Abstract
Purpose
The BK (large conductance voltage and Ca2+ activated K+) channel is a key regulator of bladder smooth muscle contractility. To our knowledge in bladder smooth muscle the BK channel pore forming α subunit BKα associates in homotetramers with 4 regulatory smooth muscle specific β1 subunits. We challenged this concept in identify whether other regulatory BKβ subunits exist in mouse and rat bladder smooth muscle.
Materials and Methods
We used a novel approach with single cell reverse transcriptase-polymerase chain reaction combined with immunocytochemical studies in freshly isolated mouse and rat bladder smooth muscle cells. Western blot was also performed.
Results
Reverse transcriptase-polymerase chain reaction identified the mRNA expression of various BK channel subunits in freshly isolated bladder smooth muscle cells. Our data indicate that, in addition to BKα and BKβ1, neuronal specific BKβ4 is expressed in mouse and rat bladder smooth muscle cells. BKβ4 expression was also revealed by Western blot. Immunocytochemistry was further applied to confirm the specific expression of BKβ4 protein directly in freshly isolated mouse and rat bladder smooth muscle cells.
Conclusions
To our knowledge we performed the first comprehensive examination of the expression of BKα and BKβ subunits in bladder smooth muscle. We identified that the bladder smooth muscle BK channel has a distinctive architecture involving pore forming BKα and regulatory BKβ1/β4. Further studies of the functional roles of BKα, BKβ1 and BKβ4 directly in human bladder smooth muscle may help the development of alternative therapeutic strategies to control bladder dysfunction. New drugs targeting specific BK channel subunits in human bladder smooth muscle may prove useful for overactive bladder.
Keywords: urinary bladder, muscle, smooth, large-conductance calcium-activated potassium channels, rats, mice
The BK channel, also known as the maxiK, KCNMA1, Slo1 or KCa1.1 channel, is a member of the Shaker related 6 transmembrane domain K+ channel superfamily that is found in various excitable and nonexcitable cells, including UBSM. In UBSM the BK channels are key regulators of cell membrane excitability, action potential formation and contractility, and they are instrumental in mediating β-adrenergic bladder relaxation.1–6 As key regulators of UBSM membrane excitability, BK channels control the opening and closing of L-type voltage gated Ca2+ channels and, therefore, the level of Ca2+ influx necessary to activate UBSM contraction.
The BK channel pore forming α subunit or BKα associates in homotetramers that can account for the basic properties of the native channels, such as conductivity and voltage/Ca2+ sensitivity. The diverse kinetic and pharmacological characteristics of the BK channel are partially mediated by alternative splicing of the Slo or KCNMA1 gene, which encodes BKα.7 In 1994 the auxiliary smooth muscle specific β1 subunit BKβ1 with modulatory function was discovered.8 BKβ1, which is particularly enriched in UBSM, increases channel Ca2+ and voltage sensitivity, slows deactivation kinetics and alters the pharmacological properties of the BK channel.5,7,9,10 In knockout mouse models genetic deletion of BKα or BKβ1 leads to increased UBSM contractility and bladder overactivity, indicating that the BK channels have a fundamental role in controlling UBSM function.2,5,11
After the initial discovery of BKβ1 in smooth muscle8 increasing evidence has suggested that the heteromultimerization of BKα with the 4 β subunits BKβ1 to BKβ4 determines the tissue specific functions of the BK channel.5,7,9,12–16 BKβ2, which is prominent in endocrine and brain tissue, negatively shifts channel activation but also results in rapid and complete channel inactivation using a ball and chain mechanism.7,13,14 BKβ3, which is abundant in the human brain and expressed strongly in the rat lung, inactivates BK currents extremely rapidly but incompletely.7,9,12–14 Neuronal specific BKβ4 has the opposite effects of BKβ1, decreasing the apparent Ca2+ sensitivity of the BK channel.7,9,13,15,17,18 On the other hand, BKβ4 was confirmed to be a down-regulator of the BK channel because it more profoundly slows the activation and inactivation kinetics of BK channels compared with BKβ1.7,9,13,17,18 Although BKβ4 is abundant in the brain, it is moderately expressed in other tissues, such as the spinal cord, kidney, lung and secretory glands.9,13,15 Knowledge about the existence of BKβ4 in smooth muscle is sparse. Only 1 group that used dot and Northern blots have reported low levels of BKβ4 mRNA expression in some smooth muscle tissues in which BKβ1 is abundant.15
The mentioned studies suggest that more than 1 type of BKβ subunit exists in a functional channel complex resulting in heteromultimeric channels composed of BKα/β1 to β4 subunits with intermediate functional properties. We challenged the current concept that in UBSM the BK channel consists only of 4 pore forming BKα and 4 smooth muscle specific regulatory BKβ1.5,8 As a prerequisite to understand the functional role of potentially novel BKβ subunits in UBSM information on their expression at the mRNA and protein levels is required. Accordingly we identified whether, in addition to BKβ1, other regulatory BKβ subunits exist in rat and mouse UBSM using molecular and immunological approaches.
