Abstract
In this greenhouse experiment we tested whether (i) ubiquitous arbuscular mycorrhizal fungi (AMF) taxa (Glomus claroideum, Glomus geosporum, Glomus intraradices, Glomus mosseae) singly and in a mixture differently affect growth and biomass production of four co-occurring grassland species (grass: Arrhenatherum elatius, non-leguminous forbs: Plantago lanceolata, Salvia pratensis and leguminous forb Trifolium pratense), and (ii) different soil sand contents alter AMF influence. We hypothesized that AMF effects on plants will increase with an increased AMF diversity and with increasing sand content. Percent AMF colonization of roots differed between plant species and AMF taxa and was higher with higher sand content. Plant growth responses to AMF were species-specific both regarding plants and AMF. Generally, biomass production of the non-leguminous forbs was the most responsive, the grass species the least and the legume intermediate both for AMF treatments and sand content. Across species, AMF influence on plant biomass increased with increasing soil sand content. Plant species growing in soil containing a mix of four AMF taxa showed similar growth responses than species in soil containing only one AMF taxon. These results suggest that both interference among AMF taxa and soil sand content can trigger the influence of AMF on plant production in grassland species.
Keywords: AMF inoculation, AMF taxa, Biomass allocation, Plant biomass production, Grassland, Soil texture, Plant growth responses, Root growth, Shoot growth
Highlights
► Ubiquitous AMF taxa of the Glomus group differently affected plant growth.► Plant growth responses were not affected by the diversity of the AMF taxa in the inoculum. ► Shoot and root growth was differently affected by different AMF taxa. ► Relative contribution of grass, forb and legume species was affected by AMF taxa. ► AMF influence on plant growth increased with increasing soil sand content.
1. Introduction
Most natural plant communities contain arbuscular mycorrhizal fungi (AMF) communities that vary from each other in their composition and species richness [1,2]. AMF form extensive hyphal networks in the soil, provide the plants with nutrients in return for assimilates [3], act as support systems for seedling establishment [4] and influence plant invasion success [5,6]. Moreover, plant diversity and plant community structure is influenced by AMF and by the composition of AMF communities [7–10]. Another way in which AMF may influence plant communities is by improving soil structure and soil aggregation by binding and enmeshing soil particles together into bigger aggregates [11–13]. Despite this broad evidence of AMF effects, relatively little is known on how different soil texture (e.g. sand content) alters AMF effects.
Although, all taxa within a AM fungal community may be able to infect the majority of plants [14,15], they have been shown to influence plants differentially [16,17]. There is good evidence to suggest a functional diversity between AMF taxa [18,19] and the identity of the plant and the fungus has been shown to determine the direction and magnitude of the response [20,21].
In the current study, we examined whether different AMF taxa of the Glomus group common to temperate grasslands and arable land affect the growth and biomass production of co-occurring plant species (grass: Arrhenatherum elatius (L.) P.Beauv., non-leguminous forbs: Plantago lanceolata L., Salvia pratensis L., leguminous forb Trifolium pratense L.) and whether different soil sand contents alter AMF-plant interactions. We hypothesized that (i) single AMF taxa would have less effect on plant growth than a mix of four AMF taxa because resource niches would be better exploited by more diverse AMF assemblages [22,23], and (ii) AMF effects would be more beneficial to plants growing in soils with a higher sand content as compared to those with a lower sand content due to the AMF-induced alleviation in nutrient limitations. The considered plant species differ in their root morphology and growth strategies and are hypothesized to also differ in their dependency on AMF symbiosis [16]. Moreover, legumes form symbioses with AMF and rhizobia, both influencing plant nutrition, and are often highly dependent on AMF to supply extra phosphorus required for nitrogen fixation [24–26].
2. Materials and methods
2.1. Experimental setup and treatments
Experiments were established in 54 plastic containers (15 × 15 × 25 cm deep, containing approx. 5 l soil) under glasshouse conditions. Growth substrate consisted of steam-sterilized (120 °C for 6 h) soil (Haplic Chernozem, silty loam, pH = 7.6, Corg = 2.2%) obtained from an arable field (Experimental farm, University of Natural Resources and Applied Life Sciences Vienna) mixed with 25%, 50% or 75% vol/vol of fire sterilized quartz sand (particle size 1.4–2.2 mm). At the bottom of each container, a small hole was made for drainage. The containers were inoculated with either 200 g inoculum containing one of four AMF taxa typically of temperate grassland and arable land [Glomus geosporum (Nicol. & Gerd.) C. Walker (La Banque Européenne des Glomales – BEG 199), Glomus intraradices N.C. Schenck & G.S. Smith BEG 163, Glomus mosseae (T.H. Nicolson & Gerdemann) Gerdemann & Trappe BEG 198, Glomus claroideum N.C. Schenck & G.S. Smith BEG 96] obtained from a commercial supplier (Symbiom, Lanskroun, Czech Republic; www.symbiom.cz); or a mixture of these four AMF isolates (50 g of each AMF taxa). Control treatments were inoculated with 200 g of steam-sterilized inoculum of these four AMF isolates. Each treatment was replicated three times. Each pot received a 100 ml filtered microbial wash from the mixed AMF treatment and 280 ml filtered washing of field soil to correct for possible differences in microbial communities between the different inocula, and to include microbial communities from the arable field [27]. To prepare the microbial wash, a total of 0.5 kg of the mixed inoculum and 2.2 kg field soil were wet-sieved through a cascade of sieves where the finest sieve had a mesh size of 10 μm.
