Overview
Hepatobiliary cancers are highly lethal. In 2008, approximately 21,370 persons in the United States were estimated to be diagnosed with liver or intrahepatic bile duct cancer and 9520 with gallbladder cancer or other biliary tract cancer. Furthermore, approximately 18,410 deaths from liver or intrahepatic bile duct cancer and 3340 deaths from gallbladder cancer or other biliary tract cancer were estimated to occur.1
The types of hepatobiliary cancers covered in these guidelines include hepatocellular carcinoma (HCC), gallbladder cancer, intrahepatic cholangio-carcinoma, and extrahepatic cholangiocarcinoma. By definition, these guidelines cannot incorporate all possible clinical variations and are not intended to replace good clinical judgment or individualization of treatments. Although not explicitly stated at every decision point of the guidelines, patient participation in prospective clinical trials is the preferred option for treatment of hepatobiliary cancers.
HCC
Risk Factors and Epidemiology
Risk factors for the development of HCC, the most common hepatobiliary malignancy, include infection with the hepatitis B (HBV) and/or C (HCV) virus, particular comorbidities or conditions, and certain external sources.2 For example, chronic HBV infection is the leading cause of HCC in Asia and Africa, whereas HCV infection is the leading cause of HCC in Europe, Japan, and North America.3,4 A retrospective analysis of patients at liver transplantation centers in the United States found that almost 50% were infected with HCV and approximately 15% with HBV, with approximately 5% of patients having markers of both.5 Conditions associated with an increased risk for HCC include relatively rare, inherited errors of metabolism, such as hereditary hemochromatosis, porphyria cutanea tarda, α1-antitrypsin deficiency, and Wilson's disease, and autoimmune hepatitis and primary biliary cirrhosis.2 Increasing evidence also shows an association between the sequelae of nonalcoholic fatty liver disease, such as nonalcoholic steatohepatitis (NASH; i.e., a spectrum of conditions characterized by histologic findings of hepatic steatosis with inflammation in individuals who consume little or no alcohol), in the setting of metabolic syndrome or diabetes mellitus6 and the development of HCC.7 Excessive alcohol intake or environmental exposure to aflatoxin, a natural product of the Aspergillus fungus found in various grains, are other known risk factors for HCC.2,4,8,9
In most cases, the risk factors for HCC are also risk factors for liver cirrhosis. An estimated 60% to 80% of persons with HCC have underlying cirrhosis,8 possibly approaching 90% in the United States.10 Although most studies evaluating the risk for development of HCC in individuals infected with HCV have focused on populations with cirrhosis, limited data show that HCC can occur in some patients infected with HCV with bridging fibrosis in the absence of overt cirrhosis.11 Importantly, certain populations chronically infected with HBV (i.e., hepatitis B carriers) have been identified as being at increased risk for HCC in the absence of cirrhosis, especially when other risk factors are present (e.g., family history of HCC);4 an estimated 30% to 50% of patients with chronic HBV infection who develop HCC do not have underlying cirrhosis.9 The presence of liver cirrhosis is usually considered to be a prerequisite for development of HCC in individuals with inherited metabolic diseases of the liver or liver disease with an autoimmune etiology.12,13 However, HCC has been reported to occur in the setting of NASH without liver cirrhosis.14,15 Although the mechanism of HCC development differs according to the underlying disease,8 HCC typically occurs in the setting of a histologically abnormal liver. Hence, the presence of chronic liver disease represents a potential risk for development of HCC.2
The incidence of HCC is increasing in the United States, particularly in the population infected with HCV. Approximately 4 million individuals in the United States are chronically infected with HCV,16 and the annual incidence rate of HCC among patients with HCV-related cirrhosis has been estimated to be between 2% and 8%.4 Although the number of cases of hepatitis C infection diagnosed per year in the United States has been reported to be declining, the observed increase in HCV-related HCC cases is likely to be associated with the often prolonged period between viral infection and the manifestation of HCC.17,18
Approximately 1.5 million people in the United States are chronically infected with HBV.16,19,20 Results from a prospective controlled study showed the annual incidence of HCC to be 0.5% in carriers of the virus without liver cirrhosis and 2.5% in those with known cirrhosis,21 although studies have shown wide variation in the annual incidence rate of HCC among individuals with chronic HBV infection.4
The prevalence of NASH in the United States is estimated to be 3% to 5%, indicating that this sizable subpopulation is at risk for cirrhosis and development of HCC.22 However, several studies suggest that HCC may be somewhat less likely to develop in the setting of NASH-associated cirrhosis compared with cirrhosis because of HCV infection.23,24
Annual incidence rates of HCC associated with certain conditions (e.g., hereditary hemochromatosis) or exposure to alcohol are not well characterized. In the former case, these conditions are uncommon; in the latter case, many of the studies evaluating the incidence rate of HCC in individuals with alcohol-induced cirrhosis have been confounded by the presence of other risk factors (e.g., viral hepatitis infection), which can interact synergistically in the pathogenesis of HCC.25,26
Screening
The purpose of a cancer screening test is to identify the presence of a specific cancer in an asymptomatic individual when early detection has the potential to favorably impact patient outcome. The panel supports the recommendation by the American Association for the Study of Liver Disease (AASLD) that HCC screening be “offered in the setting of a program or process in which screening tests and recall procedures have been standardized and in which quality control procedures are in place.”4
Support for enrolling individuals at high risk for HCC in a screening program comes from a large randomized controlled trial of 18,816 men and women with HBV infection or a history of chronic hepatitis in China. In this study, screening with serum alpha-fetoprotein (AFP) testing and ultrasonography every 6 months was shown to result in a 37% reduction in HCC mortality, even though fewer than 60% of individuals in the screening arm completed the screening program.27 A recent prospective study of 638 patients with HCC in Singapore conducted over 9 years showed that patients aged 40 years or younger were more likely than older patients to be hepatitis B carriers and to have more advanced disease at diagnosis.28 Although survival did not differ between the groups overall, a significant survival benefit was observed for younger patients when the subgroup of patients with early-stage disease was considered. These results provide support for not restricting HCC screening to older patients.
AFP and liver ultrasonography are the most widely used methods of screening for HCC.29 In a screening study involving a large population of patients in China infected with HBV or chronic hepatitis, the detection rate, false-positive rate, and positive predictive value was 84%, 2.9%, and 6.6%, respectively, for ultrasound alone; 69%, 5.0%, and 3.3%, respectively, for AFP alone; and 92%, 7.5%, and 3.0%, respectively, for the combination of AFP and ultrasound.30 These results show that ultrasound imaging alone is a better HCC screening approach than AFP testing alone. Nevertheless, because ultrasonography is highly operator-dependent, addition of AFP can increase the likelihood of detecting HCC in a screening setting.
The populations considered to be “at risk” for HCC and likely to benefit from participation in an HCC screening program are defined on page 352 (see earlier section on Risk Factors and Epidemiology).
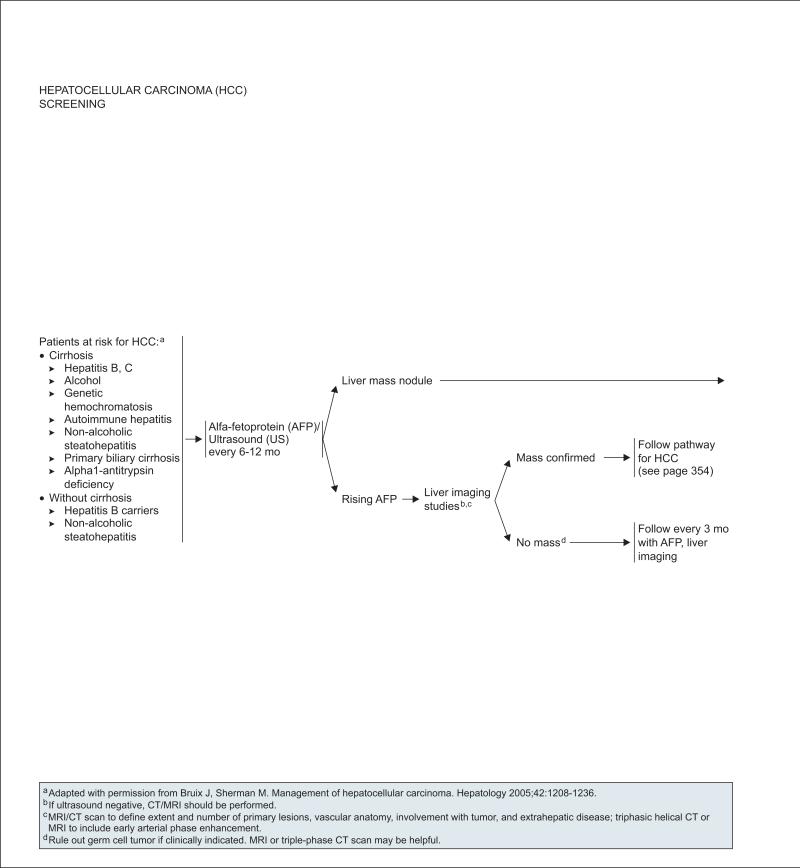
The panel recommends that patients at risk for HCC, irrespective of age, undergo periodic screening with ultrasonography and AFP testing every 6 to 12 months (see page 352). Additional imaging is recommended in the setting of a rising serum AFP or after identification of a liver mass nodule on ultrasound (see next sections on “Diagnosis” and “Initial Workup”).
Diagnosis
HCC is asymptomatic for much of its natural history. Nonspecific symptoms can include jaundice, anorexia, weight loss, malaise, and upper abdominal pain. Physical signs of HCC can include hepatomegaly and ascites.8 Paraneoplastic syndromes can also occur and include hypercholesterolemia, erythrocytosis, hypercalcemia, and hypoglycemia.8
Imaging
Recommendations for additional imaging if clinical suspicion for HCC is high (e.g., after a liver nodule is identified on ultrasonography or in the setting of rising a serum AFP level) are adapted from the guidelines outlined by the AASLD (see page 353).4 HCC lesions are characterized by arterial hypervascularity, deriving most of their blood supply from the hepatic artery, unlike the surrounding liver, which receives most of its supply of blood from the portal vein.31 Diagnostic HCC imaging involves use of one or more of the following modalities: triphasic helical CT; triphasic dynamic contrast–enhanced MRI; or contrast-enhanced ultrasonography, although the latter modality is not commonly available in the United States.4,32,33 The term triphasic refers to the 3 phases of scanning: an arterial phase, portal venous phase, and the venous phase after a delay.10 The classic imaging profile associated with an HCC lesion is characterized by intense arterial uptake or enhancement followed by contrast washout or hypointensity in the delayed venous phase.4,33,34
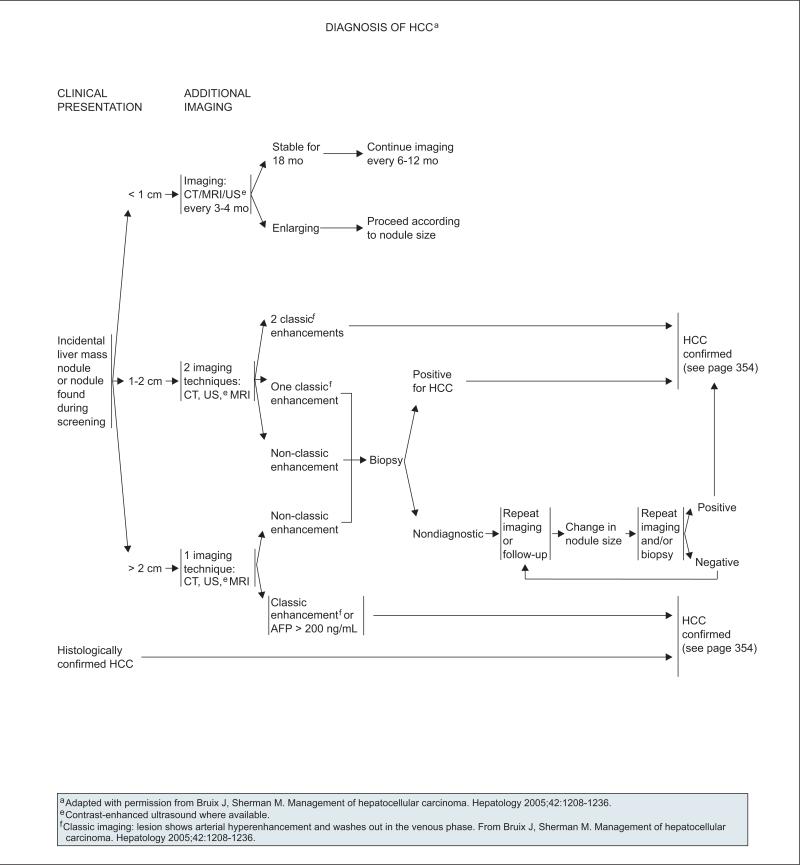
Patients with a liver mass on ultrasound should be evaluated using one or more imaging modalities with the number and type of imaging dependent on the size of the liver mass nodule (see page 353). Evaluation of liver nodules measuring 1 to 2 cm using 2 different imaging techniques from the list above is recommended. A coincidental finding of classic arterial enhancement with both modalities is considered to be diagnostic of HCC, whereas additional confirmation through tissue sampling is recommended when a classic enhancement pattern is not seen or observed with only one imaging modality. Prospective validation of this diagnostic paradigm using contrast-enhanced ultrasonography and diagnostic MRI with biopsy confirmation for patients with a well-defined single, solid nodule between 0.5 and 2.0 cm observed on screening ultrasonography has recently been presented.35 For lesions larger than 2 cm, however, only one imaging modality showing classic arterial enhancement of the lesion is needed to diagnose HCC. Finally, liver lesions smaller than 1 cm should be reevaluated with triphasic CT or MRI or contrast-enhanced ultrasonography every 3 to 4 months, with enlarging lesions evaluated according to size as described on page 353. Patients with lesions stable in size over 18 months should be followed up with imaging every 6 to 12 months.
Biopsy
HCC can be diagnosed noninvasively, in that confirmation with a tissue biopsy may not be required. For example, when evaluating liver nodules measuring 1 to 2 cm, classic arterial enhancement using 2 types of recommended imaging modalities or observed with a single recommended imaging technique for liver lesions larger than 2 cm is sufficient for diagnosing HCC (see page 353). However, a biopsy is recommended in some cases when the diagnosis of HCC is uncertain. For example, a tissue biopsy is recommended when classic arterial enhancement is not observed using any imaging method, or when the liver nodule is in the 1 to 2 cm range and classic arterial enhancement is seen on only one of the imaging tests performed. Nevertheless, use of needle biopsy to diagnose HCC is limited by several factors, including sampling error, particularly when lesions are between 1 and 2 cm.4,10 Patients for whom a non-diagnostic biopsy result is obtained should be followed up closely, and subsequent additional imaging and/or biopsy is recommended if a change in nodule size is observed.
Serum Biomarkers
Serum AFP is not a sensitive or specific diagnostic test for HCC.4,10 However, results of AFP testing can be useful in conjunction with other test results to guide management of patients believed to have HCC. For example, additional imaging studies (i.e., CT/MRI) are recommended for patients with a rising serum AFP level in the absence of a liver mass (see page 352). If no liver mass is detected after measurement of an elevated AFP level, patients should be followed up with AFP testing and liver imaging on a more frequent basis (e.g., every 3 months). In addition, an AFP level greater than 200 ng/mL in conjunction with imaging results showing the presence of a liver mass larger than 2 cm has been shown to have a high positive predictive value for HCC.36,37 Therefore, an AFP level greater than 200 ng/mL or the presence of classic arterial enhancement on triphasic CT or MRI is considered to be diagnostic of HCC when liver lesions are larger than 2 cm in size (see page 353).
