neurological disease.
In this study, we directly assessed the function of CD8+ T cells in WNV infection. Mice that lacked CD8+ T cells or classical class Ia major histocompatibility complex (MHC) molecules had sustained viremia, higher CNS viral burdens, and increased mortality rates after infection with WNV. CD8+ T cells clearly demonstrated a protective effect against WNV infection in mice. Interestingly, in the subset of CD8+-T-cell-deficient mice that survived initial viral challenge, infectious virus was recovered from CNS tissues for many weeks, suggesting that CD8+ T cells have an important function in eradicating infection.
MATERIALS AND METHODS
Viruses and cells.
The WNV strain (3000.0259) was isolated in New York in 2000 (13). The stock virus (2 × 108 PFU/ml) was propagated once in C6/36 cells and used for all cell culture and in vivo studies. For inoculation in mice, virus was diluted in Hanks balanced salt solution and 1% heat-inactivated fetal bovine serum (FBS).
Mouse experiments and tissue preparation.
C57BL/6J strain (H2KbDb) inbred wild-type and congenic CD8 α-chain−/− (CD8αtm1Mak) mice were obtained from H. Virgin (Washington University, St. Louis, Mo.). Congenic MHC class Ia-deficient (H2Kb−/− × H2Db−/−) mice (54) were obtained from T. Hansen (Washington University). All mice were bred in the animal facility of Washington University School of Medicine. Eight- to 12-week-old mice were used for all studies and were inoculated subcutaneously with 102 PFU of WNV by footpad injection. For pathological analyses, CNS tissues were harvested after perfusion with phosphate-buffered saline (PBS) and 4% paraformaldehyde, incubated in 4% paraformaldehyde for 24 h at 4°C, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined for pathological changes. Serial sections of these tissues were also stained for WNV and neuronal antigens by immunohistochemistry as described previously (9).
Quantitation of virus burden from mice.
To analyze the kinetics of virus production in the tissues and serum of infected mice, groups of mice were infected with 102 PFU and euthanized on days 2, 4, 6, 8,10, and 11 after infection. Before harvesting of organs, blood was collected by phlebotomy of the axillary vein; serum was recovered after centrifugation, aliquoted, and stored at −80°C. Organs were removed, weighed, and homogenized, and plaque assays were performed as previously described (9). Viral RNA was prepared from thawed aliquots of serum by using a Qia-Amp viral RNA recovery kit (Qiagen, Palo Alto, Calif.) and quantitated by real-time fluorogenic reverse transcriptase PCR (RT-PCR) with an ABI 7000 sequence detection system (Applied Biosystems, Foster City, Calif.) as described previously (9, 37).
Serologic analysis.
An enzyme-linked immunosorbent assay was used to measure the development of specific IgG and IgM against WNV with some modifications (9, 11). A histidine-tagged WNV envelope protein was purified from baculovirus-infected Hi-5 insect cells (M. Diamond, G. Nybakken, and D. Fremont, unpublished results) by nickel affinity chromatography and used to coat Maxi-Sorp microtiter plates (Nalge Nunc International, Rochester, N.Y.) overnight at 4°C. In parallel, plates were also coated with bovine serum albumin as a control antigen. After saturation of nonspecific sites with blocking buffer (PBS, 0.05% Tween 20, 3% bovine serum albumin, and 3% horse serum), plates were incubated with serial dilutions of heat-inactivated serum from infected mice for 2 h at room temperature. After extensive washing, plates were incubated with biotin-conjugated goat anti-mouse IgG or IgM (Sigma Chemical, St. Louis, Mo.), followed by horseradish peroxidase-conjugated streptavidin (Sigma Chemical). Signal was detected with tetramethylbenzidine substrate (Dako, Carpinteria, Calif.) and H2SO4, and plates were read in an enzyme-linked immunosorbent assay plate reader (Molecular Devices) at 450 nm. The optical density value for binding to bovine serum albumin alone was subtracted from the E-protein wells to obtain an adjusted optical density for each sample.
The titer of neutralizing antibodies was determined by a previously published protocol (9). Experiments were performed in duplicate, plaques were counted and plotted, and the plaque reduction neutralization titer for 50% inhibition was determined.
Isolation of brain lymphocytes.
To determine the kinetics of CD8+ T cells trafficking into the CNS after infection, brain leukocytes were isolated and phenotyped by flow cytometry according to a previously published protocol (29). After infection with 102 PFU, mice were euthanized on days 5, 7, 9, and 11 after infection and perfused with 30 ml of PBS. Individual brains were harvested, placed on ice in RPMI supplemented with 5% FBS, and homogenized gently by being pressed through a 100-μm-pore-size mesh tissue strainer (BD Biosciences, San Diego, Calif.). After rinsing and trituration, the cell homogenates were centrifuged (2,000 × g for 3 min) and the cell pellets were resuspended in 2 ml of RPMI with 5% FBS. The suspension was overlaid on a 70 and 30% Percoll (Pharmacia, Uppsala, Sweden) step gradient in RPMI with 5% FBS. The gradients were centrifuged (800 × g for 25 min at 25°C), and the leukocytes were collected from between the 70 and 30% interface. Leukocytes were washed two additional times, and the number of CD8+ T cells from each brain was determined after staining with CD45 (common leukocyte antigen) or CD8α (Ly-2) antibodies (BD Biosciences) for 30 min at 4°C in the presence of 5% goat serum to prevent nonspecific antibody binding. After washing, cells were fixed with 1% paraformaldehyde in PBS and analyzed with a FACscan (Becton Dickinson, San Jose, Calif.) cell sorter with CellQuest software.
CTL assays.
To avoid generating radioactive waste at the biosafety 3 level, CTL activity was determined by measuring the amount of release of calcein AM, a vital fluorochrome. MC57GL cells, derived from a fibrosarcoma in a C57BL/6J mouse (gift of H. Virgin, St. Louis, Mo.), were used as targets for CD8+-T-cell killing.
MC57GL cells that expressed the ectodomain of the E protein (MC57GLWNV-E) from the New York 1999 strain of WNV (38) were generated as follows: amino acids 1 through 402 of the E protein were amplified by PCR from an infectious clone of WNV (gift of R. Kinney, Centers for Disease Control and Prevention, Fort Collins, Colo.) and cloned into the BamHI-XhoI sites of pcDNA3.1 (Invitrogen). After sequencing, pcDNA-E or the parent vector, pcDNA3.1, was transfected into MC57GL cells by a previously described protocol (50). Forty-eight hours after transfection, cells were selected with Dulbecco modified Eagle medium (DMEM) supplemented with zeocin (0.2 mg/ml). After 10 days, clones of MC57GLWNV-E or MC57GLvector were isolated and tested for WNV E-protein antigen expression by indirect immunofluorescence and flow cytometry.
