Abstract
Nowadays, molecular analyses play an important role in studies of soil dwelling animals, for example in taxonomy, phylogeography or food web analyses. The quality of the DNA, used for later molecular analyses, is an important factor and depends on collection and preservation of samples prior to DNA extraction. Ideally, DNA samples are frozen immediately upon collection, but if samples are collected in the field, suitable preservation methods might be limited due to unavailability of resources or remote field sites. Moreover, shipping samples over long distances can cause loss of DNA quality e.g. by thawing or leaking of preservation liquid. In this study we use earthworms, a key organism in soil research, to compare three different DNA preservation methods – freezing at −20 °C, storing in 75% ethanol, and freeze drying. Samples were shipped from the United States of America to Austria. The DNA of the samples was extracted using two different extraction methods, peqGOLD™ and Chelex® 100. The DNA amplification success was determined by amplifying four DNA fragments of different length. The PCR amplification success is significantly influenced by preservation method and extraction method and differed significantly depending on the length of the DNA fragment. Freeze drying samples was the best preservation method when samples were extracted using the silica based extraction method peqGOLD™. For samples that were extracted with Chelex® 100, storage in ethanol was the best preservation method. However, the overall amplification success was significantly lower for the extraction procedure based on Chelex® 100. The detection of the small DNA fragments was higher and independent from the extraction method, while the amplification success was significantly reduced for the longer DNA fragments.
We recommend freeze drying of DNA samples, especially when they have to be shipped for longer distances. No special packaging or declaration is needed for freeze dried samples, and the risk of thawing is excluded. Storage of freeze dried samples also reduces costs because samples can be kept at room temperature in a desiccator. It should be noted, that the extraction methods showed significant differences in DNA amplification success. Thus, the extraction method should be taken into account when choosing the preservation method.
Keywords: Lyophilization, Preservation method, DNA extraction, Field samples, DNA quality
Highlights
-
•
How to best store and ship DNA samples generated in the USA for analyses in Austria?
-
•
Different preservation and extraction methods are compared by analysing tissue samples.
-
•
Freeze drying is the recommended preservation method, especially when samples have to be shipped.
-
•
The DNA amplification success is influenced by preservation and extraction method.
1. Introduction
DNA analyses have become an indispensable tool in soil zoology and soil ecology, especially in studies on trophic interactions [1], soil monitoring [2], and relationships within and among populations [3]. A critical factor for DNA analyses is the collection and the treatment of the samples prior to these analyses. Ideally, samples are taken in a laboratory under optimal conditions, where they can be processed immediately, but for most investigations on soil organisms samples have to be collected directly in the field. To avoid loss in DNA quality, it is therefore essential to optimize the preservation of samples and to find efficient and reliable transport methods. Earthworms play a major role in the composition of soil fauna, as they breakdown and recycle organic material, support plant growth due to their nutrient rich casts, improve soil quality [4], and help aerate the soil. Because of their diversity and importance in soils, earthworms have been studied for decades and molecular tools are increasingly applied to solve taxonomic and phylogenetic questions [5], [6], track predation on earthworms [7], reveal the phylogeography and mechanisms of distribution [8], or improve our understanding on litter decomposition [9]. Therefore, samples of earthworm tissue were chosen for the following experiments on preserving and shipping samples for subsequent DNA analyses. There is a variety of methods to preserve tissue and DNA samples that are well tested and frequently used including freezing, drying, and storage in ethanol or buffer [10], [11], [12]. Among the most often used preservation method of samples collected for DNA analyses is freezing. Freezing at −80 °C or in liquid nitrogen (−196 °C) [11], [13] is most often used for long term storage; for short term storage −20 to −28 °C is preferred [10], [14]. When samples are collected in the field, it is often difficult to freeze them immediately, and there is a risk of thawing during transport, especially with long distances. Alternatively, samples can be stored in absolute or in 75% ethanol. Ethanol was used successfully in various studies to preserve samples for PCR, sequence analyses or microsatellite amplification [11], [15], [16], [17], [18], [19], however, several studies observed considerable DNA degradation in tissue and environmental samples stored in 75% ethanol [10], [13]. Similarly, contradictory results are reported from drying samples [15], [20], [21]. Differences in preservation success may be explained partially by different methods of drying – air-drying, oven drying, chemical drying or freeze-drying [17], [18], [22]. Unfortunately, there are no systematic studies on stability and damage of DNA in dried samples [23] that would allow a general conclusion. In any case, both methods, preservation in ethanol or by drying, are cost efficient because storage is possible at room temperature.
Besides considering the quality of sample preservation, it may be necessary to calculate all costs and risks that are involved with transport, especially if transport requires several days. For this study we focused on three preservation methods and the effort and risks that arise if samples have to be shipped over long distances for several days. Samples were sent frozen, in 75% ethanol, and freeze-dried. Frozen samples can be shipped on ice, in liquid nitrogen or in dry ice, using special containers; however the needed equipment and the shipment service can become quite expensive, depending on the number of samples. The most cost efficient method is to send samples in maximal 2 kg dry ice. With extra insulation dry ice keeps the samples frozen for two to three days. The problem is that there is a considerable risk of thawing if the shipment is delayed due to customs.