In each container, one seedling of each of the four different plant species was planted in a constant pattern (i.e. each species had the same neighbors). Seeds germinated on moist paper towel and seedlings were transplanted into pots when about two days old. In each corner of the pot, one seedling of A. elatius, S. pratensis, P. lanceolata and T. pratense was planted in equal distance from the border and of each other (about 3 cm). This approach was chosen to avoid potential differences between treatments being confounded by neighborhood interactions and initial plant species composition [4]. Pots were randomly arranged at a greenhouse table. Pots were planted at the end of April 2008 and maintained in the greenhouse during three months. Average air temperature at night in the greenhouse was 19 °C; day temperatures varied between 23 °C and 35 °C depending on the weather conditions outside. The glass of the greenhouse was painted with white shade protection paint to avoid direct sunlight. Pots were watered three times a week with a constant amount of tap water and were not fertilized during the course of the experiment.
2.2. Measurements and harvesting
2.2.1. Plant parameters
After 12 weeks, pots were harvested and each individual plant was gently removed from the soil avoiding damage to the root systems. Plants were then divided in shoots and roots. Shoots were dried at 50 °C for at least 24 h and weighed. Roots were carefully washed free of soil, placed in a Petri-dish (diameter 15 cm) and scanned using a flatbed scanner (300 dpi). On these root images root surface was determined using the image analysis software ImageJ (vers. 1.40 g, National Institutes of Health USA, http://rsb.info.nih.gov/ij/java1.5.0_16). After scanning, roots were dried at 50 °C for at least 48 h and weighed.
2.2.2. AMF parameters
After determining root dry weight, the same roots were softened in water for 1 day, ink-stained [28] and the percentage of root length colonized by AMF was estimated for each species by the grid-line intersect method using 100 intersections per sample under the microsope [29].
2.3. Soil chemical analysis
Total nitrogen was measured by dry combustion using an elemental analyzer (ISO13878), available P and K was determined after calcium–acetate–lactate extraction (CAL method; [30]). All analyses were conducted by an external laboratory (Austrian Agency for Health and Food Safety – AGES, Vienna, Austria).
2.4. Statistical analysis
For each variable, we first performed a three-way ANOVA with plant species (four levels), sand content (three levels) and AMF (six levels) as fixed factors (Proc GLM; SPSS ver. 17). Then individually for each plant species, two-way ANOVAs with sand content and AMF and their interactions were performed. Tukey’s multiple comparison tests were performed to test which AMF treatments differed from each other within a particular sand content level. We used sequential Bonferroni adjustments to correct for multiple testing. The variables root surface, root biomass and shoot biomass were log-transformed prior to analysis to meet normalized distribution and homoscedasticity of variance among treatments. Pearson correlations were performed to test for possible relationships between AMF root colonization and plant biomass.
3. Results
3.1. Soil nutrient concentrations and AMF colonization levels
Soil mixture with 25%, 50% and 75% sand content contained 0.114 ± 0.007% Ntot, 0.102 ± 0.003% Ntot and 0.093 ± 0.014% Ntot,; 136.33 ± 1.20 mg K kg−1, 119.3 ± 0.9 mg K kg−1 and 102.7 ± 0.7 mg K kg−1 and 66.00 ± 0.58 mg P kg−1, 62.33 ± 0.33 mg P kg−1 and 56.67 ± 0.88 mg P kg−1, respectively. Soil pH averaged 7.63 ± 0.03 across the tested soil mixtures.
Percent colonization (Table 1) differed significantly between plant species (F3,113 = 12.578; P < 0.001) and was highly significantly affected by AMF treatments (F5,113 = 56.952; P < 0.001) and sand content (F2,113 = 18.023, P < 0.001; AMF × sand content interaction term: F10,113 = 3.068; P = 0.002). Comparing plant species, no clear pattern of the effects of AMF taxa on percent root colonization was detected: AMF taxa had different effects in different plant species and these effects varied with soil sand contents (Tables 1and 2). Inoculation with a single AMF taxon led to higher or lower percent colonization than inoculation with the AMF mix in all plant species (Table 1). Across plant species, G. mosseae inoculation showed the lowest percent colonization at sand contents <75%. Inoculation with G. geosporum or G. claroideum showed across sand contents the highest colonization in A. elatius, P. lancolata; G. claroideum in S. pratensis (Table 1). Across AMF taxa, there was a trend toward higher percent colonization with increasing soil sand content for A. elatius, P. lanceolata and S. pratensis but not for T. pratense where higher colonization levels where found at 50% than at 25% or 75% sand content (Table 1). Roots of the legume regularly showed root nodules, however this was not assessed quantitatively.
Table 1.