CTL assays were performed with either primary or memory CD8+-T-cell populations. (i) For primary CTL, C57BL/6J mice were infected with 106 PFU of WNV via intraperitoneal injection. At days 4, 6, and 8 after infection mice were euthanized and the spleens were isolated by dissection. Splenocytes were recovered after being pressed through a nylon mesh, and the mononuclear cells were separated by Ficoll gradient centrifugation (800 × g, 25 min at room temperature). After harvest of the gradient interface, cells were washed extensively in RPMI with 5% FBS and the CD8+ T cells were isolated by negative selection with magnetic beads according to the instructions of the manufacturer (Miltenyi Biotec, Auburn, Calif.). The phenotype was confirmed by flow cytometry with a phycoerythrin-conjugated rat anti-mouse CD8 monoclonal antibody (MAb; BD Biosciences); routinely, we obtained 90 to 95% purity of CD8+ T cells (see Fig. 6B inset). (ii) To generate memory T cells that recognized antigens derived from the WNV E protein, splenocytes were harvested from immune wild-type mice between 45 and 60 days after WNV infection. For primary stimulation, 2.5 × 107 bulk splenocytes were cultured for 7 days at 37°C with 0.5 × 106 irradiated (10,000-rad) MC57GWNV-E cells in DMEM supplemented with 5% FBS and 50 μM β-mercaptoethanol. Under these selection conditions, only the subset of WNV-specific T cells that recognize peptides derived from the WNV E protein in vivo will proliferate ex vivo. Surviving T cells were harvested and restimulated with MC57GWNV-E cells (1:1 effector/target cell [E/T] ratio) and incubated at 37°C for 7 additional days. For optimal expansion, 2.5 × 107 irradiated (2,000-rad) naïve splenocytes and 50 U of interleukin-2/ml were added to the culture. For killing assays, lymphocytes were harvested 3 days after stimulation and live cells were isolated after Ficoll gradient centrifugation. Staining with MAbs to CD8 confirmed that 99% of lymphocytes at the time of harvest were CD8+ T cells.
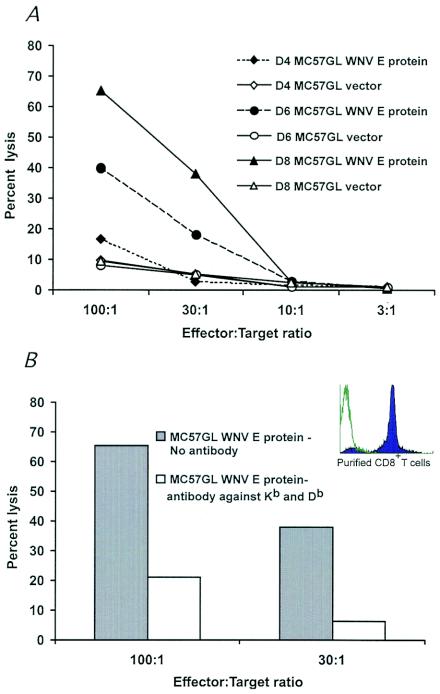
FIG. 6.
Primary CD8+-T-cell-mediated killing of WNV targets. (A) Splenocytes were harvested from mice that were infected with 106 PFU of WNV at 4, 6, and 8 days after infection. CD8+ T cells were purified by negative antibody selection and mixed with calcein AM-labeled MC57GL target cells (MC57GLWNV-E or MC57GLvector) at various E/T ratios. Target cell killing was measured by assessing the release of calcein AM with a 96-well-plate fluorimeter. Specific lysis was determined after subtracting the amount of calcein AM release for target cells that were incubated without CD8+ T cells. One representative experiment of two is shown. (B) Class I-restricted killing of MC57GLWNV-E target cells. MAbs to class I MHC molecules (Kb + Db) were added to target cells prior to the addition of effector CD8+ T cells. (Inset) Flow cytometry profile demonstrating the purity of CD8+ T cells after negative selection by antibody-coated magnetic beads.
MC57GLWNV-E or MC57GLvector cells were labeled with 10 μM vital fluorochrome calcein AM according to the instructions of the manufacturer (Molecular Probes). Cells were washed extensively and then placed in DMEM (without phenol red)-5% FBS that was supplemented with 20 μM verapamil, a calcium channel blocker that inhibits the calcium-dependent P-glycoprotein-mediated multidrug resistance channel (1); addition of verapamil was required to minimize spontaneous efflux of calcein AM from live cells. Purified CD8+ T (effector) cells and calcein AM-labeled MC57GL (target) cells were mixed at various E/T ratios, placed in individual wells (200 μl) of a 96-well U-bottomed plate in triplicate, and centrifuged (200 × g, 3 min at room temperature). In some experiments, MAbs to class I MHC molecules (Kb and Db, 1/100 dilution of mouse ascites; gift from T. Hansen, St. Louis, Mo.) were added to target cells immediately prior to the addition of effector cells. After a 4-h incubation at 37°C in a humidified 5% CO2 incubator, cells were recentrifuged, and 100 μl of supernatant was removed for measurement of fluorescence at 485 nm with a 96-well-plate SpectraFluor Plus fluorimeter (Tecan Instruments, Research Triangle Park, N.C.). Data are expressed as percentages of specific lysis as [(experimental lysis) − (spontaneous lysis)]/[total (lysis with detergent) − (spontaneous lysis)] × 100. The spontaneous release of calcein was ∼20 to 25% of the total label.
Data analysis.
For survival analysis, Kaplan-Meier survival curves were plotted using Prism software (GraphPad, San Diego, Calif.). Mortality curves were analyzed by the log rank test, and average survival times were evaluated using the Mann-Whitney test.
RESULTS
Susceptibility of CD8+-T-cell-deficient mice to WNV infection.