The shipping duration is less important for samples preserved in ethanol. However, ethanol is a flammable liquid and therefore large amounts are classified as “dangerous goods” and require special packaging and transport. For samples that require less than a total amount of 500 ml ethanol at concentrations lower than 80%, a cheaper transport service is available. Shipping of dried samples causes the fewest problems; transport is not restricted by sample size or volume, and extended shipping duration is not a risk for the samples.
In the following experiment we tested three different preservation methods using pieces of earthworm tissue. Earthworms were easily available in high numbers from a breeder. In many zoological and ecological studies the initial sample quality can be reduced due to partial digestion, e.g. in fecal pellets. We simulated this situation by treating the tissue samples with sodium hypochlorite for different periods of time. After preserving the samples by freezing, in 75% ethanol or by freeze drying they were sent from North America to Europe using one of the widely available parcel services. As it is well known that the DNA extraction method is critical for the quality of a DNA sample, we tested two different extraction procedures and finally evaluated the three preservation methods based on the success in PCR amplifications targeting four DNA fragments that differed in length. The aim of this study was to find a preservation method that is reliable and allows cost efficient long time transport of the samples.
2. Material and methods
2.1. Earthworm samples
As the source for tissue samples, we used earthworms sold as live bait and later identified morphologically as Eisenia zebra (Michaelsen 1903). Seventy-five earthworms were rinsed under water and freeze-killed. A small piece, about one-sixth of the whole worm, cut from the middle of the earthworm body, was used for further treatments.
To simulate different sample quality with partially degraded DNA, earthworm pieces were treated with bleach (1% sodium hypochlorite). Five different bleaching durations were tested: 50, 30, 15, 5, and 0 (control) minutes. Each treatment was applied to 15 earthworm pieces. Finally, the earthworm pieces were individually put into 1.5 ml reaction vials and subjected to one of three preservation methods: freezing (F), ethanol (E), or freeze dried (D), ensuring that each of the 5 different bleaching treatments was represented by 5 samples in each of the preservation methods.
2.2. Storing and shipping methods
Ethanol samples were stored in 75% ethanol at room temperature. Frozen samples were kept in the freezer at −20 °C until shipped on dry ice. Freeze dried samples were stored in the freezer at −20 °C, and put frozen into the freeze drier, where they were vacuum dried using a Virtis 12ES (SPS SCIENTIFIC, Gardiner, NY, USA), at −50 °C and 30 mTorr pressure for two days. All samples were sent at the same day through FEDEX from Athens (Georgia, USA) to Innsbruck (Austria), and arrived without damage after 3 days. In the package of the frozen samples, no dry ice was left and the samples were thawed but still cold. Upon arrival they were immediately transferred to a −80 °C freezer, while the samples preserved in ethanol were kept at 4 °C in a refrigerator, and the dried samples were stored at room temperature.
2.3. DNA extraction
For the following analyses each of the 75 earthworm pieces was homogenized with glass beads in 300 μl PBS buffer (150 mM sodium phosphate, 150 mM NaCl, pH 7,2) and 5 μl Proteinase K (20 mg/ml) for 1 min at 5.000 rpm using a Precellys® Tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). From each homogenate 100 μl were used for a Chelex® 100 extraction and an additional 100 μl for a peqGOLD™ extraction. The Chelex® 100 extraction method was chosen because it is fast, cheap, and can be adapted to extract a large number of samples simultaneously [24]. After adding 200 μl of 10% Chelex® 100 to each sample, the samples were incubated overnight at 58 °C followed by 15 min deactivation of proteinase K at 94 °C. For the second extraction method a silica based extraction kit (peqGOLD™ Tissue DNA Mini Kit, PeqLab, Erlangen, Germany) was used, following the extraction protocol of the manufactures guidelines; with an incubation time of 1 h at 50 °C. This method was selected because it delivers high quality DNA, and the equipment needed is usually available in most laboratories. Extraction methods based on chloroform or CTAB are cheaper but were not considered as they require more handling and time. All extractions were done in a pre-PCR laboratory using a UV-equipped laminar flow hood. Extraction negative controls were included to check for cross-sample contamination, and all samples were finally stored at −28 °C.
The nucleic acid concentration of all extracts was measured using NanoDrop (ND-1000, NanoDrop Technologies, Inc., Wilmington, USA), following the manufacturer's guidelines.
