Abstract
The neuropeptide calcitonin gene-related peptide (CGRP) is transiently expressed in cerebellar climbing fibers during development while its receptor is mainly expressed in astrocytes, in particular Bergmann glial cells. Here, we analyzed the effects of CGRP on astrocytic calcium signaling. Mouse cultured astrocytes from cerebellar or cerebral cortex as well as Bergmann glial cells from acutely isolated cerebellar slices were loaded with the Ca2+ sensor Fura-2. CGRP triggered transient increases in intracellular Ca2+ in astrocytes in culture as well as in acute slices. Responses were observed in the concentration range of 1 nM to 1 mM, in both the cell body and its processes. The calcium transients were dependent on release from intracellular stores as they were blocked by thapsigargin but not by the absence of extracellular calcium. In addition, after CGRP application a further delayed transient increase in calcium activity could be observed. Finally, cerebellar astrocytes from neonatal mice expressed receptor component protein, a component of the CGRP receptor, as revealed by immunofluorescence and confocal microscopy. It is thus proposed that the CGRP-containing afferent fibers in the cerebellum (the climbing fibers) modulate calcium in astrocytes by releasing the neuropeptide during development and hence possibly influence the differentiation of Purkinje cells.
Keywords: differentiation, mouse, neuron, glia transmission, receptor, receptor component protein
Introduction
It is now widely accepted that astrocytes can sense and influence synaptic transmission. Astrocytic processes are closely associated with pre- and postsynaptic elements and a number of studies have revealed that astrocytes can express a variety of receptors for transmitters, including glutamate and GABA, as well as receptors for neuropeptides such as substance P (Schipke & Kettenmann, 2004; Volterra & Steinhäuser, 2004). In order to identify neuropeptides as candidate molecules for neuron–astrocyte communication we analyzed the effects of the neuropeptide calcitonin gene-related peptide (CGRP) on astrocytic calcium signaling. CGRP has been shown to possess differentiating and proliferating activity on glial cells of the peripheral and central nervous system, as it promotes proliferation of Schwann cells (Cheng et al., 1995) and induces morphological differentiation in astrocytes (Lazar et al., 1991). Components of the receptor complexes of the CGRP family, calcitonin-like receptor, receptor activity-modifying proteins 1–3 and receptor component protein (RCP), have been detected at the mRNA level in glial cells (Moreno et al., 1999, 2002). In addition, CGRP increases cAMP levels (Moreno et al., 2002) and induces immediate–early gene expression in cultured astrocytes (Haas et al., 1990) and microglial cells (Priller et al., 1995).
Additional reasons for studying the effects of this neuropeptide on astrocytic calcium arises from previous CGRP data during cerebellar development: in a period ranging from late embryonic days to the end of the second postnatal week CGRP is transiently expressed in climbing fibers (Morara et al., 1989, 1995), where the peptide is present in vesicular structures as early as when they reach the cerebellar anlage during embryonic life (Morara et al., 2001). Moreover, in the developmental time window of peptide expression, the corresponding receptor is expressed and present on the cell surface in glial cells while, in the neuronal target of climbing fibers, the Purkinje cells, the receptor showed a significant cell surface localization only at later stages (Morara et al., 2000), a localization which is also maintained in the adult although the peptide is absent (Morara et al., 1998). Thus, it has been hypothesized that CGRP can play a role as afferent-released neuropeptide in the climbing fiber system during development, by influencing astrocyte differentiation and possibly, via a feedforward mechanism, neuronal differentiation.
CGRP can trigger Ca2+ responses in several cell types, including cardiomyocytes, smooth muscle cells, chromaffin cells and osteosarcoma cells (Aiyar et al., 1999; Drissi et al., 1999; Giniatullin et al., 1999; Herzog et al., 2002; Burns et al., 2004; Dong et al., 2005; Schiess et al., 2005). In the present study we provide evidence that CGRP triggers Ca2+ responses in astrocytes in cultures of cerebellum or cerebral cortex and in Bergmann glial cells from acutely isolated cerebellar slices, indicating that astrocytes express functional CGRP receptors in culture and in situ, supporting the hypothesis that this neuropeptide may act as modulating agent during cerebellar development.
Materials and methods
Materials
CGRP was obtained from NeoMPS (Strasbourg, France), Fura-2/AM from Molecular Probes, Inc. (Eugene, OR, USA) and thapsigargin from Sigma.
Solutions
All solutions were freshly prepared from refrigerated stock solutions. The standard bath solution for cultured cells was composed of (in mm) NaCl, 150; KCl, 5.4; CaCl2, 2; MgCl2, 1; HEPES, 5; and glucose, 10; pH adjusted to 7.4 with NaOH. The standard bath solution for brain slices (bicarbonate salt solution) was composed of (in mm) NaCl, 134.00; KCl, 2.50; CaCl2, 2.00; MgCl2, 1.30; K2HPO4, 1.25; NaHCO3, 26; and glucose, 10; pH adjusted to 7.4 with NaOH.
Preparation of cultured astrocytes
Newborn Naval Medical Research Institute (NMRI) mice (Tierzucht Schönwalde, Schönwalde, Germany) were deeply anaesthetized with carbon dioxide, decapitated and astrocytes were prepared from cerebral cortex or cerebellum as described previously (Lyons & Kettenmann, 1998). Briefly, the tissue was carefully dissected from blood vessels and meninges, rinsed with Eagle’s basal medium solution and incubated with 0.05% trypsin and 0.02% EDTA solution for 8 min at 37°C, trypsinized, and gently triturated with a fire-polished pipette in the presence of 0.05% DNase (Worthington). After washing cells twice, cells were cultured in 75-cm2 plates on poly-L-lysine-coated coverslips using Eagle’s basal culture medium with 10% fetal calf serum. One day later, cultures were washed twice with Hanks’s balanced salt solution to remove cellular debris and maintained for 3–4 days. After reaching a subconfluent state, cellular debris, microglia cells and oligodendrocytes as well as their early precursor cells were dislodged by manual shaking and removed by washing with Hanks’s balanced salt solution. The purity of the astrocytes was routinely determined by immunofluorescence using a polyclonal antibody against glial fibrillary acidic protein (GFAP; Dako, Hamburg, Germany), a specific astrocyte marker. The cultures showed > 90% cells positive for GFAP. Measurements were made from cells between days 4 and 14.