MATERIALS AND METHODS
Animal Care, UBSM Tissue and Cell Isolation Procedures
Adult mice weighing 25 to 35 gm and rats weighing 250 to 300 gm of each sex were sacrificed with CO2, followed by exsanguination. This procedure was done in accordance with the guidelines of the Animal Welfare Act, the Association for Assessment and Accreditation of Laboratory Animals, and the University of South Carolina institutional animal care and use committee (Animal Use Protocol 1426 for mice and 1482 for rats).
In RT-PCR experiments the brains, lungs, livers and bladders were removed and placed in ice-cold nominally Ca2+-free solution composed of 80 mM monosodium glutamate, 55 mM NaCl, 6 mM KCl, 10 mM glucose, 10 mM HEPES and 2 mM MgCl2 (pH 7.3, adjusted with NaOH). Fresh physiologically active UBSM cells were enzymatically isolated as previously described in mice2 and rats.4 Freshly isolated UBSM cells were left to settle at the bottom of a chamber for at least 5 minutes before individual selection based on cell morphology using an Axiovert 40CFL microscope (Carl Zeiss®) with Nomarski interference contrast. In each RT-PCR experiment 200 to 300 freshly isolated UBSM cells were collected by suction into a glass micropipette using an MP-285/ROE micromanipulator (Sutter Instruments, San Rafael, California). Collected UBSM cells were expelled into a 1.5 ml centrifuge tube with RNAlater® and then pelleted at 1,000 × gravity for 3 minutes. Pellets were prepared for an RT-PCR protocol.
For Western blot rat brain and whole bladder were cut and used for membrane protein extraction. For immunocytochemical studies fresh UBSM cells were also isolated as described and dropped on a glass coverslip to settle for 1.5 hours at room temperature before further processing, as described.
RNA Extraction, RT-PCR and Sequencing
Total RNA was isolated from brain, lung, liver, UBSM whole tissue and enzymatically isolated UBSM cells using an RNeasy™ Mini Kit. Extracted RNA was reverse transcribed into cDNA using M-MLV RT (Promega®) and oligo deoxythymidine primers. Specific primers for rat BKα, BKβ1, BKβ2 and BKβ3, and mouse BKβ, BKβ1, BKβ2, BKβ3 and BKβ4 were designed according to the rat and mouse GenBank® sequence using Primer Premier, version 5 (Premier Biosoft International, Palo Alto, California). Primer for rat BKβ4 was designed based on the sequences of multiple species in GenBank and aligned using Primer Premier, version 5. The table lists all primer pair sequences used in this study. To eliminate the contamination of genomic DNA primers were designed across exon junctions. cDNA production was PCR amplified using GoTaq® Green Master Mix and specific primers for all subunits. PCR annealing temperature for each primer pair was optimized using a Mastercycler® gradient thermocycler. Rat and mouse brain mRNA products served as a positive control for BKα, BKβ1, BKβ2 and BKβ4 to confirm the effectiveness of primers. Rat lung and mouse livers were chosen as positive controls in RT-PCR experiments because they have been reported to abundantly express BKβ3.7,12,16 Negative PCR control experiments were performed in the absence of the RT enzyme to avoid the contamination of genomic DNA. PCR products were purified using a GenElute™ PCR Clean-Up Kit and sequenced directly at our institution to confirm their identity.
Table 1.