2.4. PCR and visualization of PCR products
To test the success of DNA preservation, all extracts were tested in PCRs. As it is known from earlier studies that smaller DNA fragments can be detected even in highly digested DNA samples, four DNA fragments of different length were amplified by using appropriate general primers that were successfully used before with earthworm DNA [6] (i) a 431bp fragment of the mitochondrial 12SrDNA using the primers 12SH 10919 and 12SE1 10538 [25], (ii) a 547bp fragment of the nuclear 18SrDNA using the primers 18F3 and 18R925 [26], (iii) a 736bp fragment of the mitochondrial COI (cytochrome c oxidase subunit I) using LCO1490 and HCO2198 [27], and (iv) a 764bp fragment of the nuclear 18SrDNA using the primers 18F3 and 18S b0.5 (1197) [26], [28]. The 18srDNA is a multicopy gene and like the mitochondrial DNA it is also present in high copy numbers. Each 10 μl PCR contained 1 μl DNA extract, 1 μl of 10× Buffer (BioTherm™, GenXpress), 3 mM MgCl2, 0.2 mM dNTPs, 0.5 μM of each primer, 0.5 mg/ml bovine serum albumin (BSA) and 0.04 U/μl polymerase (BioTherm™, GenXpress). The PCR cycling protocol included 3 min at 95 °C, 35 cycles of 15 s at 95 °C, 30 s at 50 °C, 30 s at 72 °C and a final elongation of 3 min at 72 °C. One positive (DNA of Aporrectodea spp., Lumbricidae) and one negative control (PCR-grade water) were included within each PCR run to check for amplification success and DNA carry-over contamination. PCR products (1.5 μl per sample) were visualized by gel electrophoresis using GelRed™ (Genaxxon bioscience GmbH, Ulm, Germany) to stain the DNA. The quantity of PCR products was estimated based on a molecular weight marker. All samples which produced no or very weak (<10 ng/μl) PCR products were re-tested in a second PCR, to exclude false negatives.
2.5. Statistical analyses
Nucleic acid concentrations between preservation methods were compared using a Kruskal–Wallis-Test in SPSS (version 18.0.0). Samples of the two different extraction methods were calculated separately. The binomial ANOVA analysis was used to analyse the effects of bleach treatment, preservation method, extraction method, and length of target sequence on the amplification success. A Chi square test was calculated to support significances from the ANOVA. These tests were performed using R (R Development Core Team 2010; [29]). For binomial data analyses PCR results were counted as 1 for positive and as 0 for negative samples.
To compare the amplification success for the different DNA fragments, tilting confidence intervals, which adjust for bias and skewness in the bootstrap distribution and are asymmetrical, were calculated by 9999 bootstrap resamples using S-PLUS 8.1 (for Windows, TIBCO Spotfire, Somerville, USA). The tilting confidence interval was set at 95% and, accordingly, non-overlapping intervals indicate significant differences at P < 0.05.
3. Results
The ANOVA analysis revealed three parameters and their interactions that influence the amplification success – the shipping method, the extraction protocol, and the length of the fragment that was amplified during PCR.
3.1. Effect of bleach
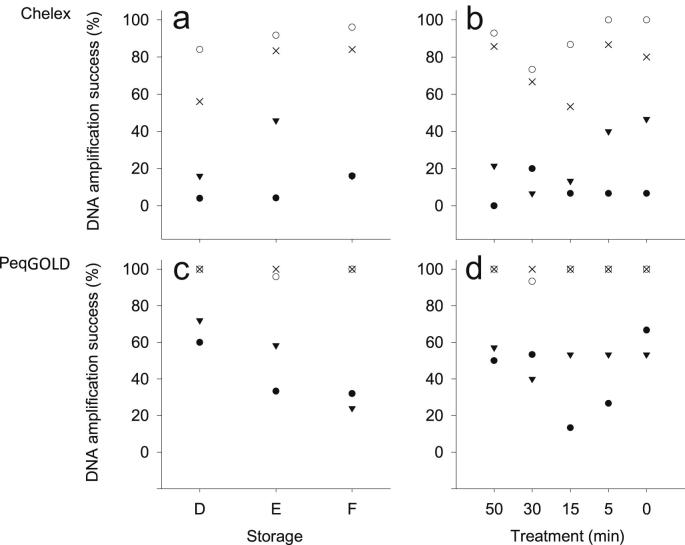
The five bleaching treatments did not show any decrease of the DNA amplification success with increasing time of bleaching. Considering the results of all PCRs (4 reactions for each of the 2 DNA extracts of a single tissue sample) 63.4%, 56.7%, 53.3%, 64.2%, and 69.2% of the 50 min, 30 min, 15 min, 5 min, and 0 min treatment, respectively, tested positive (Fig. 1b, d).
Fig. 1.
DNA amplification success of 431bp ○, 547bp ×, 736bp ●, and 764bp ▾ long DNA fragments, for Chelex® 100 extracted (a, b) and peqGOLD™ extracted (c, d) samples. Graphs a and c show the three different preservation methods: D freeze dried, E in ethanol, and F frozen. Graphs b and d show the amplification success for samples treated with bleach for different periods of time.