Tissue slices
NMRI mice, from postnatal day (P) 0 to 8, were deeply anaesthetized with carbon dioxide, decapitated and slices of 120–200 μm were prepared as previously described (Müller et al., 1992). The slices were cut perpendicular to the orientation of the Purkinje cell dendritic tree and placed in a bicarbonate salt solution (see below) continuously gassed with 5% CO2 and 95% O2.
Fluorescence imaging system
Fura-2-loaded cells were visualized under water immersion with a 20× or 40× objective from a perfusion chamber mounted on the stage of an upright microscope (Axioskop FS, Zeiss, Oberkochen, Germany). Cells were superfused with standard physiological bath solution, and cell stimulation was achieved by changing the superfusate with CGRP-containing solution. Excitation light was provided by a monochromator (Till Photonics, Munich, Germany), and the fluorescence emission was captured by a cooled CCD camera (SensiCam; PCO, Kelheim, Germany) and digitized by an image processing system (Axon Imaging Workbench; Axon). The monochromator and CCD camera were controlled by Axon Imaging Workbench software (Axon), which was used for image analysis. Ratio images were collected at intervals every 1–2 s.
Measurement of intracellular calcium
Cultured astrocytes on coverslips were incubated with 5 μm Fura-2 acetoxymethylester (AM) for 20 min in physiological buffer in the dark at 37°C. Cells were then washed with physiological buffer and stored in the dark for an additional 30 min to ensure Fura-2AM hydrolysis. Coverslips with loaded cells were transferred to the perfusion chamber and visualized under the microscope.
Slices were incubated with 5–10 μm Fura-2AM for 20–30 min in bicarbonate salt solution in the dark at 37°C for ‘bulk loading’, washed with fresh buffer and finally let recover to allow Fura-2AM de-esterification.
Fura-2, a ratiometric dye, was excited with UV light at 340 and 380 nM, and the emission was measured at 530 ± 10 nM.
CGRP was administered either by ejection from a pipette (applied locally close to the cells or in the bulk of the chamber solution away from cells) or by continuous bath perfusion.
A statistical analysis was conducted to ascertain whether CGRP-induced Ca2+ responses were significantly different from background spontaneous activity by using a Wilcoxon rank-sum test. To examine significance of the dose–response curve a Kruskal–Wallis test was used. This is a nonparametric version of ANOVA that makes no assumptions about the normal distribution of samples but requires that the populations to be compared have the same size (equal number of experiments). The results of the statistical analyses are expressed as P-values (probability of the result). These analyses have been carried out using the commercially available MATLAB (The MatWorks, Inc.) software program. The significance level was set at P = 0.05.
Immunofluorescence
In this study, cerebellar sections from 0- to 8-day-old NMRI mice (Tierzucht Schönwalde, Schönwalde, Germany) were prepared as previously described (Morara et al., 2000). In brief, after perfusion with 4% paraformaldehyde, the cerebella were soaked overnight in 20% sucrose at 4°C, frozen and cut in the frontal plane at 10 μm thickness and collected on gelatin-coated slides. Immunofluorescence was performed following previously described protocols (Morara et al., 1997) by incubating sections with chicken anti-RCP antiserum 1065 directed against the peptide EEQIEALLHTVT (1: 300–800; Pokabla et al., 2002) together with a monoclonal anti-calbindin D antibody (1: 5000–10 000; AbCam) or with a monoclonal anti-GFAP antibody (1: 450–800; Sigma), and by using Fluorescein-conjugated antichicken antibodies (Jackson) to reveal RCP and Alexa-Fluor 594-conjugated antimouse antibodies to reveal calbindin D or GFAP, and examined using a laser scanning confocal microscope (Sarastro 2000; Molecular Dynamics) equipped with a Zeiss Axioskop epifluorescence microscope. Immunolabeled sections were examined with 40× Plan-ApoChromat Zeiss objective using dual excitation wavelengths (488 nM for Alexa-Fluor 488 and 514 nM for Alexa-Fluor 594); aperture size of the pinhole was set at 50 μm, laser power was set at 12–24 mW and photomultiplier detector voltage was set in the range 600–800 V. The scanning mode format was 512 × 512 and the pixel size was 0.32 μm.
Animals were cared for in accordance with the principles of the NIH Institutional Animal Care and Use Committee Guidebook (2002).
All the present experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Results
CGRP triggered transient Ca 2+ responses in cultured astrocytes
To test for the presence of functional receptors for CGRP in astrocytes, we applied the peptide either by ejection from a pipette or by bath application while recording intracellular Ca2+ levels in Fura-2-loaded astrocytes from cultures of cerebellum or cerebral cortex. In the concentration range 250 nM to 1 mM, CGRP induced transient Ca2+ increases in cultured astrocytes from cerebellum as well as from cortex (Fig. 1A). The overall mean percentage of cells in which 1 mM CGRP, applied by continuous local (close to cells) perfusion from the pipette, induced Ca2+ transients, was 41.1% (range, 22.22–100.00%; no. of cells, 431). The response was variable in terms of intensity, response onset and percentage of responding cells: this last variability could be partially ascribed to the heterogeneous distribution of CGRP-responsive cells which were frequently detected in clusters, whereas adjacent clusters of astrocytes could be nonresponsive (not shown). On the other hand, neither confluency nor time in culture (days in vitro) influenced the responses in a significant manner. As it is our general experience that essentially all uncompromised astrocytes respond to ATP with an increase in Ca2+, we applied ATP after testing for the CGRP response and included in the analysis only the cells positive to ATP.
FIG. 1.