Other serum biomarkers being studied for the detection of HCC have been shown to have promising clinical utility.10,38,39 These biomarkers include des-gamma-carboxy prothrombin (DCP), also known as protein induced by vitamin K absence-II (PIVKA-II), and lens culinaris agglutinin-reactive AFP (AFP-L3), an isoform of AFP.
Initial Workup
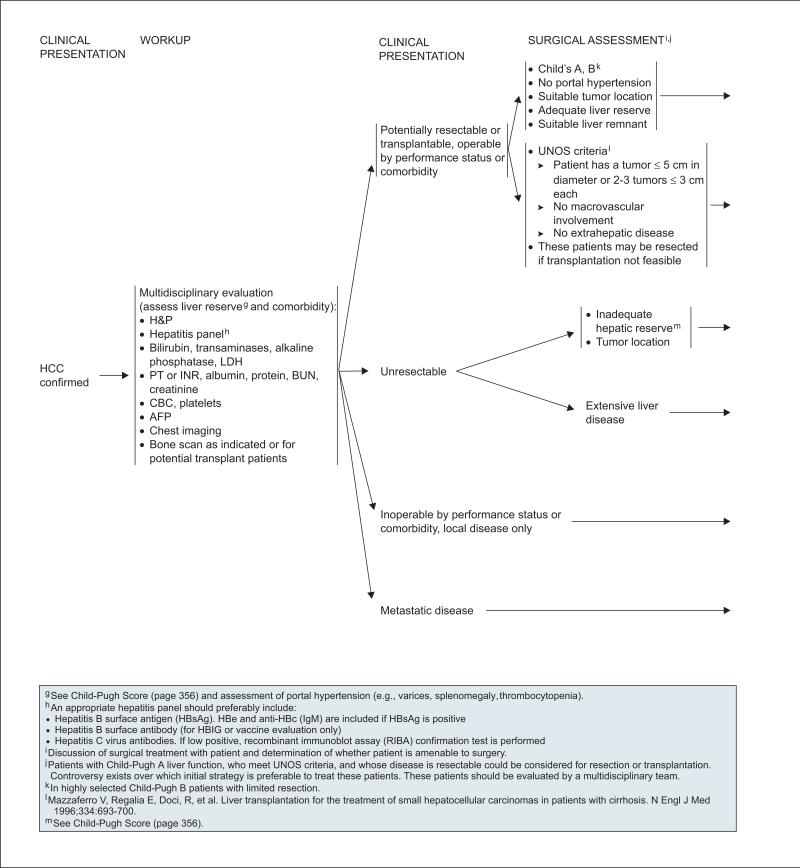
The foundation of the initial workup for HCC is a multidisciplinary evaluation involving investigations into the etiologic origin of liver disease, including a hepatitis panel for detecting HBV and/or HCV infection (see page 354) and an assessment of the presence of comorbidity; imaging studies to detect the presence of metastatic disease; and an evaluation of hepatic function, including a determination of whether portal hypertension is present.
Common sites of HCC metastasis include the lung, abdominal lymph nodes, and bone.40,41 There fore, chest imaging and a bone scan (if suspicious bone pain is present or if the patient is being considered for liver transplantation) are recommended as part of the initial workup. Triphasic CT or MRI results are also used when evaluating the HCC tumor burden; to detect the presence of metastatic disease, nodal disease, and vascular invasion; to assess whether evidence of portal hypertension is present; to provide an estimate of the size and location of HCC and the extent of chronic liver disease; and, for patients being considered for resection, to provide an estimate of the future liver remnant (FLR) in relation to the total liver volume (see section on “Partial Hepatectomy”).33
An initial assessment of hepatic function involves liver function testing, including measurement of serum levels of bilirubin, aspartate transaminase, alanine transaminase, alkaline phosphatase, lactate dehydrogenase, albumin, and protein. Other recommended tests include tests of kidney function (i.e., blood urea nitrogen and creatinine), which are established prognostic markers in patients with liver disease,42 and measurement of prothrombin time (PT)/international normalized ratio (INR) and a CBC (see page 354).
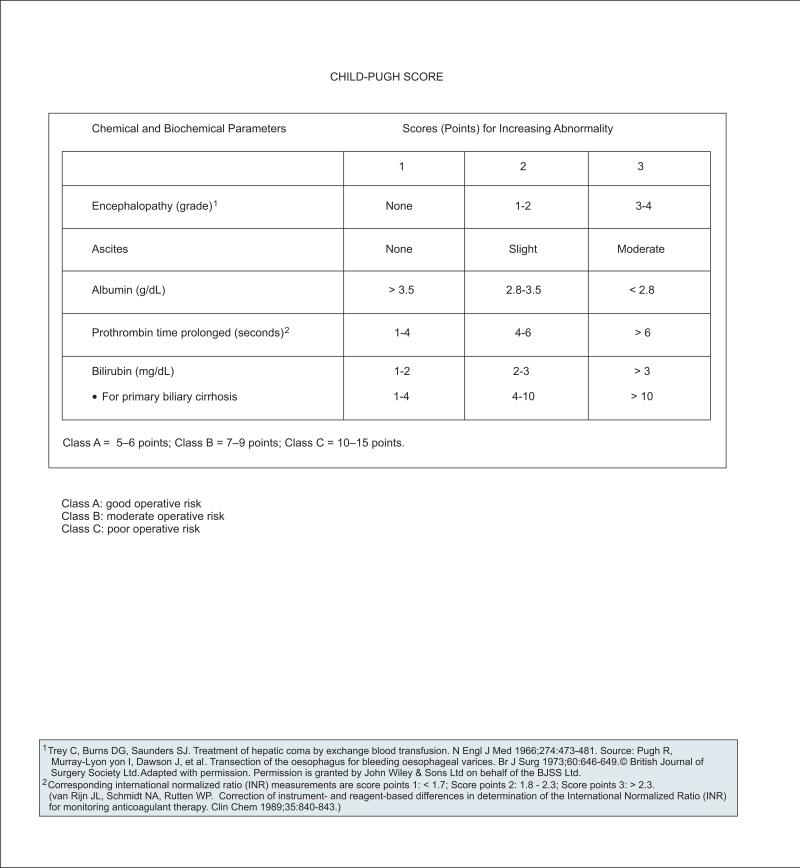
Further assessment of hepatic function or reserve in patients with chronic liver disease has traditionally been performed using the Child-Pugh score, which places patients into 1 of 3 classes (A–C) according to likelihood of survival (see page 356).43,44 The Child-Pugh classification provides a rough estimate of liver function by classifying patients as having compensated (class A) or decompensated (class B and C) cirrhosis. It is an empiric score that incorporates laboratory measurements (i.e., serum albumin, bili-rubin, PT) and more subjective clinical assessments of encephalopathy and ascites. More recently, a version of the Child-Pugh score that includes INR has been used (see page 356).
Advantages of the Child-Pugh score include ease of performance (i.e., can be assessed at bedside) and the inclusion of clinical parameters. An important additional assessment of liver function not included in the Child-Pugh score is an evaluation of signs of clinically significant portal hypertension (e.g., esophago-gastric varices, splenomegaly, abdominal collaterals, thrombocytopenia). Evidence of portal hypertension may also be evident on CT/MRI.33 Measurement of hepatic venous pressure gradient is an evolving tool for the assessment of portal hypertension.45–48
Another system for evaluating hepatic reserve is the Model for End-Stage Liver Disease (MELD) score, which is a numeric scale ranging from 6 (less ill) to 40 (gravely ill) for individuals aged 12 years or older. It is derived from an equation using 3 laboratory values (serum bilirubin, creatinine, and INR) and was originally devised to provide an assessment of mortality for patients undergoing transjugular intrahepatic portosystemic shunts.49 The MELD score has since been adopted by the United Network for Organ Sharing (UNOS) to stratify patients on the liver transplantation waiting list according to their risk for death within 3 months50 (see section on “Liver Transplantation”). The MELD score has more recently sometimes been used instead of the Child-Pugh score to assess prognosis in patients with cirrhosis.
Advantages of the MELD score include the inclusion of a measurement of renal function and an objective scoring system based on widely available laboratory tests, although clinical assessments of as-cites and encephalopathy are not included. It is currently unclear whether the MELD score is superior to the Child-Pugh score as a predictor of survival in patients with liver cirrhosis. The MELD score has not been validated as a predictor of survival in patients with cirrhosis who are not on a liver transplantation waiting list.44
Pathology and Staging
Pathology
Three gross morphologic types of HCC have been identified: nodular, massive, and diffuse.51–53 Nodular HCC is often associated with cirrhosis and is characterized by well-circumscribed nodules. The massive type of HCC, usually associated with a noncirrhotic liver, occupies a large area with or without satellite nodules in the surrounding liver. The less common diffuse type is characterized by diffuse involvement of many small indistinct tumor nodules throughout the liver.
Staging
Clinical staging systems for patients with cancer can provide a more accurate prognostic assessment before and after a particular treatment intervention, and may be used to guide treatment decision-making. Therefore, staging can have a critical impact on treatment outcome by facilitating appropriate patient selection for specific therapeutic interventions, and by providing risk stratification information after treatment.
Four main factors affect prognosis in patients with HCC: 1) stage, aggressiveness, and growth rate of the tumor; 2) general health of the patient; 3) liver function of the patient; and 4) HCC treatments administered.32 Several staging systems for patients with HCC have been devised,54,55 each including variables that evaluate 1 or more of the first 3 factors listed. For example, the Child-Pugh43 and MELD scores56 can be considered staging systems that evaluate aspects of liver function only. The American Joint Committee on Cancer (AJCC) TNM staging system (available online, in these guidelines, at www.nccn.org [ST-1]) provides information on tumor characteristics only,57 whereas the Okuda system incorporates aspects of liver function and tumor characteristics.58 The French classification (GRETCH) system incorporates the Karnofsky performance score and measurements of liver function and serum AFP.59 Several other staging systems include all parameters from other staging systems and additional parameters. For example, the Chinese University Prognostic Index system60 and the Japanese Integrated Staging (JIS)61 scores incorporate the TNM staging system. The Cancer of the Liver Italian Program (CLIP),62 Barcelona Clinic Liver Cancer (BCLC),63 SLiDe,64 and JIS systems include the Child-Pugh score (with modified versions of CLIP and JIS substituting the MELD score for the Child-Pugh score).65–67 In addition, the BCLC system also incorporates the Okuda system and other tumor characteristics, measurements of liver function, and patient performance status.4
Although some systems have been found to have use in all stages of HCC (e.g., BCLC),4,10,68 limitations have been identified in all of them. For example, the AJCC TNM classification system has limited usefulness because most patients with HCC do not undergo surgery. Several studies have shown that particular staging systems perform well for specific patient populations. Furthermore, staging systems may be used to direct treatment and/or predict survival outcomes after a particular type of therapeutic intervention. For example, the AJCC TNM system was recently shown to accurately predict survival for patients who underwent orthotopic liver transplantation.69 The CLIP and GRETCH staging systems have been shown to perform well in predicting morbidity and mortality in patient populations with advanced disease,70 and the CLIP system has been specifically identified as being useful for staging patients who underwent transarterial chemoembolization (TACE)71 and those treated in a palliative setting.72
An advantage of the BCLC system is that it stratifies patients into treatment groups, although the type of treatment is not included as a staging variable.55 Furthermore, it was recently shown to be very useful for predicting outcome in patients after radiofrequency ablation (RFA) therapy.73 A recently developed novel staging system based on a nomogram of particular clinicopathologic variable, including patient age; tumor size and margin status; postoperative blood loss; the presence of satellite lesions and vascular invasion; and serum AFP level, has been shown to perform well in predicting postoperative outcomes for patients undergoing liver re-section for HCC.74
Although a particular staging system (except the Child-Pugh score and TNM system) is not currently used in these guidelines, after an initial workup (see page 354) patients are stratified into those with disease that is 1) potentially resectable or transplantable, and operable according to performance status or comorbidity; 2) unresectable; 3) inoperable by performance status or comorbidity with local disease only; or 4) metastatic disease. The selection characteristics of these patient populations are described in more detail on pages 355, 357, and 358, and in the next section on Management.
Management
Patients with HCC should be carefully evaluated for HCC treatment consideration. It is important to reiterate that the management of patients with HCC is complicated by the presence of underlying liver disease. Furthermore, it is possible that different etiologies of HCC and their effects on the host liver may impact treatment response and outcome.75 The treatment of patients with HCC often necessitates the involvement of hepatologists, cross-sectional radiologists, interventional radiologists, transplant surgeons, pathologists, medical oncologists, and surgical oncologists, thereby requiring careful coordination of care.10
Surgery
Partial Hepatectomy
Partial hepatectomy (i.e., liver resection) is a potentially curative therapy for patients with early-stage HCC who are eligible to undergo the procedure. Partial hepatectomy for selected patients with HCC can now be performed with low operative morbidity and mortality (in the range of ≤ 5%).76,77 Results of large retrospective studies have shown 5-year survival rates of more than 50% for patients undergoing liver resection for HCC,77–79 and some studies suggest that for selected patients with preserved liver function and early-stage HCC, liver resection can achieve a 5-year survival rate of approximately 70%.79–81 However, HCC tumor recurrence rates at 5 years after liver resection have been reported to exceed 70%.4,79
Because risks associated with liver resection for patients with HCC include surgical removal of functional liver parenchyma in the setting of underlying liver disease, careful patient selection, based on patient characteristics and characteristics of the liver and HCC tumors, is essential. Assessments of patient performance status must be considered; the presence of comorbidity has been shown to be an independent predictor of perioperative mortality.82 Likewise, estimates of overall liver function, the size and function of the putative FLR, and technical considerations related to tumor and liver anatomy must be taken into account before determining that patients have potentially resectable disease (see page 355).
Resection is recommended only in the setting of preserved liver function. The Child-Pugh score provides an estimate of liver function, although it was recently suggested to be more useful as a tool to rule out patients for liver resection (i.e., identifying patients with substantially decompensated liver disease).83 An evaluation of the presence of significant portal hypertension is also an important part of the presurgical assessment (see previous section on “Initial Workup”). In general, evidence of optimal liver function in the setting of liver resection is characterized by a Child-Pugh class A score and no evidence of portal hypertension (see page 355). However, in highly selected cases, patients with a Child-Pugh class B score may be considered for limited liver re-section, particularly if liver function tests are normal and clinical signs of portal hypertension are absent.
Regarding tumor characteristics and estimates of the FLR after resection, preoperative imaging is essential for surgical planning.33 CT/MRI can be used to facilitate characterization of the number and size of HCC lesions; detect the presence of satellite nodules, extrahepatic metastasis, and tumor invasion of the portal vein or inferior vena cava; and help establish the location of the tumors with respect to vascular and biliary structures.
Optimal tumor characteristics for liver resection are solitary tumors without major vascular invasion. Although no limitation on tumor size is specified for liver resection, the risk for vascular invasion and dissemination increases with size.76,84 However, one study showed no evidence of vascular invasion in approximately one third of patients with single HCC tumors measuring 10 cm or larger.76 Nevertheless, the presence of macro- or microscopic vascular invasion is considered to be a strong predictor of HCC recurrence.76,85,86 The role of liver resection for patients with multifocal disease and/or signs of major vascular invasion is controversial,85,87 although results of a recent retrospective analysis showed a 5-year overall survival rate of 81% for selected patients with 1 tumor of 5 cm or less, or 3 or fewer tumors of 3 cm or less undergoing liver resection.88 The consensus of the panel is that resection can be considered in selected patients with these disease characteristics. The presence of extrahepatic metastasis is considered to be a contraindication for resection.