3.2. Storing and shipping methods
The overall amplification success, including results for all fragment lengths and of both extraction methods, did not differ much: the ratio of successful to unsuccessful PCR for frozen, ethanol conserved, and freeze dried samples was 117/83, 123/69, and 123/77, respectively.
The most obvious differences between the preservation methods could be observed for samples extracted with PeqGOLD™ (Fig. 1c). Here the DNA amplification success for the longest DNA fragment of 764bp, shows a significantly lower DNA amplification success for ethanol preserved and frozen samples (58.3%, χ2 = 30.42, P = 0.0216; 24.0%, χ2 = 30.42, P = 0.0372) compared to freeze dried samples. The DNA amplification success of the second longest fragment of 736bp was significantly lower for samples stored frozen (32.0%, χ2 = 32.21, P = 0.0393) than for samples stored in ethanol or freeze dried. The DNA amplification success of the two smallest fragments, 431bp and 547bp, was not significant influenced by the preservation method. The Chelex® 100 extracts did not show a similar trend (Fig. 1a). The 547bp long fragment was significantly better amplified from samples preserved in ethanol (83.3%, χ2 = 35.55, P = 0.0442) and by freezing (84.0%, χ2 = 35.55, P = 0.0367) than samples that were freeze dried. The longest fragment, 764bp, showed a significantly better DNA amplification success for samples preserved in ethanol (45.8%, χ2 = 30.42, P = 0.0288), compared to freeze dried and frozen samples (both 16%). For the Chelex® 100 extracts, the DNA amplification success of the 431bp and 736bp long fragments was not significantly influenced by the preservation method.
3.3. DNA extraction
The two extraction protocols differed significantly with respect to DNA amplification success. Only 49.7% of PCRs with samples extracted using Chelex® 100 resulted in an amplification product. PeqGOLD™ extracted samples showed a significantly better DNA amplification success – 72% (χ2 = 24.32, P = 0.0002). The difference in amplification success for the two smaller fragments (431bp and 541bp – 97.3% and 98.7% for peqGOLD™ and 90.5% and 74.3% for Chelex® 100 extracted samples) was not significant, but the two longer fragments, 736bp and 764bp, showed significantly higher DNA amplification success for samples extracted with peqGOLD™ (χ2 = 24.17, P < 0.0001, χ2 = 10.45, P = 0.0016).
The nucleic acid concentration varied between 1 and 101 ng/μl (mean 16 ng/μl) and 181 and 1857 ng/μl (mean 739 ng/μl) for PeqGOLD™ and Chelex® 100 extracted samples, respectively. Among the Chelex® 100 extracted samples those preserved in ethanol showed significantly lower nucleic acid concentrations compared to freeze dried and frozen samples. For samples extracted with PeqGOLD™, the nucleic acid concentration of the frozen samples was significantly lower than for samples freeze dried or preserved in ethanol.
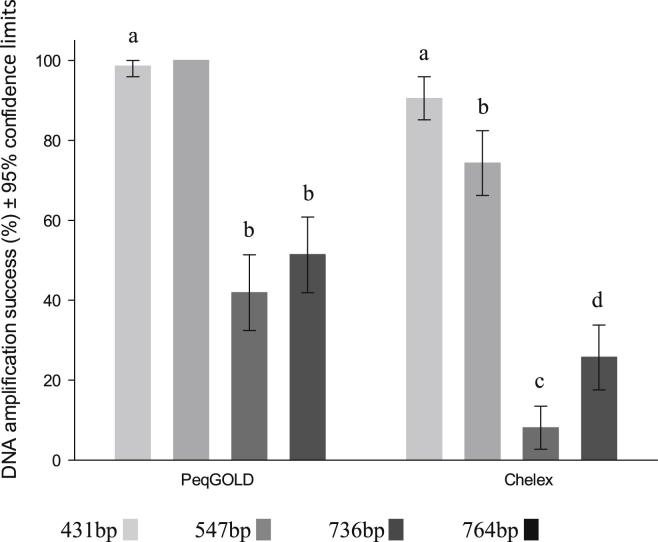
3.4. Fragment lengths
Shorter fragments had a better DNA amplification success than longer fragments (Fig. 2). The tilting confidence intervals (TCI) were calculated to support those data. The TCI of the DNA amplification success, for peqGOLD™ extracted samples, showed significantly better results for the shortest fragment (431bp) than for the longer fragments (736bp and 764bp) (notice: no TCI could be calculated for fragment 547bp as all samples were tested positive). Chelex® 100 extracted samples showed a significant difference in DNA amplification success between all fragment lengths. Mitochondrial genes are usually much more abundant in DNA extracts than nuclear genes, but this did not influence the DNA amplification success. The two longest fragments, COI (736bp) and 18SrDNA (764bp), showed comparable amplification success when extracted with PeqGOLD™. For Chelex® 100 extracted samples the amplification success for the nuclear gene was even better than for the mitochondrial one.
Fig. 2.
