Abstract
Human cytomegalovirus (CMV) utilizes a complex route of entry into cells that involves multiple interactions between viral envelope proteins and cellular receptors. Three conserved viral glycoproteins, gB, gH, and gL, are required for CMV-mediated membrane fusion, but little is known of how these proteins cooperate during entry (E. R. Kinzler and T. Compton, submitted for publication). The goal of this study was to begin defining the molecular mechanisms that underlie membrane fusion mediated by herpesviruses. We identified heptad repeat sequences predicted to form alpha-helical coiled coils in two glycoproteins required for fusion, gB and gH. Peptides derived from gB and gH containing the heptad repeat sequences inhibited virus entry when introduced coincident with virus inoculation onto cells or when mixed with virus prior to inoculation. Neither peptide affected binding of CMV to fibroblasts, suggesting that the peptides inhibit membrane fusion. Both gB and gH coiled-coil peptides blocked entry of several laboratory-adapted and clinical strains of human CMV, but neither peptide affected entry of murine CMV or herpes simplex virus type 1 (HSV-1). Although murine CMV and HSV-1 gB and gH have heptad repeat regions, the ability of human CMV gB and gH peptides to inhibit virus entry correlates with the specific residues that comprise the heptad repeat region. The ability of gB and gH coiled-coil peptides to inhibit virus entry independently of cell contact suggests that the coiled-coil regions of gB and gH function differently from those of class I, single-component fusion proteins. Taken together, these data support a critical role for alpha-helical coiled coils in gB and gH in the entry pathway of CMV.
Herpesviruses are structurally complex enveloped viruses displaying at least nine glycoproteins on their surface (4, 10, 13, 15, 16, 28). Unlike orthomyxoviruses, paramyxoviruses, filoviruses, and retroviruses that use a single glycoprotein for membrane fusion, herpesviruses employ multicomponent membrane fusion machines that comprise at least three proteins, glycoprotein B (gB), glycoprotein H (gH), and glycoprotein L (gL) (11, 24, 29). Each glycoprotein involved is conserved among the Herpesviridae family, but little is known of their structures or how their interactions promote membrane fusion. In addition to the three conserved glycoproteins, gB, gH, and gL, some herpesviruses require an additional receptor binding protein, such as glycoprotein D for herpes simplex virus (HSV) (29) or gp42 for Epstein-Barr virus (11), whereas receptor binding activity lies within gB for cytomegalovirus (CMV) and Kaposi's sarcoma-associated herpesvirus (1, 2). While much progress has been made in understanding how membrane fusion is promoted by single-component fusion proteins, little is known of how multiple components mediate fusion. Although it seems likely that multiple component fusion machines require cooperation among the fusion proteins, it remains unclear if and how herpesvirus glycoproteins interact with one another either during the assembly of virions, in fully assembled virus particles, or in virus undergoing membrane fusion during entry into host cells.
Human cytomegalovirus, a member of the betaherpesvirus subfamily, encodes homologs of gB, gH, and gL. As is true for other herpesviruses, expression of either gB or the gH/gL complex is not sufficient to promote membrane fusion, indicating that none of these individual glycoproteins is inherently fusogenic. By contrast, coexpression of gB, gH, and gL triggers syncytium formation due to cell-cell fusion (E. R. Kinzler and T. Compton, submitted for publication). Both gB and gH are highly antigenic in CMV-infected individuals, and many antibodies directed against these two glycoproteins are neutralizing to CMV, blocking infection at the level of entry (3, 9, 27, 31). To date, molecular details underlying the mechanism of CMV entry into host cells remain elusive. CMV entry into cells occurs at physiological pH and does not require receptor-mediated endocytosis (7), akin to HSV and human immunodeficiency virus (HIV). Thus, as with HSV and HIV, membrane fusion and entry of CMV is presumed to be receptor triggered. Without knowledge of specific structural domains in glycoproteins that are involved in membrane fusion, little progress can be made in understanding the molecular mechanism underlying this aspect of herpesvirus biology.
A number of studies have addressed the role of coiled coils in the entry of retroviruses, orthomyxoviruses, paramyxoviruses (all three reviewed in reference 5), and filoviruses (33). In these cases, alpha-helical coiled coils form the basis for critical protein-protein interactions within the fusogenic glycoprotein and play a pivotal role in membrane fusion. Single-component, type I fusion proteins are organized into homotrimers, with each monomer possessing two heptad repeat sequences. Typically, one is found near the membrane-spanning domain of the protein while the other is located distal to the membrane, near the fusion peptide at the mature amino terminus of the protein. Upon triggering of the fusion protein, the membrane-distal heptad repeat sequences of each monomer within the trimer interact with one another to form triple-stranded coiled coils. Each monomer then folds back on itself, allowing the membrane-proximal heptad repeat sequences to pack along grooves on the membrane-distal coiled coil. The resulting hairpin structure is energetically stable, and the free energy released upon formation of this bundle of helices is thought to contribute to the merging of the cell membrane with the viral envelope. Importantly, when soluble peptides comprising the heptad repeat sequences are presented to virus during inoculation onto cells, entry of the virus is significantly impaired due to a dominant-negative interaction between the heptad repeat-containing peptide and the virus fusion protein.