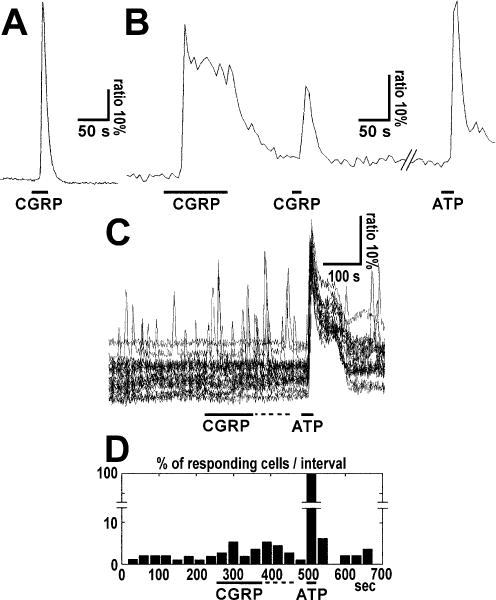
Types of calcium responses induced in cultured cerebellar astrocytes by (A) pipette application of CGRP at medium to high concentrations or (B and C) by bath perfusion at low concentrations. (A) Simple transient calcium response to CGRP application. Ratiometric Fura-2 fluorescence recordings were used to determine changes in intracellular Ca2+ concentration. Note that the onset of decay occurred in the presence of the peptide. The bar indicates local pipette application of 250 nM CGRP. (B) Peak plus sustained plateau calcium responses in a single cultured cerebellar astrocyte after CGRP application. Note that the sustained calcium plateau was maintained in the presence of CGRP and Ca2+ returned to the basal level when the peptide was washed out. The bar indicates local pipette application of CGRP (50 μM) or ATP (100 μM) as control. (C) Ratiometric traces and (D) histogram of the percentage of responding cells per time interval are shown. CGRP (1 nM) and ATP (100 μM) were applied as indicated by bars. Only cells that responded at least once to CGRP were included. In D the columns represent the percentage of astrocytes which exhibited transient calcium responses in each 30 s time interval. The statistical analysis conducted by comparing two groups of seven time intervals corresponding to (i) the total CGRP period (from 240 to 450 s, i.e. CGRP application period plus the following 90 s) and (ii) the pre-CGRP application period (from 30 to 240 s) by means of the Wilcoxon signed-rank test showed that the increase in spike frequency was significant (P = 0.0313).
The response to CGRP consisted either of a simple peak that showed a rapid transient Ca2+ concentration increase (Fig. 1A) or of a rapid peak followed by a sustained plateau similar to that described for ATP responses in astrocytes (Fig. 1B). The latter response was triggered by high (50 μM–1 mM) CGRP concentrations when given by local pipette application. The sustained plateau occurred only in the presence of CGRP, as Ca2+ returned to the basal level when the peptide was washed out (Fig. 1B).
In order to obtain kinetic measurements of CGRP-induced calcium transients, the peptide was given by local pipette application at 1 mM. In these experiments (n = 18; no. of cells, 431; see above) Ca2+ responses increased to peak within 4–10 s (mean 5.8 s); this was similar to ATP responses. However, the onset of the CGRP-induced Ca2+ transients showed a delay when compared to ATP: when ATP was puffed from a micropipette placed close to the cells, the cells responded within 4 s (mean; range, 1–10 s), while the CGRP response (at 1 mm concentration) occurred typically with a delay of 15 s on average (range, 3–44 s) after onset of application. Moreover, when the first type of response (simple peak) was detected the CGRP-induced responses lasted for 9–85 s (mean, 40.3 s), whereas the duration of the second type (peak plus plateau) was dependent on the presence of the peptide, as described above.
When applying CGRP by bath perfusion at 1–100 nM concentration for 30–120 s, we observed an increase in calcium transients in a subpopulation of astrocytes and this increase could persist for a few minutes after cessation of CGRP application (no. of experiments = 3; no. of cells = 374). As the percentage of responding cells was lower than at higher concentrations, and astrocytes could exhibit spontaneous Ca2+ increases in the absence of a ligand, we compared the percentage of cells showing calcium spikes during CGRP application with that prior to CGRP application (Fig. 1C and D). We therefore counted the number of Ca2+ peaks per cell within a given time interval (30 s) and compared two periods (each of them comprising several intervals): the CGRP application (including an additional 90 s) period and the pre-CGRP application period. The analysis showed that CGRP induced a statistically significant increase in calcium transients at 1 nM (Wilcoxon signed-rank test; P = 0.0313).
As at low (nanomolar) concentration the peptide required longer incubation times to get a reproducible and statistically significant response, and as response onset was variable, we performed a dose– response analysis by using pipette application of CGRP in the bulk of the chamber solution (away from cells) without bath perfusion and compared the spike frequency in 10 intervals (25 s each) before and during CGRP application. At the end of each experiment, we also checked calcium activity after CGRP washout, but no significant increase over spontaneous background activity was observed (not shown). During CGRP application, the increase in spike frequency over background spontaneous activity (within each experiment) was 2.18 ± 2.63 at 20 nM (no. of experiments = 16; no. of cells = 426), 3.50 ± 3.62 at 200 nM (no. of experiments = 16; no. of cells = 468), 7.32 ± 9.90 at 2 μM (no. of experiments = 16; no. of cells = 407) and 10.17 ± 11.82 at 20 μM (no. of experiments = 16; no. of cells = 444) and a statistical analysis revealed that at each concentration the increase in spike frequency following CGRP was significant (Table 1; Fig. 2A). As our measurements did not follow a normal distribution (as revealed by Lilliefors test; not shown; indeed they frequently showed rather asymmetric distributions) the statistical analysis of the difference between concentrations was performed using the Kruskal– Wallis test, a nonparametric version of anova that does not make assumption about normal distribution of samples and test medians. Our populations had the following median values: 0 at 20 nM, 2.08 at 200 nM, 2.79 at 2 μM and 6.41 at 20 μM. This analysis revealed that the increases in spike frequency per cell between 200 nM and 2 μM (P = 0.0270) or between 2 and 20 μM (P = 0.0217) were significant. In contrast, the difference between 20 and 200 nM was not significant (Fig. 2A). It is worth mentioning that as this test compares medians rather than means even considerably overlapping distributions can provide significantly low P-values.
Table 1.
Dose–response analysis of cerebrocortical astrocytes to application of CGRP
| CGRP concentration | Spike frequency per cell
|
Wilcoxon signed-rank test (P-value) | Spike frequency per cell: difference during vs. before CGRP | |
|---|---|---|---|---|
| Before CGRP | During CGRP | |||
| 20 nM | 0.72 ± 1.25 | 2.90 ± 3.52 | 0.0156* | 2.18 ± 2.63 |
| 200 nM | 0.88 ± 1.58 | 4.39 ± 5.16 | 0.0313* | 3.50 ± 3.62 |
| 2 μM | 2.21 ± 5.15 | 9.54 ± 14.76 | 0.0059* | 7.32 ± 9.90 |
| 20 μM | 0.881 ± 1.457 | 11.05 ± 13.05 | 0.002* | 10.17 ± 11.82 |
Values are mean ± SD. The analysis was conducted by counting the number of spikes per cell in 10 intervals of 25 s before and during CGRP application by pipette in the bulk of the chamber solution. At each concentration the mean spike frequency during CGRP application was significantly different from the background spontaneous activity (Wilcoxon signed-rank test);
P = 0.05.
FIG. 2.