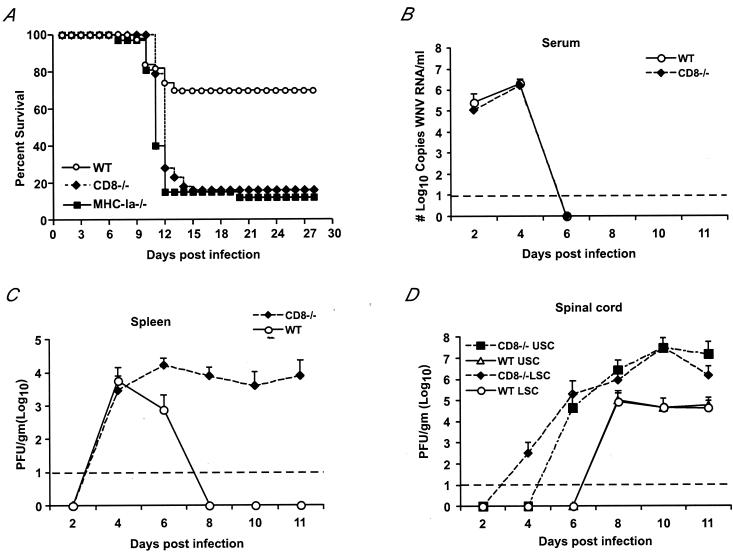
Previously, we demonstrated that RAG1 mice that lack both T and B cells were vulnerable to lethal WNV infection (9). In passive transfer studies, although immune antibody completely prevented infection in wild-type mice, its effect was not durable in RAG1 mice; many eventually succumbed to infection weeks to months after the initial exposure. Thus, antibody, by itself, did not completely eliminate WNV reservoirs in host tissues (16). Because of these experiments, we hypothesized that CD8+ CTL may control and/or eliminate virus from infected cells. To directly assess the function of CD8+ T cells in the control of WNV infection, we compared morbidity and mortality after subcutaneous infection in wild-type and congenic CD8+-T-cell-deficient mice. After inoculation with 102 PFU of virus, all mice showed clinical signs of infection after day 8 that included reduced activity, weight loss, hair ruffling, and hunchbacked posture. Some wild-type and CD8−/− mice also displayed hind limb paralysis, a sign of CNS involvement. Additional signs of CNS infection were observed at greater frequency in CD8−/− mice, including hemiplegia, tremor, and seizures. The onset of these severe manifestations of CNS disease invariably predicted death, which followed within 12 to 24 h. Overall, survival rates were markedly lower after infection in CD8−/− mice: 16% in CD8−/− mice compared to 70% in congenic wild-type mice (Fig. 1A, P < 0.0001). Thus, by morbidity and mortality analyses, an absence of CD8+ T cells was shown to cause more severe WNV infection with adverse clinical outcomes.
FIG. 1.
Survival data and viral burden data for wild-type (WT), CD8−/−, and MHC class Ia−/− C57BL/6J mice inoculated with WNV. (A) Wild-type, CD8−/−, and MHC class Ia−/− mice were inoculated via footpad with 102 PFU of WNV and monitored for 28 days. The survival curves were constructed using data from three to five independent experiments. The numbers of animals were n = 50 for wild-type, n = 43 for CD8−/−, and n = 32 for MHC class Ia−/− mice. Survival differences between wild-type and CD8−/− or MHC class Ia−/− mice were statistically significant (P < 0.0001). (B) Levels of viral RNA in serum. Viral RNA levels were determined from serum of wild-type or CD8−/− mice after WNV infection at the indicated days by a real-time fluorogenic RT-PCR assay. Data are expressed as genomic equivalents of WNV RNA per milliliter of serum and reflect the average of five independent mice per time point. The dashed line represents the limit of sensitivity of the assay. (C to E) Infectious virus levels in tissues. Virus levels were measured from the spleen (C), upper (USC) and lower (LSC) halves of the spinal cord (D), and brain (E) of wild-type and CD8−/− mice by a viral plaque assay in BHK21 cells after tissues were harvested at the indicated days after inoculation. Data are shown as the average PFU per gram of tissue or milliliter of serum and reflect five mice per time point for either wild-type or CD8−/− mice. The dashed line represents the limit of sensitivity of the assay.
WNV burden in CD8−/− mice.
To elucidate the mechanism by which a deficiency in CD8+ T cells made mice vulnerable to lethal infection by WNV, wild-type and CD8−/− mice were infected with 102 PFU of WNV and viral loads were measured at 2, 4, 6, 8, 10, and 11 days after infection in serum, spleen, spinal cord, and brain by plaque assays (Fig. 1B to E).
(i) Viremia.
In both wild-type and CD8−/− mice, viremia was below the level of detection by direct plaque assay throughout the time course (data not shown). However, when viral RNA in serum was measured by a more sensitive fluorogenic RT-PCR assay (9, 37), additional information was obtained. The kinetics and magnitude of viremia were virtually identical between wild-type and CD8−/− mice; viral RNA was detected from day 2 to day 4 after infection but was cleared from circulation by day 6 (Fig. 1B).
(ii) Spleen.
A different pattern was observed between wild-type and CD8−/− mice in the spleen. Although similar levels of infectious virus were detected in both groups at 4 days after infection, in wild-type mice, infectious WNV levels decreased by day 6 and were absent at day 8. In contrast, there was no clearance phase in CD8−/− mice, as levels of virus (104 PFU/g) persisted in the spleen after day 6 (Fig. 1C). Thus, a lack of CD8+ T cells resulted in a failure to rapidly clear virus infection from the spleen.
(iii) CNS.
(a) Spinal cord. WNV was detected earlier and in greater levels in the spinal cord of CD8−/− mice (Fig. 1D). At day 4 after infection, 25% of CD8−/− mice had detectable levels of infectious virus in the inferior spinal cord. By day 6, 30% of CD8−/− mice had significant levels (∼105 PFU/g) in both the inferior and superior spinal cord. In contrast, infectious virus was not detected in wild-type mice until 8 days after infection. By the latter stages of the time course, the magnitude of viral infection in the spinal cord was dramatically different: at day 10 after infection in CD8−/− mice there were ∼500-fold-higher levels in the inferior and superior spinal cord than in the wild-type counterparts. (b) Brain. A similar pattern of infection was observed in the brain (Fig. 1E). Tenfold-higher levels of WNV were detected in brains of CD8−/− mice on day 6 after infection. As the time course progressed, the gap in viral burden in the brain widened such that by day 10 after infection there were ∼1,000-fold-higher levels of infectious virus in the CD8−/− mice. Overall, the virologic analysis demonstrates that CD8+ T cells are critically important for the control of WNV infection in the CNS.
Histopathology and immunohistochemistry after WNV infection in the CNS.
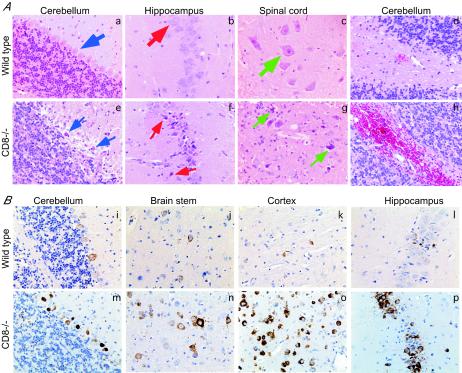
To understand the basis for increased mortality in CD8−/− mice, we examined CNS tissues for histopathological changes following infection and compared them to those of wild-type mice. Brains and spinal cords were harvested from equivalently moribund CD8−/− and wild-type mice on days 10 and 11 after infection. Hematoxylin and eosin staining of brain sections from CD8−/− mice revealed a larger number of dying or injured neurons than those for wild-type mice (Fig. 2A). Although neuronal destruction was patchy and showed some tissue variability, there was consistent evidence of greater neuronal injury in the Purkinje and granular neurons of the cerebellum and in hippocampal and spinal cord neurons from CD8−/− mice (Fig. 2A, panels a to c compared with e to g). Many of the neurons appeared pyknotic with evidence of altered morphology or frank degeneration. Another interesting feature of the pathological analysis in CD8−/− mice was an increased frequency of CNS focal hemorrhage (Fig. 2A, panel h). In contrast, in wild-type mice, a lower level of neuronal degeneration and hemorrhage was seen (Fig. 2A, panel d) (63).