BK channel subunits RT-PCR primers
| Subunits | Sense | Antisense | Production (bp) |
|---|---|---|---|
| Rat: | |||
| BKα | ATGTCTACAGTGGGTTACGG | TGGGTGGTAGTTCTTTATGG | 504 |
| BKβ1 | TGACTGTTGCCTCCTGTG | TCCCGAGTGTCTTCTGTG | 314 |
| BKβ2 | TTACAGACACGACGAGAAA | CGACACTCACAAGGGACA | 449 |
| BKβ3 | CTGGACTTTGCCTTCACC | CCTCCCAGCAATGTCAGTA | 344 |
| BKβ4 | GCGTTCTCATTGTGGTCC | TGTGCCTGTTTCTGTTGC | 239 |
| Mouse: | |||
| BKα | GCTGTTGATGGGTGTTCG | CGCAAGCCAAAGTAGAGG | 775 |
| BKβ1 | CCTGGGAGTGGCAATGGT | CCCGAGTGTCTTCCGTGT | 239 |
| BKβ2 | CGGACCTCTTCATCTTACA | CACTGGGCTTCTTCTGTC | 230 |
| BKβ3 | CATCCCTGTCCAAATCACG | TCCTGGCAGCTACCCTCA | 318 |
| BKβ4 | ATCGGTTCCCAGCCATTC | CGACTTCTTTGAGGGTTTCC | 485 |
Protein Isolation and Western Blot
Brain and UBSM tissues were homogenized with standard RIPA buffer containing protease inhibitors (Thermo Fisher Scientific, Waltham, Massachusetts). The homogenate was vortexed for 60 seconds, sonicated for 5 seconds at low power and incubated on ice for 45 minutes. The mixture was centrifuged at 12,000 × gravity for 30 minutes at 4C. Supernatant was collected and the pellet was resuspended in RIPA buffer and centrifuged again. The 2 supernatants were mixed and centrifuged at 45,000 × gravity for 30 minutes. Supernatant was collected and the protein concentration was determined with a BCA protein assay kit (Thermo Fisher Scientific). Protein was stored at −80C until use for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot.
For Western blot protein was mixed with 5 × Laemmli buffer (1:4) and denaturized for 5 minutes at 95C. Subsequently equal amounts of brain and UBSM proteins (approximately 50 µg) were loaded into adjacent lanes, subjected to 4% to 20% pre-case sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 2.5 hours at 20 mA and transferred to a polyvinylidene fluoride membrane at 40 mA for 2 hours using semidry blot. The membrane was blocked with 3% bovine serum albumin/TBS-Tween 20 buffer for 2 hours at room temperature. The blots were incubated with affinity purified polyclonal BKβ4 antibody (Alomone Labs, Jerusalem, Israel) (1:100) overnight at 4C. The membrane was washed with tris buffered saline- Tween 20, 4 times and incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (1:2,500) in blocking buffer for 1 hour at room temperature. Bound antibodies were detected by an echochemiluminescence substrate kit (Amersham, Piscataway, New Jersey) according to manufacturer instructions. Staining specificity was verified by pre-incubation of antibodies with a competing peptide.
Immunocytochemistry
Enzymatically isolated UBSM cells were fixed with pre-warmed (37C) 4% paraformaldehyde for 10 minutes. UBSM cells were then washed twice in PBS, blocked and permeabilized for 30 minutes in PBS containing 10% normal donkey serum and 0.1% Triton X-100. UBSM cells were washed again in PBS and incubated with primary antibody, that is rabbit polyclonal anti-sloβ4 (KCNMB4) BKβ4 antibody (Alomone Labs) (1:100) at 37C for 1 hour. Subsequently UBSM cells were washed twice in PBS and labeled with secondary antibodies, that is Cy3-conjugated anti-rabbit IgG (1:200) and PBS/3% normal donkey serum/0.01% Triton X-100 (Jackson ImmunoResearch, West Grove, Pennsylvania) for 1 hour in the dark. After labeling UBSM cells were washed with PBS and incubated with phalloidin, which stains F-actin green, for 2 hours in the dark. UBSM cells were then washed twice more with PBS, incubated with 4′,6-diamidino-2-phenylindole, which stains nuclei in blue, for 15 minutes and washed again, then mounted on slides with Dabco®. Control treatments included 1) omission of primary antibody to confirm secondary antibody specificity and 2) absorption of primary antibody by a competing peptide to confirm primary antibody specificity. Images were acquired at 63× with a Carl Zeiss LSM 510 META confocal microscope.