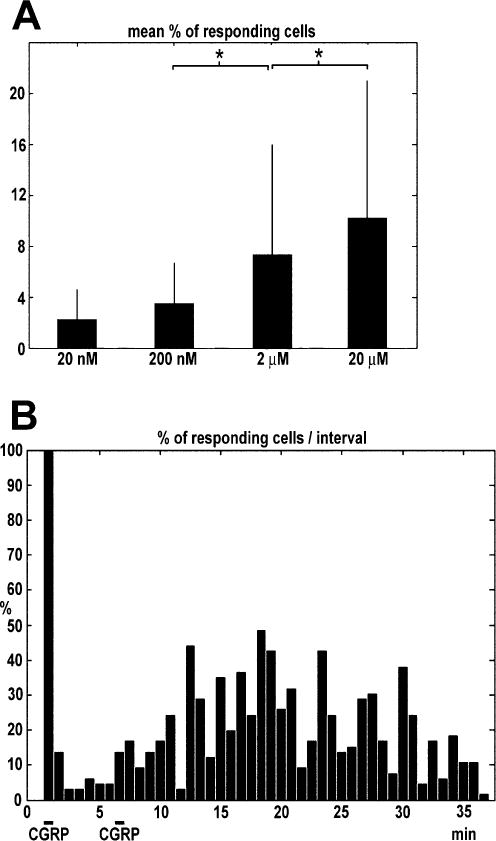
(A) Dose–response analysis of the effect of CGRP on astrocytic calcium and (B) biphasic response to CGRP. (A) Histogram representing the mean + SD percentage of responding cells recorded in 10 intervals (25 s each) during application of CGRP (20, 200 nM, 2 or 20 μM) by pipette in the bulk of the chamber solution. The mean background (spontaneous) calcium activity during 10 intervals before CGRP application has been subtracted. A statistical analysis of the comparison between spike frequencies recorded at different concentrations were carried out using the Kruskal–Wallis test, a nonparametric test for medians that was used because the samples did not follow a normal distribution. The test showed that medians were significantly different between 20 nM and 2 μM (P = 0.0270) and between 2 and 20 μM (P = 0.0217), whereas between 2 and 20 nM the difference was not significant (P = 0.9536). *P = 0.05. The number of experiments was 16 at each concentration. Numbers of cells: 20 nM, 426; 200 nM, 468; 2 μM, 407; 20 μM, 444. (B) The columns represent the percentage of astrocytes which exhibited transient calcium responses in each 30 s time interval. Note the two-phases type biphasic response the second application of CGRP. Bar indicates local pipette applications of CGRP (1 mM).
The histograms of the temporal pattern of CGRP-induced calcium transients in the astrocyte population (at both low and high peptide concentrations) showed a tendency to appear as a double bell-shaped curve (see e.g. Fig. 1D), displaying the presence of a second, delayed, transient increase in calcium activity: when CGRP was applied once, this delayed effect could last a few minutes whereas when it was applied twice it could last up to 30 min (Fig. 2B; it is worth mentioning that in this experiment local pipette application of 1 mM CGRP resulted in a simple peak response; not shown). In another set of experiments when the competitive CGRP antagonist CGRP 8–37 (300 μM) was applied before and during application of 1 nM CGRP, we did not observe any change in Ca2+ transient activity (not shown).
In general it was possible to apply CGRP repetitively without much desensitization of the receptor, whereas desensitization could be observed when peptide was applied (local pipette application) at high concentrations (50 μM–1 mM) for times > 40–50 s. In five experiments (total no. of cells = 229) 1 mM CGRP was applied twice for 50 s with an interval ranging from 250 to 350 s (no differences were detected within this time range): during the first application 45.41% of cells responded on average (range 27–100%) and showed a 31.3% increase in intensity ratio (range 21–56%), whereas during the second application responding cells were only 31.44% (range 6–100%) and showed a 15.1% increase in intensity ratio (range 6–25%).
Finally, we compared responses obtained within different cell compartments by selecting regions at the soma and at the radial processes, and recorded ratiometric fluorescence changes in response to CGRP. We observed responses with similar time course and response onset at soma and processes in cultured cerebellar (or cerebrocortical) astrocytes (Fig. 3B and C), although the amplitude of the Ca2+ increase was higher in the soma than in the processes. This suggests that CGRP receptors are expressed in both domains of the cell, in agreement with previous immunofluorescence data (Morara et al., 2000).
FIG. 3.
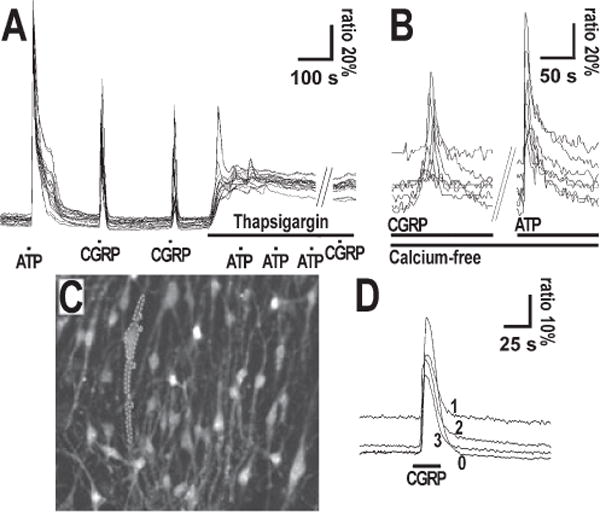
(A and B) Cellular mechanism and (C and D) localization of calcium responses to CGRP in cultured astrocytes. (A) Calcium responses of a cultured cerebrocortical astrocytic population to the application of CGRP before and after thapsigargin and ATP application. Ratiometric Fura-2 fluorescence recordings were used to determine changes in intracellular Ca2+ concentration. CGRP (10 μM), ATP (100 μM) and thapsigargin (125 μM) were locally applied by pipette as indicated by bars. (B) Calcium responses of a cultured cerebrocortical astrocytic population to the application of CGRP in Ca2+-free medium. CGRP was applied by pipette in the bulk of the chamber solution (away from cells) at 2 μM concentration and ATP at 4 μM, as indicated by bars. (C and D) Calcium responses in the soma and in processes from a single astrocyte cultured from cerebellum. In C the fluorescence image of the Fura-2-loaded astrocyte culture is shown. Four regions were selected (0, cell body; 1 and 2, proximal part of processes; 3, distal part of process) as indicated and the corresponding ratiometric Fura-2 fluorescence recordings are shown in D. Bar indicates 1 mm CGRP local application by pipette.