AMF root colonization of four grassland plant species in pots containing different sand contents inoculated with different AMF taxa of the genus Glomus. Different letters after the means indicate significant differences between treatments within each plant species growing in a particular soil type (Tukey-HSD test with Bonferroni correction). Means ± SE, n = 3.
| Parameter | Soil sand content |
||
|---|---|---|---|
| 25% sand | 50% sand | 75% sand | |
| Arrhenatherum elatius | |||
| No AMF | 3.3 ± 2.3b | 0.0 ± 0.0b | 0.0 ± 0.0c |
| G. claroideum | 9.3 ± 0.9a | 50.2 ± 14.8a | 44.2 ± 14.3a |
| G. geosporum | 28.2 ± 11.6a | 48.5 ± 11.5a | 55.3 ± 8.7a |
| G. intraradices | 10.5 ± 5.8a | 11.0 ± 0.2b | 27.2 ± 13.7b |
| G. mosseae | 0.8 ± 0.6b | 9.3 ± 0.5b | 27.8 ± 3.5b |
| AMF mix | 24.7 ± 7.5a | 5.9 ± 1.0b | 23.0 ± 6.8b |
| Plantago lanceolata | |||
| No AMF | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c |
| G. claroideum | 28.5 ± 10.4a | 54.4 ± 2.5a | 59.3 ± 3.4a |
| G. geosporum | 35.7 ± 15.2a | 36.3 ± 14.1a | 59.9 ± 1.1a |
| G. intraradices | 29.3 ± 16.9a | 50.7 ± 1.2a | 53.0 ± 15.7a |
| G. mosseae | 12.3 ± 7.8b | 8.2 ± 3.4b | 42.3 ± 1.9a |
| AMF mix | 31.5 ± 4.5a | 12.8 ± 2.4b | 32.0 ± 5.7b |
| Salvia pratensis | |||
| No AMF | 1.4 ± 1.0b | 0.0 ± 0.0c | 0.2 ± 0.1b |
| G. claroideum | 38.9 ± 9.4a | 73.7 ± 0.5a | 61.0 ± 10.5a |
| G. geosporum | 25.8 ± 8.4a | 56.1 ± 14.3a | 64.1 ± 2.0a |
| G. intraradices | 28.7 ± 10.3a | 42.8 ± 13.5a | 48.5 ± 11.5a |
| G. mosseae | 8.5 ± 5.7b | 14.0 ± 3.0b | 44.8 ± 5.5a |
| AMF mix | 29.5 ± 6.5a | 70.5 ± 1.2a | 59.3 ± 1.6a |
| Trifolium pratense | |||
| No AMF | 0.0 ± 0.0c | 0.0 ± 0.0d | 0.4 ± 0.3c |
| G. claroideum | 34.6 ± 1.2a | 53.8 ± 6.4b | 57.3 ± 3.9a |
| G. geosporum | 38.7 ± 0.9a | 58.3 ± 6.5b | 65.7 ± 4.5a |
| G. intraradices | 24.1 ± 3.6b | 76.2 ± 1.6a | 53.0 ± 1.6a |
| G. mosseae | 0.0 ± 0.0c | 33.6 ± 0.7c | 55.9 ± 12.6a |
| AMF mix | 44.6 ± 7.1a | 86.8 ± 0.5a | 40.8 ± 0.8b |
Table 2.
ANOVA table (F-values) of the effects of AMF inoculation and soil sand content on percent AMF colonization, plant biomass parameters and root growth of pots and for each plant species investigated. Significant effects (P < 0.05) after sequential Bonferroni adjustments are in bold.
| Dependent variable | Source of variation |
||
|---|---|---|---|
| AMF treatment F-value | Sand content F-value | AMF × Sand F-value | |
| Total pot measures | |||
| AMF colonization | 35.532 | 14.015 | 1.710 |
| Total plant biomass | 2.869 | 1.217 | 1.002 |
| Shoot biomass | 1.543 | 5.638 | 0.472 |
| Root biomass | 0.449 | 3.805 | 0.297 |
| Shoot:root ratio | 1.607 | 1.251 | 1.122 |
| Root surface | 2.851 | 5.726 | 1.342 |
| Arrhenatherum elatius | |||
| AMF colonization | 8.301 | 5.146 | 1.425 |
| Total biomass | 0.106 | 0.275 | 0.263 |
| Shoot biomass | 0.108 | 0.170 | 0.219 |
| Root biomass | 0.172 | 0.370 | 0.419 |
| Shoot:root ratio | 1.455 | 0.114 | 0.859 |
| Root surface | 0.351 | 0.660 | 0.549 |
| Plantago lanceolata | |||
| AMF colonization | 8.197 | 4.499 | 0.838 |
| Total biomass | 1.951 | 3.546 | 0.556 |
| Shoot biomass | 2.281 | 4.081 | 0.701 |
| Root biomass | 2.375 | 2.745 | 0.452 |
| Shoot:root ratio | 3.194 | 0.076 | 0.494 |
| Root surface | 2.355 | 3.907 | 0.657 |
| Salvia pratensis | |||
| AMF colonization | 17.211 | 11.889 | 1.465 |
| Total biomass | 1.924 | 9.619 | 0.962 |
| Shoot biomass | 2.236 | 7.332 | 1.161 |
| Root biomass | 2.007 | 9.627 | 1.030 |
| Shoot:root ratio | 2.037 | 1.969 | 1.013 |
| Root surface | 0.876 | 3.129 | 0.711 |
| Trifolium pratense | |||
| AMF colonization | 31.483 | 27.730 | 5.386 |
| Total biomass | 1.659 | 7.795 | 1.495 |
| Shoot biomass | 2.817 | 8.596 | 1.334 |
| Root biomass | 1.426 | 6.070 | 1.409 |
| Shoot:root ratio | 2.104 | 2.642 | 1.006 |
| Root surface | 0.733 | 2.426 | 0.893 |
3.2. Plant biomass production and root surface
Plant biomass production differed significantly between species (F3,124 = 3.078; P = 0.030) and sand content (F2,124 = 9.672; P < 0.001) and was marginally significantly affected by AMF treatments (F5,124 = 2.217; P = 0.057; AMF × sand interaction: F10,124 = 1.227; P = 0.280).