To better understand the molecular underpinnings that govern herpesvirus entry into host cells, we sought to examine the role of alpha-helical coiled coils in CMV entry. We hypothesized that heptad repeat regions predicted to form coiled coils would be present in CMV fusion proteins and that they would be fundamentally important in membrane fusion. Using an algorithm to detect potential coiled coils, we identified heptad repeat regions in gB and gH and generated peptides that correspond to the predicted coiled-coil regions. We demonstrate that the gB and gH coiled-coil peptides inhibit both uptake of virion tegument into fibroblast cells and immediate-early (IE) gene expression in a dose-dependent and sequence-specific manner. The peptides have no effect on binding of the virus to the cell surface and do not impair IE gene expression when added to cells immediately following infection. In contrast, when added to virus before or during inoculation onto fibroblast cells, the peptides effectively inactivate virus and block its uptake into cells. Furthermore, neither peptide impaired entry of murine CMV (MCMV) into mouse fibroblast cells or entry and replication of HSV type 1 (HSV-1) in human fibroblast cells. While the gB and gH homologs of MCMV and HSV-1 have heptad repeat regions, the amino acid residues that comprise these regions differ from the human CMV sequences. Thus, the peptides show specificity for the virus of origin. These data support a critical role for alpha-helical coiled coils in gB and gH in the pathway of entry of CMV into fibroblast cells. The observation that the peptides can inhibit virus entry independently of cell contact suggests that the coiled coils of CMV glycoproteins function differently than do those of single-component, class I fusion proteins. For example, heptad repeat-containing peptides derived from the SV5 F protein (25) or HIV gp41 (19) must be present following receptor binding but before membrane fusion in order to inhibit entry of these viruses.
MATERIALS AND METHODS
Viruses and cells.
Human CMV strains were propagated as previously described (6). Strain Toledo was a generous gift from Ed Mocarski. Strain Gerry was isolated from bronchial lavage fluid at the University of Wisconsin Hospital and Clinics. MCMV(RVG102), marked with enhanced green fluorescent protein (GFP) under the control of the native IE 1/3 promoter, was propagated in mouse fibroblast (NIH 3T3) cells (12). Wild-type HSV-1(KOS) and HSV-1(KOS)gL86, marked with the Escherichia coli lacZ gene, were a kind gift from Rebecca Montgomery. HSV-1(KOS)gL86 was propagated in 79VB4 cells.
Antibodies and peptides.
Monoclonal antibody 1203, which recognizes the IE gene products of CMV, was purchased from the Rumbaugh-Goodwin Institute for Cancer Research, Inc. (Plantation, Fla.). A monoclonal antibody raised against the major tegument protein, pp65, was purchased from Advanced Biotechnologies, Inc. (Columbia, Md.). Monoclonal antibody 27-78, which recognizes antigenic domain 1 of gB, was a kind gift from William Britt (26). Fluorescein-conjugated goat anti-mouse secondary antibody and horseradish peroxidase-conjugated goat anti-mouse secondary antibody were purchased from Pierce (Rockford, Il.). The linear peptides gB-COIL, gB-COIL-30, gB-COIL-scrambled, gH-COIL, and gH-COIL-scrambled (sequences shown in Fig. 1B) were synthesized at the Peptide Synthesis Facility at the University of Wisconsin Biotechnology Center (University of Wisconsin, Madison). Peptide gB-COIL-30 was synthesized with an acetylated amino terminus and an amidated carboxy terminus, while all other peptides were synthesized with free amino and carboxy termini. All peptides were purified by reverse-phase high-performance liquid chromatography, and their identities were confirmed by mass spectrometry.
FIG. 1.
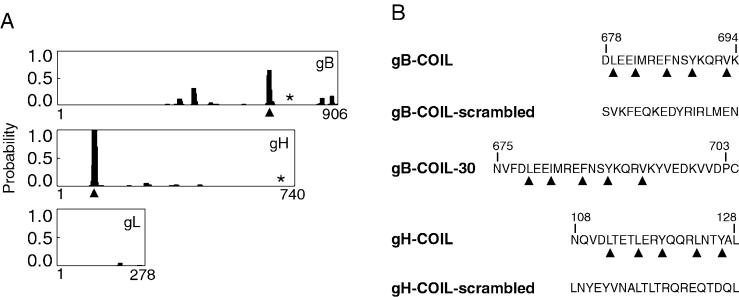
Identification of coiled-coil domains in CMV fusogenic glycoproteins. (A) Probability plots for alpha-helical coiled coils were generated using the algorithm by Lupas et al. (21) (http://www.ch.embnet.org/software/COILS_form.html). The horizontal axis represents the primary sequence of each protein, and the vertical axis represents the probability for forming alpha-helical coiled coils. Identified heptad repeat regions, predicted to result in coiled-coil structures, are marked with arrowheads. Both gB and gH are predicted to have a single alpha-helical coiled-coil domain with a probability of at least 60%. The membrane-spanning domain of each protein is marked with an asterisk. Note that gL has no membrane-spanning domain but is covalently associated with gH. (B) Peptides including the heptad repeat sequences of the predicted coiled-coil regions of gB and gH were synthesized. Heptad repeat residues are marked with arrowheads, and the amino acids are numbered according to their position within the primary sequence of each protein. Note that peptide gB-COIL-30 has a cysteine residue at its carboxy terminus that is not derived from gB sequence. This cysteine residue was added to facilitate potential chemical modifications to the peptide. Peptides gB-COIL-scrambled and gH-COIL-scrambled contain the same amino acids as gB-COIL and gH-COIL, respectively, but the amino acids have been randomized to eliminate the heptad repeat predicted to give rise to a coiled-coil structure.
Virus entry assay.
For dose-response experiments, subconfluent NHDF cells were grown on glass coverslips in 12-well plates. Human CMV(AD169) was added to cells at a multiplicity of infection (MOI) of approximately 1 PFU/cell at 37°C for 90 min in the presence or absence of 10 μM, 100 μM, or 500 μM peptide diluted into serum-free Dulbecco's modified Eagle's medium (DMEM). Nonpenetrated virus was inactivated with low-pH citrate buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl [pH 3.0]). The cells were incubated for 18 to 22 h at 37°C in DMEM supplemented with 10% bovine calf serum (BCS). Immunofluorescence analysis was performed as previously described (8) with either mouse anti-IE monoclonal antibody 1203 or mouse anti-pp65 monoclonal antibody, followed by detection with a fluorescein-conjugated goat anti-mouse secondary antibody. Nuclei were stained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI). Experiments were performed in triplicate with a minimum of 500 cells scored per coverslip.