CGRP triggered Ca 2+ release from internal stores
To study the mechanism of the CGRP-induced Ca2+ release, we used a protocol established previously (Kirischuk et al., 1995b; Tuschick et al., 1997) to test responses to 10 μM CGRP after depleting the internal Ca2+ stores. This was achieved by applying ATP two to three times in the presence of thapsigargin, a blocker of Ca2+ uptake into endoplasmic stores. To verify whether CGRP was able to elicit repetitive responses, CGRP was applied several times before thapsigargin application. In four experiments (total no. of cells, 131) 50 μM CGRP was applied, twice before and once after thapsigargin application. The first CGRP application produced a response in 58.0% of cells, the second application in 43.6% and the third (after thapsigargin) in 2.2% (three cells). A representative example of this experiment is illustrated in Fig. 3A, which shows that CGRP elicited two responses before thapsigargin and ATP application. After applying the store-depletion protocol, CGRP no longer induced Ca2+ responses: thus, CGRP-induced Ca2+ transients were dependent on Ca2+ release from intracellular stores.
Finally, in order to check whether CGRP-induced calcium responses were also dependent on the presence of external calcium, we applied 2 μM CGRP in nominally Ca2+-free buffer: pipette application of CGRP in the bulk of the chamber solution resulted in Ca2+ peaks even in absence of external calcium, as illustrated in Fig. 3B (no. of experiments, 3; no. of cells, 174). Similar results were also obtained by local application via pipette under constant perfusion (not shown). Based on the above findings we propose that CGRP-induced Ca2+ transients are dependent on Ca2+ release from intracellular stores.
CGRP elicited Ca 2+ responses in Bergmann glial cells from acute slices
We have previously developed a procedure (Kirischuk et al., 1995a) to preferentially load Bergmann glial cells in acute cerebellar slices with the Ca2+ sensor Fura-2. When recording Ca2+ responses from these Bergmann glial cells (no. of experiments, 16), 1 nM–500 μM CGRP triggered similar responses to those observed in cultured astrocytes, namely simple transient Ca2+ increases (as shown in Fig. 4A) or rapid peaks followed by an additional plateau phase (Fig. 4B): typically, however, the kinetic of the responses (onset, and duration of decay phase) was slower than for cultured astrocytes. Moreover, in Bergmann glial cells from acute slices we observed responses, at the soma and processes, with response onset and time course (Fig. 4C and D) as well as response variability and desensitization similar to those found in cultured astrocytes. However, unlike cultured cerebellar astrocytes, Bergmann glial cells from acute slices did not show a second, delayed, transient increase in calcium activity. Within the developmental period analyzed [postnatal day (P) 0–8], which comprises the period of maximal CGRP expression in climbing fibers and the initial phase of declining expression of the peptide, Bergman glial cells showed similar responses (not shown).
FIG. 4.
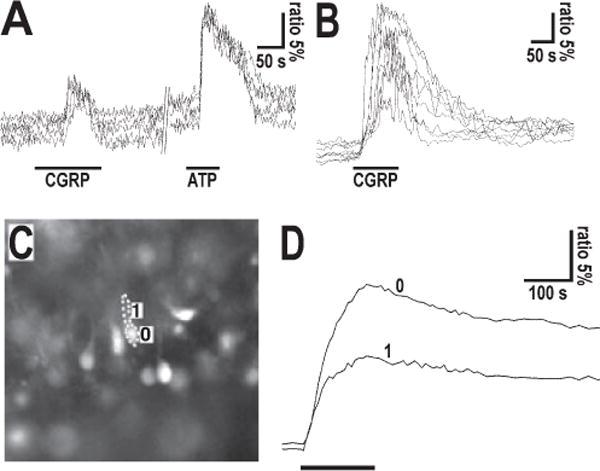
Calcium responses obtained in Bergmann glial cells from acute cerebellar slices. (A) Simple peak-type calcium responses in Fura-2-loaded Bergmann glial cells to local pipette application of CGRP (2.5 μM) and ATP (100 μM). Note that the onset of decay of the CGRP response occurreds in the presence of the peptide. The slice was prepared from a P4 mouse. (B) Peak plus plateau-type calcium responses in Bergmann glial cells to local pipette application of 50 μM CGRP. The slice was prepared from a P6 mouse cerebellum. Note that recovery from the plateau starts with cessation of CGRP application. (C, D) Calcium responses recorded from cell body and process of a single Bergmann glia cell from a cerebellar slice of a P3 mice. In (C) the fluorescence photograph of a Fura-2 loaded Bergmann glia cells is shown. The cell body and radial process (Bergmann glia fiber) of the analyzed cell has been separately outlined: 0 = cell body; 1 = radial process. In (D) the ratiometric traces of calcium concentration in the corresponding cell domains following local pipette application of CGRP (50 μM; indicated by bar) is shown.
Expression of RCP (a component of the CGRP receptor) in Bergmann glial cells
To verify the expression of CGRP receptors in Bergmann glial cells we immunolabelled cerebellar slices from P7 mice with an antibody directed against RCP, a component of the CGRP receptor that is the functional link to the cAMP cascade, and analyzed the distribution of immunofluorescence by confocal microscopy. In the Purkinje cell layer, the labeling was detected in the somata of Purkinje neurons (identified by double labeling with Calbindin D, a marker of Purkinje cells), and in small somata in the close vicinity of Purkinje neurons, reminiscent of Bergmann glial cell (Fig. 5A). In double immunofluorescence experiments with GFAP, a marker for astrocytes and also Bergmann glial, these cells were colabelled, indicating that Bergmann glial cells express RCP (Fig. 5B). While in Bergmann glial cells immunoreactivity gave the impression of also labeling plasma membranes, in Purkinje cells immunoreactivity was confined to regions within the cytoplasm and, rarely, within dendrites (Fig. 5). To test for functional Ca2+-coupled CGRP receptors in Purkinje cells we performed Ca2+ imaging experiments in which, by prolonging Fura-2 loading, Purkinje cells were also loaded with the indicator: in contrast to Bergmann glial cells, CGRP did not evoke Ca2+ responses in Purkinje cells (not shown). Finally, cultured astrocytes displayed RCP immunoreactivity, as astrocytes in situ, although at variable levels (a finding that may contribute to the variability in CGRP responsiveness) and also displayed CGRP-induced cAMP increases (not shown), confirming previous results (Lazar et al., 1991; Moreno et al., 2002).
FIG. 5.