Another critical preoperative assessment includes evaluation of the postoperative FLR as an indicator of postoperative liver function. CT is used to measure the FLR directly and calculate estimates of the total liver volume. The ratio of FLR to total liver volume (subtracting tumor volume) is then determined.89 The panel recommends that this ratio be at least 20% in patients without cirrhosis and at least 30% to 40% in those with a Child-Pugh A score.90 For patients with an estimated FLR to total liver volume ratio below recommended values who are otherwise suitable candidates for liver resection, preoperative portal vein embolization should be considered. It is a safe and effective procedure for redirecting blood flow toward the portion of liver that will remain after surgery. Hypertrophy is induced in these segments of the liver while the embolized portion of the liver undergoes atrophy.91
Liver Transplantation
Liver transplantation is an attractive, potentially curative therapeutic option for patients with early HCC. It removes both detectable and undetectable tumor lesions, treats underlying liver cirrhosis, and avoids surgical complications associated with a small FLR. In a landmark study published in 1996, Mazzaferro et al.92 showed that 4-year overall and recurrence-free survival rates of 85% and 92%, respectively, were obtained when liver transplantation was restricted to a subgroup of patients with unresectable HCC meeting specific selection criteria (i.e., Milan criteria). Furthermore, these results have been supported by more recent studies in which patient selection for liver transplantation was based on these criteria.93 These selection criteria were adopted by UNOS (and include radiologic evidence of a single tumor ≤ 5 cm in diameter, or 2–3 tumors ≤ 3 cm in diameter, and no evidence of macrovascular involvement or extrahepatic disease)94 because they identify a subgroup of patients with HCC for whom liver transplantation results are similar to those in patients who underwent liver transplantation for end-stage cirrhosis without HCC.
The UNOS criteria also specify that patients eligible for liver transplantation should not be candidates for liver resection.94 Therefore, liver transplantation generally has been considered the preferred initial treatment for patients with early-stage HCC and moderate to severe cirrhosis (i.e., patients with Child-Pugh B and C scores), with partial hepatectomy generally accepted as the best option for first-line treatment of patients with early-stage HCC and Child-Pugh class A scores when tumor location is amenable to resection. However, because no studies compare the effectiveness of liver resection and transplantation for the latter group of patients, the optimal initial strategy for this population is controversial.95–98 The NCCN panel consensus is that initial treatment with either partial hepatectomy or transplantation can be considered for patients with liver function characterized by a Child-Pugh class A score who fit UNOS criteria. In addition, patients must have operable disease based on performance status and comorbidity (see page 357).99
The MELD score as a measure of liver function (see previous section “Initial Workup”) is also used as a measure of pretransplant mortality. In 2002 it was adopted by UNOS to provide an estimate of risk for death within 3 months for patients on the waiting list for cadaveric liver transplant. According to the current UNOS policy, patients with T2 HCC tumors (defined as 1 nodule measuring 2–5 cm or 2 or 3 nodules all < 3 cm) receive additional 22 priority MELD points (also called a MELD-exception).50 In a retrospective analysis of data provided by UNOS of 15,906 patients undergoing first-time liver transplantation during 1997 to 2002, 4.6% of patients had HCC compared with 26% among 19,404 patients undergoing the procedure during 2002 to 2007, with most patients in the latter group receiving an HCC MELD-exception.100
In 2002 to 2007, patients with an HCC MELD-exception had similar survival to patients without HCC. Important predictors of poor posttransplantation survival for patients with HCC were MELD score 20 or greater and serum AFP level 455 ng/mL or greater,100 although the reliability of the MELD score as a measure of posttransplantation mortality is controversial. Survival was also significantly lower for the subgroup of patients with HCC tumors ranging from 3 to 5 cm.
Expansion of the Milan/UNOS criteria to provide patients who have marginally larger HCC tumors with liver transplant eligibility is an active area of debate.4,93,101,102 An expanded set of criteria, including patients with a single HCC tumor 6.5 cm or smaller with a maximum of 3 total tumors with no tumor larger than 4.5 cm (and cumulative tumor size < 8 cm) as liver transplant candidates, has been proposed by a group at the University of California at San Francisco (UCSF).103
Studies evaluating the posttransplantation survival of patients who exceed the Milan criteria but meet the UCSF criteria show wide variation in 5-year survival rates (range, 38%–93%).101,102,104–106 An argument favoring expanding the Milan/UNOS criteria includes the general recognition that many patients with HCC tumors exceeding the Milan criteria can be cured with liver transplantation.102 Opponents of expanding the Milan/UNOS criteria cite the increased risk for vascular invasion and tumor recurrence associated with larger tumors and higher HCC stage, and the shortage of donor organs.93,101,104 Some support for the former objection comes from a large retrospective analysis of the UNOS database showing significantly lower survival for patients with tumors measuring 3 to 5 cm than for those with smaller tumors.100
Bridge Therapy
Several studies have investigated the role of locoregional treatment of HCC as a bridge to liver transplantation in patients undergoing evaluation for this procedure.4,107 These studies include use of RFA,108,109 chemoembolization,110 and radioembolization as bridge therapies.111
Locoregional Therapy
Local approaches to the treatment of HCC are directed toward inducing selective tumor necrosis and involve either ablation or embolization. The effectiveness of locoregional approaches in treating HCC has not been established as comparable to that of liver resection or transplantation.83,112 The consensus of the panel is that these methods should not be used in place of liver resection or transplantation for patients who meet surgical selection criteria (see pages 355 and 358).
Ablation
HCC tumor necrosis can be induced through direct exposure of the tumor to a particular chemical substance (e.g., ethanol, acetic acid) or an alteration in temperature (e.g., RFA, microwave ablation, cryoablation).29 Any ablative therapy can be performed using laparoscopic, percutaneous, or open approaches. The 2 most commonly used methods of ablation therapy are RFA and percutaneous ethanol injection (PEI) therapy. Patients meeting selection criteria for ablative therapy include those with local disease only characterized as being completely amenable to ablative therapy according to size and location of the tumors. The complication rate associated with ablative therapy in the treatment of HCC has been reported to be relatively low. For example, a randomized controlled trial comparing treatment with RFA or PEI showed major complication and mortality rates of 4.8% and 0%, respectively.113
The extent of tumor necrosis induced by ablative therapy is typically approximated by dynamic CT/MRI at a specified time after treatment (as opposed to a histologic assessment).10,114 The absence of contrast uptake within the tumor compared with imaging findings before treatment is interpreted as indicative of no residual vascularity and complete tumor necrosis.
Studies have shown that ablative therapy is most effective on smaller HCC tumors.108,109,115,116 Panel consensus is that ablation therapy alone for treating HCC is optimal when tumors are 3 cm or less, and that lesions between 3 and 5 cm may be treated using a combination of ablation and embolization methods (see sections on “Bland Embolization and Chemoembolization” and “Combinations of Local Therapies”). Furthermore, the panel considers percutaneous ablation a very good option for well-selected patients with small tumors who are not candidates for surgery.
In a retrospective analysis, 40 mostly Child-Pugh class A or B patients with HCC liver nodules were treated with RFA, PEI, or a combination while awaiting liver transplantation. The results of this study showed complete and partial necrosis rates of 46.7% and 53.3%, respectively, when RFA therapy was used, and 23.1% and 46.1%, respectively, after PEI therapy, with 30.8% of tumors showing no evidence of necrosis with PEI therapy. The overall rate of complete necrosis was 53.1% for HCC tumors smaller than 3 cm and 14.3% for tumors 3 cm or larger (P = .033). However, this rate increased to 61.9% for the subset of tumors smaller than 3 cm treated with RFA.108
The study by Mazzaferro et al.109 also shows that tumor size is a critical factor in determining the effectiveness of ablation therapy in treating HCC. In this prospective study of 50 consecutive patients with liver cirrhosis undergoing RFA while awaiting liver transplantation, the rate of complete tumor necrosis was 55% overall and 63% when only tumors 3 cm or smaller were considered.
Several randomized controlled trials have also compared the effectiveness of RFA and PEI therapy in the treatment of patients with HCC and Child-Pugh class A cirrhosis.117–119 RFA was shown to be superior to PEI with respect to complete response rate (65.7% for RFA vs. 36.2% for PEI; P = .0005)118 and rate of local recurrence.117,119 In addition, one study showed that patients in the RFA arm required fewer treatment sessions.119 However, 2 of these studies showed the benefit of RFA on overall survival compared with PEI,117,119 but a third showed no significant overall survival differences between the treatment arms.118 RFA has also been compared with liver resection in a prospective randomized controlled study.114 No differences in recurrence-free or overall survival were found when treatment arms were compared.
A wide range of local recurrence rates after ablative therapy for HCC have been reported that may reflect differences in patient selection criteria and treatment protocols. For example, Shiina et al.119 estimated 4-year recurrence rates were 70% and 85% in the RFA and PEI arms, respectively, for patients with 3 or fewer small tumors (≤ 3 cm). However, another study found that fewer than 3% of patients with single HCC tumors measuring 2 cm or less who underwent repeated applications of RFA therapy experienced disease recurrence at 31 months.116
Results of some long-term studies show survival rates of more than 50% at 5 years for patients with successful HCC tumor necrosis after ablative therapy.120,121 Nevertheless, reported rates of overall survival vary widely across studies of patients with HCC treated with ablation.114,117,119,121,122 This variation is likely to reflect differences in specific disease characteristics (e.g., size and number of tumors) and, perhaps more importantly, the extent of underlying liver function in the patient populations studied.121,122
Regarding tumor location, lesions in certain portions of the liver (e.g., dome) may not be accessible to a percutaneous approach, ablative treatment of tumors associated with the liver capsule may cause organ rupture, and major vessels in proximity to the tumor can absorb large amounts of heat when techniques such as RFA are performed.10 The panel emphasizes that caution should be exercised when ablating lesions near major blood vessels, major bile ducts, and other intra-abdominal organs (see page 358).
Embolization
Arterial embolization therapy (chemoembolization, bland embolization, radioembolization) in the treatment of HCC is based on selective catheter-based infusion of particles targeted to the arterial branch of the hepatic artery feeding the portion of the liver where the tumor is located. Embolization therapy is made possible by the dual blood supply to the liver; although most of the blood supply to normal liver tissue comes from the portal vein, blood flow to liver tumors is mainly from the hepatic artery.31 Furthermore, HCC tumors are characterized by hypervascularity, resulting in increased blood flow to the tumor relative to normal liver tissue.
Before embolization, a careful evaluation of the arterial anatomy of the liver is necessary. Because non-target embolization of the liver can result in serious injury, arterial embolization is limited to a segment, subsegment, or lobe of the liver. All HCC tumors, irrespective of location, may be amenable to embolization therapy if the arterial blood supply to the tumor can be isolated.123–126 Tumor necrosis induced by ablative therapy is typically estimated according to the extent to which contrast uptake on dynamic CT/MRI is diminished at some specified point after treatment when compared with pretreatment imaging findings.
General patient selection criteria for embolization procedures include unresectable/inoperable disease with tumors not amenable to ablation therapy only, and the absence of extrahepatic disease (see pages 355 and 358). Performance status and liver function (i.e., Child-Pugh score) should also be evaluated. In addition, more individualized patient selection that is specific to the particular embolization procedure being considered is necessary to avoid significant treatment-related toxicity (see following sections on “Bland Embolization and Chemoembolization” and “Radioembolization”).
The panel recommends that patients with unresectable/inoperable disease who are eligible to undergo embolization therapy and have tumor lesions larger than 5 cm be treated using arterial embolic approaches. Patients with lesions measuring 3 to 5 cm can be considered for combination therapy with ablation and arterial embolization (see page 358 and section on “Combinations of Local Therapies”).
Bland Embolization and Chemoembolization
The principle of bland embolization, also called transarterial embolization (TAE) and TACE, is a reduction in blood flow to the tumor resulting in tumor ischemia followed by tumor necrosis. Gelatin sponge particles, polyvinyl alcohol particles, and polyacryl-amide microspheres have been used to block arterial flow.124,127,128 TACE is distinguished from TAE by the catheter-based administration of a concentrated dose of chemotherapy (e.g., doxorubicin, cisplatin) combined with an emulsifying agent, usually administered before the embolic particles.127 Results of 2 randomized clinical trials have shown a survival benefit associated with TACE therapy versus supportive care in patients with unresectable HCC.126,129 One study thatrandomly assigned patients to TAE, TACE, and supportive care treatment arms126 showed 1- and 2-year survival rates of 82%, and 63%, 75% and 50%, and 63% and 27%, respectively. Most patients in the study had liver function classified as Child-Pugh A, performance status of 0, and main tumor nodule size of approximately 5 cm. The group of evaluable patients undergoing either TACE or TAE therapy showed approximately 30% and 1% partial and complete response rates sustained for at least 6 months, respectively. Limitations of this study include its early termination and lack of power to detect a difference between TACE and TAE treatment arms.124
Many clinical studies evaluating the effectiveness of TAE and/or TACE therapies in the treatment of patients with HCC are confounded by use of a wide range of treatment strategies, including types of embolic particles, chemotherapy, and emulsifying agent (for studies involving TACE), and number of treatment sessions.124,128 A recent retrospective analysis of patients undergoing TAE therapy for treatment of HCC in which a standardized technique was used showed 1-, 2-, and 3-year overall survival rates of 66%, 46%, and 33%, respectively. These rates increased to 84%, 66%, and 51%, respectively, when only the subgroup of patients without extra-hepatic spread or portal vein involvement by tumor was considered.124
In the study by Maluccio et al.,124 predictors of poor prognosis on multivariate analysis after TAE therapy were tumor size of 5 cm or greater, 5 or more tumors, and extrahepatic disease; portal vein occlusion was not found to be an independent predictor of survival.124 However, evidence shows that portal vein obstruction,130,131 liver function categorized as Child-Pugh class C,131 and a total serum bilirubin level of greater than 3 mg/mL132 are significant predictors of poor prognosis in patients treated with TACE therapy. Hence, the panel considers main portal vein thrombosis to be a contraindication for TACE therapy, and recommends against its use in patients with liver function characterized as Child-Pugh class C. Because TAE therapy can increase the risk for hepatic necrosis and liver abscess formation in patients with biliary obstruction,127 the panel recommends that a total bilirubin level greater than 3 mg/mL be considered a relative contraindication for TACE or TAE therapy unless segmental injections can be performed (see page 358). Furthermore, patients with previous biliary-enteric bypass have an increased risk for intrahepatic abscess after TACE therapy.127
Complications of TAE and TACE therapy can include acute portal vein thrombosis, cholecystitis, and bone marrow suppression, in addition to other toxicities,29,133 although the reported frequencies of serious adverse events vary across studies. A postembolization syndrome involving fever, abdominal pain, and intestinal ileus has been reported to be relatively common.29,133 Reported rates of TAE and TACE treatment–associated mortality are usually less than 5%.29,124,126,133
Radioembolization
Radioembolization is a newer embolization method that provides for the internal delivery of high-dose radiation to the tumor-associated capillary bed.134 This is accomplished through the catheter-based administration of microspheres in which yttrium-90, an emitter of beta radiation, is embedded. This method allows for limited penetration of radiation, thereby sparing the normal liver tissue. The microspheres are available in 2 formulations: TheraSpheres (glass microspheres) and SIR-Spheres (resin microspheres). Although radioembolization, like TAE and TACE, involves some level of particle-induced vascular occlusion, experts have proposed that this occlusion is more likely to be microvascular than macrovascular, and that the resulting tumor necrosis is more likely to be induced by radiation than ischemia.125
A partial response rate of 42.2% was observed in phase II study of 108 patients with unresectable HCC with and without portal vein thrombosis treated with radioembolization therapy and followed up for up to 6 months. Grade 3/4 adverse events were more common in patients with main portal vein thrombosis. However, patients with branch portal vein thrombosis experienced a similar frequency of adverse events related to elevated bilirubin levels as those without portal vein thrombosis. Reported complications of radioembolization therapy include cholecystitis and abscess formation.125,135 Randomized controlled studies of the use of radioembolization therapy in the treatment of patients with HCC are needed.