For experiments in which the order of addition of peptide was varied, cells were treated in one of four ways. For “virus pretreatment,” approximately 2 × 104 PFU of CMV was incubated in the presence of 500 μM gB-COIL-30 or 500 μM gH-COIL for 30 min at 37°C. The virus and peptide suspensions were diluted with serum-free DMEM so that the concentration of each peptide was 80 μM prior to inoculation onto cells. The treatment that served as a no-peptide control was diluted in the same manner to control for dilution effects on virus entry. For “cell pretreatment,” NHDF cells were treated with 500 μM gB-COIL-30 or 500 μM gH-COIL for 30 min at 4°C. Peptide was removed, and the cells were washed with phosphate-buffered saline (PBS) before being challenged with AD169 at an MOI of approximately 0.5 PFU/cell at 37°C for 40 min. For “cotreatment,” NHDF cells were challenged with AD169 at an MOI of approximately 0.5 PFU/cell in the presence of 500 μM gB-COIL-30 or 500 μM gH-COIL for 40 min at 37°C. For “posttreatment,” NHDF cells were challenged with AD169 at an MOI of approximately 0.5 PFU/cell at 37°C for 20 min. Five hundred micromolar gB-COIL-30 or 500 μM gH-COIL was added to the inoculum, followed by an additional 20 min of incubation at 37°C. For all treatments, nonpenetrated virus was inactivated with low-pH citrate buffer after the 40-min incubation with cells at 37°C. The cells were incubated 18 to 24 h at 37°C in DMEM supplemented with 10% BCS, and immunofluorescence analysis was performed as described above using anti-IE monoclonal antibody 1203. Experiments were performed in triplicate with a minimum of 500 cells scored per coverslip.
For virus specificity experiments, NHDF cells were challenged with human CMV strain AD169, Toledo, Towne, or Gerry at an MOI of approximately 0.5 PFU/cell in the presence or absence of 500 μM gB-COIL-30 at 37°C for 40 min. Nonpenetrated virus was inactivated with low-pH citrate buffer, cells were incubated for 18 to 24 h at 37°C in DMEM supplemented with 10% BCS, and immunofluorescence analysis was performed as described above using anti-IE monoclonal antibody 1203. Experiments were performed in triplicate with a minimum of 500 cells scored per coverslip.
MCMV-GFP was inoculated onto NIH 3T3 cells at an MOI of approximately 3 PFU/cell in the presence or absence of 500 μM gB-COIL-30 or 500 μM gH-COIL at 37°C for 60 min. Nonpenetrated virus was inactivated with low-pH citrate buffer, and cells were incubated for 18 to 24 h at 37°C in DMEM supplemented with 10% BCS. Fluorescence microscopy was used to score GFP-positive infected cells, and the percent infection was measured by comparing the number of GFP-positive cells to total nuclei, stained with 300 nM DAPI. Experiments were performed in duplicate with a minimum of 1,000 cells scored per treatment.
HSV-1(KOS)gL86 was inoculated onto NHDF cells for 60 min in the presence or absence of 500 μM gB-COIL-30, 500 μM gH-COIL, or 50 μg of soluble heparin/ml diluted into serum-free DMEM. Nonpenetrated virus was inactivated with low-pH citrate buffer. The cells were incubated for 6 h at 37°C in DMEM supplemented with 10% BCS prior to lysis (100 mM sodium phosphate, 10 mM KCl, 1 mM magnesium sulfate, 0.1% NP-40 [pH 7.4]). β-Galactosidase activity was measured by addition of o-nitrophenyl-β-d-galactopyranoside, and the absorbance at 420 nm was monitored. Experiments were performed in triplicate.
Cell-based enzyme-linked immunosorbent assay.
NHDF cells were grown in 96-well plates and inoculated with AD169 at an MOI of approximately 2 PFU/cell in the presence or absence of 500 μM gB-COIL-30, 500 μM gH-COIL, or 50 μg of soluble heparin/ml for 90 min at 4°C. Unbound virus was removed, and the cells were washed with PBS and fixed with 3% paraformaldehyde. Bound CMV was detected using monoclonal antibody 27-78, horseradish peroxidase-conjugated goat anti-mouse secondary antibody, and the ImmunoPure TMB substrate kit (Pierce). Absorbance was measured at 450 nm, and experiments were performed in triplicate.
HSV-1 plaque assay.
HSV-1(KOS) was inoculated onto NHDF cells in the presence or absence of 500 μM gB-COIL-30, 500 μM gB-COIL-scrambled, 500 μM gH-COIL, or 500 μM gH-COIL-scrambled for 60 min at 37°C. Nonpenetrated virus was inactivated with citrate buffer, and cells were washed with PBS and overlaid with Eagle's minimal essential medium plus 0.5% agar. Plaques were visualized 3 days postinfection by crystal violet staining.
RESULTS
Identification of coiled-coil domains in gB and gH.
Considering the large number of distantly related viruses that utilize alpha-helical coiled coils for important protein-protein interactions within their fusogenic glycoprotein, we analyzed the amino acid sequence of the three CMV glycoproteins essential for membrane fusion, gB, gH, and gL, for the presence of coiled coils. Using an algorithm described by Lupas et al. (21), we identified a single heptad repeat region in gB and in gH. The heptad repeat region of gB is predicted to form an alpha-helical coiled coil with a probability of approximately 60%, while the heptad repeat region of gH has a probability of approximately 99% for forming an alpha-helical coiled coil (Fig. 1A). The heptad repeat region of gB is found between amino acids 679 and 693 in the carboxy-terminal, membrane-anchored fragment of the protein. These amino acids lie downstream of immunodominant antigenic domain 1 of gB and precede a hydrophobic stretch of approximately 30 amino acids immediately upstream of the membrane-spanning domain (amino acids 750 to 770). The heptad repeat region of gH is found between amino acids 112 and 126, near the amino terminus of the protein. This region lies within an antigenic domain of gH defined by amino acids 15 to 142, and antibodies raised to this region of gH neutralize CMV infection (30). We generated peptides for gB and gH that include the heptad repeat sequences of the predicted coiled coil as well as flanking sequences (Fig. 1B). For each coiled-coil peptide, we also generated a scrambled version in which the amino acid sequence was randomized. The randomized peptides specifically lack a heptad repeat.