DNA amplification success including the 95% tilting confidence intervals (TCI) for DNA fragments differing in length (431bp, 547bp, 736bp, and 764bp). Samples extracted with peqGOLD™ or Chelex® 100 are shown separately (No TCI could be calculated for fragment 547bp as all samples were tested positive).
4. Discussion
The aim of the presented experiment was to find an optimal DNA preservation method with respect to risks and costs for shipping samples for several days over long distances. Interestingly, the best preservation method for tissue samples evaluated by PCR amplification success depended on the extraction method: the freeze-dried samples performed best when a silica based extraction kit was used, whereas the ethanol preserved samples worked best when samples were extracted with Chelex® 100. Considering the requirements and risks for shipping samples, freeze drying is the easiest and cheapest preservation method, followed by preservation with ethanol, which is restricted to a maximum volume of 500 ml ethanol or would require special shipment conditions for flammable goods. In contrast to frozen samples, freeze-dried and ethanol preserved samples are not at risk due to unexpected delays in transport. Working in remote areas usually requires transport of samples to a laboratory for further treatments. As this short distance transport is anyway necessary, it is likely to find a laboratory that provides the equipment needed for freeze drying. Thus, including all arguments, we recommend using freeze drying as the preferred method of sample preservation for long distance shipping.
Previous studies showed that short time storage (up to 1 year) of freeze- dried samples for later DNA extraction and PCR analyses, was successfully used for medical samples [30], microbial communities [31], algae and seagrass [32], or for feces [33], [34], [35]. Due to the recovery of ancient DNA from bones and other fossils it is known that DNA can survive in dried samples for up to 50,000 years [36], [37], [38], [39]. However, these ancient DNA samples also show considerable degrees of DNA degradation [37], [40]. Systematic experiments on the stability and degradation of DNA in dried tissue samples during long term storage are rare. Matsuo and co-authors [14] found only slight degradation of DNA and no significant influence on PCR analysis in freeze-dried liver tissue samples that had been stored for up to four years. Recent efforts to understand the degradation processes concentrate on samples of extracted DNA, with the main aim to reduce costs that arise for storing samples at −80 °C. According to these studies the two main factors increasing DNA breakdown even in well dried samples are humidity and oxygen [41], [42].
The most common way of preservation, freezing at −20 to −80 °C, did not perform best in our experiment. This is most likely a consequence of the temperature increase during shipment. Several studies found that singular thawing of samples, or recurring thawing and freezing of samples can lead to DNA degradation [43], [44], [45]. It is likely that a shorter transportation time or a better insulation of the package that ensures constantly frozen samples may lead to an overall better performance of this preservation method. Therefore, the decision against freezing as the preferred preservation method is guided mainly by handling convenience.
PCR analyses have become an important tool in ecological studies, especially because detection of minute amounts of DNA is possible. Analysing trophic interactions, distribution of individuals, migration, and relationships among populations is possible from samples that are small or even digested like feces, prey remains, shed skin cells, saliva or gut content [46], [47], [48], [49]. To simulate partially digested samples we treated the earthworm pieces with bleach. Bleach has been used in several studies to break down DNA remains, in bones and teeth or on the surface of maggots, and spotted pink ladybug [50], [51], [52]. Based on these studies we selected the treatments of five to 50 min with 1% active sodium hypochlorite. There was no effect observed in our study. Obviously longer incubation times and a higher concentration would be needed to significantly degrade DNA in tissue samples.
The nucleic acid concentration differed considerably between the extraction methods, which is due to limited binding capacity of the silica-based matrix. The storage methods did show an impact on the nucleic acid concentration but there was no correlation between nucleic acid content and DNA amplification success. This can be explained due to the sensitivity of our PCR assays that has been tested successfully with samples containing less than 1 ng/μl of DNA.
For studies with high sample numbers, fast and cost efficient DNA extraction methods are an advantage. Therefore, the Chelex® 100 extraction protocol is still used frequently, although it is known that the amplification rate of the DNA extracts is reduced compared to samples extracted with other extraction methods [53]. In our experiment the storage of samples in ethanol performed best for samples treated with Chelex® 100. It is worth noticing, that for short DNA fragments there is no difference in the DNA amplification success between the two extraction methods. When choosing an optimal preservation and extraction method, it is important to consider the length of target sequence in the subsequent DNA analyses. Numerous studies on trophic interactions and sequence analyses of ancient DNA prove that small DNA fragments can survive degradation processes during digestion, decomposition, or fossilization [36], [54], [55], [56]. Thus the choice of preservation method has to be considered with respect of the research question.
5. Conclusion
In conclusion we recommend freeze drying samples, especially when they are sensitive to thawing, and have to be shipped for longer distances. Freeze- dried samples do not need any special packaging or declaration of dangerous goods, the risk of DNA degradation for samples that are delayed due to transport is minimal, and storage at room temperature is cheaper than freezing samples. Storing freeze dried samples up to a year at room temperature is possible [30] but it is recommended to use a desiccator. It also would be an asset if the choice of the extraction method is made before the samples are taken, to find the best storage method and thus ensure the best DNA amplification success.