Combinations of Local Therapies
Recently, several studies have evaluated the effectiveness of using a combination of local therapies in the treatment of patients with unresectable/inoperable HCC. For example, the principle behind combination RFA and TAE is that the focused heat delivery of RFA may be enhanced by vessel occlusion through TAE because blood circulation inside the tumor may interfere with the transfer of heat to the tumor.
A retrospective review of selected patients with a single HCC tumor up to 7 cm treated with either combination TAE and ablation or liver resection showed 1-, 3-, and 5-year actuarial survival rates of 97%, 77%, and 56%, respectively, for patients undergoing combination therapy, and 81%, 70%, and 58%, respectively, for patients undergoing surgery.123 In another study of similar design, the 1-, 3-, and 5-year survival rates of patients with tumors meeting UNOS criteria in terms of number and size were 98%, 94%, and 75%, respectively, for the combina tion group, and 97%, 93%, and 81%, respectively, for the surgery group.88
The panel consensus is that patients with HCC tumors measuring 3 to 5 cm who are not eligible for liver resection or transplantation may be treated with a combination of RFA and embolization.
Conformal or Stereotactic Radiation Therapy
External-beam radiotherapy (3-dimensional conformal or stereotactic) allows focal administration of high-dose radiation to HCC tumors while sparing surrounding liver tissue, thereby limiting the risk for radiation-induced liver damage in patients with unresectable/inoperable liver disease.136
Conformal or stereotactic radiation therapy is listed as an option for patients with unresectable disease characterized as extensive or otherwise not suitable for liver transplantation, and those with local disease only who are not operable because of performance status or comorbidity (see page 355). It is not included in the guidelines as an option for patients with metastatic disease (see page 355).
Systemic Therapy
Most patients diagnosed with HCC have advanced disease, and many are not eligible for potentially curative therapies. Furthermore, with the wide range of ablative and embolization techniques available to treat patients with unresectable HCC confined to the liver, often only patients with very advanced disease are referred for systemic therapy.
Clinical studies evaluating the use of chemo-therapy (e.g., doxorubicin) in the treatment of patients with advanced HCC have typically reported low response rates to therapy, and evidence for a favorable impact of chemotherapy on overall survival in patients with HCC is lacking.137–139 The panel recommends that systemic single-agent or combination chemotherapy, intra-arterial chemotherapy, and combination chemotherapy and radiation therapy be given to patients with unresectable HCC only in the context of a clinical trial (see page 355).
Sorafenib, an oral multikinase inhibitor that suppresses tumor cell proliferation and angiogenesis, has been evaluated in 1 phase II trial and 2 randomized, placebo-controlled phase III trials for the treatment of patients with advanced or metastatic HCC.75,107,128,140–142
In the phase III Sorafenib in Advanced Hepatocellular Carcinoma (SHARP) trial, 602 patients with advanced HCC were randomly assigned to sorafenib or best supportive care. In this study, advanced HCC was defined as patients not eligible for or those who experienced disease progression after surgical or locoregional therapies.140 Approximately 70% of patients in the study had macroscopic vascular invasion, extrahepatic spread, or both. Nevertheless, most of the patients had preserved liver function (i.e., ≥ 95% classified as Child-Pugh A) and good performance status (i.e., > 90% had ECOG performance status of 0 or 1) to limit confounding causes of death. Disease etiology for the enrolled patients was varied, with HCV, alcohol, and HBV determined to be the cause of HCC in 29%, 26%, and 19% of patients, respectively. Median overall survival was significantly longer in the sorafenib arm (10.7 vs. 7.9 months in the placebo group; HR, 0.69; 95% CI, 0.55–0.87; P < .001).
The Asia-Pacific study, another phase III trial with a similar design to the SHARP study, randomly assigned 226 patients to sorafenib (n = 150) or placebo (n = 76) arms.141 Although inclusion/exclusion criteria were similar in the Asia-Pacific and SHARP trials, as was the percentage of patients with Child-Pugh A liver function (97%), significant differences in patient and disease characteristics were seen between the trials. Only Asian patients were enrolled in the Asia-Pacific study and they were more likely to be younger and have HBV–related disease (i.e., > 70%), symptomatic disease, and a higher number of tumor sites than patients in the SHARP study. The HR for the sorafenib arm compared with the placebo arm (HR, 0.68; 95% CI, 0.50–0.93; P = .014) was nearly identical to that reported in the SHARP study, although median overall survival was lower in both treatment and placebo groups in the Asia-Pacific study (6.5 vs. 4.2 months).
Using data from the SHARP trial, several analyses have been performed to investigate the efficacy of sorafenib in particular patient subgroups. Results of these analyses suggest that sorafenib has a survival benefit in patients with ECOG performance status of 1 to 2,143 and those with alcohol-related144 and HCV– related HCC.145 Sorafenib was well tolerated in both randomized clinical trials. Adverse sorafenib-related events in the SHARP trial included diarrhea, weight loss, and hand-foot skin reaction.140
Data on the efficacy of sorafenib in patients with Child-Pugh class B liver function are limited because almost all patients in the randomized trials were characterized as having preserved liver function (Child-Pugh class A). However, approximately 28% of the 137 patients enrolled in a phase II trial evaluating sorafenib in the treatment of HCC had Child-Pugh class B liver function.142 A subgroup analysis of data from this study showed lower overall survival for patients in the Child-Pugh class B group compared with those in Child-Pugh class A (14 vs. 41 weeks).146 In addition, liver function impairment may impact sorafenib dosing and toxicity. Abou-Alfa et al.146 found higher levels of hyperbilirubinemia, encephalopathy, and ascites in the group with Child-Pugh class B, although separating the extent to which treatment drug and underlying liver function contributed to these disease manifestations is difficult.146 A pharmacokinetic phase I study of sorafenib in patients with hepatic and renal dysfunction showed an association between elevated bilirubin levels and possible hepatic toxicity.147 Furthermore, a grade 3/4 toxicity rate of 34% was seen in a study of sorafenib in a poor-risk patient population characterized by Child-Pugh class B or higher liver function, and extensive portal vein thrombosis in 26% and 50% of patients, respectively.148
Based on the results of these trials, sorafenib is recommended as a category 1 option for selected patients with Child-Pugh class A or B with disease characterized as unresectable and extensive/not suitable for liver transplantation; local disease only in patients who are not operable because of performance status or comorbidity; or metastatic. Nevertheless, the panel considers the data on safety and dosing of sorafenib to be inadequate in patients with liver function characterized as Child-Pugh class B, and recommends extreme caution when considering use of sorafenib in patients with elevated bilirubin levels (see page 355).
Best Supportive Care
The panel recommends that best supportive care measures be administered to patients with unresectable/inoperable disease who are not candidates for other therapies (see page 355).
Surveillance
Although data on the role of surveillance in patients with resected HCC are very limited, recommendations are based on the consensus that earlier identification of disease may facilitate patient eligibility for investigational studies or other forms of treatment. The panel recommends high-quality cross-sectional imaging every 3 to 6 months for 2 years, then annually. AFP levels, if initially elevated, should be measured every 3 months for 2 years, then every 6 months. Re-evaluation according to the initial workup should be considered in the event of disease progression (see page 355).
Gallbladder Cancer
Risk Factors
Risk factors for gallbladder cancer, of which cholelithiasis is the most prevalent, are associated with the presence of chronic inflammation. Calcification of the gallbladder (porcelain gallbladder), a result of chronic inflammation of the gallbladder, has also been associated with gallbladder cancer.149
Diagnosis and Initial Workup
Gallbladder cancer is often diagnosed at an advanced stage because of the aggressive nature of the tumor, which can spread rapidly. Another factor contributing to late diagnosis of gallbladder cancer is a clinical presentation that mimics that of biliary colic or chronic cholecystitis.149 Hence, it is not uncommon for a diagnosis of gallbladder cancer to be an incidental finding at surgery or on pathologic review after cholecystectomy for symptomatic cholelithiasis. (See section on “Management of Gallbladder Cancer” for recommendations on surgical assessment and postoperative workup of patients diagnosed at or after surgery.)
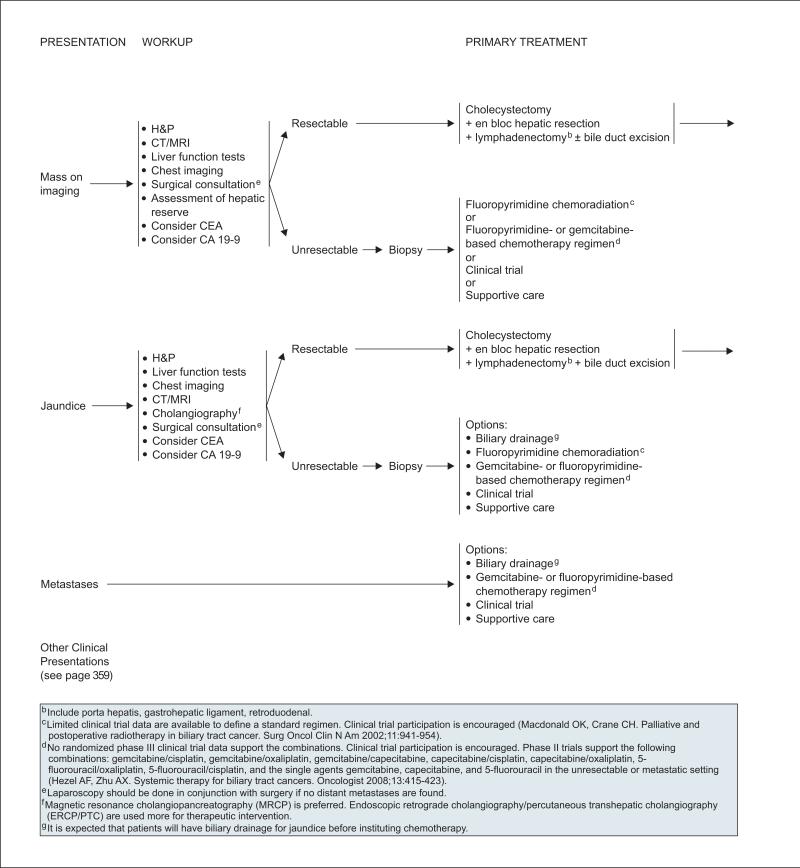
Other possible clinical presentations of gallbladder cancer include a suspicious mass detected on ultrasound, or jaundice. Initial workup of these patients should include liver function tests and an assessment of hepatic reserve. CEA and CA 19-9 testing can be considered, although these markers are not specific for gallbladder cancer.149 High-quality imaging is recommended to evaluate tumor penetration within the wall of the gallbladder, detect direct tumor invasion of other organs/biliary system, determine whether major vascular invasion is present, and evaluate for the presence of nodal and distant metastases.33 In addition, patients should undergo chest imaging and laparoscopy should be performed in conjunction with surgery if no distant metastasis is found. For patients presenting with jaundice, additional workup should include cholangiography to evaluate for hepatic and biliary invasion of tumor.33 Noninvasive MR cholangiography (MRCP) is preferred over endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC), unless a therapeutic intervention is planned. Although the role of PET scanning has not been established in the evaluation of patients with gallbladder cancer, emerging evidence indicates that it is useful for detecting the presence of distant metastatic disease in patients with otherwise potentially resectable disease.150,151
Pathology and Staging
Approximately 80% of gallbladder cancers are adenocarcinomas.149,152 Gallbladder cancer is often characterized by early spread to lymph tissue and the bloodstream.149,153
The AJCC TNM staging criteria for gallbladder cancer is available online, in these guidelines, at www.nccn.org (ST-2). A review of approximately 2500 patients with gallbladder cancer from hospital cancer registries throughout the United States showed tumor stage to be closely associated with survival; 5-year survival rates were 60%, 39%, 15%, 5%, and 1% for patients with stage 0 through IV disease, respectively.154 Results from a recent retrospective single-center analysis showed a 10.3 month median survival for the overall population of patients diagnosed with gallbladder cancer.152 Median survival was 12.0 and 5.8 months for those with stage Ia through III and stage IV disease, respectively.152
Management of Gallbladder Cancer
Surgery remains the only curative modality for gallbladder cancer. In a retrospective review covering 1995 to 2005, 123 of 435 patients treated for gallbladder cancer at a single center underwent curative resection, and 47% were diagnosed with gallbladder cancer as an incidental finding during laparoscopic cholecystectomy.152
Although initial management of patients found to have gallbladder cancer at cholecystectomy or on pathologic review after cholecystectomy differs from that of those diagnosed with gallbladder cancer before surgery (see later discussion), the surgical approach for patients found to have resectable gallbladder cancer is the same, provided the gallbladder was not removed. In all cases, surgery to treat gallbladder cancer should be performed by a surgeon prepared to do a cancer operation.
Factors determining gallbladder tumor resect-ability include the stage of the tumor according to AJCC TNM staging criteria (available online, in these guidelines, at www.nccn.org [ST-2])and tumor location.149 Staging laparoscopy has a high yield and is recommended before laparotomy for a potentially curative resection of gallbladder cancer.
An analysis of prospective data collected on 104 patients undergoing surgery for gallbladder cancer from 1990 to 2002 showed that, although major hepatectomy and common bile duct excision significantly increased the surgical complication rate, they were not independently associated with survival, leading the authors to conclude that these procedures should be performed only when necessary to remove disease.155 The panel recommends that patients considered to have resectable gallbladder cancer undergo treatment with cholecystectomy, en bloc hepatic resection, and lymphadenectomy with or without bile duct excision.149,156 Lymphadenectomy should include lymph nodes in the porta hepatis, gastrohepatic ligament, and retroduodenal regions. Nodal disease outside of this area (most commonly celiac, retropancreatic or in the interaortocaval groove) should be considered unresectable.