Heptad repeat-containing gB and gH peptides inhibit CMV entry into human fibroblast cells.
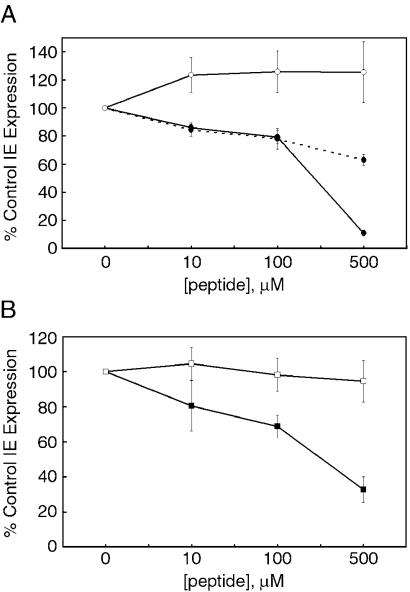
To test if the coiled-coil regions of gB and gH are functional in virus entry, we inoculated CMV onto fibroblast cells at 37°C in the presence or absence of each peptide. Expression of viral IE genes was measured 18 to 22 h postinfection. Since these gene products are the first to be expressed during CMV infection, they are frequently used as a readout of infectious virus entry. The gB peptide of 17 amino acids, called gB-COIL, and the longer gB peptide of 30 amino acids, called gB-COIL-30, showed a dose-dependent inhibition of CMV entry in this assay. In contrast, the randomized gB peptide, gB-COIL-scrambled, had no effect on CMV infection (Fig. 2A). The longer gB peptide, gB-COIL-30, showed greater entry inhibition than gB-COIL at the highest dose tested, resulting in approximately 90% inhibition of CMV IE gene expression. The observation that longer coiled-coil peptides are more potent inhibitors of virus entry has been shown for other systems as well (34). It is possible that the longer gB peptide contains additional sequence important for interacting with its target and thus leading to greater inhibition. An alternate explanation is that the longer gB peptide is capable of forming a more stable secondary structure than the shorter gB peptide. For example, it is well appreciated that longer peptides form more stable alpha helices than do short peptides (22) due in part to the greater hydrogen bonding potential within a longer helix. While it is not clear if the CMV peptides are alpha helical in their active conformation, this explanation is consistent with the behavior of heptad repeat-containing peptides from other virus fusion proteins with respect to secondary structure and length dependence of potency (20, 34).
FIG. 2.
gB and gH peptides inhibit IE gene expression in fibroblast cells. CMV was inoculated onto NHDF cells at an MOI of approximately 1 PFU/cell in the presence or absence of gB and gH peptides and allowed to bind and penetrate for 90 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated for 18 to 24 h at 37°C. Immunofluorescence was performed to detect IE gene products, and the percentage of IE-positive cells was scored for each treatment. Data are reported in triplicate, and error bars represent standard deviations. (A) The 17-amino-acid peptide, gB-COIL (dotted line, filled circles) and the 30-amino-acid peptide, gB-COIL-30 (solid line, filled circles) showed a dose-dependent inhibition of CMV entry in this assay compared to the randomized gB peptide, gB-COIL-scrambled (solid line, open circles), which had no effect on CMV entry. The longer gB peptide showed a more potent inhibition at the highest dose tested. (B) The 21-amino-acid peptide, gH-COIL (filled squares), also showed a dose-dependent inhibition of CMV entry, while the randomized version of this peptide, gH-COIL-scrambled (open squares) had no effect on CMV entry.
The 21-amino-acid gH peptide also showed a dose-dependent inhibition of CMV entry, whereas the randomized gH peptide had no effect. Inhibition was approximately 70% at the highest dose of peptide tested (Fig. 2B). This level of inhibition is intermediate compared to the same dose of gB-COIL (approximately 40% inhibition) and gB-COIL-30 (approximately 90% inhibition). The positive correlation between peptide length and inhibitory activity supports the hypothesis that the active conformation of the peptides is a secondary structure sensitive to peptide length, such as alpha helix. However, structural analysis of the gB and gH coiled-coil peptides in aqueous solution by circular dichroism spectroscopy revealed a random coil configuration (data not shown), which is also consistent with the behavior of certain heptad repeat-containing peptides from other virus fusion proteins (20).
To confirm that the coiled-coil peptides were inhibiting CMV infection at the level of virus entry and not during postentry transcription or translation, we monitored delivery of a virus structural component, the major tegument protein pp65, into cells following infection with CMV. In these experiments, gB-COIL-30 and gH-COIL showed inhibition of pp65 delivery into cells following inoculation with CMV that is comparable to the observed levels of IE inhibition (Fig. 3). These results indicate that the peptides are indeed targeting the virus at the level of entry.
FIG. 3.
gB and gH peptides inhibit pp65 uptake into fibroblast cells. CMV was inoculated onto NHDF cells at an MOI of approximately 0.05 PFU/cell in the presence or absence of gB and gH peptides and allowed to bind and penetrate for 90 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated for approximately 18 h at 37°C. Immunofluorescence analysis was performed to detect pp65, and the percentage of pp65-positive cells was scored for each treatment. (A) In addition to inhibiting expression of IE gene products, gB-COIL-30 and gH-COIL inhibited uptake and trafficking of the major tegument protein pp65 to the nucleus of infected cells. Since delivery of pp65 to infected cells is an earlier marker for virus entry than IE gene expression, these data support the hypothesis that the inhibiting peptides act during virus entry and not at the level of transcription or translation of IE gene products. Data are reported in duplicate, and error bars represent the range. (B) Representative immunofluorescence images for mock, no peptide, or gB-COIL-30 treatment showing pp65 in the nucleus of infected fibroblast cells.
gB and gH peptides do not impair binding of CMV to cells.