RESULTS
mRNA Detection
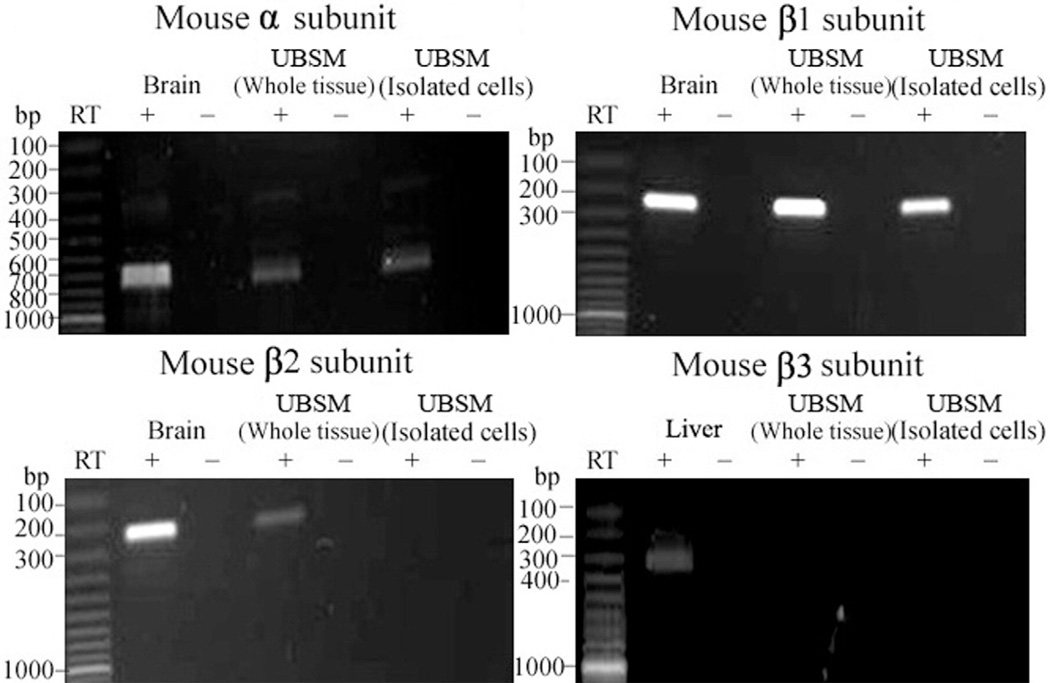
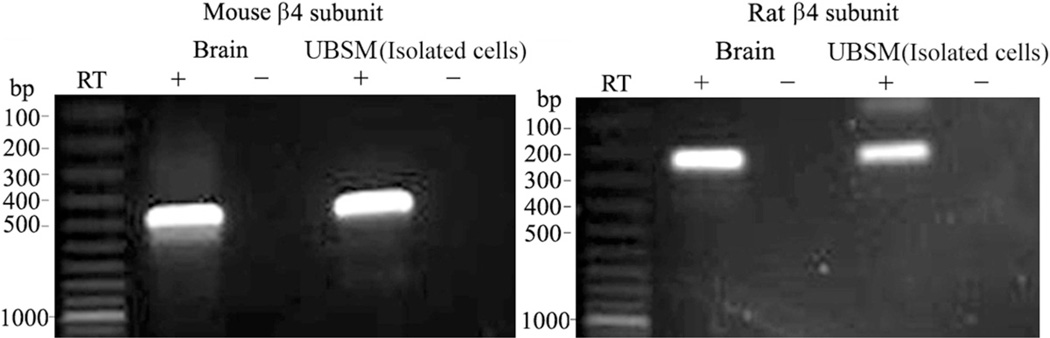
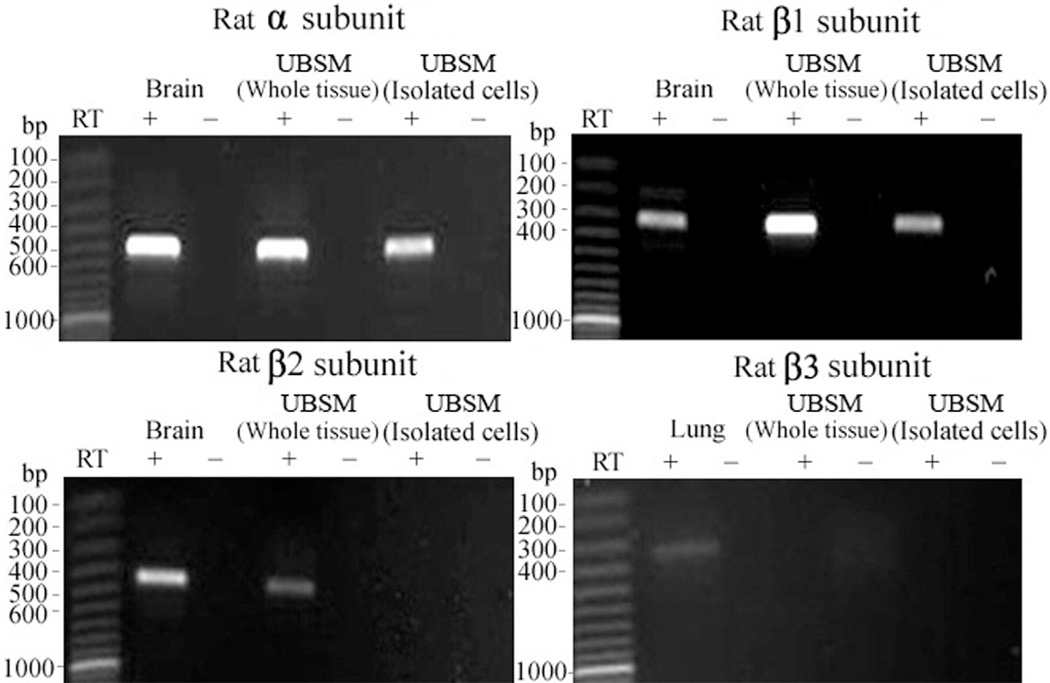
To detect BK channel subunit mRNA expression RT-PCR experiments were performed in mouse and rat UBSM whole tissue, enzymatically isolated UBSM cells and various other tissues that served as positive controls, as described. Subunit specific primers were used to determine the expression of BKα, BKβ1, BKβ2, BKβ3 and BKβ4 (see table and figs. 1 to 3). BKα, BKβ1, BKβ2 and BKβ4 mRNA expression was detected in UBSM whole tissue and in brain tissue, which served as a positive control (figs. 1 to 3). As expected, the BKβ3 mRNA message was detected in rat lung and mouse liver but not in UBSM whole tissue even when subjected to a second round of PCR amplification (figs. 1 and 2). Negative control experiments demonstrated absent genomic DNA contamination (figs. 1 to 3). While the expression of mRNA messages in UBSM whole tissue for pore forming BKα and smooth muscle specific BKβ1 was expected, the detection of BKβ2 and BKβ4 was surprising. The presence of other cell types in the detrusor muscle layer, such as neurons, vascular myocytes, endothelial cells and fibroblasts, may lead to the detection of subunits expressed in cell types other than UBSM cells. To address this issue we performed single cell RT-PCR experiments in freshly isolated