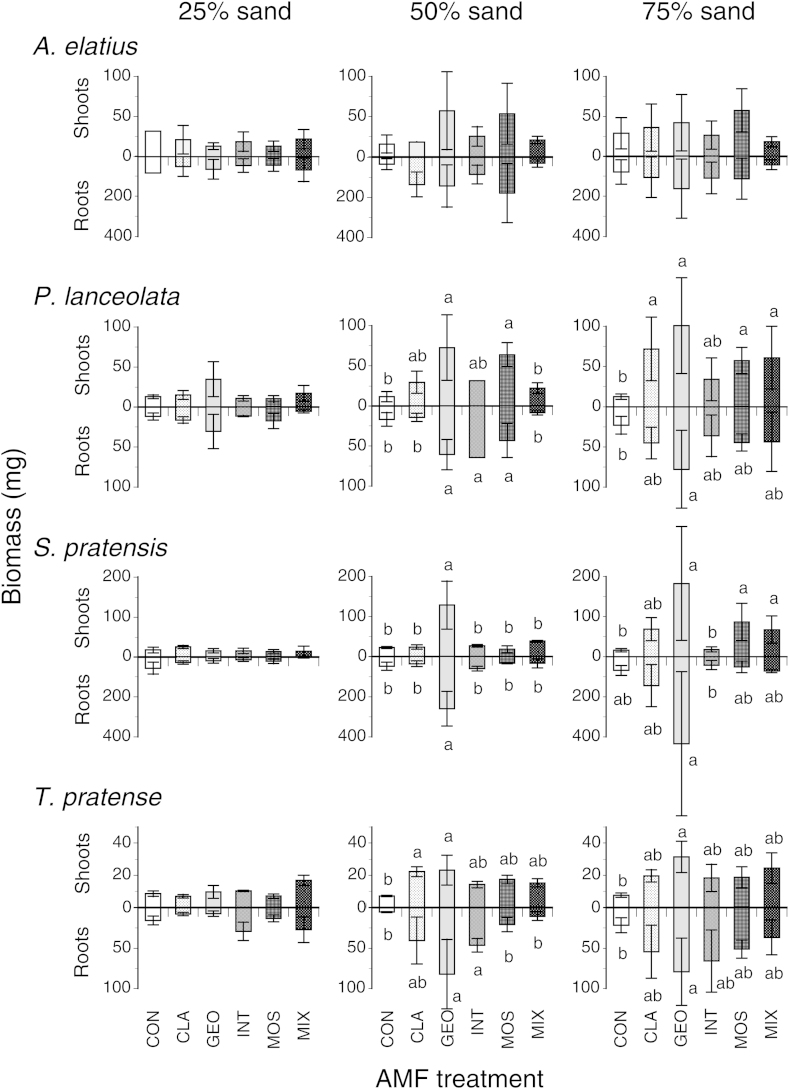
A detailed analysis of plant species responses (Fig. 1, Table 2) showed that biomass production of the grass A. elatius was neither affected by AMF treatment nor sand content. Total biomass, shoot, and root biomass of P. lanceolata, S. pratensis and T. pratense significantly increased with increasing soil sand content (Fig. 2, Table 2). Across sand contents, AMF taxa only significantly affected shoot biomass of T. pratense (Table 2). When individually testing effects of AMF taxa at each soil sand content we saw that at 25% sand content AMF taxa generally had no effect on shoot or root biomass of the tested plant species (Fig. 1). However, at 50% and 75% sand content AMF taxa had significant effects on both shoot and root production on all species except A. elatius (Fig. 1). Although the response patterns are heterogenous, there was a tendency toward higher shoot and root production in soil with 50% and 75% sand content inoculated with G. geosporum compared with pots inoculated with other AMF taxa (Fig. 1). No interactions between AMF and soil sand content on biomass production were found (Table 2).
Fig. 1.
Shoot and root biomass production in pots containing no AMF (CON), inoculum of the single AMF taxon Glomus claroideum (CLA), G. geosporum (GEO), G. intraradices (INT), G. mosseae (MOS) or a mixture of the four Glomus taxa (MIX) in soil containing either 25% (left column), 50% (middle column) or 75% sand (right column). Different letters indicate significant differences of AMF treatments within a particular soil type (Tukey-HSD test, α < 0.05). Means ± SE, n = 3.
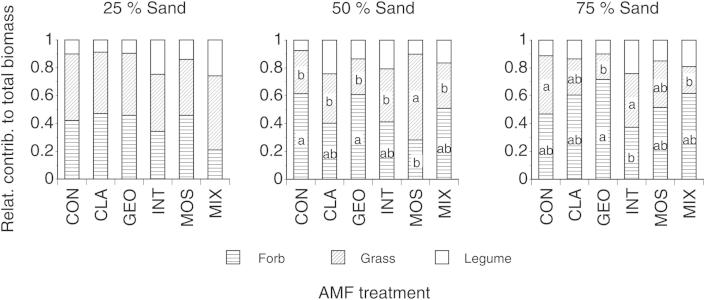
Fig. 2.