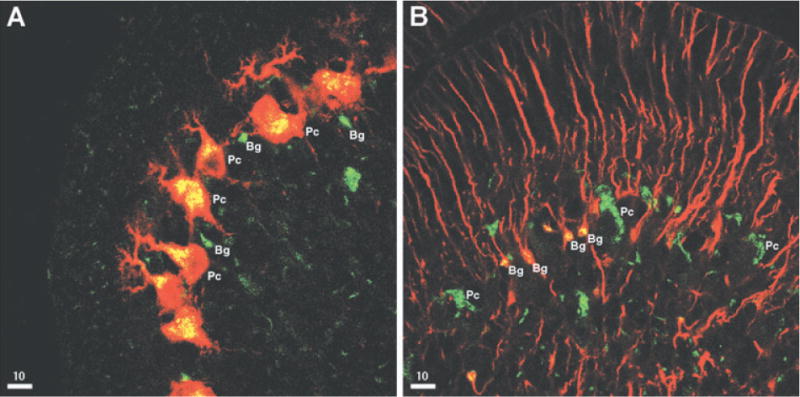
Confocal images of immunoreactivity for RCP (green) and Calbindin D (A, red) or GFAP (B, red) from frontal sections of P7 cerebellum. Bergmann glia cell bodies (Bg) and Purkinje cell bodies (Pc) are indicated. The yellow color corresponds to colocalization.
Discussion
CGRP triggers Ca 2+ responses in astrocytes
CGRP affected astrocytic calcium signaling over a large range of peptide concentrations, from the low nanomolar up to the millimolar range. Only a small population of cultured astrocytes responded to the low nanomolar CGRP concentrations, while almost half of the astrocytes tested responded to CGRP concentrations in the high micromolar to millimolar range. The Bergmann glial cells, which are often used as a model for astrocytes in situ despite their atypical astrocyte morphology, responded reliably to CGRP; this is in agreement with previous findings on high expression levels of CGRP receptor in Bergmann glia during development (Morara et al., 2000).
We recorded CGRP-induced calcium transients at different locations within a given cell but did not find any differences with respect to signal onset. We assume that either the Ca2+ responses spread too fast for our detection system or the receptors and their subsequent Ca2+ signaling cascades are not locally confined. This latter conclusion is supported by our previous findings, on the expression of CGRP receptors in Bergmann glial cell bodies and radial processes, obtained by immunofluorescence and confocal microscopy (Morara et al., 2000).
The responses were variable in terms of both responding cell number and response intensity although, for example, in the dose– response curve increases in spike frequency at different peptide concentrations were statistically significant. The variability could be ascribed to at least two factors: (i) the heterogeneous distribution of responding cells (often distributed in clusters) which might affect the percentage of responding cells per field; and (ii) the variability in RCP (the functional link of the receptor to cAMP cascade) expression levels, which might produce different levels of receptor activation and hence response amplitude.
CGRP triggers distinct Ca 2+ responses in astrocytes
We found that the Ca2+ responses to CGRP were either a simple rapid calcium transient which declined independently of the presence of the peptide, or a rapid peak followed by a sustained plateau that continued as long as CGRP was present in the bath. The latter response occurred mainly following local pipette applications of high peptide concentrations (high micromolar–millimolar range) and thus it might be triggered by a local excess of CGRP.
In addition to these rapid effects a second delayed increased frequency of Ca2+ spikes was observed, suggesting that CGRP triggers two types of responses. This implies that CGRP could also produce a longer-lasting effect on the astrocyte properties. Similar kinetics in CGRP receptor signaling have been observed in endogenous CGRP receptors in the hair cells of the lateral line organ of Xenopus laevis and for heterologous CGRP receptors expressed in Xenopus laevis oocytes (Adams et al., 1987; Luebke et al., 1996). In these previous studies CGRP receptor signaling was measured by afferent nerve firing rates and cAMP production, respectively. Thus, using three different readouts of CGRP receptor function, a similar signaling response to CGRP was observed: a rapid large response, followed by a delayed prolonged diminished response. Altogether these results suggest that signaling at the CGRP receptor may involve two components.
Finally, when CGRP-induced calcium transients were compared to ATP-induced calcium transients CGRP showed a delayed response onset. This observation suggest that timing and the second transient calcium activity are two features that can allow astrocytes to distinguish between peptide-induced and ATP-induced calcium increases and thus react differently to the two stimuli.
It remains to be explained how stimulation of CGRP receptors can promote Ca2+ increase. The effect of CGRP on Ca2+ homeostasis has been accounted for by both cAMP-independent and cAMP-dependent mechanisms, according to the type of CGRP receptors involved. In osteoblastic cells, cAMP mediates CGRP-stimulated calcium increase by opening calcium channels in MG-63 cells (Burns et al., 2004), or a promiscuous coupling of the CGRP receptors with heterotrimeric G proteins other than Gs was reported in OHS-4 (cells that are unable to elicit cAMP increases upon CGRP stimulation; Drissi et al., 1998). In HEK-293 cells expressing recombinant CGRP receptors, not only did forskolin not induce a Ca2+ increase but cholera toxin pretreatment was even reported to cause permanent activation of adenylyl cyclase with inhibition of CGRP-mediated Ca2+ release (Aiyar et al., 1999). The situation is clearly very different in astrocytes, in which not only are calcium channels not involved in CGRP-induced calcium increases, but also prominent cAMP elevation is observed upon CGRP stimulation (Lazar et al., 1991; Moreno et al., 2002; not shown). Interestingly, phospholipase Cɛ (PLCɛ), an enzyme responsible for the cAMP-induced Ca2+ release from intracellular stores, has been identified (Schmidt et al. 2001) and is thought to contribute to Ca2+ elevation in astrocytes (Di Cesare et al., 2006). In fact, Ca2+ elevation was reported in astrocytes after exposure to a series of protocols increasing cAMP or direct activation of PLCɛ. Based on these findings, it can be assumed that most of the Ca2+ responses, if not all, are to be ascribed to CGRP-induced cAMP elevation with ensuing activation of PLCɛ and Ca2+ release from intracellular stores.