Acknowledgements
The research was supported by the Austrian Science Fund (FWF – P21629-B19). We thank Dr. John C. Maerz, Dr. Paul F. Hendrix, Thomas R. Maddox (Univ. of Georgia, Athens, USA), Dr. Julia Seeber, Dr. Michael Traugott (Univ. of Innsbruck, Austria) and our working group for valuable discussion and comments on the manuscript.
Handling editor: Stefan Schrader
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Daniela Straube, Email: Daniela.Straube@uibk.ac.at, Daniela.Straube@gmx.net.
Anita Juen, Email: Anita.Juen@uibk.ac.at.
References
- 1.Staudacher K., Wallinger C., Schallhart N., Traugott M. Detecting ingested plant DNA in soil-living insect larvae. Soil Biology and Biochemistry. 2011;43:346–350. doi: 10.1016/j.soilbio.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X.Y., Daniell T.J., Neilson R., O'Flaherty V., Griffiths B.S. A comparison of molecular methods for monitoring soil nematodes and their use as biological indicators. European Journal of Soil Biology. 2010;46:319–324. [Google Scholar]
- 3.Beebee T., Rowe G. Oxford University Press; 2008. An Introduction to Molecular Ecology. [Google Scholar]
- 4.Bohlen P.J., Parmelee R.W., McCartney D.A., Edwards C.A. Earthworm effects on carbon and nitrogen dynamics of surface litter in corn agroecosystems. Ecological Applications. 1997;7:1341–1349. [Google Scholar]
- 5.Iglesias Briones M.J., Morán P., Posada D. Are the sexual, somatic and genetic characters enough to solve nomenclatural problems in lumbricid taxonomy? Soil Biology and Biochemistry. 2009;41:2257–2271. [Google Scholar]
- 6.Klarica J., Kloss-Brandstatter A., Traugott M., Juen A. Comparing four mitochondrial genes in earthworms – implications for identification, phylogenetics, and discovery of cryptic species. Soil Biology and Biochemistry. 2012;45:23–30. [Google Scholar]
- 7.Brown D.S., Jarman S.N., Symondson W.O.C. Pyrosequencing of prey DNA in reptile faeces: analysis of earthworm consumption by slow worms. Molecular Ecology Resources. 2012;12:259–266. doi: 10.1111/j.1755-0998.2011.03098.x. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Losada M., Breinholt J.W., Porto P.G., Aira M., Dominguez J. An earthworm riddle: systematics and phylogeography of the Spanish lumbricid Postandrilus. PLoS One. 2011;6:e28153. doi: 10.1371/journal.pone.0028153. 28151–28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapp B.A., Podmirseg S.M., Seeber J., Meyer E., Insam H. Diet-related composition of the gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning. Soil Biology and Biochemistry. 2009;41:2299–2307. [Google Scholar]
- 10.Seutin G., White B.N., Boag P.T. Preservation of avian blood and tissue samples for DNA analyses. Canadian Journal of Zoology. 1991;69:82–90. [Google Scholar]
- 11.Michaud C.L., Foran D.R. Simplified field preservation of tissues for subsequent DNA analyses. Journal of Forensic Sciences. 2011;56:846–852. doi: 10.1111/j.1556-4029.2011.01771.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagy Z.T. A hands-on overview of tissue preservation methods for molecular genetic analyses. Organisms Diversity & Evolution. 2010;10:91–105. [Google Scholar]
- 13.Rissanen A.J., Kurhela E., Aho T., Oittinen T., Tiirola M. Storage of environmental samples for guaranteeing nucleic acid yields for molecular microbiological studies. Applied Microbiology and Biotechnology. 2010;88:977–984. doi: 10.1007/s00253-010-2838-2. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo S., Sugiyama T., Okuyama T., Yoshikawa K., Honda K., Takahashi R., Maeda S. Preservation of pathological tissue specimens by freeze-drying for immunohistochemical staining and various molecular biological analyses. Pathology International. 1999;49:383–390. doi: 10.1046/j.1440-1827.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 15.Dillon N., Austin A.D., Bartowsky E. Comparison of preservation techniques for DNA extraction from hymenopterous insects. Insect Molecular Biology. 1996;5:21–24. doi: 10.1111/j.1365-2583.1996.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 16.Frantzen M.A.J., Silk J.B., Ferguson J.W.H., Wayne R.K., Kohn M.H. Empirical evaluation of preservation methods for faecal DNA. Molecular Ecology. 1998;7:1423–1428. doi: 10.1046/j.1365-294x.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 17.Quick D.L.J., Belshaw R., Lopez-Vaamonde C. Preservation of hymenopteran specimens for subsequent molecular and morphological study. Zoologica Scripta. 1999;28:261–267. [Google Scholar]