UBSM cells from mice and rats. The single cell RT-PCR approach eliminates any contamination from other cell types, as described. Freshly isolated UBSM cells were confirmed to express mRNA for BKα, BKβ1 and BKβ4 subunits in rats and mice (figs. 1 to 3). The BKβ2 mRNA message was not detected in freshly isolated UBSM cells even after the initial RCR products were subjected to a second round of amplification (figs. 1 and 2). A lack of genomic DNA contamination was also confirmed using the negative control reactions lacking the RT enzyme. All RT-PCR purified products from intact UBSM tissues and isolated UBSM cells were sequenced to confirm their identity. Results demonstrated that freshly isolated UBSM cells from rats and mice expressed BKα, BKβ1 and BKβ4 mRNA.
Figure 1.
BKα, BKβ1, BKβ2 and BKβ3 mRNA expression in mouse brain, liver, UBSM whole tissue and freshly isolated UBSM cells showed 775, 239, 230 and 318 bp mRNA message, respectively. No products were observed in negative controls (−RT) with RT left out of reaction. Results were verified in total of 6 preparations in 2 mice.
Figure 3.
BKβ4 mRNA expression in mouse and rat brain, and freshly isolated UBSM cells showed 485 and 239 bp mRNA messages, respectively. No products were observed in negative controls (−RT) with RT left out of reaction. Results were verified in total of 9 preparations each in 3 rats and 3 mice.