The CGRP receptor is expressed during cerebellar development
RCP (a component of the CGRP receptor) is a cytoplasmic protein which, by binding the receptor complex, provides the functional link to the cAMP signaling cascade (Evans et al., 2000; Prado et al., 2001). At the end of the first postnatal week, when CGRP is still expressed by climbing fibers, RCP was found to be expressed in cerebellar astrocytes, in particular by Bergmann glial cells. Strong immunoreactivity was also found in Purkinje cells, in particular in the cell bodies and proximal dendrites, but immunoreactivity was largely confined to the cytoplasm. This finding is in perfect agreement with a previous confocal analysis carried out with a different antibody directed against a purified CGRP receptor showing that in Purkinje cells the CGRP receptor is first localized in the cytoplasm, presumably associated with the synthetic compartment, and is later on progressively displaced within proximal dendrites, probably directed toward cell surface presentation (Morara et al., 1998, 2000). Moreover, in calcium experiments with Fura-2-loaded Purkinje cells, we failed to record any calcium changes in response to CGRP at the end of the first postnatal week. At this developmental stage, CGRP receptors in Purkinje neurons are mainly restricted to the cytoplasm. Thus, confocal analysis seems to support calcium imaging findings that, at this stage, the cellular target of CGRP in the developing cerebellum would be the astrocytes, and not the Purkinje cells, the neuronal target of the fibers from which CGRP is released.
The development of the cerebellar cortex follows a series of complex events that comprise Purkinje cells proliferation and radial migration toward the cerebellar anlage surface (Altman, 1982), where they contact climbing fibers (their major early input; Morara et al., 2001), granule cell migration, proliferation and radial inward travel (Ramón y Cajal, 1911; Rakic, 1971; Altman, 1982; Komuro & Yacubova, 2003), Purkinje cell dendrite elongation and retraction (Ramón y Cajal, 1911; Armengol & Sotelo, 1991) and climbing fiber axon and terminal settlement on Purkinje cells, and retraction (Ramón y Cajal, 1911; Mason et al., 1990; Chedotal & Sotelo, 1993; Morara et al., 2001). During these developmental processes, Bergmann glial cells have been mainly considered a riding trail for radial migration of granule cells (Ramón y Cajal, 1911; Rakic, 1971; Altman, 1982; Komuro & Yacubova, 2003), but their involvement in Purkinje cell differentiation is receiving increasing attention (see Yamada & Watanabe, 2002; Lordkipanidze & Dunaevsky, 2005; for recent reviews). In addition, postnatal ablation of cerebellar astrocytes causes severe disruption of cerebellar development including marked secondary effects on Purkinje cell and granule cell differentiation (Delaney et al., 1996), suggesting further developmental roles for cerebellar astrocytes, in particular for Bergmann glial cells.
The present findings on the effects of CGRP on cerebellar astrocytes led us to conclude that Bergmann glial cells are an important target of the CGRP-containing climbing fibers during development; they could, in turn, also provide a feedforward mechanism in neuronal differentiation. The present observation, that CGRP is able to stimulate calcium transients in astrocytes, adds another signaling molecule for neuron–glia communication which, by modulating astrocytic calcium, could play roles in the regulation of neuron–glia transmission and also in neural differentiation that occurs during development.
Acknowledgments
S.M. is supported by the FIRB and the FISR projects of the Italian Ministry of University and Research, H.K. is supported by the German Research Council, I.M.D. is supported by NIH DK52328 and F.G. is supported by the Italian Ministry of Research (PRIN-2006054051) and by the Italian Telethon Foundation (GGP05141). We thank Irene Haupt and Brigitte Gerlach for excellent technical assistance, Laura Zambusi for excellent technical assistance and preparation of illustrations, Anja Hoffman for helpful discussions on statistical analysis, the whole lab personnel of the NFC Unit at DIBIT-HSR for excellent technical assistance, helpful discussion and support, and Deutsche Forschungsgemeinschaft for support.
Abbreviations
- CGRP
calcitonin gene-related peptide
- GFAP
glial fibrillary acidic protein
- NMRI
Naval Medical Research Institute
- P
postnatal day
- RCP
receptor component protein
References
- Adams JC, Mroz EA, Sewell WF. A possible neurotransmitter role for CGRP in a hair-cell sensory organ. Brain Res. 1987;419:347–351. doi: 10.1016/0006-8993(87)90606-8. [DOI] [PubMed] [Google Scholar]
- Aiyar N, Disa J, Stadel JM, Lysko PG. Calcitonin gene-related peptide receptor independently stimulates 3′,5′-cyclic adenosine monophosphate and Ca2+ signaling pathways. Mol Cell Biochem. 1999;197:179–185. doi: 10.1023/a:1006962221332. [DOI] [PubMed] [Google Scholar]
- Altman J. Morphological development of the rat cerebellum and some of its mechanisms. In: Palay SL, Chan-Palay V, editors. The Cerebellum New Vistas. Springer Verlag; Berlin-Heidelberg: 1982. pp. 8–46. [Google Scholar]
- Armengol JA, Sotelo C. Early dendritic development of Purkinje cells in the rat cerebellum. A light and electron microscopic study using axonal tracing in ‘in vitro’ slices. Dev Brain Res. 1991;64:95–114. doi: 10.1016/0165-3806(91)90213-3. [DOI] [PubMed] [Google Scholar]
- Burns DM, Stehno-Bittel L, Kawase T. Calcitonin gene-related peptide elevates calcium and polarizes membrane potential in MG-63 cells by both cAMP-independent and -dependent mechanisms. Am J Physiol Cell Physiol. 2004;287:C457–C467. doi: 10.1152/ajpcell.00274.2003. [DOI] [PubMed] [Google Scholar]
- Chedotal A, Sotelo C. The ‘creeper stage’ in cerebellar climbing fiber synaptogenesis precedes the ‘pericellular nest’ – ultrastructural evidence from parvalbumin immunocytochemistry. Dev Brain Res. 1993;76:207–220. doi: 10.1016/0165-3806(93)90209-s. [DOI] [PubMed] [Google Scholar]
- Cheng L, Khan M, Mudge AW. Calcitonin gene-related peptide promotes Schwann cell proliferation. J Cell Biol. 1995;129:789–796. doi: 10.1083/jcb.129.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney CL, Brenner M, Messing A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J Neurosci. 1996;16:6908–6918. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Del Piccolo P, Zacchetti D, Grohovaz F. EP2 receptor stimulation promotes calcium responses in astrocytes via activation of the adenylyl cyclase pathway. Cell Mol Life Sci. 2006;63:2546–2553. doi: 10.1007/s00018-006-6262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YL, Vegiraju S, Yallampalli C. Ca2+ signaling in human fetoplacental vasculature: effect of CGRP on umbilical vein smooth muscle cytosolic Ca2+ concentration. Am J Physiol Heart Circ Physiol. 2005;289:H960–H967. doi: 10.1152/ajpheart.00059.2005. [DOI] [PubMed] [Google Scholar]