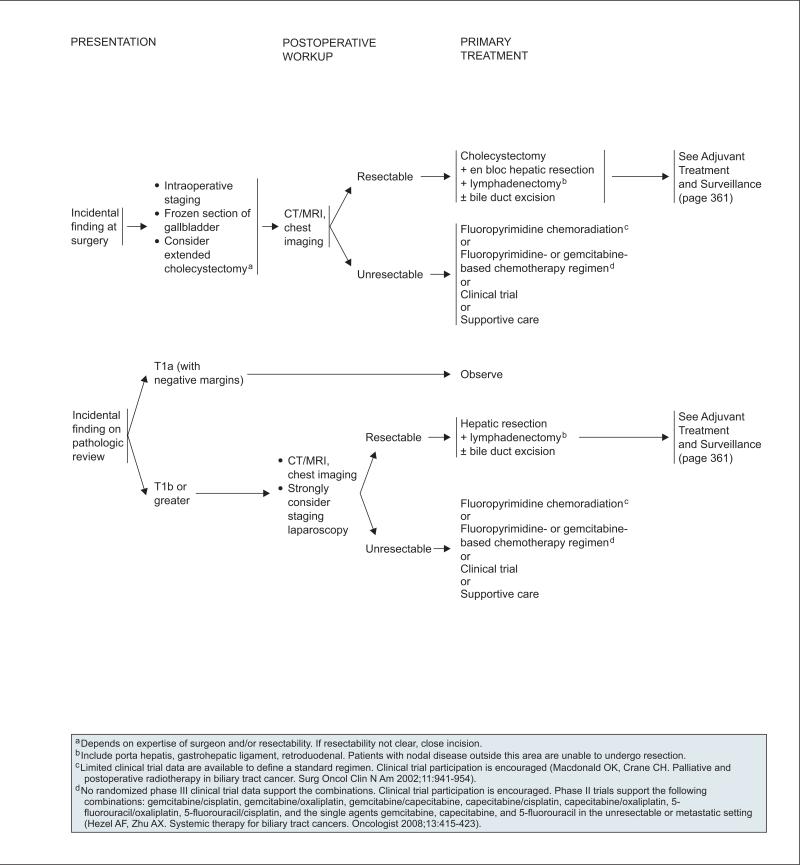
In a retrospective analysis of patients with gall-bladder cancer treated at a single institution, 74% who underwent surgical re-exploration because of an incidental diagnosis of gallbladder cancer after laparoscopic cholecystectomy were found to have residual cancer.152 If gallbladder cancer is found at surgery, the panel recommends intraoperative staging and procurement of a frozen section of gallbladder. An extended cholecystectomy can be considered depending on the expertise of the surgeon and the establishment of disease resectability. Among patients with an incidental finding of gallbladder cancer on pathologic review, those with T1a lesions may be observed if the tumor margins are negative. For patients with T1b or greater lesions, surgery is recommended for resectable lesions after CT/MRI, chest imaging, and laparoscopy confirm the absence of metastatic disease. If the lesions are resectable, patients should undergo hepatic resection and lymphadenectomy with or without bile duct excision.157
Panel consensus is that surgery should not be performed when disease resectability has not been established nor should it be performed by surgeons untrained in this operation (see page 359). Although the optimal treatment strategy for patients with resected gallbladder cancer has not been determined, options include consideration of fluoropyrimidine chemoradiation (except T1b, N0) and fluoropyrimidine or gemcitabine chemotherapy (see page 361 and later section on “Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
For patients with unresectable disease after pre-operative evaluation, the diagnosis should be confirmed with biopsy. In patients with unresectable or metastatic gallbladder cancer and jaundice, biliary drainage is an appropriate palliative procedure and should be performed before instituting chemotherapy if technically feasible (see page 360). Biliary drainage followed by chemotherapy can result in improved quality of life.158 Other options for these patients include chemoradiation (in patients with localized disease) and chemotherapy (see section on “Chemo-radiation and Chemotherapy for Treatment of Gall-bladder Cancer and Cholangiocarcinoma), participation in a clinical trial, and best supportive care.
Surveillance
No data support aggressive surveillance after resection of gallbladder cancer; determination of an appropriate follow-up schedule/imaging should include a careful patient/physician discussion. The panel recommends follow-up of patients undergoing an extended cholecystectomy for gallbladder cancer include consideration of imaging studies every 6 months for 2 years. Reevaluation according to the initial workup should be considered in the event of disease progression (see page 361).
Cholangiocarcinomas
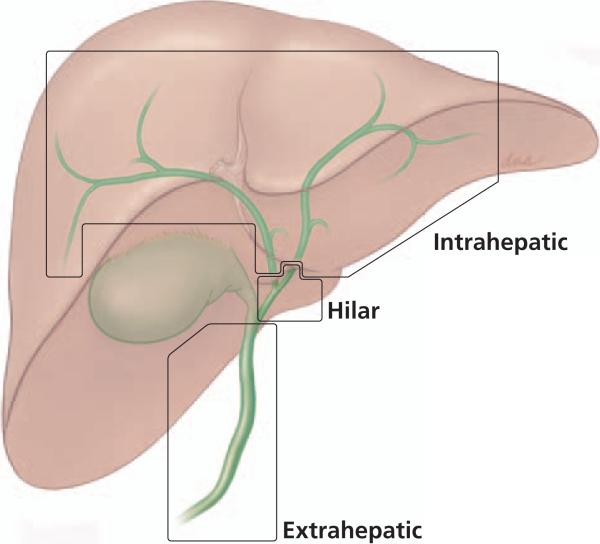
The term cholangiocarcinoma encompasses all tumors originating in the epithelium of the bile duct.159,160 Although cholangiocarcinomas are diagnosed throughout the biliary tree, they are distinguished by anatomic site and typically classified as either intrahepatic or extrahepatic cholangiocarcinoma. Intrahepatic cholangiocarcinomas have also been called peripheral cholangiocarcinomas and are located within the hepatic parenchyma (see Figure 1). In these guidelines, extrahepatic cholangiocarcinomas include hilar cholangiocarcinomas (also called Klatskin tumors), which occur at or near the junction of the right and left hepatic ducts. Therefore, cholangiocarcinomas occurring anywhere within the common hepatic duct, region of the junction of the right and left hepatic ducts, or common bile duct (including the intrapancreatic portion of the common bile duct) are classified as extrahepatic (see Figure 1). Extrahepatic cholangiocarcinomas are more common than intrahepatic cholangiocarcinomas, and hilar cholangiocarcinoma is the most common type of extrahepatic choloangiocarcinoma.161
Figure 1.
Classification of cholangiocarcinoma.
Risk Factors
No predisposing factors have been identified in most patients diagnosed with cholangiocarcinoma,162 al though evidence shows that particular risk factors may be associated with the disease in some patients. These risk factors, like those for gallbladder cancer, are associated with the presence of chronic inflammation and include chronic calculi of the bile duct, choledochal cysts, and liver fluke infections.160,163 Unlike gallbladder cancer, however, cholelithiasis is not believed to be closely linked with the etiology of cholangiocarcinoma.149 Recently, however, intrahepatic cholangiocarcinoma has been associated with HCV infection,164 and this may be responsible for the increased incidence of intrahepatic cholangio-carcinoma observed at some centers.165
Diagnosis and Initial Workup
Early-stage cholangiocarcinomas are typically asymptomatic. Patients with intrahepatic cholangiocarcinoma are more likely to present with nonspecific symptoms such as fever, weight loss, and/or abdominal pain; symptoms of biliary obstruction are uncommon. Alternatively, intrahepatic cholangio-carcinoma may be detected incidentally as an isolated intrahepatic mass on imaging.33 In contrast, the patient with extrahepatic cholangiocarcinoma is likely to present with jaundice followed by evidence of a biliary obstruction or abnormality on subsequent imaging.149
The initial workup of these patients should include liver function tests. CEA and CA 19-9 testing can be considered, although these markers are not specific for cholangiocarcinoma.149 Early surgical consultation with a multidisciplinary team is recommended as part of the initial workup for assessing resectability in both types of cholangiocarcinomas (see section on “Management of Cholangiocarcinoma”).
Delayed-contrast CT/MRI is recommended as part of the workup of patients with intrahepatic cholangiocarcinoma. Although no pathognomonic CT/MRI features are associated with it, CT/MRI is used to help determine tumor resectability by characterizing the primary tumor, its relationship to nearby major vessels and the biliary tree, the presence of satellite lesions and distant metastases in the liver, and any lymph node involvement.33 In addition, patients should undergo chest imaging, and laparoscopy may be performed in conjunction with surgery if no distant metastasis is found. The panel emphasized that a multidisciplinary review of imaging studies involving experienced radiologists and surgeons is necessary to stage the disease and determine potential treatment options (i.e., resection or other approach).
Delayed-contrast CT/MRI to assess disease involvement of the liver, major vessels, nearby lymph nodes, and distant sites is also recommended in the initial workup of patients for whom there is a suspicion of extrahepatic cholangiocarcinoma.33 Since many of these patients present with jaundice, additional workup should include cholangiography to evaluate for hepatic and biliary invasion of tumor.33 Because MRCP is noninvasive, it is considered a safer alternative to direct cholangiography, and therefore is preferred over ERCP or PTC unless a therapeutic intervention is planned. Although the role of PET scanning has not been established in the evaluation of patients with cholangiocarcinoma, emerging evidence indicates that it is useful for detecting the presence of lymph node involvement and distant metastatic disease in patients with otherwise potentially resectable disease.150,151,166,167
Pathology and Staging
More than 90% of cholangiocarcinomas are adenocarcinomas.168 Cholangiocarcinomas can be divided into 3 types depending on macroscopic appearance: mass-forming, periductal, and intraductal.159,169
The AJCC has developed staging systems for cholangiocarcinomas (see staging tables, available online, in these guidelines, at www.nccn.org [ST-1 and ST-2]), and the AJCC staging system for intrahepatic cholangiocarcinoma is the same one used for HCC staging (see ST-1 online, in these guidelines, at www.nccn.org). However, this staging system does not include predictive clinicopathologic features that are specific to intrahepatic cholangiocarcinoma.33 Other more practical staging systems for intrahepatic cholangiocarcinoma have been used.170,171 The AJCC staging system for extrahepatic cholangiocarcinoma (available online, in these guidelines, at www.nccn.org [ST-3]) is based on pathologic criteria but is not useful for determining resectability or predicting outcome.33 Jarnagin et al.172 developed a useful preoperative staging system for hilar cholangiocarcinoma that predicts resectability, likelihood of metastatic disease, and survival.
Management of Cholangiocarcinoma
Intrahepatic Cholangiocarcinoma
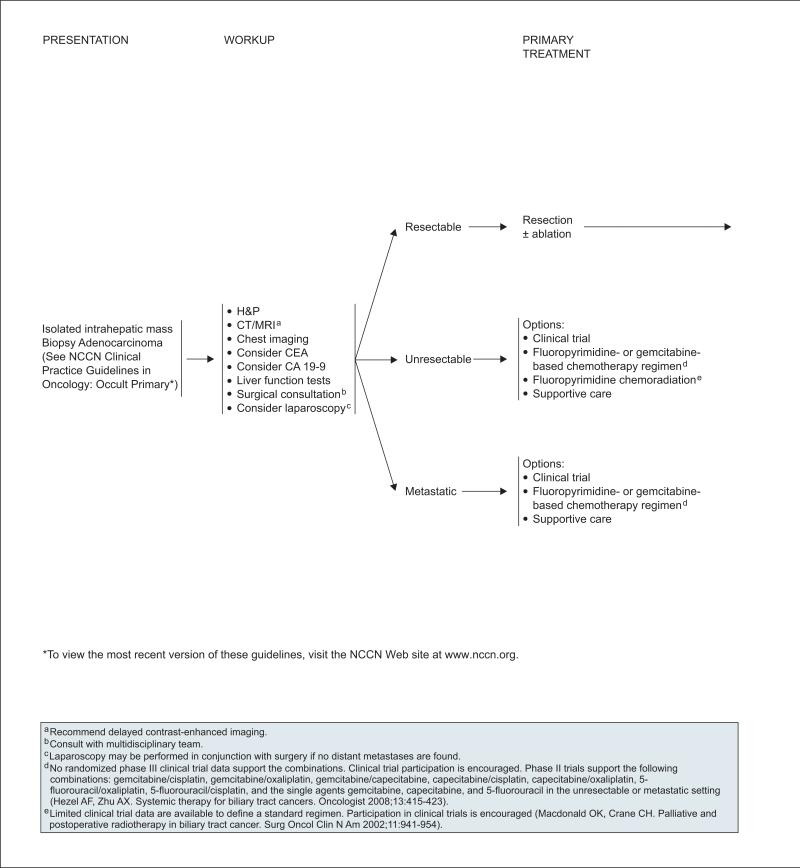
Complete re-section is the only potentially curative therapy for patients with intrahepatic cholangiocarcinoma, although most patients are not candidates for surgery because of advanced disease at diagnosis. Surgery involves removal of the involved hepatic lobe or segment along the bile duct in which the tumor is located.173 Patient selection for surgery is facilitated by careful preoperative staging, which may include laparoscopy to identify patients with unresectable or metastatic disease. Five-year survival rates ranging from 20% to 43% have been reported.174–177
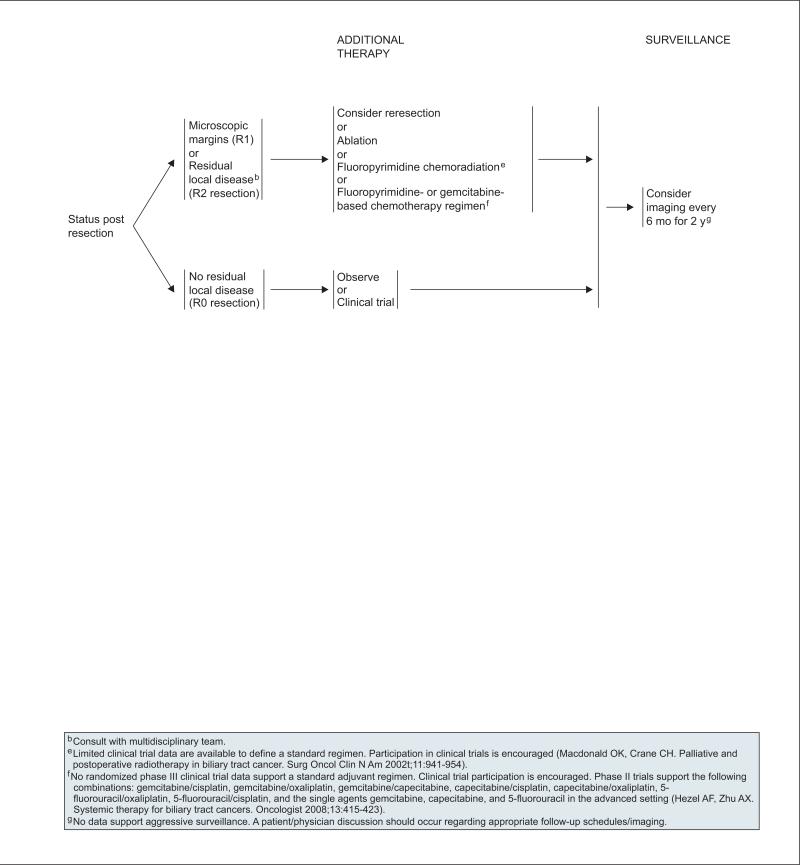
Patients who have undergone an R0 resection with or without ablation may be followed up with observation alone. Adjuvant chemotherapy can be administered if appropriate clinical trials are available.