FIG. 2.
Histopathology and immunohistochemistry after infection with WNV (A) Histopathology of CNS tissue from wild-type and CD8−/− mice. CNS tissues from equivalently moribund wild-type (a to d) and CD8−/− (e to h) mice were harvested at day 10 after infection with 102 PFU of WNV, sectioned, and stained with hematoxylin and eosin. Typical sections from the cerebellum, hippocampus, and spinal cord are shown after review of more than 10 independent brains. In samples from wild-type infected mice (thick arrows) the Purkinje neurons of the cerebellum, the CA1 neurons of the hippocampus, and the anterior horn motor neurons are identified with blue, red, and green arrows, respectively. In samples from CD8−/− mice (thin arrows) these neurons are again delineated; however, significantly more neuronal degeneration is observed. (B) Detection of WNV infection in the CNS by immunohistochemistry in wild-type and CD8−/− mice. The brains of wild-type (i to l) and CD8−/− (m to p) mice were harvested 10 days after infection with WNV, sectioned, and stained with rat anti-WNV polyclonal serum or a control negative polyclonal rat serum. Typical sections are shown from the cerebellum (i and m), brain stem (j and n), cerebral cortex (k and o), and hippocampus (l and p) after review of more than 10 independent brains from either wild-type or CD8−/− mice.
Because there was a 500- to 1,000-fold-higher viral titer in the CNS of CD8−/− mice by plaque assay, we questioned whether there was a difference in the tropism of infection. In CD8−/− mice, as with wild-type mice, high levels of WNV antigen were detected only in cells that stained positive for neuronal antigens (Fig. 2B) (63; also data not shown). The cortex, brain stem, base of the brain, hippocampus, and cerebellum were the principal sites of WNV infection in the brain (Fig. 2B). However, compared to comparably sickened wild-type mice, more intense viral antigen staining was observed, consistent with the higher viral load in CD8−/− mice. Many of the heavily infected neurons showed evidence of neuronal injury with altered morphology (Fig. 2B, panels m to p). Similarly, in the spinal cord, larger numbers of neurons were infected in CD8−/− mice than in equivalently sickened wild-type mice (data not shown). Thus, an absence of CD8+ T cells resulted in increased CNS viral load because of increased infection in neurons throughout the brain and spinal cord.
CD8+ T-cell trafficking into the brain.
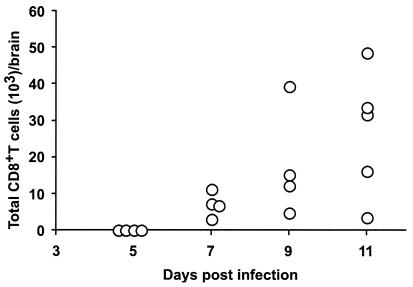
In a prior study (63), we observed larger numbers of CD45+ lymphocytes in the CNS of mice infected with WNV. To determine whether CD8+ T cells entered into the brain after infection, inflammatory cells from the brains of infected mice were isolated by density gradient centrifugation and phenotyped with MAbs (Fig. 3). Virtually no lymphocytes were detected in the brains of uninfected mice or of WNV-infected mice before day 5. Because infectious WNV was not recovered from the brains of wild-type mice until 6 days after infection, CD8+ T cells appear to traffic into the brain after viral infection. The number of CD8+ T cells in the brain increased over time, with the highest levels being observed at our latest time point (day 11) of analysis.
FIG. 3.
Trafficking of CD8+ T cells into the brain of WNV-infected mice. Groups of wild-type mice were infected with 102 PFU of WNV subcutaneously. At the indicated days, brain leukocytes were recovered by Percoll gradient centrifugation and phenotyped with phycoerythrin-conjugated anti-CD8 antibodies. The data are expressed as a scatter plot and reflect the total number of brain CD45+ leukocytes recovered after Percoll gradient centrifugation of individual brains multiplied by the percentage that expressed CD8α (Ly-2) chain antigen as detected by flow cytometry.
Anti-WNV antibody response in CD8−/− mice.
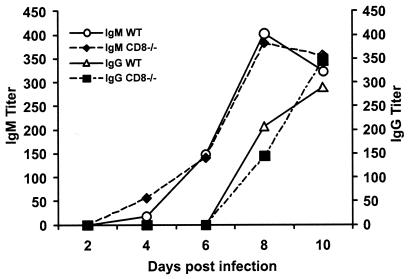
Because we have previously shown that a depressed anti-WNV IgM or IgG response results in earlier and increased dissemination of WNV in the CNS (9, 11), we sought to eliminate the possibility that a compromised antibody response could explain the increased susceptibility in CD8−/− mice. As expected, the kinetics and magnitude of the anti-WNV IgM and IgG response were virtually identical in wild-type and CD8−/− mice: IgM was detected within 4 days of infection and peaked at day 8, and IgG was detected from day 8 onward (Fig. 4). Notably, there was no difference in neutralization titers between wild-type and CD8−/− mice at day 4, 6, or 10 after infection (data not shown). Thus, the increased susceptibility and CNS viral burden were likely due to the direct absence of a protective effect by CD8+ T cells.
FIG. 4.
Development of specific antibodies to WNV in wild-type and CD8−/− mice. Serum was collected from wild-type (WT) or CD8−/− mice at the indicated days after infection with 102 PFU of WNV. The development of specific IgM or IgG antibodies to WNV was determined after incubating serum with adsorbed control or purified WNV E protein. Data are the averages of 5 to 10 mouse experiments per time point performed in duplicate.
WNV infection in MHC class Ia-deficient mice.
To confirm that the absence of antigen-specific CD8+ T-cell interaction with WNV-infected cells caused the increased susceptibility to infection, congenic C57BL/6 mice that were deficient in MHC class Ia (Kb and Db) molecules (54) were infected with WNV and compared to wild-type and CD8−/− mice. These mice lack classical class Ia MHC molecules and show a profound (>95%) reduction in their peripheral CD8+-T-cell repertoire but still express nonclassical class Ib MHC molecules (54).
Similar to CD8−/− mice, MHC class Ia−/− mice were highly susceptible to WNV infection (Fig. 1A). Only 12% of MHC class Ia−/− mice survived infection: this value was significantly different from that for wild-type mice (P < 0.0001) but similar to that of CD8−/− mice (16% survival for CD8−/− mice). In addition, a significant (∼20%) percentage of the MHC class Ia−/− mice had prominent hemiplegia and seizure activity, clinical signs that were similar to those observed with CD8−/− mice. To determine whether the similar pattern of susceptibility between CD8−/− and MHC class Ia−/− mice correlated with the viral burden, we compared levels of infectious virus in tissues at day 10 after infection. This time was chosen because it represented the point at which peak viral titers were observed in the CNS of CD8−/− mice. Strikingly, in a pattern similar to that of the CD8−/− mice, none of the MHC class Ia−/− mice had cleared WNV from the spleen (data not shown). Moreover, a 1,000-fold-higher virus burden was detected in the brain and spinal cord of both CD8−/− and MHC class Ia−/− mice than in those of wild-type mice, and a similar pattern of increased viral antigen staining was observed after immunohistochemical analysis (data not shown).
