To the Editor
Epidemiologic and genetic studies suggest that bipolar disorder (BP) and schizophrenia (SCZ) may share common susceptibility genes (1). Volumetric neuroanatomical studies, however, do not indicate similarities between these two disorders. The hippocampus appears to be central to the pathophysiology of SCZ (2), and loss of gray matter in the hippocampus is often reported (3, 4). In contrast, there appears to be little change in hippocampal volume in BP. Rather, structural studies suggest that global measures of total cerebral and gray and white matter volumes in BP are largely normal (5). However, volumes of the amygdala are sometimes abnormal, compared to controls (CON) or SCZ patients (4, 5). Studying surface shape patterns can identify more subtle structural abnormalities not evident by volumetric studies. Surface shape abnormalities of the hippocampus have been reported in SCZ (6, 7), but no such studies have been conducted in BP.
We used magnetic resonance imaging and a FreeSurfer-initiated fully automated brain segmentation method involving Large Deformation Diffeomorphic Metric Matching (8) to compare the volume and surface shape of the hippocampus and amygdala in SCZ and BP. We hypothesized that SCZ and BP would show similar shape patterns in the hippocampus and amygdala. Further, if SCZ and BP differed in the degree, but not the pattern, of structural irregularity, there would be an ordered relationship in surface shape among the SCZ, BP, and CON groups.
Participants included individuals with bipolar I disorder (n=12) and SCZ (n=11), both based on DSM-IV criteria, as well as CON participants (n= 12). Participants were matched for age, gender, race, and handedness. Exclusion criteria included recent (within three months) substance dependence, mental retardation, and history of severe head injury. Principal component (PC) analysis was used to reduce the high dimensionality of structure surfaces, yielding an orthonormal set of PCs representing shape variation. Group differences in structure shapes were assessed using MANOVA with the weights from the first 10 PCs (> 82% shape variance) used as dependent variables. To test for an ordered variation in surface shape across the groups, we performed a canonical analysis using a general linear model with the PC scores as dependent variables and group as the predictor variable. The canonical analysis was designed to score BP subjects along a dimension of surface shape variation for each structure that ‘maximized’ the difference between SCZ and CON (9).
There was a trend-level significant effect for hippocampal volume [F(2,34) = 2.9, p = 0.07]. Post-hoc analysis of hippocampal volumes showed significant group difference on the left [F(2,34) = 3.5, p = 0.04] but not the right [F(2,34) = 2.0, p = 0.15]. Further analysis of the left hippocampus showed smaller volumes [mm3 (SD)] in SCZ [2,164 (370)] compared to BP [2,453 (174)] (p = 0.019) and CON [2,395 (252)] (p = 0.052). There was a significant hemisphere effect for hippocampal volume [right > left: F(1,34) = 91.7, p < 0.0001], but no hemisphere × diagnosis interaction. Covarying for cerebral volume eliminated the hemisphere × diagnosis interaction for the hippocampus. There were no group effects for amygdala volume [F(2,34) = 0.81, p = 0.45]. Significant hemispheric effects for amygdala volume were observed [right > left: F(1,34) = 16.5, p = 0.003], but no hemisphere × diagnosis interaction.
MANOVA applied to the PC scores summarizing hippocampal surface variation, with hemisphere as a repeated factor, indicated a significant effect of group status (Wilks’ = 0.04, p = 0.004). Group comparisons based on the canonical shape score of the hippocampus did not show significant differences between BP and CON, while there were significant differences between BP and SCZ (p < 0.05). A visual representation of hippocampal shape in SCZ and BP compared to CON is shown in Figure 1. MANOVA applied to the PC scores summarizing amygdala shape variation did not indicate an effect of group status (Wilks’ = 0.23, p = 0.83).
Fig. 1.
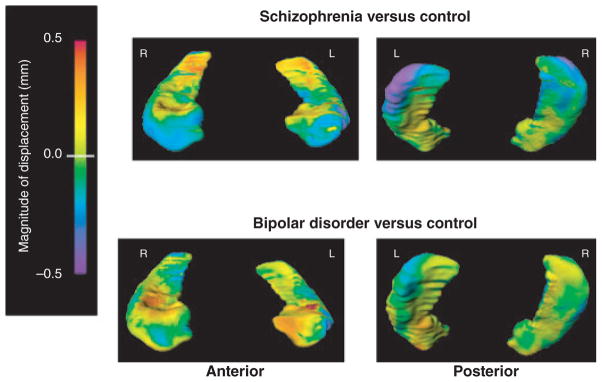
Hippocampal shape pattern in schizophrenia and bipolar disorder. Figures represent mean estimated displacement between subject groups. Surface displacement maps were obtained by first computing for the surface-normal component of the displacement of each surface point relative to the average surface of a superset of subjects. The mean of these displacements for each group (and surface point) was then computed, and the difference of means between the two selected groups displayed as a color map (overlaid onto the mean surface of control subjects). Purple-to-blue shading denotes regions of inward deformation compared with controls.
Our results demonstrated significant group differences in hippocampal shape and only a trend-level group effect in hippocampal volume. Contrary to our hypothesis, our results did not suggest similarities in structure volumes or shapes in SCZ and BP. Regional decrease of the hippocampal head observed in SCZ in our study was similar to that from our earlier studies (6), and our findings of additional regional reduction in a region of the left tail have been described by other authors (7). BP appeared to have hippocampal surface shapes different from those of SCZ. BP also had similar shape scores to controls, which further suggests significant shape dissimilarities between BP and SCZ. We did not find amygdala structure differences across groups.
A limitation of the current study in estimating structural abnormalities is the low statistical power due to the number of subjects used. Also, the study does not take into account the potential confounding role of psychotropic medications and recreational substances on brain morphology. A larger study may allow for detecting more subtle abnormalities in the amygdala, as well as studying brain structure separately in psychotic and nonpsychotic subtypes of BP. The psychotic bipolar BP subtype may be genetically more closely related to schizophrenia (10), which could be manifest in morphological similarities.
Acknowledgments
This research was funded by federal NIH grant P50 MH071616 (Conte Center for the Neuroscience of Mental Disorders) and MH59534.
Footnotes
All authors report no competing interests between financial supports and the interests of this manuscript.
References
- 1.Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 2.Harrison P. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 3.Lawrie SM, Abukmeil SS, Chiswick A, Egan V, Santosh CG, Best JJ. Qualitative cerebral morphology in schizophrenia: a magnetic resonance imaging study and systematic literature review. Schizophr Res. 1997;25:155–166. doi: 10.1016/S0920-9964(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 4.Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 5.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatr. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 7.Styner M, Lieberman JA, Pantazis D, Gerig G. Boundary and medial shape analysis of the hippocampus in schizophrenia. Med Image Anal. 2004;8:197–203. doi: 10.1016/j.media.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. Neuroimage. 2008;41:735–746. doi: 10.1016/j.neuroimage.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamah D, Harms MP, Wang L, et al. Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biol Psychiatry. 2008;64:111–120. doi: 10.1016/j.biopsych.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park N, Juo SH, Cheng R, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]