For patients found to have microscopic positive tumor margins (R1) or residual local disease (R2) after resection, a multidisciplinary team must review the available options on an individual basis. Although the optimal treatment strategy has not been determined, options include 1) additional resection, 2) ablative therapy, 3) fluoropyrimidine chemoradiation, or 4) fluoropyrimidine- or gemcitabine-based chemotherapy (see page 363; see section on “Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
For patients with unresectable disease, options include 1) clinical trial, 2) fluoropyrimidine-based or gemcitabine-based chemotherapy, 3) fluoropyrimi-dine chemoradiation, or 4) best supportive care (see page 362). The same primary treatment options are recommended for patients with metastatic disease, except chemoradiation (see section on “Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
Extrahepatic Cholangiocarcinoma
Complete re-section is the main curative therapy for patients with extrahepatic cholangiocarcinoma. The surgical procedures for resectable disease are based on the portion of the extrahepatic biliary tree in which the lesion resides. Hilar resection with lymphadenectomy and en bloc liver resection is recommended for lesions in the proximal third or extrahepatic biliary tree. In this situation, caudate resection is strongly encouraged. Major bile duct excision with lymphadenectomy with frozen section assessment of bile duct margins, and pancreaticoduodenectomy with lymphadenectomy are recommended for lesions in the mid third and distal third of the extrahepatic biliary tree, respectively.149 Very rare cases of small mid bile duct tumors can be resected with an isolated bile duct resection and lymphadenopathy. Five-year survival rates ranging from 20% to 40% have been reported for patients treated for hilar cholangiocarcinoma172,178,179 and 37% for bile duct cancers in the distal third of the extrahepatic biliary tree.159
Patient selection for surgery is facilitated by careful preoperative staging, which may include surgical exploration and laparoscopy to identify patients with unresectable or metastatic disease. However, the consensus of the panel is that surgery may be performed without a biopsy if the index of suspicion is high. Panel consensus is that biliary drainage should be considered before surgery, although controversy exists about the risks and benefits of this approach.180,181 Preoperative biliary drainage is accomplished with ERCP or PTC.
Patients who have undergone an R0 resection and have negative regional nodes may be followed up with observation alone or undergo fluoropyrimi-dine chemoradiation or fluoropyrimidine or gemcitabine chemotherapy. However, limited clinical trial data define a standard regimen, and patient enrollment in a clinical trial is encouraged. For patients found to have microscopic positive tumor margins (R1), residual local disease (R2), carcinoma in situ, or positive regional lymph nodes after resection, a multidisciplinary team must review the available options on an individual basis. Although the optimal treatment strategy has not been determined, options include: 1) fluoropyrimidine chemoradiation (brachytherapy or external beam) followed by additional fluoropyrimidine or gemcitabine chemotherapy or 2) fluoropyrimidine- or gemcitabine-based chemotherapy for patients with positive regional nodes (see page 365). Data supporting particular chemoradiation and chemotherapy regimens are limited (see section on “Chemo-radiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
Liver transplantation is the only other potentially curative option for patients with extrahepatic cholangiocarcinoma.182,183 This procedure is only recommended for highly selected patients with either unresectable disease with otherwise normal biliary and hepatic function or underlying chronic liver disease precluding surgery. Retrospective evidence shows that selected patients with hilar cholangio-carcinoma undergoing preoperative chemoradiation therapy followed by liver transplantation have significantly improved overall survival compared with patients undergoing resection.184 Nevertheless, substantial differences were seen in the characteristics of these groups of patients. The panel encourages continuation of clinical research in this area.
For distal strictures in which a diagnosis is needed or palliation is indicated, an ERCP is performed to allow for complete imaging of the duct and stenting of the obstruction. In addition, brushes of the duct can be obtained for pathologic evaluation. Hilar strictures can be managed with PTC. Endoscopic ultrasound may be useful for distal common bile duct cancers for defining a mass or abnormal thickening, which can direct biopsies. Direct visualization of the duct with directed biopsies is the ideal technique for the workup of cholangiocarcinoma.
Patients with unresectable disease should undergo biliary drainage using either surgical bypass or an endoscopic (i.e., ERCP) or percutaneous approach (i.e., PTC), most often involving biliary stent placement.149,185–187 A biopsy is also recommended to confirm diagnosis before initiation of further treatment. Additional treatment options include 1) clinical trial, 2) fluoropyrimidine chemoradiation, 3) fluoropyrimidine- or gemcitabine-based chemotherapy, and 4) best supportive care (see page 364). Data supporting particular chemoradiation and chemotherapy regimens are limited (see section on “Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
Those with metastatic disease should undergo biliary drainage through stent placement using an endoscopic or percutaneous approach. A biopsy is also recommended to confirm diagnosis before initiation of further treatment. Additional treatment options include clinical trial, fluoropyrimidine- or gemcitabine-based chemotherapy, and best supportive care (see page 364). Data supporting particular chemoradiation and chemotherapy regimens are limited (see section on “Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma”).
Photodynamic therapy is a relatively new therapy for the local treatment of cholangiocarcinoma. It is an ablative method involving intravenous injection of a photosensitizing drug followed by selective irradiation with light of a specific wavelength to initiate localized drug activation, and has been used for palliation of cholangiocarcinoma.188,189 Two small randomized clinical trials have shown the combination of photodynamic therapy with biliary stenting to significantly improve the overall survival of patients with unresectable cholangiocarcinoma.190,191
Surveillance
No data support aggressive surveillance in patients undergoing resection of cholangiocarcinoma; determination of appropriate follow-up schedule/imaging should include a careful patient/physician discussion. The panel recommends that follow-up of patients undergoing resection of cholangiocarcinoma include consideration of imaging studies every 6 months for 2 years. Re-evaluation according to the initial workup should be considered in the event of disease progression (see pages 363 and 365).
Chemoradiation and Chemotherapy for Treatment of Gallbladder Cancer and Cholangiocarcinoma
Because of the low incidence of biliary tract cancers (i.e., gallbladder cancer and cholangiocarcinomas), most trials evaluating the efficacy and safety of chemotherapeutic agents administered either alone or concurrently with radiation therapy in these cancers represent single-institution phase II trials. Most of these studies are not randomized, often combine gall-bladder cancers with intrahepatic and extrahepatic cholangiocarcinoma, and involve small numbers of patients, making definitive conclusions difficult.
Some recommendations included in the guidelines, particularly those relating to the use of chemo-radiation, are primarily based on practice patterns at NCCN member institutions and single-center experiences from retrospective studies. Despite the challenges associated with accruing large numbers of patients with biliary tract cancer for randomized phase III trials, it is widely recognized that efforts should be made to conduct these studies so that the individual disease entities are evaluated separately.192 Nevertheless, because of limited data and the heterogenous patient populations in many of the published studies, recommendations in these guidelines on use of chemotherapy or chemoradiation therapy are in most cases not specific to the particular type of biliary tract cancer.
Chemotherapy and Chemoradiation in the Adjuvant Setting
The role of adjuvant chemotherapy/chemoradiation in patients with resected biliary cancer is poorly defined. A recent retrospective review covering 1995 to 2005 at a single institution showed that among patients treated for biliary tract cancer, only 6.5% of patients underwent adjuvant chemotherapy alone, 6.5% underwent adjuvant chemoradiation alone, and 6.5% underwent both adjuvant chemoradiation and systemic chemo-therapy.152 In another retrospective analysis using the Surveillance Epidemiology and End Results (SEER) database to investigate patients diagnosed with gallbladder cancer during 1992 to 2002, only 17% of the 2325 patients in the surgical cohort underwent adjuvant chemoradiation.193
Few studies have evaluated the use of adjuvant chemotherapy alone in patients with biliary tract cancer. No clear benefit of adjuvant chemotherapy alone was seen in 2 large retrospective analyses of patients with biliary tract cancer,152,194 although 1 had a very limited number of patients who underwent this therapy,152 and chemotherapy did not include newer agents in the latter study which covered the period from1988 to 1997.194
A phase III trial evaluated adjuvant chemo-therapy in patients with resected pancreaticobiliary cancer.195 Approximately 50% of the eligible patients in this study were diagnosed with either gallbladder cancer or cholangiocarcinoma. Patients were randomly assigned to adjuvant chemotherapy with 5-FU/mitomycin C or a control arm. Subgroup analyses showed a significantly better 5-year survival in the chemotherapy group for patients with gallbladder cancer, although no significant differences between the treatment arms were observed when the subgroup of patients with biliary tract cancer was considered. A retrospective analysis of 177 patients with resected biliary tract cancer showed that initial recurrence involving a distant site occurred in 85% and 41% of patients with gallbladder cancer and hilar cholangio-carcinoma, respectively, arguing for the development of active adjuvant systemic therapy in gallbladder cancer.153 Because of very limited data on chemotherapy use in the adjuvant setting, specific recommendations for fluoropyrimidine- or gemcitabine-based chemotherapy listed in the guidelines primarily represent an extrapolation from studies of patients with advanced disease (see pages 361, 363, and 365).
A primary limitation for cure in patients with biliary tract cancer after surgery is local failure, thereby providing an important justification for use of adjuvant radiation therapy. Useful reviews on the use of radiation therapy in biliary tract cancers are available and include specific citations to several relevant studies.196,197 A retrospective study of 2325 patients who had undergone surgery for gallbladder cancer from the SEER database during 1992 to 2002 showed a median survival of 14 months in the group undergoing adjuvant chemoradiation versus 8 months in the one that did not (P < .0001). The survival benefit of adjuvant chemoradiation was even more pronounced (16 vs. 5 months; P < .0001) when only the group of patients with positive regional lymph nodes was considered.193 Retrospective analyses from single-center experiences for patients with resected extrahepatic cholangiocarcinoma who underwent fluoropyrimidine-based chemoradiation therapy also suggested that chemoradiation may offer a local control benefit, although distant failure was commonly observed.198,199 A multivariate Cox proportional hazards model, developed to make individualized predictions of survival from the addition of radiation therapy after gallbladder cancer resection, showed that the greatest benefit of radiation therapy was seen in patients with T2 or higher-stage tumors and node-positive disease.200 Results of these studies support omitting adjuvant chemoradiation in the postsurgical treatment of patients with gallbladder cancer characterized asT1b, N0 (see page 361).
A retrospective analysis of patients in the SEER database provides some support for adjuvant chemo-radiation in the treatment of patients with intrahepatic cholangiocarcinoma.201 In this study, overall survival was significantly improved when patients underwent chemoradiation in addition to surgery (P = .014). In a retrospective study of patients with extrahepatic cholangiocarcinoma, no significant survival differences were seen when patients with R0 margins after surgery who did not undergo adjuvant therapy were compared with patients with R1 margins after surgery who underwent chemoradiation, suggesting that chemoradiation may have a survival benefit in the latter group.202 In another retrospective analysis of patients with extrahepatic cholangiocarcinoma, the addition of maintenance chemotherapy after adjuvant chemoradiation was found to have a survival benefit (i.e., 3-year overall survival of 75.6% with addition of maintenance chemotherapy vs. 34.8% with no maintenance chemo-therapy; P = .001).203 These results provide support for recommending consideration of fluoropyrimidine chemoradiation followed by additional fluoropyrimi-dine or gemcitabine chemotherapy for patients with extrahepatic cholangiocarcinoma with either positive margins or positive regional lymph nodes (see page 365).
Most collective experiences of chemoradiation in biliary tract cancer involve concurrent chemoradiation and 5-FU.196,197 More recently, concurrent chemo-radiation with capecitabine has also been used.202,204 Concurrent chemoradiation with gemcitabine is not recommended because of the limited experience and toxicity associated with this treatment.205
Chemotherapy and Chemoradiation in the Advanced Setting
Although no standard chemotherapy regimens have been defined for the first-line treatment of advanced biliary tract cancers, a consensus is beginning to emerge. A recent review summarizes many of the studies addressing this issue.192
The survival benefit of chemotherapy in patients with advanced biliary tract cancer was suggested in a trial comparing 5-FU/leucovorin/etoposide with best supportive care.206 A subsequent phase III trial evaluating patients with advanced biliary tract cancer randomly assigned to receive either 5-FU/leucov-orin/etoposide or 5-FU/cisplatin/epirubicin did not show one regimen to be significantly superior with respect to overall survival (12.03 vs. 9.02 months, respectively), although the trial was underpowered to detect this difference.207
Several other chemotherapy combinations and single agents have been evaluated in clinical studies for the treatment of advanced biliary tract cancers192 (see Hezel and Zhu192 and references therein). Examples of chemotherapy combinations shown in phase II trials to have activity in the treatment of advanced biliary tract cancers include gemcitabine/cisplatin;208–210 gemcitabine/capecitabine;211,212 gemcitabine/oxaliplatin;213 capecitabine/oxaliplatin;214 capecitabine/cisplatin;208 and 5-FU/cisplatin.215
Results of a recent pooled analysis of 104 trials of patients with advanced biliary tract cancers showed that the subgroup of patients receiving combination gemcitabine and platinum-based agents experienced the greatest benefit.216 Gemcitabine as an anchor drug for the treatment of advanced biliary tract cancers is further supported by a retrospective review of 304 patients with advanced biliary tract cancer who received gemcitabine, a cisplatin-based regimen, or a fluoropyrimidine-based regimen.217 In that study, patients receiving a gemcitabine-based regimen were shown to have a lower risk for death. However, no differences in response rate, disease control rate, or survival were observed for fluoropyrimidine- and gemcitabine-based regimens in another recent retrospective study involving 243 patients with unresectable biliary tract cancer, and the only significant benefit associated with the addition a platinum-containing agent to these regimens was an increase in the disease control rate.218
Based on the experiences from phase II studies, the following gemcitabine- and fluoropyrimidine-based chemotherapy options are recommended for the treatment of advanced biliary tract cancer: single-agent 5-FU, capecitabine, gemcitabine, or combination regimens of gemcitabine/cisplatin; gemcitabine/oxaliplatin; gemcitabine/capecitabine; capecitabine/cisplatin; capecitabine/oxaliplatin; 5-FU/cisplatin; and 5-FU/ oxaliplatin. The combination of gemcitabine/5-FU is not included because of the increased toxicity and decreased efficacy observed with this regimen219 compared with the gemcitabine/capecitabine regimen in the setting of advanced biliary tract cancer.192
Chemoradiation in the setting of advanced biliary tract cancer can provide control of symptoms caused by local tumor effects and may prolong overall survival. Useful reviews on the use of radiation therapy in biliary tract cancers are available and include specific citations to several relevant studies.196,197 In a retrospective analysis of 37 patients with inoperable extrahepatic cholangiocarcinoma who underwent chemoradiation, actuarial overall survival at 1 and 2 years was 59% and 22%, respectively, although effective local control was observed in most patients during this period (actuarial local control rates of 90% and 61% at 1 and 2years, respectively).220 The most extensively investigated chemotherapeutic agent for use in concurrent chemoradiation in the treatment of biliary tract cancers has been 5-FU,196,197 although capecitabine has been substituted for 5-FU in some studies.204 The panel recommends that chemotherapy administered concurrently with radiation be limited to either 5-FU or capecitabine, and that this treatment be restricted to patients without evidence of metastatic disease. Concurrent chemoradiation with gemcitabine is not recommended because of the limited experience and toxicity associated with this treatment.
Summary
Although many patients with hepatobiliary cancers are diagnosed at an advanced stage, all patients should be evaluated for treatment. Nevertheless, careful patient selection for treatment and active multidisciplinary cooperation are essential. Very few high-quality randomized clinical trials of patients with hepatobiliary cancers exist, and patient participation in prospective clinical trials is the preferred option for treatment of patients with all stages of disease.