Figure 2.
BKα, BKβ1, BKβ2 and BKβ3 mRNA expression in rat brain, lung, UBSM whole tissue and freshly isolated UBSM cells demonstrated 504, 314, 449 and 344 bp mRNA message, respectively. No products were observed in negative controls (−RT) with RT left out of reaction. Results were verified in total of 9 preparations in 3 rats.
Western Blot Detection
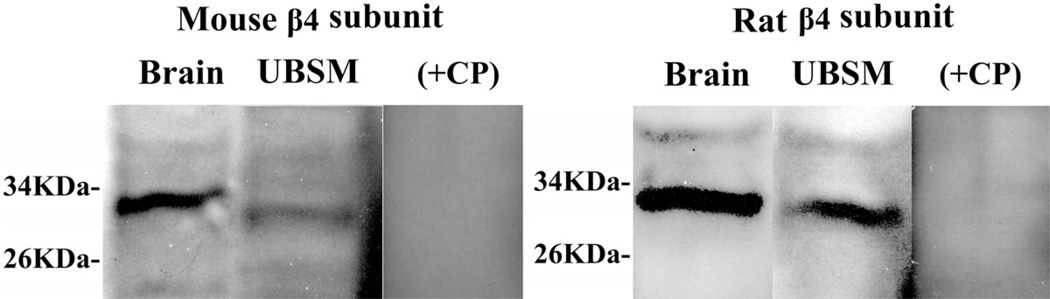
While the expression of BKα and BKβ1 mRNA messages in freshly isolated mouse and rat UBSM cells was expected, to our knowledge the detection of BKβ4 was a novel finding. To confirm the presence of BKβ4 protein in mouse and rat UBSM cells we applied Western blot. The presence of BKβ4 protein in rat and mouse UBSM tissue was confirmed by BKβ4 specific antibody (fig. 4). Pre-absorption of primary antibody with its antigenic competing peptide indicated the specificity of the antibody for its intended epitope.
Figure 4.
Western blot demonstrates BKβ4 protein expression in mouse and rat brain and UBSM tissue. Immunoreactive band was eliminated by competing peptide (+CP). Results were verified in 3 Western blot reactions using proteins isolated from 3 mice and 4 rats. KDa, kDa.
Immunocytochemical Detection
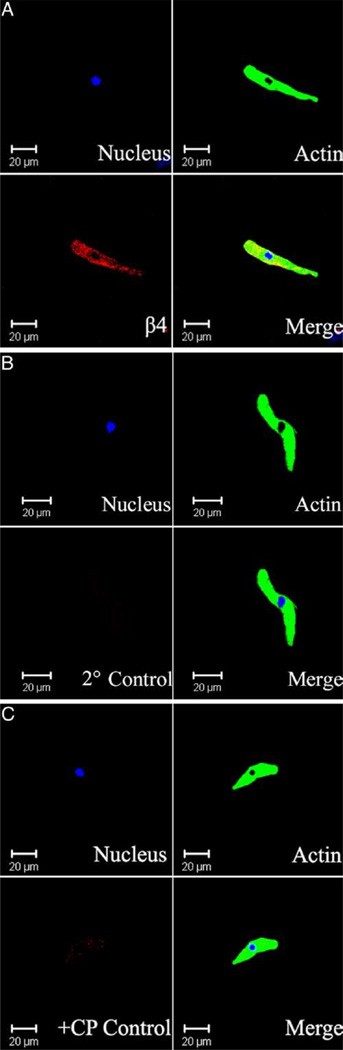
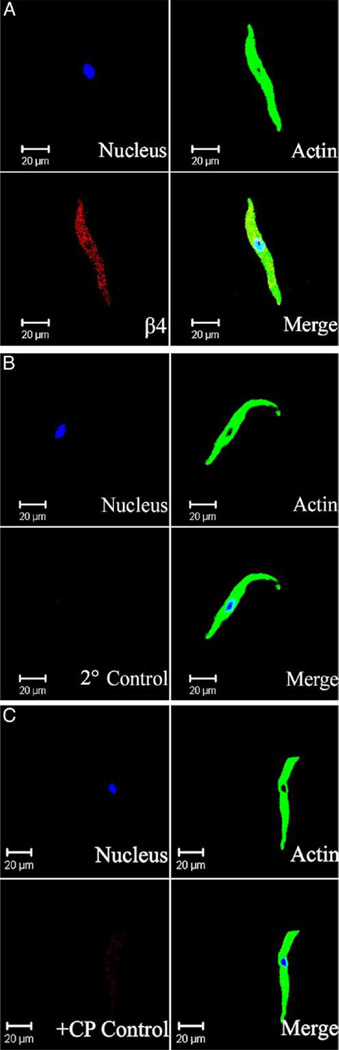
Immunocytochemical labeling was further applied to confirm the specific expression of BKβ4 protein directly in freshly isolated mouse and rat UBSM cells. Freshly isolated mouse and rat UBSM cells had bright, distinct edges when observed with a phase contrast confocal microscope. BKβ4 antibody specifically labeled UBSM cells isolated from mouse and rat bladders (figs. 5 and 6). Results were carefully controlled for specificity using omission of the primary antibody or absorption of the primary antibody by a competing peptide. BKβ4 antibody specificity has also been previously verified.16,19 Immunocytochemical experiments confirmed that mouse and rat UBSM cells expressed BKβ4.