- Drissi H, Lasmoles F, Le Malley V, Marie PJ, Lieberherr M. Activation of phospholipase C-b1 via Galphaq/11 during calcium mobilization by calcitonin gene-related peptide. J Biol Chem. 1998;273:20168–20174. doi: 10.1074/jbc.273.32.20168. [DOI] [PubMed] [Google Scholar]
- Drissi H, Lieberherr M, Hott M, Marie PJ, Lasmoles F. Calcitonin gene-related peptide (CGRP) increases intracellular free Ca2+ concentrations but not cyclic AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4) Cytokine. 1999;11:200–207. doi: 10.1006/cyto.1998.0415. [DOI] [PubMed] [Google Scholar]
- Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Di Angelantonio S, Marchetti C, Sokolova E, Khiroug L, Nistri A. Calcitonin gene-related peptide rapidly downregulates nicotinic receptor function and slowly raises intracellular Ca2+ in rat chromaffin cells in vitro. J Neurosci. 1999;19:2945–2953. doi: 10.1523/JNEUROSCI.19-08-02945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CA, Streit WJ, Kreutzberg GW. Rat facial motoneurons express increased levels of calcitonin gene-related peptide mRNA in response to axotomy. J Neurosci Res. 1990;27:270–275. doi: 10.1002/jnr.490270305. [DOI] [PubMed] [Google Scholar]
- Herzog M, Scherer EQ, Albrecht B, Rorabaugh B, Scofield MA, Wangemann P. CGRP receptors in the gerbil spiral modiolar artery mediate a sustained vasodilation via a transient cAMP-mediated Ca2+-decrease. J Membr Biol. 2002;189:225–236. doi: 10.1007/s00232-002-1017-5. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Möller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci. 1995a;15:7861–7871. doi: 10.1523/JNEUROSCI.15-12-07861.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol. 1995b;483(PT 1):41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–1098. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar P, Reddington M, Streit W, Raivich G, Kreutzberg GW. The action of calcitonin gene-related peptide on astrocyte morphology and cyclic AMP accumulation in astrocyte cultures from neonatal rat brain. Neurosci Lett. 1991;130:99–102. doi: 10.1016/0304-3940(91)90237-n. [DOI] [PubMed] [Google Scholar]
- Lordkipanidze T, Dunaevsky A. Purkinje cell dendrites grow in alignment with Bergmann glia. Glia. 2005;51:229–234. doi: 10.1002/glia.20200. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Dahl GP, Roos BA, Dickerson IM. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc Natl Acad Sci USA. 1996;93:3455–3460. doi: 10.1073/pnas.93.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Mason CA, Christakos S, Catalano SM. Early climbing fiber interactions with Purkinje cells in the postnatal mouse cerebellum. J Comp Neurol. 1990;297:77–90. doi: 10.1002/cne.902970106. [DOI] [PubMed] [Google Scholar]
- Morara S, Provini L, Rosina A. CGRP expression in the rat olivocerebellar system during postnatal development. Brain Res. 1989;504:315–319. doi: 10.1016/0006-8993(89)91376-0. [DOI] [PubMed] [Google Scholar]
- Morara S, Sternini C, Provini L, Rosina A. Developmentally regulated expression of α- and β-CGRP mRNA and CGRP-immunoreactivity in the rat inferior olive. J Comp Neurol. 1995;354:27–38. doi: 10.1002/cne.903540104. [DOI] [PubMed] [Google Scholar]
- Morara S, Marcotti W, Provini L, Rosina A. Neuropeptide Y (NPY) expression is up-regulated in the rat inferior olive during development. Neuroreport. 1997;8:3743–3747. doi: 10.1097/00001756-199712010-00017. [DOI] [PubMed] [Google Scholar]
- Morara S, Wimalawansa SJ, Rosina A. Monoclonal antibodies reveal expression of the CGRP receptor in Purkinje cells, interneurons and astrocytes of rat cerebellar cortex. Neuroreport. 1998;9:3755–3759. doi: 10.1097/00001756-199811160-00034. [DOI] [PubMed] [Google Scholar]
- Morara S, Rosina A, Provini L, Forloni G, Caretti A, Wimalawansa SJ. Calcitonin gene-related peptide receptor expression in the neurons and glia of developing rat cerebellum: an autoradiographic and immunohistochemical analysis. Neuroscience. 2000;100:381–391. doi: 10.1016/s0306-4522(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Morara S, van der Want JJL, De Weerd H, Provini L, Rosina A. Ultrastructural analysis of climbing fiber-Purkinje cell synaptogenesis in the rat cerebellum. Neuroscience. 2001;108:655–671. doi: 10.1016/s0306-4522(01)00433-x. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Cohen Z, Stanimirovic DB, Hamel E. Functional calcitonin gene-related peptide type 1 and adrenomedullin receptors in human trigeminal ganglia, brain vessels, and cerebromicrovascular or astroglial cells in culture. J Cereb Blood Flow Metab. 1999;19:1270–1278. doi: 10.1097/00004647-199911000-00012. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Terron JA, Stanimirovic DB, Doods H, Hamel E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Müller T, Möller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Pokabla MJ, Dickerson IM, Papka RE. Calcitonin gene-related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides. 2002;23:507–514. doi: 10.1016/s0196-9781(01)00638-6. [DOI] [PubMed] [Google Scholar]
- Prado MA, Evans-Bain B, Oliver KR, Dickerson IM. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides. 2001;22:1773–1781. doi: 10.1016/s0196-9781(01)00517-4. [DOI] [PubMed] [Google Scholar]
- Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglial cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus rhesus J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Ramón y, Cajal S. Histologie du Système Nerveux de l’Homme et de Vertébrés. II. Maloine; Paris: 1911. [Google Scholar]
- Schiess MC, Poindexter BJ, Brown BS, Bick RJ. The effects of CGRP on calcium transients of dedifferentiating cultured adult rat cardiomyocytes compared to noncultured adult cardiomyocytes: possible protective and deleterious results in cardiac function. Peptides. 2005;26:525–530. doi: 10.1016/j.peptides.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Kettenmann H. Astrocyte responses to neuronal activity. Glia. 2004;47:226–232. doi: 10.1002/glia.20029. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Oude Weernink PA, vom Dorp F, Rehmann H, Lomasney JW, Jacobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;21:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- Volterra A, Steinhaüser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–257. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77:94–108. doi: 10.1046/j.0022-7722.2002.00021.x. [DOI] [PubMed] [Google Scholar]