WNV persistence in surviving CD8-deficient mice.
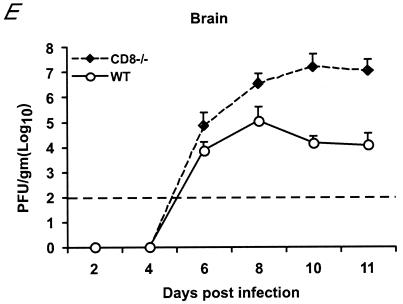
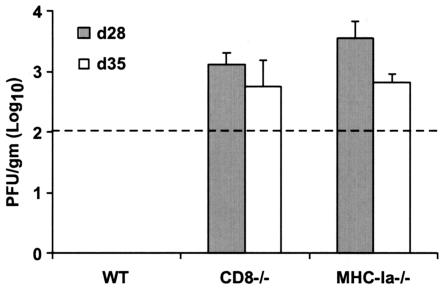
An absence of CD8+ T cells may result in increased tissue viral burdens because antigen-specific CD8+-T-cell activity is required for the direct or indirect targeting of infected cells. In the CNS, CD8+ T cells are believed to clear viral infections from neurons by both cytolytic and noncytolytic mechanisms (5, 6, 25). Although deficiencies in CD8+ T cells resulted in significantly increased vulnerability to lethal WNV infection, consistently a small number (∼15%) of mice survived infection. To assess the effect of CD8+-T-cell activity on kinetics of viral clearance, levels of infectious virus in the CNS were measured in wild-type and CD8−/− mice that survived the initial infection (Fig. 5). In wild-type mice, no infectious virus was detected in the brain after 21 days, whereas significant levels (103 PFU/g) were detected in CD8−/− mice. Even 35 days after infection, infectious virus was still recovered from the brain of CD8−/− mice. Similar levels of viral infection were seen after day 28 in MHC class Ia−/− mice (Fig. 5). Thus, the absence of CD8+ T-cell activity against WNV resulted in a failure to clear virus from infected cells in the CNS.
FIG. 5.
Delayed clearance of WNV from the brains of surviving CD8−/− and MHC-Ia−/− mice. Wild-type (WT), CD8−/−, and MHC class Ia−/− mice were infected with 102 PFU of WNV. Surviving animals were euthanized at 28 or 35 days after infection, and brains were analyzed for infectious virus by plaque assay as described in the Fig. 2 legend. Data are shown as the average PFU per gram of tissue and reflect three to five mice per time point.
Cytolytic activity of CD8+ T cells.
To confirm that CD8+ T cells played an important role in directly controlling virus infection, we assessed the cytolytic activity of purified CD8+ T cells against target cells that expressed WNV antigens (Fig. 6A). Previous studies by others had suggested that lymphocytes that were isolated from WNV-infected mice were capable of killing WNV target cells (23, 44), although purified CD8+ T cells were not used. Target cells (MC57GLWNV-E or MC57GLvector) were generated by stably transfecting the ectodomain of the 1999 New York strain of WNV or the parent vector (pcDNA3.1) into MC57GL cells, a syngeneic KbDb-expressing fibroblast tumor cell line. Target cells were labeled with calcein AM, a vital fluorochrome, and killing was assayed fluorimetrically by measuring the amount of calcein released from dying cells.
Primary CD8+ T cells were obtained directly from the spleens of mice 4, 6, and 8 days after infection and separated by negative antibody selection (90 to 95% purity; Fig. 6B, inset). Relatively high (30:1 or greater) E/T ratios were required to detect efficient killing of MC57GLWNV-E targets (Fig. 6A), likely reflecting a low number of WNV-specific CD8+ T cells in the spleen. Little killing (<10%) was observed after incubation with MC57GLvector target cells even at very high (100:1) E/T ratios. Whereas low levels of killing were observed at day 4, CD8+ splenic T cells isolated from mice at days 6 and 8 after WNV infection demonstrated higher levels of killing (Fig. 6A). The temporal increase in cytolytic activity after day 4 correlated with an expansion of CD8+ T cells (data not shown) and clearance of WNV from the spleen (Fig. 1C). Cytolysis by primary CD8+ T cells was antigen restricted as MAbs against class I (Kb + Db) MHC molecules inhibited lysis of WNV target cells (Fig. 6B). Memory CD8+ T cells that were isolated from the spleen of WNV-immune mice and expanded ex vivo more efficiently killed MC57GLWNV-E but not MC57GLvector target cells. CD8+-T-cell-mediated killing was observed at lower (3:1) E/T ratios and was inhibited with MAbs to class I MHC molecules and reproduced with WNV-infected but not uninfected MC57GL cells (data not shown).
DISCUSSION
Using a variety of experimental approaches, we demonstrate that CD8+ T cells contribute to clearance and recovery from WNV infection. CD8+ T cells that are isolated from WNV-infected mice lyse target cells that express WNV antigens in a class I MHC-restricted manner. Mice that lack CD8+ T cells or classical class Ia MHC molecules had higher CNS viral burdens and increased mortality rates after infection. Finally, in the subset of CD8+-T-cell-deficient mice that survived initial infection, infectious virus was recovered from the CNS for several weeks, suggesting that an absence of CD8+ T cells can cause a prolonged state of viral persistence.
Neuropathology and CD8+ T cells.
Neuropathological and immunohistochemical examination of CD8−/− mice revealed large numbers of infected neurons that appeared to be injured or dying. Similar to wild-type mice (9, 63), infection in the brain was observed in the cortex, brain stem, basal ganglia, hippocampus, and cerebellum, and infection in the spinal cord was predominantly in anterior horn motor neurons. However, compared to comparably ill wild-type mice, greater viral antigen staining was observed, consistent with the higher viral load in CD8−/− mice.
Viral burden and CD8+ T cells.