Figure 5.
Wide field confocal microscopy reveals immunocytochemical detection of BKβ4 in freshly isolated mouse UBSM cells. Results were verified in total of 9 cells freshly isolated from 3 mice. Note results using BKβ4 specific antibody (A). In control experiments primary antibody was omitted and cells were incubated with secondary antibody only (2° Control) (B). Note results after absorption of primary antibody with competing peptide (+CP Control) (C). Red areas indicate BKβ4 detection (A). Blue areas indicate cell nuclei. Green areas indicate F-actin. Merged images show nucleus, actin and β4 subunit overlap.
Figure 6.
Wide field confocal microscopy shows immunocytochemical detection of BKβ4 in freshly isolated rat UBSM cells. Results were verified in total of 12 cells freshly isolated from 4 rats. Note results using BKβ4 specific antibody (A). In control experiments primary antibody was omitted and cells were incubated with secondary antibody only (2° Control) (B). Note results after absorption of primary antibody with competing peptide (+CP Control) (C). Red areas indicate BKβ4 detection. Blue areas indicate cell nuclei. Green areas indicate F-actin. Merged images indicate nucleus, actin and β4 subunit overlap.
DISCUSSION
To our knowledge in the current study we provide the first comprehensive examination of the expression of BKα and BKβ subunit mRNA in mouse and rat UBSM combined with molecular and immunocytochemical studies in freshly isolated UBSM cells. The molecular biological data presented indicate that, in addition to BKα and BKβ1, neuronal specific BKβ4 is expressed in rat and mouse UBSM cells. To our knowledge this finding demonstrates for the first time that the UBSM BK channel expresses regulatory BKβ4, which distinguishes the architecture of the UBSM BK channel. Our novel findings are con- sistent with a previous study in which dot and Northern blots were used, suggesting the possible presence of transcripts encoding BKβ4 in some smooth muscle tissues.15
However, to date conclusions concerning BKβ4 expression in native UBSM cells have not been drawn due to the methodological limitations of molecular biological techniques. In our series native UBSM BKβ4 was identified by applying novel complementary techniques to reveal the expression of mRNA encoding the BK channel subunits and the subunit proteins themselves. In the current study RT-PCR was used to identify BKβ4 expression in freshly isolated UBSM cells at the mRNA level. Immunocytochemistry instead of traditional immunohistochemistry was performed to identify the specific expression of BKβ4 protein directly in freshly isolated UBSM cells from mice and rats. This combination of novel approaches minimized the possibility of artifactual identification of a subunit because of 1) contaminating mRNA derived from cell types in the detrusor muscle layer, such as neurons, vascular myocytes, endothelial cells and fibroblasts or 2) nonspecific immunolabeling by BKβ4 antibody. Furthermore, the specificity of the BKβ4 antibody has been previously confirmed by examining it in brain sections isolated from BKβ4−/− knockout mice.16,19 Those investigators reported that no specific staining was observed in BKβ4 deficient animals. The BKβ4−/− knockout mouse model used by Brenner et al17 may prove useful to further identify the functional and regulatory role of BKβ4 in mouse UBSM.