Contribution of non-leguminous forbs, the grass and the leguminous forb to the total biomass production in pots containing no AMF (CON), inoculum of the single AMF taxon Glomus claroideum (CLA), G. geosporum (GEO), G. intraradices (INT), G. mosseae (MOS) or a mixture of the four Glomus taxa (MIX) in soil containing different sand content. Different capital letters indicate significant differences of AMF treatments within a particular soil type on total plant biomass, different lower case letters indicate significant differences of plant functional groups (Tukey-HSD test, α < 0.05). Means ± SE, n = 3.
Shoot:root ratios varied significantly between the tested species (F3,124 = 13.139; P < 0.001), were significantly higher in AMF-inoculated pots than in pot without AMF (F5,124 = 6.350; P < 0.001) but unaffected by sand content (data not shown). Only shoot:root ratio of P. lanceolata was significantly affected by AMF treatments with higher shoot production with AMF inoculation compared to the control pots (Table 2).
Total plant biomass production per pot was on average 341 ± 64 mg (mean ± SE) and differed significantly between AMF treatments but was unaffected by sand contents (Table 2). Total shoot or root biomass per pot was unaffected by AMF treatments but increased significantly with increasing sand content (Table 2, Fig. 1). The contribution of plant functional groups to total biomass production per pot was significantly influenced by AMF taxa only in pots >25% sand (Fig. 2). In pots containing 50% sand, total biomass production was significantly different between functional groups (F2,32 = 7.399; P = 0.002) and AMF treatment (F5,32 = 3.473; P = 0.013) with pots without AMF inoculation and pots inoculated with G. geosporum showing a significantly higher forb mass than pots inoculated with G. mosseae (F5,12 = 3.779; P = 0.027; Fig. 2). In pots containing 75% sand, total biomass production was significantly different between functional groups (F2,34 = 4.105; P = 0.025) and significantly affected by AMF treatment (F5,34 = 3.953; P = 0.033) with pots inoculated with G. geosporum showing the highest and pots inoculated with G. intraradices showing the least biomass of non-leguminous forbs (F5,12 = 4.655; P = 0.019); while the biomasses of the legume and the grass were similar among AMF treatments.
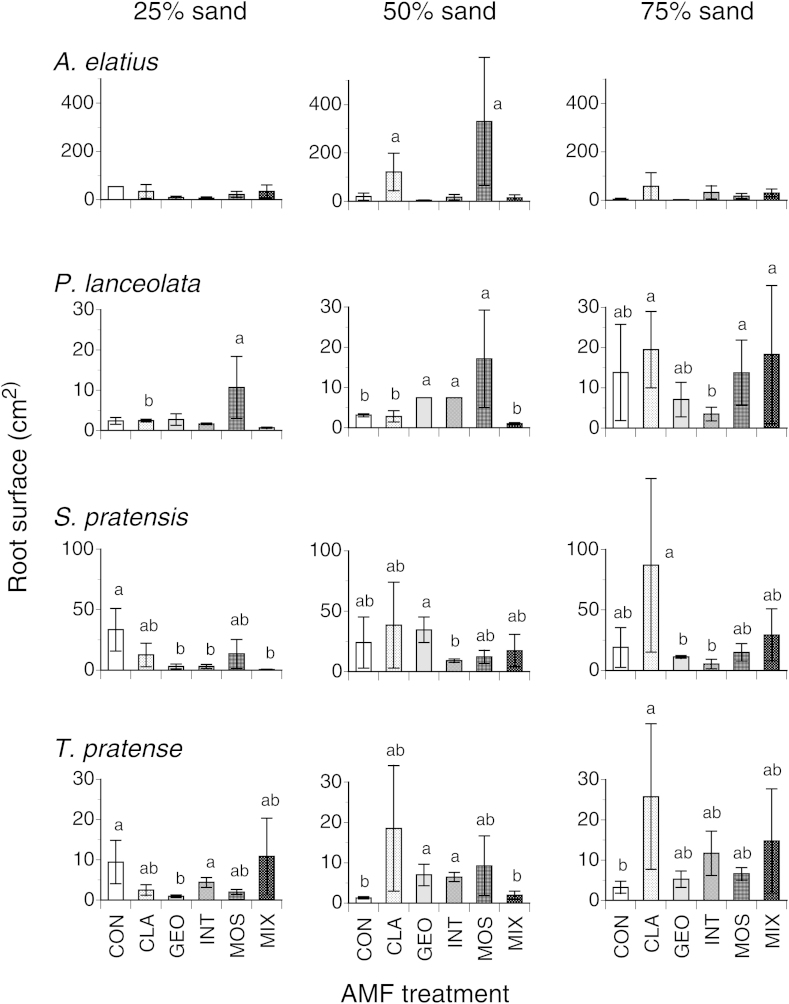
Root surface (Fig. 3) differed significantly between species (F3,118 = 3.162; P = 0.027) and sand content (F2,118 = 3.869; P = 0.024) but was unaffected by AMF taxa in the general analysis (F3,118 = 1.551; P = 0.179; AMF × sand interaction: F2,118 = 1.248; P = 0.268). However, analyses at particular sand contents showed across plant species a trend toward higher root surfaces in soils inoculated with AMF taxa and several significant effects of specific AMF taxa on root surface (Fig. 3). Only the root surface of P. lanceolata was significantly affected by sand content (F2,30 = 3.907; P = 0.031), while root surfaces of the other species remained unaffected by either AMF or sand treatments (Table 2).