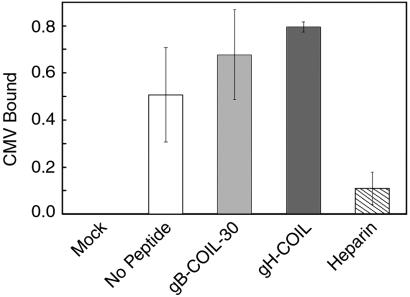
To test the possibility that the coiled-coil peptides affect binding of the virus to the cell surface, we performed a cell-based enzyme-linked immunosorbent assay to measure binding of CMV to fibroblast cells in the presence or absence of each coiled-coil peptide. gB-COIL-30 and gH-COIL were each tested at 500 μM, the highest dose used in the inhibition experiments, and neither peptide impaired the ability of CMV to bind to the cell surface (Fig. 4). In contrast to the gB and gH coiled-coil peptides, soluble heparin blocked the ability of CMV to bind to fibroblasts in this assay. Soluble heparin is known to interfere with binding of CMV to the cell surface, likely due to the requirement of CMV for binding heparan sulfate proteoglycans during entry (8). Thus, the gB and gH coiled-coil peptides block CMV entry at a postattachment step.
FIG. 4.
gB and gH peptides do not impair attachment of CMV to fibroblast cells. CMV was inoculated onto NHDF cells at an MOI of approximately 2 PFU/cell in the presence or absence of 500 μM gB-COIL-30, 500 μM gH-COIL, or 50 μg of heparin/ml for 90 min at 4°C. Unbound virus was removed, and bound virus was detected with anti-gB monoclonal antibody 27-78, an HRP-conjugated secondary antibody, and a colorimetric substrate. Experimental treatments were normalized to the mock treatment, data are reported in triplicate, and error bars represent standard deviations. Neither 500 μM gB-COIL-30 (light gray) nor 500 μM gH-COIL (dark gray) impaired the ability of CMV to bind to NHDF cells, in contrast to soluble heparin (hatched bar), which blocked virus attachment.
gB and gH peptides inactivate virions independently of cell contact.
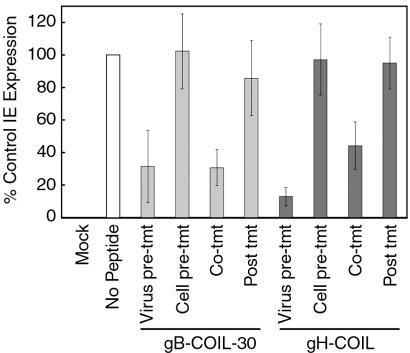
Given that CMV is competent to bind to cells in the presence of the coiled-coil peptides, we wanted to determine at which step in the entry pathway the peptides inhibit and whether they affect viral proteins, cell surface proteins, or both. To this end, we performed an experiment in which the order of addition of peptide and virus to fibroblast cells was varied (Fig. 5). Cells that were pretreated with either 500 μM gB-COIL-30 or 500 μM gH-COIL peptide and thoroughly washed prior to inoculation with CMV showed normal levels of virus entry, as did cells that had been inoculated with CMV for 20 min in the absence of peptide and then challenged with peptide for an additional 20 min. These data indicate that the peptides do not irreversibly block a cell surface receptor and that a majority of the entry events occurred in the first 20 min following inoculation, by which point the virus is unaffected by addition of the coiled-coil peptides. The coiled-coil peptides were able to significantly inhibit virus entry when added concurrent with virus inoculation, consistent with results shown in Fig. 2. Interestingly, virus entry was also significantly inhibited when virus was preincubated with 500 μM gB-COIL-30 or 500 μM gH-COIL peptide and subsequently diluted to 80 μM, a peptide concentration at which virus is poorly inhibited. These data indicate that the gB and gH coiled-coil peptides inhibit virus entry by interacting with and inactivating virus either before cell contact or during virus entry.
FIG. 5.
gB and gH peptides inactivate virions independently of cell contact. For virus pretreatment, approximately 2 × 104 PFU of CMV was incubated in the presence of 500 μM gB-COIL-30 or 500 μM gH-COIL for 30 min at 37°C prior to dilution and addition to cells. For cell pretreatment, 500 μM gB-COIL-30 or 500 μM gH-COIL was added to cells for 30 min at 4°C and then washed out prior to inoculation with CMV. For cotreatment, 500 μM gB-COIL-30 or 500 μM gH-COIL was added to cells concurrent with inoculation with CMV. For posttreatment, 500 μM gB-COIL-30 or 500 μM gH-COIL was added to cells 20 min after inoculation with CMV. In each treatment, inoculation with CMV was carried out for a total of 40 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated for 18 to 24 h. Immunofluorescence analysis was performed to detect IE gene products, and the percentage of IE-positive cells was scored for each treatment. Data are reported in triplicate, and error bars represent standard deviations.
Virus specificity of gB and gH peptide inhibitors.