Our previous studies in a BKβ1 transgenic knockout mouse model revealed that in UBSM BKβ1 is exclusively expressed and functionally associated with pore forming BKα to control UBSM function.5 However, to our knowledge the existence of a BKα/β1/β4 heteromultimeric channel complex in mouse and rat UBSM is a novel finding. The current study provides direct molecular evidence for the presence of heteromultimeric channels composed of BKα/β1/β4 in native, freshly isolated mouse and rat UBSM cells. The apparent Ca2+ affinity of the BKα/β4 channel is decreased dramatically at lower Ca2+ concentrations, whereas BKβ1 increases Ca2+ affinity without Ca2+ concentration dependence.9,10 In addition, BKα/β4 channels cannot be blocked by charybdotoxin or iberiotoxin at concentrations that typically block BKα/β1 channels.13,15,20 Interestingly our previous studies in a BKβ1 knockout mouse model showed changes in the effects of iberiotoxin on UBSM contractility after genetic deletion of BKβ1.5 Further knowledge about the specific properties of BKβ4 may prove useful to elucidate the correlation of BKα/β1 and BKα/β4 complexes in UBSM.
CONCLUSIONS
To our knowledge we provide the first evidence for the expression of BKβ4 mRNA and protein in freshly isolated mouse and rat UBSM cells. It is reasonable to speculate that BKβ4 is a regulatory component of the UBSM BK channel. However, future studies are required to determine the functional roles of this auxiliary BKβ4 in UBSM and elucidate the pharmacological properties of the UBSM BK channel in BKβ4−/− knockout mice.17 Confirming the unique architecture of the UBSM BK channel involving pore forming BKα and regulatory BKβ1/β4 would provide innovative insight into the function of the BK channel in UBSM cells. Further knowledge about the functional and regulatory roles of UBSM BKα, BKβ1 and BKβ4 may be helpful for developing alternative therapeutic strategies to control overactive bladder. For example, drugs targeting specific UBSM BK channel subunits may prove useful for some forms of bladder dysfunction.1 However, we must first obtain information about BK channel structure and function directly in human UBSM, as knowledge about this fundamental channel is limited.3
ACKNOWLEDGMENTS
Dr. Kiril Hristov, University of South Carolina assisted with cell isolation and collection. Dr. Jennifer G. Schnellmann, Medical University of South Carolina assisted with the article. PCR products were sequenced at the Environmental Genomics Core Facility, University of South Carolina. The BKβ4−/− knockout mouse model was created at University of Texas Health Science Center, San Antonio, Texas.17
Supported by National Institutes of Health DK-070909 (GVP).
Abbreviations and Acronyms
- BK
large conductance voltage and Ca2+ activated K+
- BKα
BK channel α subunit
- BKβ1
BK channel β1 subunit
- BKβ2
BK channel β2 subunit
- BKβ3
BK channel β3 subunit
- BKβ4
BK channel β4 subunit
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- UBSM
bladder smooth muscle
Footnotes
Study received University of South Carolina institutional animal care and use committee approval.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 2.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, et al. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol. 2008;295:F1149. doi: 10.1152/ajprenal.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hristov K, Cui X, Kellett WF, Rovner ES, Petkov GV. Evidence for the presence of large conductance Ca2+-activated K+ channels in native human urinary bladder smooth muscle. J Urol. 2008;179 [Google Scholar]
- 4.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large conductance Ca2+-activated K+ (BK) channels. Am J Physiol Cell Physiol. 2008;295:C1344. doi: 10.1152/ajpcell.00001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1255. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- 7.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 8.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921. [PubMed] [Google Scholar]
- 9.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 10.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol. 2000;116:411. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 12.Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- 14.Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, et al. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 15.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol. 2007;293:F350. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- 17.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. J Gen Physiol. 2006;127:449. doi: 10.1085/jgp.200509436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piwonska M, Wilczek E, Szewczyk A, Wilczynski GM. Differential distribution of Ca(2+)-activated potassium channel beta4 subunit in rat brain: immunolocalization in neuronal mitochondria. Neuroscience. 2008;153:446. doi: 10.1016/j.neuroscience.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 20.Gan G, Yi H, Chen M, Sun L, Li W, Wu Y. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels. J Biol Chem. 2008;283:24177. doi: 10.1074/jbc.M800179200. [DOI] [PMC free article] [PubMed] [Google Scholar]