Fig. 3.
Root surface of plants growing in pots containing no AMF (CON), inoculum of the single AMF taxon Glomus claroideum (CLA), G. geosporum (GEO), G. intraradices (INT), G. mosseae (MOS) or a mixture of the four Glomus taxa (MIX) in soil containing either 25% (left column), 50% (middle column) or 75% sand (right column). Different letters indicate significant differences of AMF treatments within a particular soil type (Tukey-HSD test, α < 0.05). Means ± SE, n = 3.
3.3. Correlations between AMF colonization and plant biomass
Across sand contents AMF root colonization was significantly positively correlated with either total pot shoot mass (r = 0.425, P = 0.001), total pot root mass (r = 0.358, P = 0.008) or total pot plant biomass (r = 0.374, P = 0.005). However, no correlations between AMF root colonization and total pot plant biomass could be found for each individual sand content (data not shown).
4. Discussion
The results presented in this paper show that, (i) AMF taxa of the Glomus group common to temperate grasslands and arable land can differ considerably in their effects on plant biomass production, (ii) soil inoculation with a single AMF taxon showed similar effects on plant biomass production than inoculation with a mix of four AMF taxa and (iii) plant species response to AMF inoculation varied significantly with soil sand content. The general lack of significant interactions between AMF and sand treatments implies that the impact of AMF is independent of soil sand content.
4.1. Sand content determines AMF effects
To the best of our knowledge, so far no study experimentally examined whether soil sand content affects the influence of specific AMF taxa on plant growth. We observed that AMF stimulated the growth of the four plant species only in systems with 50% and more sand content, while AMF had little influence in soil containing 25% sand. This pattern could be explained by alleviated nutrient limitation by AMF in soil containing more sand. However, in the current experiment soil nutrient contents differed only slightly and biomass production in the control pots without AMF was unaffected by sand content. For the non-leguminous forbs, the increasing AMF effect with increasing sand content was related to a higher root area and a higher shoot:root ratio with higher sand content. This suggests that forbs with the help of AMF allocated more biomass into shoots while growing a more extensive root system with increasing sand content. The weak but general correlation between AMF root colonization and total plant biomass production additionally backs this explanation. Perhaps this finding is also associated with a higher P acquisition in the AMF-inoculated pots paired with a higher biomass [23], although this was not tested in the current experiment. Our current finding is in line with observational studies showing that plants in sandy soil are usually highly mycorrhizal [31,32], however, this is perhaps not a universal pattern [33].
4.2. AMF diversity and plant growth
Increased mycorrhizal diversity (four compared with one AMF taxon) did not result in higher plant biomass in the current experiment. This is in accordance with results of other short-term studies [6,34] and a two year study when the number of AMF taxa increased from one to four (experiment 1 by [23]). But the current result is in contrast to an experiment where plant productivity increased when the number of AMF taxa increased from 1 to 16 (experiment 2 in [23]). However, in the latter study four functionally diverse AMF genera were present in contrast to this study and work previously published ([6,34] or experiment 1 in [23]). Our current finding thus also questions whether an ecosystem will per se benefit from higher AMF taxa richness as frequently suggested [22,23]. The high variability within the same AMF group [39] observed in the current study corroborates recent work showing that different AMF isolates of the same AMF taxon can induce highly variable plant growth responses [35,36]. Generally, differences in AMF effects can be explained by different speed of root colonization [37], amount of root colonization and spore production [3], the frequency of hyphal fusions and the integrity of hyphal networks [38], the physiological activities of nutrient uptake and transport pathways [40,41] or from interactions with other soil organisms and climatic factors [42–44]. Interestingly, soils inoculated with G. geosporum showed the highest plant biomass with little influence on root growth. As root colonization by this AMF taxon was not substantially higher than that of other AMF taxa this indicates the support of a more effective nutrient foraging in a given soil volume than the other AMF taxa tested or than roots. Our data also suggest that AMF taxa can affect root morphology with significant effects on root surface (e.g. G. claroideum vs. G. geosporum for Salvia at 75% sand content) but little influence on root biomass.
4.3. Plant AMF specificity
There is ample evidence that different plant species do not profit equally from AMF and some plants acquire more nutrients from AMF than others [3], which may, in turn, lead to changes in their biomass production and competitive relationships [45–47]. When testing 64 plant species growing with a single fungal species it was shown that AMF are just as likely to produce positive and negative effects, and the final outcome depends on the identity of the plant species [17]. In the current study we found that grass, forb and legume species did not benefit to the same extent from AMF, and among those that did, the extent of benefit depended upon the identity of the fungus (see also [8]). Our data suggest that sand content also influenced AMF dependency of plant species: with increasing sand content AMF dependency increased; this was most pronounced in the legume species. Mycorrhizal dependency has been shown to be related to P from AMF [48], although P supply is not always coupled with plant production [47]. Our current finding which shows that AMF effects are altered by sand content indicates that AMF dependency of a plant is not a fixed trait but depend on substrate conditions.
With the current experimental setup it is not possible to test AMF effects on competitive interactions of plants, however, the specific AMF-plant responses suggest that the biomass structure of co-existing non-leguminous forbs and grasses will be determined by AMF identity [6,34,45,49]. Moreover, our results suggest that both interference among AMF taxa and soil sand content can trigger the influence of AMF on plant production in grassland species.