Although prior studies have demonstrated that γδ T cells also mediate control of WNV infection in mice (67), the role of CD8+ T cells in vivo has remained unclear. Here we demonstrate that the absence of CD8+ T cells or classical class Ia MHC molecules resulted in increased and sustained WNV infection in both peripheral and CNS tissues. Compared to wild-type mice, similar levels of virus were initially observed in the spleen of CD8−/− and class Ia−/− mice; however, mice that lacked CD8+-T-cell function did not clear infectious virus during the early course of infection. In CNS tissues, a distinct pattern was observed. In the spinal cord and brain, a deficiency of CD8+ T cells was associated with markedly higher viral titers. At 10 days after infection, 1,000-fold-increased titers were demonstrated in the CNS of both CD8−/− and class Ia MHC−/− mice compared to congenic wild-type mice. Our results, which demonstrate an essential role for CD8+ T cells in the clearance of WNV from the CNS, agree with some of those from a recently published paper (68). In that study, intravenous administration of a low dose (103 PFU) of the mouse-adapted Sarafend WNV was associated with increased viral load in the brain and mortality of wild-type C57BL/6 mice that were depleted of CD8+ T cells with antibodies or genetically deficient in β2-microglobulin (68). However, this study also showed that intravenous administration of a high (108 PFU), nonphysiologic dose of WNV resulted in decreased mortality in β2-microglobulin-deficient mice with an increase in average survival time. The authors suggested that under these conditions the improved survival indicates that CD8+ T cells can have adverse immunopathological effects in the CNS after WNV infection (68). In our own studies with higher doses of virus, we did not observe a similar pathological effect of CD8+ T cells: inoculation of 106 PFU of New York 1999 WNV into CD8−/− mice was associated with increased morbidity, mortality, and viral burden in the CNS (B. Shrestha and M. Diamond, unpublished results). Further study of the potential pathological function of CD8+ T cells may be warranted as the disparity in our results could reflect the route of inoculation, the strain of virus, and the genetically deficient strain of mouse.
Although increased levels of virus were detected in the CNS of CD8-deficient mice, this was not due to increased hematogenous spread. Virtually identical levels of viral RNA were detected in the serum of wild-type and CD8-deficient mice; viral RNA was detected in both wild-type and CD8−/− mice at days 2 and 4 after infection but subsequently was cleared. Combining this information with that from our previous studies (9, 11), antibody (primarily anti-WNV IgM) appears to control hematogenous spread of WNV to the CNS and terminate viremia. In contrast, once WNV reaches the CNS, CD8+ T cells play an essential function in controlling viral replication and clearing WNV from infected neurons. Our experiments suggest that at least part of this clearance may occur through a cytolytic mechanism, as purified CD8+ T cells (both newly expanded and memory populations) kill syngeneic targets that express WNV antigens in a class I MHC-restricted manner. These results agree with prior studies that demonstrated that cytolytic lymphocytes proliferate, kill, and release inflammatory cytokines after incubation with WNV-infected targets (12, 23, 24, 30, 44). They are also consistent with our recent experiments that show that mice that lack perforin granules have increased mortality and higher CNS viral burdens after WNV infection (B. Shrestha and M. S. Diamond, unpublished data).
CD8+ T cells may control infection in the CNS through class I MHC-restricted killing of WNV-infected cells. In vivo, in the CNS, WNV predominantly infects neurons (9, 14, 39, 40, 53, 63, 70), although in vitro, oligodendrocytes (21) and astrocytes (45, 46) have been infected. Neurons, however, basally express few class I MHC molecules in vivo (36, 51) with the exception of some brain stem and spinal motor neurons and after certain inflammatory stimuli (8, 42, 43). Although WNV infection upregulates class I MHC molecules on many cell types (27, 28, 47), it remains unclear whether expression on selected neuron populations occurs in vivo. If CD8+ T cells cannot directly recognize WNV-infected neurons, they may indirectly limit WNV infection by noncytolytic clearance mechanisms such as through the production of IFN-γ (5, 6) or by activating microglia or macrophages to phagocytose infected neurons (27). Our data confirm that primary and memory CD8+ T cells kill targets that express class I MHC molecules and WNV antigens. Studies with cultured neurons and syngeneic WNV-specific CD8+ T cells are under way to directly address the mechanism by which CD8+ T cells control WNV in the CNS.
Viral persistence in vivo.
Our findings establish the importance of CD8+ T cells in preventing sustained WNV infection in peripheral and CNS compartments. Mice that lack CD8+ T cells or class Ia MHC molecules demonstrated persistent infectious WNV in the brain for several weeks after the initial infection despite normal anti-WNV antibody responses. Flavivirus persistence occurs when the duration of production of infectious virus exceeds that which is expected (2 to 3 weeks) in acute uncomplicated flavivirus encephalitis (7). The observation of viral persistence in CD8−/− mice is consistent with our previous studies that suggest that antibody, by itself, cannot eliminate WNV reservoirs in host tissues, as an intact cellular immune response is required for viral clearance (16), and with other studies that demonstrate that treatment with immunosuppressive agents during flavivirus infection can lead to viral persistence (49). Persistent WNV infection in the CNS has been observed in monkeys (56) and hamsters (70) and with other flaviviruses including Saint Louis encephalitis (64), tick-borne (55, 57-59), and louping ill (71) viruses. Finally, the failure of CD8−/− mice to clear virus from infected cells is also consistent with studies with neuroadapted Sindbis virus in which CD8+ T cells were required for clearance of viral RNA from neurons (5, 6). Based on its occurrence in some wild-type animals, flavivirus persistence may be a function of several independent immunologic variables, one of which is the interaction of virus-specific CD8+ T cells with infected targets in the CNS.
Studies with CD8−/− and other immunodeficient (3, 9, 11, 16, 17, 67, 68) mice have provided insight into the mechanism of pathogenesis of and protection against WNV infection (10). In the mouse model, CD8+ T cells are required to clear virus from infected cells. These findings may have implications for treatment of human patients with WNV infection. Patients with genetic or acquired deficiencies in T-cell function may have prolonged WNV CNS persistence and thus require extended therapy to minimize the chance for disease recrudescence.
Acknowledgments
We thank O. Kanagawa, T. Chambers, A. Pekosz, D. Leib, L. Morrison, P. Olivo, P. Stuart, and members of their laboratories for experimental advice. We thank H. Virgin and T. Hansen for the CD8−/− and class Ia MHC−/− mice, the Ophthalmology Core Facilities at Washington University for technical assistance with the pathological sections, and D. Leib for critical reading of the manuscript.
The work was supported by grants from the Edward Mallinckrodt Jr. Foundation, the Ellison Medical Foundation, the National Institutes of Health (U54 AI057160-01), and the Centers for Disease Control and Prevention (U50/CCU720545-02).