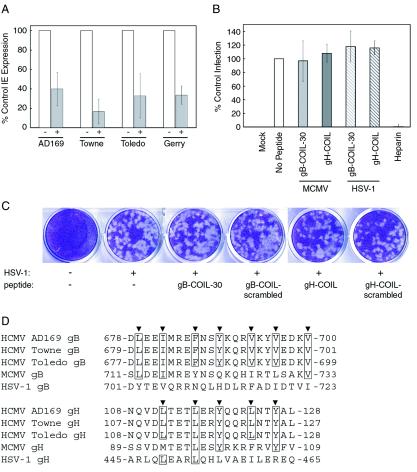
To test the specificities of the gB and gH peptide inhibitors against other viruses, we examined the ability of gB-COIL-30 to inhibit the entry of three human CMV strains in addition to AD169. Each strain was inoculated onto fibroblast cells in the presence or absence of 500 μM gB-COIL-30, and IE gene expression was measured as a readout of virus entry. Both laboratory-adapted strains, AD169 and Towne, as well as two clinical isolates tested, Toledo and Gerry, were susceptible to inhibition by gB-COIL-30 (Fig. 6A). To further test specificity and rule out nonspecific toxicity of the peptides, we measured the effects of gB-COIL-30 and gH-COIL on entry of MCMV into NIH 3T3 mouse fibroblast cells and entry of HSV-1 into human fibroblast cells.
FIG. 6.
Virus specificity of gB and gH peptides. (A) Various strains of CMV were inoculated onto NHDF cells at an MOI of approximately 0.5 PFU/cell in the presence or absence of 500 μM gB-COIL-30 and allowed to bind and penetrate for 40 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated 18 to 24 h. Immunofluorescence analysis was performed to detect IE gene products, and the percentage of IE-positive cells was scored for each treatment. Data are reported in triplicate, and error bars represent standard deviations. All strains of CMV tested were vulnerable to the inhibiting activity of 500 μM gB-COIL-30. (B) MCMV-GFP was inoculated onto NIH 3T3 cells at an MOI of approximately 3 PFU/cell in the presence or absence of 500 μM gB-COIL-30 or 500 μM gH-COIL for 60 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated for 22 to 24 h at 37°C. GFP expression was detected by fluorescence microscopy, and the percentage of GFP-positive cells was scored for each treatment. Data are reported in duplicate, and error bars represent the range. HSV-1(KOS)gL86 was inoculated onto NHDF cells in the presence or absence of 500 μM gB-COIL-30 or 500 μM gH-COIL for 60 min at 37°C. Nonpenetrated virus was inactivated, and the cells were incubated 6 h at 37°C. β-Galactosidase expression was measured using the colorimetric substrate o-nitrophenyl-β-d-galactopyranoside. Data are reported in triplicate, and error bars represent standard deviations. Neither peptide inhibited MCMV entry into NIH 3T3 cells (light gray bar represents gB-COIL-30; dark gray bar represents gH-COIL) or HSV-1 entry into NHDF cells (light-gray hatched bar represents gB-COIL-30; dark-gray hatched bar represents gH-COIL). In contrast, soluble heparin efficiently blocked entry of HSV-1 into human fibroblast cells (rightmost bar). (C) HSV-1 was inoculated onto NHDF cells in the presence or absence of 500 μM gB-COIL-30, gB-COIL-scrambled, gH-COIL, or gH-COIL-scrambled. Plaques were visualized 3 days postinfection by crystal violet staining. (D) The amino acid sequence of the predicted coiled-coil regions of gB and gH from human CMV strains AD169, Towne, and Toledo, as well as MCMV and HSV-1, are aligned. Heptad repeat residues are indicated by arrowheads above each alignment, and amino acids are numbered according to their position within the primary sequence of each protein. Note that the heptad repeat sequences are identical among the three human CMV strains for both gB and gH, while the MCMV and HSV-1 strains show both conservative and nonconservative substitutions at the heptad repeat sequences of gB and gH compared to the human CMV strains. The conservation of the heptad repeat sequences, or lack thereof, positively correlates with the ability of the human CMV strain AD169-derived gB and gH peptides to inhibit virus entry.
For these experiments we used an MCMV strain genetically marked with enhanced GFP under the control of the native IE 1/3 promoter (12). Mouse fibroblast cells were inoculated with MCMV-GFP in the presence or absence of 500 μM gB-COIL-30 or 500 μM gH-COIL, and GFP expression was measured as a readout of virus entry and infection. In this assay, neither the gB nor the gH coiled-coil peptide impaired the ability of MCMV-GFP to enter NIH 3T3 cells (Fig. 6B). The HSV-1 strain, HSV-1(KOS)gL86, is commonly used as a reporter for HSV-1 entry (23). Human fibroblast cells were inoculated with HSV-1(KOS)gL86 in the presence or absence of 500 μM gB-COIL-30 or 500 μM gH-COIL, and β-galactosidase expression was measured as a readout of virus entry and infection. In this assay, neither the gB nor the gH coiled-coil peptide impaired the ability of HSV-1 to enter human fibroblast cells (Fig. 6B). These results indicate that the peptides are not generally detrimental to enveloped viruses but are specific to the virus of origin.
Peptides were also tested for cytotoxicity, which could potentially account for the observed virus entry inhibition. Neither 500 μM gB-COIL-30 nor 500 μM gH-COIL was cytotoxic to fibroblast cells as measured by trypan blue dye exclusion (data not shown) and by visual inspection of cellular morphology by light microscopy (data not shown). Furthermore, neither the gB nor the gH coiled-coil peptide impaired the ability of HSV-1 to enter and form plaques in NHDF cells (Fig. 6C). The ability of HSV-1 to replicate in NHDF cells treated with the gB and gH peptides indicates that neither peptide compromised cellular functions critical for viability.