- 18.Murphy M.A., Waits L.P., Kendall K.C., Wasser S.K., Higbee J.A., Bogden R. An evaluation of long-term preservation methods for brown bear (Ursus arctos) faecal DNA samples. Conservation Genetics. 2002;3:435–440. [Google Scholar]
- 19.Bubb A., Ehlers K., Kotze A., Grobler J.P. The effect of sample age and storage method on DNA yield and microsatellite amplification from baboon (Papio ursinus) faecal samples. European Journal of Wildlife Research. 2011;57:971–975. [Google Scholar]
- 20.Roon D.A., Waits L.P., Kendall K.C. A quantitative evaluation of two methods for preserving hair samples. Molecular Ecology Notes. 2003;3:163–166. [Google Scholar]
- 21.Wasser S.K., Houston C.S., Koehler G.M., Cadd G.G., Fain S.R. Techniques for application of faecal DNA methods to field studies of Ursids. Molecular Ecology. 1997;6:1091–1097. doi: 10.1046/j.1365-294x.1997.00281.x. [DOI] [PubMed] [Google Scholar]
- 22.Bainard L.D., Klironomos J.N., Hart M.M. Differential effect of sample preservation methods on plant and arbuscular mycorrhizal fungal DNA. Journal of Microbiological Methods. 2010;82:124–130. doi: 10.1016/j.mimet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Anchordoquy T.J., Molina M.C. Preservation of DNA. Cell Preservation Technology. 2007;5:180–188. [Google Scholar]
- 24.Casquet J., Thebaud C., Gillespie R.G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Molecular Ecology Resources. 2012;12:136–141. doi: 10.1111/j.1755-0998.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson B.G.M., Tillier S., Tillier A., Justine J.-L., Ling E., James S., McDonald K., Hugall A.F. Phylogeny of the Megascolecidae and Crassiclitellata (Annelida, Oligochaeta): combined versus partitioned analysis using nuclear (28S) and mitochondrial (12S, 16S) rDNA. Zoosystema. 2002:707–734. [Google Scholar]
- 26.Struck T., Hessling R., Purschke G. The phylogenetic position of the Aeolosomatidae and Parergodrilidae, two enigmatic oligochaete-like taxa of the ‘Polychaeta’, based on molecular data from 18S rDNA sequences. Journal of Zoological Systematics and Evolutionary Research. 2002;40:155–163. [Google Scholar]
- 27.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- 28.Whiting M.F. Molecular Systematics and Evolution: Theory and Practice, Birkhauser Verlag, Basel. 2002. Phylogeny of the holometabolous insect orders based on 18S ribosomal DNA: when bad things happen to good data; pp. 69–83. [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2010. R: A Language and Environment for Statistical Computing.http://www.R-project.org [Google Scholar]
- 30.Leboeuf C., Ratajczak P., Zhao W.L., Plassa L.F., Court M., Pisonero H., Murata H., Cayuela J.M., Ameisen J.C., Garin J., Janin A. Long-term preservation at room temperature of freeze-dried human tumor samples dedicated to nucleic acids analyses. Cell Preservation Technology. 2008;6:191–197. [Google Scholar]
- 31.Simister R.L., Schmitt S., Taylor M.W. Evaluating methods for the preservation and extraction of DNA and RNA for analysis of microbial communities in marine sponges. Journal of Experimental Marine Biology and Ecology. 2011;397:38–43. [Google Scholar]
- 32.Pearson G., Lago-Leston A., Valente M., Serrao E. Simple and rapid RNA extraction from freeze-dried tissue of brown algae and seagrasses. European Journal of Phycology. 2006;41:97–104. [Google Scholar]
- 33.Ruiz R., Rubio L.A. Lyophilisation improves the extraction of PCR-quality community DNA from pig faecal samples. Journal of the Science of Food and Agriculture. 2009;89:723–727. [Google Scholar]
- 34.C. Somgird, U. Kasetsart, DNA Extraction and Amplification from Freeze-dried Elephant Feces with Freeze-dryer, in: Proceedings of the 44th Kasetsart University Annual Conference, Animals, Veterinary Medicine, 2006, pp. 561–568.