Individual Disclosures for the NCCN Hepatobiliary Cancers Panel
| Panel Member | Clinical Research Support | Advisory Boards, Speakers Bureau, Expert Witness, or Consultant | Patent, Equity, or Royalty | Other | Date Completed |
|---|---|---|---|---|---|
| Thomas A. Abrams, MD | None | Bayer HealthCare | None | None | 7/23/08 |
| Edgar Ben-Josef, MD | None | None | None | None | 5/28/08 |
| Al B. Benson III, MD | Abbott Laboratories; Amgen Inc.; AstraZeneca Pharmaceuticals LP; Bayer HealthCare; Bristol-Myers Squibb Company; Eli Lilly and Company; Enzon Pharmaceuticals; Genentech, Inc.; General Electric; Genomic Health, Inc.; ImClone Systems Incorporated; National Cancer Institute; Novartis Pharmaceuticals Corporation;Onyx Pharmaceuticals, Inc.; Pfizer Inc.; Roche Laboratories, Inc.; sanofi-aventis U.S.; and Taiho Parmaceuticals Co., Ltd. | None | None | None | 10/26/08 |
| P. Mark Bloomston, MD | None | None | None | None | 5/11/08 |
| Jean F. Botha, MB, BCh | None | None | None | None | 9/8/08 |
| Bryan M. Clary, MD | None | Covidien AG; Onyx Pharmaceuticals, Inc.; and sanofi-aventis U.S. | None | None | 7/24/08 |
| Anne M. Covey, MD | None | None | None | None | 7/27/08 |
| Steven A Curley, MD | None | None | None | None | 8/1/08 |
| Michael I. D'Angelica, MD | None | None | None | None | 5/28/08 |
| Rene Davila, MD | None | None | None | None | 5/10/08 |
| William D. Ensminger, MD, PhD | None | None | None | None | 5/16/08 |
| John F. Gibbs, MD, FACS, MHCM | None | None | None | None | 12/1/08 |
| Daniel Laheru, MD | None | Genentech, Inc. | None | Concordia | 9/9/08 |
| Mokenge P. Malafa, MD, FACS | National Cancer Institute; and Davos Life Sciences, Singapore | None | Biogene, Singapore | None | 11/30/08 |
| Jorge Marrero, MD | Bayer HealthCare | Onyx Pharmaceuticals, Inc. | None | None | 9/8/08 |
| Steven G. Meranze, MD | None | None | None | None | 9/5/08 |
| Sean J. Mulvihill, MD | National Cancer Institute | None | None | None | 9/15/08 |
| James O. Park, MD | None | None | None | None | 5/12/08 |
| James A. Posey, MD | Bayer HealthCare | Bayer HealthCare | None | None | 10/3/08 |
| Jasgit Sachdev, MD | None | None | None | None | 12/23/08 |
| Riad Salem, MD, MBA | MDS Nordion | MDS Nordion | None | None | 6/3/08 |
| Elin R. Sigurdson, MD, PhD | None | sanofi-aventis U.S. | None | None | 1/2/08 |
| Constantinos Sofocleous, MD | National Cancer Institute | None | None | None | 11/7/08 |
| Jean-Nicolas Vauthey, MD | None | None | None | None | 1/2/09 |
| Alan P. Venook, MD | Amgen Inc.; Genentech, Inc.; Novartis Pharmaceuticals Corporation; and Pfizer Inc. | Amgen Inc.; Genentech, Inc.; Novartis Pharmaceuticals Corporation; and Pfizer Inc. | None | None | 7/1/08 |
| Laura Williams Goff, MD | Bristol-Myers Squibb Company; ImClone Systems Incorporated; OSI Pharmaceuticals, Inc.; and sanofi-aventis | Daiichi-Sankyo Co.; and OSI Pharmaceuticals, Inc. | None | None | 3/29/09 |
| Yun Yen, MD, PhD, FACP | None | Bayer HealthCare; and Onyx Pharmaceuticals, Inc. | None | None | 12/15/08 |
| Andrew X Zhu, MD, PhD | Bayer HealthCare; and Genentech, Inc. | Bayer HealthCare; and Genentech, Inc. | None | None | 8/3/08 |
The NCCN guidelines staff have no conflicts to disclose.
NCCN Categories of Evidence and Consensus.
Category 1: The recommendation is based on high-level evidence (e.g., randomized controlled trials) and there is uniform NCCN consensus.
Category 2A: The recommendation is based on lower-level evidence and there is uniform NCCN consensus.
Category 2B: The recommendation is based on lower-level evidence and there is nonuniform NCCN consensus (but no major disagreement).
Category 3: The recommendation is based on any level of evidence but reflects major disagreement.
All recommendations are category 2A unless otherwise noted.
NCCN Hepatobiliary Cancers Panel Members
*Al B. Benson III, MD/Chair†
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
Thomas A. Abrams, MD†
Dana-Farber/Brigham and Women's Cancer Center
Edgar Ben-Josef, MD§
University of Michigan Comprehensive Cancer Center
P. Mark Bloomston, MD¶
Arthur G. James Cancer Hospital & Richard J. Solove Research Institute at The Ohio State University
Jean F. Botha, MB, BCh¶
UNMC Eppley Cancer Center at The Nebraska Medical Center
Bryan M. Clary, MD¶
Duke Comprehensive Cancer Center
Anne Covey, MD§
Memorial Sloan-Kettering Cancer Center
Steven A. Curley, MD¶
The University of Texas M. D. Anderson Cancer Center
*Michael I. D'Angelica, MD¶
Memorial Sloan-Kettering Cancer Center
Rene Davila, MDþ
St. Jude Children's Research Hospital/University of Tennessee Cancer Institute
William D. Ensminger, MD, PhD†
University of Michigan Comprehensive Cancer Center
John F. Gibbs, MD¶
Roswell Park Cancer Institute
Daniel Laheru, MD†þ
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
Mokenge P. Malafa, MD¶
H. Lee Moffitt Cancer Center & Research Institute
Jorge Marrero, MDþ
University of Michigan Comprehensive Cancer Center
Steven G. Meranze, MD§
Vanderbilt-Ingram Cancer Center
Sean J. Mulvihill, MD¶
Huntsman Cancer Institute at the University of Utah
James O. Park, MD¶
Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance
James A. Posey, MD†
University of Alabama at Birmingham Comprehensive Cancer Center
Jasgit Sachdev, MD†
St. Jude Children's Research Hospital/University of Tennessee Cancer Institute
*Riad Salem, MD, MBA§
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
Elin R. Sigurdson, MD, PhD¶
Fox Chase Cancer Center
Constantinos Sofocleous, MD, PhD§
Memorial Sloan-Kettering Cancer Center
Jean-Nicolas Vauthey, MD¶
The University of Texas M. D. Anderson Cancer Center
*Alan P. Venook, MD†‡
UCSF Helen Diller Family Comprehensive Cancer Center
Laura Williams Goff, MD‡
Vanderbilt-Ingram Cancer Center
Yun Yen, MD, PhD‡
City of Hope Comprehensive Cancer Center
*Andrew X. Zhu, MD, PhD
Massachusetts General Hospital Cancer Center
KEY:
*Writing Committee Member
Specialties: †Medical Oncology; §Radiotherapy/Radiation Oncology/Interventional Radiology; ¶Surgery/Surgical Oncology; þInternal Medicine; ‡Hematology/Hematology Oncology
Footnotes
Clinical trials: The NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Please Note
These guidelines are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult these guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient's care or treatment. The National Comprehensive Cancer Network makes no representation or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way.
These guidelines are copyrighted by the National Comprehensive Cancer Network. All rights reserved. These guidelines and the illustrations herein may not be reproduced in any form without the express written permission of the NCCN © 2009.
Disclosures for the NCCN Hepatobiliary Cancers Guidelines Panel
At the beginning of each NCCN guidelines panel meeting, panel members disclosed any financial support they have received from industry. Through 2008, this information was published in an aggregate statement in JNCCN and on-line. Furthering NCCN's commitment to public transparency, this disclosure process has now been expanded by listing all potential conflicts of interest respective to each individual expert panel member.
Individual disclosures for the NCCN Hepatobiliary Cancers Guidelines Panel members can be found on page 391. (To view the most recent version of these guidelines and accompanying disclosures, visit the NCCN Web site at www.nccn.org.)
These guidelines are also available on the Internet. For the latest update, please visit www.nccn.org.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Takamatsu S, Noguchi N, Kudoh A, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:609–614. [PubMed] [Google Scholar]
- 8.Bartlett DL, Di Bisceglie AM, Dawson LA. Cancer of the liver. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 8th ed. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 1129–1156. [Google Scholar]
- 9.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 10.Volk ML, Marrero JA. Early detection of liver cancer: diagnosis and management. Curr Gastroenterol Rep. 2008;10:60–66. doi: 10.1007/s11894-008-0010-2. [DOI] [PubMed] [Google Scholar]
- 11.Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeoman AD, Al-Chalabi T, Karani JB, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: implications for follow-up and screening. Hepatology. 2008;48:863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 13.Beaton MD, Adams PC. Prognostic factors and survival in patients with hereditary hemochromatosis and cirrhosis. Can J Gastroenterol. 2006;20:257–260. doi: 10.1155/2006/428048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichikawa T, Yanagi K, Motoyoshi Y, et al. Two cases of non-alcoholic steatohepatitis with development of hepatocellular carcinoma without cirrhosis. J Gastroenterol Hepatol. 2006;21:1865–1866. doi: 10.1111/j.1440-1746.2006.04282.x. [DOI] [PubMed] [Google Scholar]
- 15.Guzman G, Brunt EM, Petrovic LM, et al. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services Centers for Disease Control and Prevention. [February 19, 2009];National Health and Nutrition Examination Survey. Available at: www.cdc.gov/nchs/data/nhanes/databriefs/viralhep.pdf.
- 17.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1:559–568. doi: 10.1016/s1089-3261(05)70321-4. [DOI] [PubMed] [Google Scholar]
- 18.Ryder SD, Irving WL, Jones DA, et al. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl 1):S1–3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein ST, Zhou F, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 21.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM. Review article: current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:2–12. doi: 10.1111/j.1365-2036.2008.03710.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 24.Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2008;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 25.Asare GA, Bronz M, Naidoo V, Kew MC. Synergistic interaction between excess hepatic iron and alcohol ingestion in hepatic mutagenesis. Toxicology. 2008;254:11–18. doi: 10.1016/j.tox.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–772. doi: 10.1097/MCG.0b013e3180381584. [DOI] [PubMed] [Google Scholar]
- 27.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang PE, Ong WC, Lui HF, Tan CK. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol. 2008;43:881–888. doi: 10.1007/s00535-008-2238-x. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–110. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 31.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 32.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller G, Schwartz LH, D'Angelica M. The use of imaging in the diagnosis and staging of hepatobiliary malignancies. Surg Oncol Clin N Am. 2007;16:343–368. doi: 10.1016/j.soc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Marrero JA, Hussain HK, Nghiem HV, et al. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281–289. doi: 10.1002/lt.20357. [DOI] [PubMed] [Google Scholar]
- 35.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 36.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 37.Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206–209. doi: 10.1097/00000658-200108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 39.Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta. 2008;395:19–26. doi: 10.1016/j.cca.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Katyal S, Oliver JH, III, Peterson MS, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 41.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 42.Cooper GS, Bellamy P, Dawson NV, et al. A prognostic model for patients with end-stage liver disease. Gastroenterology. 1997;113:1278–1288. doi: 10.1053/gast.1997.v113.pm9322523. [DOI] [PubMed] [Google Scholar]
- 43.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 44.Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease--should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 45.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 46.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 47.Boyer TD. Changing clinical practice with measurements of portal pressure. Hepatology. 2004;39:283–285. doi: 10.1002/hep.20037. [DOI] [PubMed] [Google Scholar]
- 48.Thalheimer U, Mela M, Patch D, Burroughs AK. Targeting portal pressure measurements: a critical reappraisal. Hepatology. 2004;39:286–290. doi: 10.1002/hep.20061. [DOI] [PubMed] [Google Scholar]
- 49.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 50.Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39:3169–3174. doi: 10.1016/j.transproceed.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Eggel H. Uber das primare Carcinoma der Leber. Beitr Pathol Anat Allg Pathol. 1901;30:506–604. [Google Scholar]
- 52.Clavien PA, Fong Y, Lyerly HK, et al. Malignant Liver Tumors: Current and Emerging Therapies. 2nd ed. Jones and Barlett; Sudbury: 2003. [Google Scholar]
- 53.Yuki K, Hirohashi S, Sakamoto M, et al. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174–2179. doi: 10.1002/1097-0142(19901115)66:10<2174::aid-cncr2820661022>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Dohmen K. Many staging systems for hepatocellular carcinoma: evolution from Child-Pugh, Okuda to SLiDe. J Gastroenterol Hepatol. 2004;19:1227–1232. doi: 10.1111/j.1440-1746.2004.03538.x. [DOI] [PubMed] [Google Scholar]
- 55.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 56.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 57.Greene F, Page D, Fleming I, Fritz A. AJCC Cancer Staging Manual. 6th ed. Springer-Verlag; New York: 2002. [Google Scholar]
- 58.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 59.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 60.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 61.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 62.The Cancer of the Liver Italian Program (CLIP) investigators A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 63.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 64.Omagari K, Honda S, Kadokawa Y, et al. Preliminary analysis of a newly proposed prognostic scoring system (SLiDe score) for hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:805–811. doi: 10.1111/j.1440-1746.2004.03350.x. [DOI] [PubMed] [Google Scholar]
- 65.Huo TI, Lin HC, Huang YH, et al. The model for end-stage liver disease-based Japan Integrated Scoring system may have a better predictive ability for patients with hepatocellular carcinoma undergoing locoregional therapy. Cancer. 2006;107:141–148. doi: 10.1002/cncr.21972. [DOI] [PubMed] [Google Scholar]
- 66.Limquiaco JL, Wong GL, Wong VW, et al. Evaluation of Model for End Stage Liver Disease (MELD)-based systems as prognostic index for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;24:63–69. doi: 10.1111/j.1440-1746.2008.05701.x. [DOI] [PubMed] [Google Scholar]
- 67.Nanashima A, Sumida Y, Abo T, et al. Modified Japan Integrated Staging is currently the best available staging system for hepatocellular carcinoma patients who have undergone hepatectomy. J Gastroenterol. 2006;41:250–256. doi: 10.1007/s00535-005-1751-4. [DOI] [PubMed] [Google Scholar]
- 68.Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma—survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–1006. doi: 10.1016/j.ejca.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 69.Vauthey JN, Ribero D, Abdalla EK, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg. 2007;204:1016–1027. doi: 10.1016/j.jamcollsurg.2006.12.043. discussion 1027–1018. [DOI] [PubMed] [Google Scholar]
- 70.Huitzil FD, Capanu M, O'Reilly E, et al. Ranking and improvement of staging systems in advanced hepatocellular carcinoma [abstract].. Presented at the 2008 Gastrointestinal Cancers Symposium; Orlando, Florida. January 25–27, 2008; Abstract 210. [Google Scholar]
- 71.Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352–361. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- 72.Collette S, Bonnetain F, Paoletti X, et al. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117–1126. doi: 10.1093/annonc/mdn030. [DOI] [PubMed] [Google Scholar]
- 73.Guglielmi A, Ruzzenente A, Pachera S, et al. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol. 2008;103:597–604. doi: 10.1111/j.1572-0241.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 74.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281–291. doi: 10.1016/j.jamcollsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 75.Kelley RK, Venook AP. Sorafenib in hepatocellular carcinoma: separating the hype from the hope. J Clin Oncol. 2008;26:5845–5848. doi: 10.1200/JCO.2008.19.7996. [DOI] [PubMed] [Google Scholar]
- 76.Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–457. doi: 10.1001/archsurg.140.5.450. discussion 457–458. [DOI] [PubMed] [Google Scholar]
- 77.Chok KS, Ng KK, Poon RT, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87. doi: 10.1002/bjs.6358. [DOI] [PubMed] [Google Scholar]
- 78.Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am. 2003;12:51–63. doi: 10.1016/s1055-3207(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 79.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 80.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo DD, Lee HC, Jang MK, et al. Preoperative portal vein embolization and surgical resection in patients with hepatocellular carcinoma and small future liver remnant volume: comparison with transarterial chemoembolization. Ann Surg Oncol. 2007;14:3501–3509. doi: 10.1245/s10434-007-9553-y. [DOI] [PubMed] [Google Scholar]
- 82.Wei AC, Tung-Ping Poon R, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 83.Ribero D, Curley SA, Imamura H, et al. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol. 2008;15:966–992. doi: 10.1245/s10434-007-9731-y. [DOI] [PubMed] [Google Scholar]
- 84.Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 85.Abdalla EK, Denys A, Hasegawa K, et al. Treatment of large and advanced hepatocellular carcinoma. Ann Surg Oncol. 2008;15:979–985. doi: 10.1245/s10434-007-9727-7. [DOI] [PubMed] [Google Scholar]
- 86.Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 87.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 88.Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008;247:260–266. doi: 10.1148/radiol.2471070818. [DOI] [PubMed] [Google Scholar]
- 89.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 90.Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 91.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 93.Mazzaferro V, Chun YS, Poon RT, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. [February 19, 2009];United Network for Organ Sharing: Organ Donation and Transplantation. Available at: www.unos.org.