Acknowledgments
We are grateful to the Austrian Science Fund for supporting this research (FWF grant no. P20171-B16). Help by Lina Weissengruber, Lisa Kargl, Birgit Putz, Barbara Heiner and Norbert Schuller in the greenhouse and laboratory is gratefully acknowledged. We thank Pia Euteneuer, Karl Refenner, Helmut Wagentristl and Rolf Mistlberger of BOKU Experimental Farm for providing technical and logistical support. Marcel van der Heijden and anonymous reviewers provided helpful comments on an earlier version of this manuscript.
Handling editor: Christoph Tebbe
References
- 1.Helgason T., Daniell T.J., Husband R., Fitter A.H., Young J.P.Y. Ploughing up the wood-wide web? Nature. 1998;394:431. doi: 10.1038/28764. [DOI] [PubMed] [Google Scholar]
- 2.Helgason T., Merryweather J.W., Denison J., Wilson P., Young J.P.W., Fitter A.H. Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J. Ecol. 2002;90:371–384. [Google Scholar]
- 3.Smith S.E., Read D.J. third ed. Academic Press; London: 2008. Mycorrhizal Symbiosis. [Google Scholar]
- 4.van der Heijden M.G.A. Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol. Lett. 2004;7:293–303. [Google Scholar]
- 5.Klironomos J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- 6.Stampe E.D., Daehler C.C. Mycorrhizal species identity affects plant community structure and invasion: a microcosm study. Oikos. 2003;100:362–372. [Google Scholar]
- 7.Gange A.C., Brown V.K., Farmer L.M. A test of mycorrhizal benefit in an early successional plant community. New. Phytol. 1990;115:85–91. [Google Scholar]
- 8.van der Heijden M.G.A., Boller T., Wiemken A., Sanders I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology. 1998;79:2082–2091. [Google Scholar]
- 9.Harnett D.C., Wilson W.T. Mycorrhizae influence plant community structure and diversity in tall grass prairie. Ecology. 1999;80:1187–1195. [Google Scholar]
- 10.O’Connor P.J., Smith S.E., Smith F.A. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New. Phytol. 2002;154:209–218. [Google Scholar]
- 11.Oades J.M. The role of biology in the formation, stabilization and degradation of soil structure. Geoderma. 1993;56:377–400. [Google Scholar]
- 12.Miller R.M., Jastrow J.D. Mycorrhizal fungi influence soil structure. In: Kapulnik Y., Douds D.D., editors. Arbuscular Mycorrhizae: Molecular Biology and Physiology. Kluwer Academic Press; Dordrecht, The Netherlands: 2000. pp. 3–18. [Google Scholar]
- 13.Rillig M.C., Mummey D.L. Mycorrhizas and soil structure. New. Phytol. 2006;171:41–53. doi: 10.1111/j.1469-8137.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 14.Allen M.F. The ecology of arbuscular mycorrhizas: a look back into the 20th century and a peek into the 21st. Mycol. Res. 1996;100:769–782. [Google Scholar]
- 15.Vandenkoornhuyse P., Husband R., Daniell T.J., Watson I.J., Duck J.M., Fitter A.H., Young J.P.W. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol. Ecol. 2002;11:1555–1564. doi: 10.1046/j.1365-294x.2002.01538.x. [DOI] [PubMed] [Google Scholar]
- 16.Klironomos J.N., McCune J., Hart M., Neville J. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol. Lett. 2000;3:137–141. [Google Scholar]
- 17.Hart M.M., Klironomos J.N. Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In: van der Heijden M.G.A., Sanders I.R., editors. Mycorrhizal Ecology. Springer-Verlag; Heidelberg, Germany: 2002. pp. 225–242. [Google Scholar]
- 18.Cavagnaro T.R., Smith F.A., Smith S.E., Jakobsen I. Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant. Cell. Environ. 2005;28:642–650. [Google Scholar]
- 19.Maherali H., Klironomos J.N. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science. 2007;316:1746–1748. doi: 10.1126/science.1143082. [DOI] [PubMed] [Google Scholar]
- 20.Ravnskov S., Jakobsen I. Functional compatibility in arbuscular mycorrhizas measured as hyphal P transport to the plant. New. Phytol. 1995;129:611–618. [Google Scholar]
- 21.Klironomos J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- 22.Grime J.P., Mackey J.M.L., Hillier S.H., Read D.J. Floristic diversity in a model system using experimental microcosms. Nature. 1987;328:420–422. [Google Scholar]
- 23.van der Heijden M.G.A., Klironomos J.N., Ursic M., Moutoglis P., Streitwolf-Engel R., Boller T., Wiemken A., Sanders I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- 24.Asimi S., Gianinazii-Pearson V., Gianinazzi S. Influence of increasing soil phosphorus levels on interactions between vesicular–arbuscular mycorrhizae and rhizobium in soybeans. Can. J. Bot. 1980;58:2200–2205. [Google Scholar]
- 25.Azcón R., Rubio R., Barea J.M. Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New. Phytol. 1991;117:399–404. doi: 10.1111/j.1469-8137.1991.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 26.Werner D. Chapman & Hall; London, UK: 1992. Symbiosis of Plants and Microbes. [Google Scholar]