- 35.Murphy M.A., Waits L.P., Kendall C. Quantitative evaluation of fecal drying methods for brown bear DNA analysis. Wildlife Society Bulletin. 2000;28:951–957. [Google Scholar]
- 36.Pruvost M., Schwarz R., Correia V.B., Champlot S., Braguier S., Morel N., Fernandez-Jalvo Y., Grange T., Geigl E.M. Freshly excavated fossil bones are best for amplification of ancient DNA. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:739–744. doi: 10.1073/pnas.0610257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofreiter M., Serre D., Poinar H.N., Kuch M., Paabo S. Ancient DNA. Nature Reviews Genetics. 2001;2:353–359. doi: 10.1038/35072071. [DOI] [PubMed] [Google Scholar]
- 38.Orlando L., Bonjean D., Bocherens H., Thenot A., Argant A., Otte M., Hanni C. Ancient DNA and the population genetics of cave bears (Ursus spelaeus) through space and time. Molecular Biology and Evolution. 2002;19:1920–1933. doi: 10.1093/oxfordjournals.molbev.a004016. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro B., Hofreiter M. Analysis of ancient human genomes: using next generation sequencing, 20-fold coverage of the genome of a 4,000-year-old human from Greenland has been obtained, BioEssays: news and reviews in molecular. Cellular and Developmental Biology. 2010;32:388–391. doi: 10.1002/bies.201000026. [DOI] [PubMed] [Google Scholar]
- 40.Briggs A.W., Stenzel U., Johnson P.L.F., Green R.E., Kelso J., Prufer K., Meyer M., Krause J., Ronan M.T., Lachmann M., Paabo S. Patterns of damage in genomic DNA sequences from a Neandertal. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colotte M., Coudy D., Tuffet S., Bonnet J. Adverse effect of air exposure on the stability of DNA stored at room temperature. Biopreservation and Biobanking. 2011;9:47–50. doi: 10.1089/bio.2010.0028. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 43.Farnert A., Arez A.P., Correia A.T., Bjorkman A., Snounou G., do Rosario V. Sampling and storage of blood and the detection of malaria parasites by polymerase chain reaction. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:50–53. doi: 10.1016/s0035-9203(99)90177-3. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki H., Inoue K., Turvy C.G., Guengerich F.P., Shimada T. Effects of freezing, thawing, and storage of human liver samples on the microsomal contents and activities of cytochrome P450 enzymes. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 1997;25:168–174. [PubMed] [Google Scholar]
- 45.Lahiri D.K., Schnabel B. DNA isolation by a rapid method from human blood samples: effects of MgCI2, EDTA, storage time, and temperature on DNA yield and quality. Biochemical Genetics. 1993;31:321–328. doi: 10.1007/BF02401826. [DOI] [PubMed] [Google Scholar]
- 46.Smiley T., Spelman L., Lukasik-Braum M., Mukherjee J., Kaufman G., Akiyoshi D.E., Cranfield M. Noninvasive saliva collection techniques for free-ranging mountain gorillas and captive eastern gorillas. Journal of Zoo and Wildlife Medicine. 2010;41:201–209. doi: 10.1638/2009-0015R.1. [DOI] [PubMed] [Google Scholar]
- 47.Kohn M.H., Wayne R.K. Facts from feces revisited. Trends in Ecology & Evolution. 1997;12:223–227. doi: 10.1016/s0169-5347(97)01050-1. [DOI] [PubMed] [Google Scholar]
- 48.Swanson B.J., Kelly B.P., Maddox C.K., Moran J.R. Shed skin as a source of DNA for genotyping seals. Molecular Ecology Notes. 2006;6:1006–1009. [Google Scholar]
- 49.King R.A., Read D.S., Traugott M., Symondson W.O.C. Molecular analysis of predation: a review of best practice for DNA-based approaches. Molecular Ecology. 2008;17:947–963. doi: 10.1111/j.1365-294X.2007.03613.x. [DOI] [PubMed] [Google Scholar]
- 50.Greenstone M.H., Weber D.C., Coudron T.C., Payton M.E. Unnecessary roughness? Testing the hypothesis that predators destined for molecular gut-content analysis must be hand-collected to avoid cross-contamination. Molecular Ecology Resources. 2011;11:286–293. doi: 10.1111/j.1755-0998.2010.02922.x. [DOI] [PubMed] [Google Scholar]
- 51.Kemp B.M., Smith D.G. Use of bleach to eliminate contaminating DNA from the surface of bones and teeth. Forensic Science International. 2005;154:53–61. doi: 10.1016/j.forsciint.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 52.Linville J.G., Wells J.D. Surface sterilization of a maggot using bleach does not interfere with mitochondrial DNA analysis of crop contents. Journal of Forensic Sciences. 2002;47:1055–1059. [PubMed] [Google Scholar]
- 53.Desloire S., Moro C.V., Chauve C., Zenner L. Comparison of four methods of extracting DNA from D. gallinae (Acari: Dermanyssidae) Veterinary Research. 2006;37:725–732. doi: 10.1051/vetres:2006031. [DOI] [PubMed] [Google Scholar]
- 54.Prost S., Smirnov N., Fedorov V.B., Sommer R.S., Stiller M., Nagel D., Knapp M., Hofreiter M. Influence of climate warming on arctic mammals? New insights from ancient DNA studies of the Collared Lemming Dicrostonyx torquatus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vural H.C., Tirpan A.A. Species determination of ancient bone DNA from fossil skeletal remains of Turkey using molecular techniques. Scientific Research and Essays. 2010;5:2250–2256. [Google Scholar]
- 56.Pääbo S., Higuchi R.G., Wilson A.C. Ancient DNA and the polymerase chain reaction – the emerging field of molecular Aarchaeology. Journal of Biological Chemistry. 1989;264:9709–9712. [PubMed] [Google Scholar]