- 95.Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatocellular carcinoma: clues for the best strategy. Hepatology. 2000;31:1019–1021. doi: 10.1053/he.2000.6959. [DOI] [PubMed] [Google Scholar]
- 96.Facciuto ME, Koneru B, Rocca JP, et al. Surgical treatment of hepatocellular carcinoma beyond Milan criteria. Results of liver resection, salvage transplantation, and primary liver transplantation. Ann Surg Oncol. 2008;15:1383–1391. doi: 10.1245/s10434-008-9851-z. [DOI] [PubMed] [Google Scholar]
- 97.Poon RT. Optimal initial treatment for early hepatocellular carcinoma in patients with preserved liver function: transplantation or resection? Ann Surg Oncol. 2007;14:541–547. doi: 10.1245/s10434-006-9156-z. [DOI] [PubMed] [Google Scholar]
- 98.Poon RT, Fan ST, Lo CM, et al. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 100.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 101.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–846. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 102.Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–509. doi: 10.1097/SLA.0b013e318148c704. discussion 509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 104.Volk M, Marrero JA. Liver transplantation for hepatocellular carcinoma: who benefits and who is harmed? Gastroenterology. 2008;134:1612–1614. doi: 10.1053/j.gastro.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 105.Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 106.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 107.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 108.Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 109.Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Richard HM, III, Silberzweig JE, Mitty HA, et al. Hepatic arterial complications in liver transplant recipients treated with pretransplantation chemoembolization for hepatocellular carcinoma. Radiology. 2000;214:775–779. doi: 10.1148/radiology.214.3.r00mr31775. [DOI] [PubMed] [Google Scholar]
- 111.Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 112.Vivarelli M, Guglielmi A, Ruzzenente A, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 116.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 117.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 118.Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 119.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 120.Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 121.Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 122.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 123.Maluccio M, Covey AM, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005;16:955–961. doi: 10.1097/01.RVI.0000161377.33557.20. [DOI] [PubMed] [Google Scholar]
- 124.Maluccio MA, Covey AM, Porat LB, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19:862–869. doi: 10.1016/j.jvir.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 125.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 126.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 127.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211–221. doi: 10.1016/s1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 128.O'Neil BH, Venook AP. Hepatocellular carcinoma: the role of the North American GI Steering Committee Hepatobiliary Task Force and the advent of effective drug therapy. Oncologist. 2007;12:1425–1432. doi: 10.1634/theoncologist.12-12-1425. [DOI] [PubMed] [Google Scholar]
- 129.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 130.Stuart K, Stokes K, Jenkins R, et al. Treatment of hepatocellular carcinoma using doxorubicin/ethiodized oil/gelatin powder chemoembolization. Cancer. 1993;72:3202–3209. doi: 10.1002/1097-0142(19931201)72:11<3202::aid-cncr2820721112>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 131.Llado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 132.Doan PL, O'Neil BH, Moore DT, Bernard SA. Predictors of survival in patients with hepatocellular carcinoma treated with transarterial chemoembolization [abstract].. Presented at the 2008 Gastrointestinal Cancers Symposium; Orlando, Florida. January 25–27, 2008; Abstract 204. [Google Scholar]
- 133.Molinari M, Dixon E, Kachura JR, et al. Chemoembolization for unresectable hepatocellular carcinoma?. Presented at the 2004 Gastrointestinal Cancers Symposium; Orlando, Florida. January 22–24, 2004; Abstract 167. [Google Scholar]
- 134.Ibrahim SM, Lewandowski RJ, Sato KT, et al. Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol. 2008;14:1664–1669. doi: 10.3748/wjg.14.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics. 2008;28:81–99. doi: 10.1148/rg.281065721. [DOI] [PubMed] [Google Scholar]
- 136.Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 2006;106:1653–1663. doi: 10.1002/cncr.21811. [DOI] [PubMed] [Google Scholar]
- 137.Ikeda M, Okusaka T, Ueno H, et al. Hepatic arterial infusion chemotherapy with epirubicin in patients with advanced hepatocellular carcinoma and portal vein tumor thrombosis. Oncology. 2007;72:188–193. doi: 10.1159/000112805. [DOI] [PubMed] [Google Scholar]
- 138.Thomas MB, O'Beirne JP, Furuse J, et al. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008–1014. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 139.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 140.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 141.Cheng A, Kang YK, Chen CL, et al. Randomized phase III trial of sorafenib versus placebo in Asian patients with advanced hepatocellular carcinoma [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 4509. [Google Scholar]
- 142.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 143.Raoul JL, Santoro A, Beaugrand M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to ECOG performance status: a subanalysis from the SHARP trial [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 4587. [Google Scholar]
- 144.Craxi A, Porta C, Sangiovanni A, et al. Efficacy and safety of sorafenib in patients with alcohol-related hepatocellular carcinoma: a sub-analysis from the SHARP trial [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 15591. [Google Scholar]
- 145.Bolondi L, Caspary J, Bennouna B, et al. Clinical benefit of sorafenib in hepatitis C patients with hepatocellular carcinoma: subgroup analysis of the SHARP trial [abstract].. Presented at the 2008 Gastrointestinal Cancers Symposium; Orlando, Florida. January 25–27, 2008; Abstract 129. [Google Scholar]
- 146.Abou-Alfa G, Amadori D, Santoro A, et al. Is sorafenib safe and effective in patients with hepatocellular carcinoma and Child-Pugh B cirrhosis? J Clin Oncol. 2008;26(Suppl 1) [abstract] Abstract 4518. [Google Scholar]
- 147.Miller AA, Murry DJ, Owzar K, et al. Pharmacokinetic and phase I study of sorafenib for solid tumors and hematologic malignancies in patients with hepatic or renal dysfunction: CALGB 60301 [abstract]. J Clin Oncol. 2007;25(Suppl 1) Abstract 3538. [Google Scholar]
- 148.Yau T, Chan P, Ng KK, et al. Efficacy and tolerability of single agent sorafenib in poor risk advanced hepatocellular carcinoma patients [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 15513. [Google Scholar]
- 149.Bartlett DL, Ramanthan RK, Ben-Josef E. Cancers of the biliary tree. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. 8th ed Wolters Kluwer; Lippincott Williams & Wilkins: 2008. pp. 1156–1186. [Google Scholar]
- 150.Petrowsky H, Wildbrett P, Husarik DB, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 151.Corvera CU, Blumgert LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 153.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 154.Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989–1995. Cancer. 1998;83:2618–2628. doi: 10.1002/(sici)1097-0142(19981215)83:12<2618::aid-cncr29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 155.D'Angelica M, Dalal KM, Dematteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. doi: 10.1245/s10434-008-0189-3. in press. [DOI] [PubMed] [Google Scholar]
- 156.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671–681. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 157.Jensen EH, Abraham A, Habermann EB, et al. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2008 doi: 10.1007/s11605-008-0772-8. in press. [DOI] [PubMed] [Google Scholar]
- 158.Daines WP, Rajagopalan V, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: a comprehensive update, part 2. Oncology (Williston Park) 2004;18:1049–1059. discussion 1060, 1065–1046, 1068. [PubMed] [Google Scholar]
- 159.Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–867. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 161.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10(Suppl 4):308–311. [PubMed] [Google Scholar]
- 163.Rajagopalan V, Daines WP, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: a comprehensive update, part 1. Oncology (Williston Park) 2004;18:889–896. [PubMed] [Google Scholar]
- 164.Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 166.Kim JY, Kim MH, Lee TY, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 167.Seo S, Hatano E, Higashi T, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery. 2008;143:769–777. doi: 10.1016/j.surg.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 168.Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181:819–827. doi: 10.2214/ajr.181.3.1810819. [DOI] [PubMed] [Google Scholar]
- 169.Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging. 2004;29:540–547. doi: 10.1007/s00261-004-0187-2. [DOI] [PubMed] [Google Scholar]
- 170.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 171.Pawlik TM, Nathan H, Aloia T, et al. A simplified staging system for intrahepatic cholangiocarcinoma [abstract].. Presented at the 2008 Gastrointestinal Cancers Symposium; Orlando, Florida. January 25–27, 2008; Abstract 194. [Google Scholar]
- 172.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lieser MJ, Barry MK, Rowland C, et al. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998;5:41–47. doi: 10.1007/pl00009949. [DOI] [PubMed] [Google Scholar]
- 174.Yeh CN, Jan YY, Yeh TS, et al. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004;11:606–611. doi: 10.1245/ASO.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 175.Nakagohri T, Asano T, Kinoshita H, et al. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–293. doi: 10.1007/s00268-002-6696-7. [DOI] [PubMed] [Google Scholar]
- 176.Isaji S, Kawarada Y, Taoka H, et al. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6:108–116. doi: 10.1007/s005340050092. [DOI] [PubMed] [Google Scholar]
- 177.Berdah SV, Delpero JR, Garcia S, et al. A Western surgical experience of peripheral cholangiocarcinoma. Br J Surg. 1996;83:1517–1521. doi: 10.1002/bjs.1800831108. [DOI] [PubMed] [Google Scholar]
- 178.Silva MA, Tekin K, Aytekin F, et al. Surgery for hilar cholangiocarcinoma; a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 179.Havlik R, Sbisa E, Tullo A, et al. Results of resection for hilar cholangiocarcinoma with analysis of prognostic factors. Hepatogastroenterology. 2000;47:927–931. [PubMed] [Google Scholar]
- 180.Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 181.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. doi: 10.1001/archsurg.134.3.261. [DOI] [PubMed] [Google Scholar]
- 182.Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10:S65–68. doi: 10.1002/lt.20266. [DOI] [PubMed] [Google Scholar]
- 183.Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 184.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 186.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 187.Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835–841. doi: 10.1067/mge.2002.129868. [DOI] [PubMed] [Google Scholar]
- 188.American Society for Gastrointestinal Endoscopy Technology Status Report. Photodynamic therapy for gastrointestinal disease. Gastrointest Endosc. 2006;63:927–932. doi: 10.1016/j.gie.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 189.Dumoulin FL, Gerhardt T, Fuchs S, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2003;57:860–867. doi: 10.1016/s0016-5107(03)70021-2. [DOI] [PubMed] [Google Scholar]
- 190.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 191.Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. doi: 10.1111/j.1572-0241.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 192.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 193.Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol. 2007;96:8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]
- 194.Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007;110:572–580. doi: 10.1002/cncr.22815. [DOI] [PubMed] [Google Scholar]
- 195.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 196.Macdonald OK, Crane CH. Palliative and postoperative radiotherapy in biliary tract cancer. Surg Oncol Clin N Am. 2002;11:941–954. doi: 10.1016/s1055-3207(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 197.Czito BG, Anscher MS, Willett CG. Radiation therapy in the treatment of cholangiocarcinoma. Oncology (Williston Park) 2006;20:873–884. discussion 886–878, 893–875. [PubMed] [Google Scholar]
- 198.Nelson JW, Ghafoori AP, Willett CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73:148–153. doi: 10.1016/j.ijrobp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68:178–182. doi: 10.1016/j.ijrobp.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 200.Wang SJ, Fuller CD, Kim JS, et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 201.Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 202.Borghero Y, Crane CH, Szklaruk J, et al. Extrahepatic bile duct adenocarcinoma: patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone. Ann Surg Oncol. 2008;15:3147–3156. doi: 10.1245/s10434-008-9998-7. [DOI] [PubMed] [Google Scholar]
- 203.Lim K, Oh E, Chie E, et al. Which is better in patients with curatively resected extrahepatic biliary tract cancer? Adjuvant concurrent chemoradiation alone versus CCRT followed by maintenance chemotherapy [abstract]. J Clin Oncol. 2008;26(Suppl 1) Abstract 15659. [Google Scholar]
- 204.Das P, Wolff RA, Abbruzzese JL, et al. Concurrent capecitabine and upper abdominal radiation therapy is well tolerated. Radiat Oncol. 2006;1:41. doi: 10.1186/1748-717X-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lin LL, Picus J, Drebin JA, et al. A phase II study of alternating cycles of split course radiation therapy and gemcitabine chemotherapy for inoperable pancreatic or biliary tract carcinoma. Am J Clin Oncol. 2005;28:234–241. doi: 10.1097/01.coc.0000156920.11091.12. [DOI] [PubMed] [Google Scholar]
- 206.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 207.Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer. 2005;92:1650–1654. doi: 10.1038/sj.bjc.6602576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol. 2003;14:1115–1120. doi: 10.1093/annonc/mdg281. [DOI] [PubMed] [Google Scholar]
- 209.Doval DC, Sekhon JS, Gupta SK, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516–1520. doi: 10.1038/sj.bjc.6601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16:279–281. doi: 10.1093/annonc/mdi046. [DOI] [PubMed] [Google Scholar]
- 211.Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 212.Koeberle D, Saletti P, Borner M, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2008;26:3702–3708. doi: 10.1200/JCO.2008.16.5704. [DOI] [PubMed] [Google Scholar]
- 213.Andre T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 214.Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kobayashi K, Tsuji A, Morita S, et al. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and low-dose consecutive (Cisplatin) in advanced biliary tract carcinoma. BMC Cancer. 2006;6:121. doi: 10.1186/1471-2407-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yonemoto N, Furuse J, Okusaka T, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 218.Kim MJ, Oh DY, Lee SH, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008;8:374. doi: 10.1186/1471-2407-8-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil. And leucovorin in advanced biliary tract and gallbladder carcinoma. A North Central Cancer Treatment Group phase II trial. Cancer. 2005;103:111–118. doi: 10.1002/cncr.20753. [DOI] [PubMed] [Google Scholar]
- 220.Ghafoori AP, Nelson JW, Willett C, et al. Chemoradiation for inoperable extrahepatic cholangiocarcinoma [abstract].. Presented at the 2008 Gastrointestinal Cancers Symposium; Orlando, Florida. January 25–27, 2008; Abstract 166. [Google Scholar]