A sequence alignment of the heptad repeat regions of the gB and gH homologs from three human CMV strains, MCMV, and HSV-1, shows the degree of conservation of these domains (Fig. 6D). The heptad repeat region of gB is invariant among human CMV strains AD169, Towne, and Toledo, positively correlating with the ability of gB-COIL-30 to inhibit entry of these strains. The heptad repeat region of gH is also invariant among the three human CMV strains analyzed. However, variation exists between the human CMV, MCMV, and HSV-1 strains at the heptad repeat regions of both gB and gH. Although the gB and gH homologs of MCMV and HSV-1 contain heptad repeat regions predicted to form coiled coils, the amino acid sequences that comprise the heptad repeats differ from those of human CMV strains. Among the heptad repeat residues of gB, only three of seven are identical between the human and murine CMV strains and none are identical compared with HSV-1. Only three of five heptad repeat residues are identical among the human and murine CMV strains in the gH heptad repeat region, while two heptad repeat residues are identical among the human CMV strains and HSV-1. A single heptad repeat leucine residue is conserved among all four gH homologs. This invariant residue may be involved in a key interaction between the heptad repeat region of gH and its binding target. In the case of both the gB and gH heptad repeat regions, residues flanking the heptad repeat residues also show variation. The variation in heptad repeat sequence between the human CMV, MCMV, and HSV-1 strains may explain the inability of the human CMV-derived peptides to inhibit entry of MCMV and HSV-1. We speculate that the heptad repeat regions of MCMV and HSV-1 gB and gH, as well as the analogous regions of other herpesvirus gB and gH homologs, are also functional in the entry of those viruses. The specific amino acid sequences that comprise the heptad repeat regions likely play a role in the protein-protein interactions that the coiled coils facilitate, thus explaining the strain specificity of the coiled-coil peptides. Taken together, these data support the hypothesis that the heptad repeat regions of gB and gH are functional in CMV entry and may represent critical protein interaction domains.
DISCUSSION
In this study we provide evidence for the importance of alpha-helical coiled coils in gB and gH in CMV entry. Predicted coiled-coil regions were identified in both glycoproteins (Fig. 1A), and peptides comprising the heptad repeat sequences were generated (Fig. 1B) and tested for their effects on virus entry. Both gB and gH coiled-coil peptides inhibited IE gene expression in fibroblast cells in a dose-dependent, sequence-specific manner (Fig. 2) and inhibited uptake of the CMV structural protein, pp65 (Fig. 3). In contrast to soluble heparin, neither the gB nor the gH peptide had an effect on binding of virus to the cell surface (Fig. 4), suggesting that inhibition is at a postattachment step. Treatment of cells with either peptide following incubation with virus had no effect on entry (Fig. 5), indicating that the inhibitory peptides act at a step during virus entry and have no effect once virus has penetrated the cell membrane. Pretreatment of cells with either peptide, followed by its removal, also did not impair the ability of CMV to enter cells (Fig. 5), suggesting that the peptides do not irreversibly block a cell surface receptor. Efficient inhibition was observed only when the gB or the gH peptides were incubated with virus at the same time as inoculation onto fibroblasts or when the virus was preincubated with peptide before being inoculated onto fibroblasts (Fig. 5). Taken together, these data point to a critical role for the coiled-coil domains of gB and gH in the entry of CMV into fibroblast cells.
Although the importance of alpha-helical coiled coils seems to be conserved in the fusion proteins of a broad range of enveloped viruses, in which CMV can now be included, there is an important difference in the manner in which coiled coils seem to function in CMV entry compared to the case with viruses that employ a single, class I fusion protein for entry. With respect to preincubation of virus and peptide prior to inoculation onto cells, our observations contrast with inhibition seen in paramyxovirus and retrovirus entry using similarly derived peptides. In these cases, peptides can inhibit infection only when present during virus entry, not when added to virus before cell contact. It is thought that the binding surface for the paramyxovirus and retrovirus peptides is hidden in the native, resting conformation of the fusion protein. This surface is only exposed following receptor activation, allowing peptides to interact with and inhibit the fusion protein. In the case of CMV, our data suggest that the binding surface is exposed on native, resting virions, allowing for efficient inhibition of virus independently of cell contact. According to this proposed mechanism of inhibition, gB and gH coiled-coil peptides interact with virion components and block necessary conformational changes and/or protein-protein interactions required for the assembly of a functional membrane fusion machine.
An alternate explanation for the ability of the gB and gH peptides to block CMV entry independently of cell contact is that the peptides may prematurely trigger the virus envelope glycoproteins for fusion, rendering them incompetent for subsequent membrane fusion following cell binding. Although the exact nature of the trigger for CMV-mediated membrane fusion is unknown, it is conceivable that the gB and gH coiled-coil peptides interact with virion components to form a complex that resembles a fusion-triggered intermediate. By prematurely triggering the envelope glycoproteins, the peptides could render the virus unable to respond to the appropriate fusion trigger upon binding to the cell surface. The molecular basis for gB and gH coiled-coil peptide inhibition of CMV entry will be the focus of future studies.
The distribution of heptad repeat sequences in gB and in gH may have important ramifications for how these sequences function in the context of CMV entry. In class I fusion proteins, two heptad repeat sequences are found at opposing termini within a single protein. This arrangement is fundamentally important for the mechanics of membrane fusion mediated by this class of molecules. Specifically, the association of the two opposing heptad repeat regions into a hairpin structure increases the proximity of the adjacent membranes. Among the CMV fusion glycoproteins, however, there is a single heptad repeat sequence in two of the three proteins required for fusion, gB and gH. While it is not yet clear if these sequences interact to form an alpha-helical coiled-coil complex during membrane fusion, we speculate that the heptad repeat sequences of gB and gH probably are involved in protein-protein interactions critical for fusion and that the heptad repeat-containing gB and gH peptides block this interaction. Such an interaction may involve intramolecular contacts within gB and/or gH during the assembly of a competent fusion complex or perhaps intermolecular interactions between gB, gH, and cellular receptors.