REFERENCES
- 1.Ambudkar, S. V., S. Dey, C. A. Hrycyna, M. Ramachandra, I. Pastan, and M. M. Gottesman. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39:361-398. [DOI] [PubMed] [Google Scholar]
- 2.Asnis, D. S., R. Conetta, A. A. Teixeira, G. Waldman, and B. A. Sampson. 2000. The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin. Infect. Dis. 30:413-418. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Nathan, D., I. Huitinga, S. Lustig, N. van Rooijen, and D. Kobiler. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141:459-469. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Nathan, D., S. Lustig, G. Tam, S. Robinzon, S. Segal, and B. Rager-Zisman. 2003. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 188:5-12. [DOI] [PubMed] [Google Scholar]
- 5.Binder, G. K., and D. E. Griffin. 2003. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 5:439-448. [DOI] [PubMed] [Google Scholar]
- 6.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, T. J., and M. S. Diamond. 2003. Pathogenesis of flavivirus encephalitis. Adv. Virus Res. 60:273-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corriveau, R. A., G. S. Huh, and C. J. Shatz. 1998. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21:505-520. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond, M. S., B. Shrestha, E. Mehlhop, E. Sitati, and M. Engle. 2003. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 16:259-278. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, M. S., E. Sitati, L. Friend, B. Shrestha, S. Higgs, and M. Engle. 2003. Induced IgM protects against lethal West Nile virus infection. J. Exp. Med. 198:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas, M. W., A. M. Kesson, and N. J. King. 1994. CTL recognition of West Nile virus-infected fibroblasts is cell cycle dependent and is associated with virus-induced increases in class I MHC antigen expression. Immunology 82:561-570. [PMC free article] [PubMed] [Google Scholar]
- 13.Ebel, G. D., A. P. Dupuis III, K. Ngo, D. Nicholas, E. Kauffman, S. A. Jones, D. Young, J. Maffei, P. Y. Shi, K. Bernard, and L. Kramer. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldadah, A. H., and N. Nathanson. 1967. Pathogenesis of West Nile virus encephalitis in mice and rats. II. Virus multiplication, evolution of immunofluorescence, and development of histological lesions in the brain. Am. J. Epidemiol. 86:776-790. [DOI] [PubMed] [Google Scholar]
- 15.Eldadah, A. H., N. Nathanson, and R. Sarsitis. 1967. Pathogenesis of West Nile virus encephalitis in mice and rats. 1. Influence of age and species on mortality and infection. Am. J. Epidemiol. 86:765-775. [DOI] [PubMed] [Google Scholar]
- 16.Engle, M., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against West Nile virus infection in wild type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halevy, M., Y. Akov, D. Ben-Nathan, D. Kobiler, B. Lachmi, and S. Lustig. 1994. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch. Virol. 137:355-370. [DOI] [PubMed] [Google Scholar]
- 18.Heinz, F. X., R. Berger, W. Tuma, and C. Kunz. 1983. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology 126:525-537. [DOI] [PubMed] [Google Scholar]
- 19.Henchal, E. A., L. S. Henchal, and J. J. Schlesinger. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 69:2101-2107. [DOI] [PubMed] [Google Scholar]
- 20.Hubalek, Z., and J. Halouzka. 1999. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan, I., T. Briese, N. Fischer, J. Y. Lau, and W. I. Lipkin. 2000. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J. Infect. Dis. 182:1214-1217. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman, B. M., P. L. Summers, D. R. Dubois, W. H. Cohen, M. K. Gentry, R. L. Timchak, D. S. Burke, and K. H. Eckels. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41:576-580. [DOI] [PubMed] [Google Scholar]
- 23.Kesson, A. M., R. V. Blanden, and A. Mullbacher. 1987. The primary in vivo murine cytotoxic T cell response to the flavivirus, West Nile. J. Gen. Virol. 68:2001-2006. [DOI] [PubMed] [Google Scholar]
- 24.Kesson, A. M., R. V. Blanden, and A. Mullbacher. 1988. The secondary in vitro murine cytotoxic T cell response to the flavivirus, West Nile. Immunol. Cell Biol. 66:23-32. [DOI] [PubMed] [Google Scholar]
- 25.Kimura, T., and D. E. Griffin. 2000. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J. Virol. 74:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura-Kuroda, J., and K. Yasui. 1988. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J. Immunol. 141:3606-3610. [PubMed] [Google Scholar]
- 27.King, N. J., and A. M. Kesson. 2003. Interaction of flaviviruses with cells of the vertebrate host and decoy of the immune response. Immunol. Cell Biol. 81:207-216. [DOI] [PubMed] [Google Scholar]
- 28.King, N. J., and A. M. Kesson. 1988. Interferon-independent increases in class I major histocompatibility complex antigen expression follow flavivirus infection. J. Gen. Virol. 69:2535-2543. [DOI] [PubMed] [Google Scholar]
- 29.Klein, R. S., L. Izikson, T. Means, H. D. Gibson, E. Lin, R. A. Sobel, H. L. Weiner, and A. D. Luster. 2004. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J. Immunol. 172:550-559. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni, A. B., A. Mullbacher, and R. V. Blanden. 1991. In vitro T-cell proliferative response to the flavivirus, West Nile. Viral Immunol. 4:73-82. [DOI] [PubMed] [Google Scholar]
- 31.Kurane, I., M. A. Brinton, A. L. Samson, and F. A. Ennis. 1991. Dengue virus-specific, human CD4+ CD8− cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J. Virol. 65:1823-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurane, I., and F. A. Ennis. 1994. Cytotoxic T lymphocytes in dengue virus infection. Curr. Top. Microbiol. Immunol. 189:93-108. [DOI] [PubMed] [Google Scholar]
- 33.Kurane, I., B. L. Innis, C. H. Hoke, Jr., K. H. Eckels, A. Meager, J. Janus, and F. A. Ennis. 1995. T cell activation in vivo by dengue virus infection. J. Clin. Lab. Immunol. 46:35-40. [PubMed] [Google Scholar]
- 34.Kurane, I., B. L. Innis, A. Nisalak, C. Hoke, S. Nimmannitya, A. Meager, and F. A. Ennis. 1989. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J. Clin. Investig. 83:506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lad, V. J., A. K. Gupta, M. K. Goverdhan, V. L. Ayachit, J. J. Rodrigues, and L. V. Hungund. 1993. Susceptibility of BL6 nude (congenitally athymic) mice to Japanese encephalitis virus by the peripheral route. Acta Virol. 37:232-240. [PubMed] [Google Scholar]
- 36.Lampson, L. A., and W. F. Hickey. 1986. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from “histologically normal” to that showing different levels of glial tumor involvement. J. Immunol. 136:4054-4062. [PubMed] [Google Scholar]
- 37.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 39.Leis, A. A., J. Fratkin, D. S. Stokic, T. Harrington, R. M. Webb, and S. A. Slavinski. 2003. West Nile poliomyelitis. Lancet Infect. Dis. 3:9-10. [DOI] [PubMed] [Google Scholar]