- 27.Koide R.T., Li M. Appropriate controls for vesicular–arbuscular mycorrhiza research. New. Phytol. 1989;111:35–44. [Google Scholar]
- 28.Vierheilig H., Coughlan A.P., Wyss U., Piche Y. Ink and vinegar, a simple staining technique for arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovanetti M., Mosse B. An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New. Phytol. 1980;84:489–500. [Google Scholar]
- 30.VDLUFA . Datenblatt Vdlufa I, P und K, CAL–löslich, A 6.2.1.1. In: Blume H.-P., Deller B., Leschber R., Paetz A., Schmidt S., Wilke B.-M., editors. Handbuch der Bodenuntersuchung – Terminologie, Verfahrensvorschriften und Datenblätter. Physikalische, Chemische, Biologische Untersuchungsverfahren. Gesetzliche Regelwerke - Grundwerk. Beuth; Berlin, Germany: 2009. [Google Scholar]
- 31.Koske R.E., Gemma J.N. Mycorrhizae and succession in plantings of beachgrass in sand dunes. Am. J. Bot. 1997;84:118–130. [Google Scholar]
- 32.Rodriguez-Echeverria S., Freitas H. Diversity of AMF associated with Ammophila arenaria ssp arundinacea in Portuguese sand dunes. Mycorrhiza. 2006;16:543–552. doi: 10.1007/s00572-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 33.Cuenca G., De Andrade Z., Lovera M., Fajardo L., Meneses E. Mycorrhizal response of Clusia pusilla growing in two different soils in the field. Trees. 2003;17:200–206. [Google Scholar]
- 34.van der Heijden M.G.A., Wiemken A., Sanders I.R. Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plants. New. Phytol. 2003;157:569–578. doi: 10.1046/j.1469-8137.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- 35.Hart M.M., Read D.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New. Phytol. 2002;153:335–344. [Google Scholar]
- 36.Munkvold L., Kjoller R., Vestberg M., Rosendahl S., Jakobsen I. High functional diversity within species of arbuscular mycorrhizal fungi. New. Phytol. 2004;164:357–364. doi: 10.1111/j.1469-8137.2004.01169.x. [DOI] [PubMed] [Google Scholar]
- 37.Hart M.M., Reader R.J. Host plant benefit from association with arbuscular mycorrhizal fungi: variation due to differences in size of mycelium. Biol. Fertil. Soils. 2002;36:19–42. [Google Scholar]
- 38.Giovannetti M., Azzolini D., Citernesi A.S. Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 1999;65:5571–5575. doi: 10.1128/aem.65.12.5571-5575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton J.B., Benny G.L. Revised classifiction of arbuscular mycorrhizal fungi (Zygomycetes): a new order, glomales, two new suborders, Glominae and Gigasporinae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon. 1990;37:471–491. [Google Scholar]
- 40.Boddington C.L., Dodd J.C. Evidence that differences in phosphate metabolism in mycorrhizas formed by species of Glomus and Gigaspora might be related to their life-cycle strategies. New. Phytol. 1999;142:531–538. [Google Scholar]
- 41.Burleigh S.H., Cavagnaro T., Jakobsen I. Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J. Exp. Bot. 2002;53:1593–1601. doi: 10.1093/jxb/erf013. [DOI] [PubMed] [Google Scholar]
- 42.Zaller J.G., Caldwell M.M., Flint S.D., Scopel A.L., Sala O., Ballaré C.L. Solar UV-B radiation affects belowground parameters in a fen ecosystem in Tierra del Fuego, Argentina: implications of stratospheric ozone depletion. Glob. Change Biol. 2002;8:867–871. [Google Scholar]
- 43.Wurst S., Dugassa-Gobena D., Langel R., Bonkowski M., Scheu S. Combined effects of earthworms and vesicular–arbuscular mycorrhizas on plant and aphid performance. New. Phytol. 2004;163:169–176. doi: 10.1111/j.1469-8137.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhauer N., König S., Sabais A.C.W., Renker C., Buscot F., Scheu S. Impacts of earthworms and arbuscular mycorrhizal fungi (Glomus intraradices) on plant performance are not interrelated. Soil. Biol. Biochem. 2009;41:561–567. [Google Scholar]
- 45.Scheublin T.R., Van Logtestijn R.S.P., Van der Heijden M.G.A. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 2007;95:631–638. [Google Scholar]
- 46.Sanders I.R., Fitter A.H. The ecology and functioning of vesicular–arbuscular mycorrhizas in co-existing grassland species. II. Nutrient uptake and growth of vesicular–arbuscular mycorrhizal plants in a semi-natural grassland. New. Phytol. 1992;120:525–533. [Google Scholar]
- 47.Smith S.E., Smith F.A., Jakobsen I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New. Phytol. 2004;162:511–524. [Google Scholar]
- 48.van der Heijden M.G.A. Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search for underlying mechanisms and general principles. In: van der Heijden M.G.A., Sanders I.R., editors. Mycorrhizal Ecology. Springer-Verlag; Heidelberg, Germany: 2002. pp. 243–265. [Google Scholar]
- 49.Oliveira R.S., Castro P.M.L., Dodd J.C., Vosatka M. Different native arbuscular mycorrhizal fungi influence the coexistence of two plant species in a highly alkaline anthropogenic sediment. Plant. Soil. 2006;287:209–221. [Google Scholar]