Several cell surface molecules are known to play roles in the entry pathway of CMV. Heparan sulfate proteoglycans serve as initial docking sites and may function to recruit virus to the surface of cells (8, 14). Recently, Wang et al. showed that CMV gB bound to and activated epidermal growth factor receptor (EGFR) (32). While it is likely that EGFR is a cellular receptor for CMV, it remains unknown how this interaction is involved in mediating uptake of infectious virus. One possibility is that the interaction of gB with EGFR promotes conformational changes involved in regulating membrane fusion. Additional cell surface molecules probably play a role in CMV entry, such as a putative 92.5-kDa cellular receptor that interacts with gH (17). Overall, it is unclear how interactions between CMV fusion proteins and identified cell surface receptors trigger membrane fusion. Understanding the role of cellular receptors in assembling a functional membrane fusion machine will be the focus of future work.
The observation that gB and gH coiled-coil peptides can inhibit virus entry independent of cell contact raises the possibility that these peptides may be efficacious at inhibiting CMV infection in vivo. In addition to inhibiting two laboratory-adapted strains of human CMV, we have also shown that the gB coiled-coil peptide can block entry of two clinical human CMV strains (Fig. 6A). The inability of the gB and gH peptides to affect entry of MCMV and entry and replication of HSV-1 (Fig. 6B and C) suggests that the peptides are not generally toxic to enveloped viruses but rather show specificity to CMV strains that display the amino acid sequence represented by the peptides. However, the concentrations of peptide required for efficient inhibition of CMV entry are higher than those used in some viral systems (18, 25, 34). One possible explanation is that a greater concentration of peptide is required for inhibition in the context of a single cycle of virus entry, while the assay systems used for HIV and paramyxovirus inhibitory peptides utilized multiple cycles of virus entry. Similar to the coiled-coil peptides of CMV, peptides derived from the coiled-coil domains of Ebola virus glycoprotein must be present at high concentrations to inhibit infectivity of vesicular stomatitis virus pseudotyped with Ebola glycoprotein (33). The assay system used in that study also relied on a single round of virus entry and required approximately 700 μM peptide to inhibit 50% infectivity.
Given the high degree of sequence conservation between the gB and gH heptad repeat regions of many CMV strains, these peptides, or analogs thereof, may prove useful in the development of anti-CMV therapeutics. The current success of gp41-based peptide inhibitors that block entry and infection of HIV in humans (18) lends support to the exploitation of CMV entry with respect to rational drug design.
Acknowledgments
This research was funded by U.S. Public Health Service grants RO1 AI-34998 and RO1 AI-44203 to T.C.
We are grateful to Gary Case of the Peptide Synthesis Facility at the University of Wisconsin Biotechnology Center for synthesis of the peptides used in this investigation and to the members of the Compton lab for critical review of the manuscript. We also thank Jasbir Singh for assistance with the HSV-1 plaque assay.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 72:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 4.Chang, C. P., D. H. Vesole, J. Nelson, M. B. Oldstone, and M. F. Stinski. 1989. Identification and expression of a human cytomegalovirus early glycoprotein. J. Virol. 63:3330-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 6.Compton, T. 1993. An immortalized human fibroblast cell line is permissive for human cytomegalovirus infection. J. Virol. 67:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 8.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 9.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gretch, D. R., B. Kari, L. Rasmussen, R. C. Gehrz, and M. F. Stinski. 1988. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J. Virol. 62:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 12.Henry, S. C., K. Schmader, T. T. Brown, S. E. Miller, D. N. Howell, G. G. Daley, and J. D. Hamilton. 2000. Enhanced green fluorescent protein as a marker for localizing murine cytomegalovirus in acute and latent infection. J. Virol. Methods 89:61-73. [DOI] [PubMed] [Google Scholar]
- 13.Huber, M. T., and T. Compton. 1997. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kari, B., and R. Gehrz. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kari, B., and R. Gehrz. 1993. Structure, composition and heparin binding properties of a human cytomegalovirus glycoprotein complex designated gC-II. J. Gen. Virol. 74:255-264. [DOI] [PubMed] [Google Scholar]
- 16.Kari, B., Y. N. Liu, R. Goertz, N. Lussenhop, M. F. Stinski, and R. Gehrz. 1990. Structure and composition of a family of human cytomegalovirus glycoprotein complexes designated gC-I (gB). J. Gen. Virol. 71:2673-2680. [DOI] [PubMed] [Google Scholar]
- 17.Keay, S., T. C. Merigan, and L. Rasmussen. 1989. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 86:10100-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 19.Koshiba, T., and D. C. Chan. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J. Biol. Chem. 278:7573-7579. [DOI] [PubMed] [Google Scholar]
- 20.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 21.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 22.Marqusee, S., V. H. Robbins, and R. L. Baldwin. 1989. Unusually stable helix formation in short alanine-based peptides. Proc. Natl. Acad. Sci. USA 86:5286-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 24.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76:4390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 20:4024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoppel, K., E. Hassfurther, W. Britt, M. Ohlin, C. A. Borrebaeck, and M. Mach. 1996. Antibodies specific for the antigenic domain 1 of glycoprotein B (gpUL55) of human cytomegalovirus bind to different substructures. Virology 216:133-145. [DOI] [PubMed] [Google Scholar]
- 27.Simpson, J. A., J. C. Chow, J. Baker, N. Avdalovic, S. Yuan, D. Au, M. S. Co, M. Vasquez, W. J. Britt, and K. L. Coelingh. 1993. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J. Virol. 67:489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaderna, S., H. Blessing, E. Bogner, W. Britt, and M. Mach. 2002. Identification of glycoprotein gpTRL10 as a structural component of human cytomegalovirus. J. Virol. 76:1450-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban, M., W. Britt, and M. Mach. 1992. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J. Virol. 66:1303-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban, M., M. Klein, W. J. Britt, E. Hassfurther, and M. Mach. 1996. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 77:1537-1547. [DOI] [PubMed] [Google Scholar]
- 32.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe, S., A. Takada, T. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2000. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol. 74:10194-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao, Q., and R. W. Compans. 1996. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 223:103-112. [DOI] [PubMed] [Google Scholar]