- 40.Leis, A. A., D. S. Stokic, J. L. Polk, V. Dostrow, and M. Winkelmann. 2002. A poliomyelitis-like syndrome from West Nile virus infection. N. Engl. J. Med. 347:1279-1280. [DOI] [PubMed] [Google Scholar]
- 41.Licon Luna, R. M., E. Lee, A. Mullbacher, R. V. Blanden, R. Langman, and M. Lobigs. 2002. Lack of both Fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J. Virol. 76:3202-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linda, H., H. Hammarberg, S. Cullheim, A. Levinovitz, M. Khademi, and T. Olsson. 1998. Expression of MHC class I and β2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp. Neurol. 150:282-295. [DOI] [PubMed] [Google Scholar]
- 43.Linda, H., H. Hammarberg, F. Piehl, M. Khademi, and T. Olsson. 1999. Expression of MHC class I heavy chain and β2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J. Neuroimmunol. 101:76-86. [DOI] [PubMed] [Google Scholar]
- 44.Liu, Y., R. V. Blanden, and A. Mullbacher. 1989. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J. Gen. Virol. 70:565-573. [DOI] [PubMed] [Google Scholar]
- 45.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1989. Flavivirus infection up-regulates the expression of class I and class II major histocompatibility antigens on and enhances T cell recognition of astrocytes in vitro. J. Neuroimmunol. 21:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, Y., N. King, A. Kesson, R. V. Blanden, and A. Mullbacher. 1988. West Nile virus infection modulates the expression of class I and class II MHC antigens on astrocytes in vitro. Ann. N. Y. Acad. Sci. 540:483-485. [DOI] [PubMed] [Google Scholar]
- 47.Lobigs, M., A. Mullbacher, and M. Regner. 2003. MHC class I up-regulation by flaviviruses: immune interaction with unknown advantage to host or pathogen. Immunol. Cell Biol. 81:217-223. [DOI] [PubMed] [Google Scholar]
- 48.Mason, P. W., J. M. Dalrymple, M. K. Gentry, J. M. McCown, C. H. Hoke, D. S. Burke, M. J. Fournier, and T. L. Mason. 1989. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J. Gen. Virol. 70:2037-2049. [DOI] [PubMed] [Google Scholar]
- 49.Mathur, A., K. L. Arora, S. Rawat, and U. C. Chaturvedi. 1986. Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J. Gen. Virol. 67:381-385. [DOI] [PubMed] [Google Scholar]
- 50.McCown, M., M. S. Diamond, and A. Pekosz. 2003. The utility of siRNA transcripts produced by RNA polymerase I in down regulating viral gene expression and replication of negative- and positive-strand RNA viruses. Virology 313:514-524. [DOI] [PubMed] [Google Scholar]
- 51.Mucke, L., and M. B. Oldstone. 1992. The expression of major histocompatibility complex (MHC) class I antigens in the brain differs markedly in acute and persistent infections with lymphocytic choriomeningitis virus (LCMV). J. Neuroimmunol. 36:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murali-Krishna, K., V. Ravi, and R. Manjunath. 1996. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J. Gen. Virol. 77:705-714. [DOI] [PubMed] [Google Scholar]
- 53.Nathanson, N., and G. A. Cole. 1971. Immunosuppression: a means to assess the role of the immune response in acute virus infections. Fed. Proc. 30:1822-1830. [PubMed] [Google Scholar]
- 54.Perarnau, B., M. F. Saron, B. R. San Martin, N. Bervas, H. Ong, M. J. Soloski, A. G. Smith, J. M. Ure, J. E. Gairin, and F. A. Lemonnier. 1999. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur. J. Immunol. 29:1243-1252. [DOI] [PubMed] [Google Scholar]
- 55.Pogodina, V. V., N. G. Bochkova, and L. S. Levina. 1984. Persistence of tick-borne encephalitis virus in monkeys. VII. Some features of the immune response. Acta Virol. 28:407-415. [PubMed] [Google Scholar]
- 56.Pogodina, V. V., M. P. Frolova, G. V. Malenko, G. I. Fokina, G. V. Koreshkova, L. L. Kiseleva, N. G. Bochkova, and N. M. Ralph. 1983. Study on West Nile virus persistence in monkeys. Arch. Virol. 75:71-86. [DOI] [PubMed] [Google Scholar]
- 57.Pogodina, V. V., M. P. Frolova, G. V. Malenko, G. I. Fokina, L. S. Levina, L. L. Mamonenko, G. V. Koreshkova, and N. M. Ralf. 1981. Persistence of tick-borne encephalitis virus in monkeys. I. Features of experimental infection. Acta Virol. 25:337-343. [PubMed] [Google Scholar]
- 58.Pogodina, V. V., L. S. Levina, G. I. Fokina, G. V. Koreshkova, G. V. Malenko, N. G. Bochkova, and O. E. Rzhakhova. 1981. Persistence of tick-borne encephalitis virus in monkeys. III. Phenotypes of the persisting virus. Acta Virol. 25:352-360. [PubMed] [Google Scholar]
- 59.Pogodina, V. V., G. V. Malenko, G. I. Fokina, L. S. Levina, G. V. Koreshkova, O. E. Rzhakhova, N. G. Bochkova, and L. L. Mamonenko. 1981. Persistence of tick-borne encephalitis virus in monkeys. II. Effectiveness of methods used for virus detection. Acta Virol. 25:344-351. [PubMed] [Google Scholar]
- 60.Roehrig, J. T., L. A. Staudinger, A. R. Hunt, J. H. Mathews, and C. D. Blair. 2001. Antibody prophylaxis and therapy for flaviviral encephalitis infections. Ann. N. Y. Acad. Sci. 951:286-297. [DOI] [PubMed] [Google Scholar]
- 61.Schlesinger, J. J., M. W. Brandriss, and E. E. Walsh. 1985. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 135:2805-2809. [PubMed] [Google Scholar]
- 62.Schlesinger, J. J., and S. Chapman. 1995. Neutralizing F(ab′)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 76:217-220. [DOI] [PubMed] [Google Scholar]
- 63.Shrestha, B., D. I. Gottlieb, and M. S. Diamond. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77:13203-13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slavin, H. B. 1943. Persistence of the virus of Saint Louis encephalitis in the central nervous system of mice for over five months. J. Bacteriol. 46:113-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takada, K., H. Masaki, E. Konishi, M. Takahashi, and I. Kurane. 2000. Definition of an epitope on Japanese encephalitis virus (JEV) envelope protein recognized by JEV-specific murine CD8+ cytotoxic T lymphocytes. Arch. Virol. 145:523-534. [DOI] [PubMed] [Google Scholar]
- 66.Tsai, T. F., F. Popovici, C. Cernescu, G. L. Campbell, and N. I. Nedelcu. 1998. West Nile encephalitis epidemic in southeastern Romania. Lancet 352:767-771. [DOI] [PubMed] [Google Scholar]
- 67.Wang, T., E. Scully, Z. Yin, J. H. Kim, S. Wang, J. Yan, M. Mamula, J. F. Anderson, J. Craft, and E. Fikrig. 2003. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J. Immunol. 171:2524-2531. [DOI] [PubMed] [Google Scholar]
- 68.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 77:13323-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner, L. P., G. A. Cole, and N. Nathanson. 1970. Experimental encephalitis following peripheral inoculation of West Nile virus in mice of different ages. J. Hyg. (London) 68:435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao, S. Y., H. Guzman, H. Zhang, A. P. Travassos da Rosa, and R. B. Tesh. 2001. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 7:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zlotnik, I., G. B. Carter, and D. P. Grant. 1971. The persistence of louping ill virus in immunosuppressed guinea-pigs. Br. J. Exp. Pathol. 52:395-407. [PMC free article] [PubMed] [Google Scholar]