Abstract
Endothelial cells have the ability to change their complement of cell surface proteins in response to inflammatory cytokines. We hypothesized that the expression of the coxsackievirus-adenovirus receptor (CAR), a viral receptor and putative cell-cell adhesion molecule, may be altered during the response of endothelial cells to inflammation. To test this hypothesis, we evaluated CAR protein and mRNA levels in human umbilical vein endothelial cells after they were exposed to tumor necrosis factor alpha, gamma interferon, or a combination of the two cytokines. Flow cytometric and Western blot analyses indicated that cytokine treatment led to a synergistic decrease in CAR protein expression. A Western blot analysis showed that CAR levels decreased to 16% ± 4% or 1% ± 4% of the CAR protein levels in untreated cells with either 24 or 48 h of cytokine treatment, respectively. Quantitative reverse transcription-PCR demonstrated that the combination treatment caused CAR mRNA levels to decrease to 21% ± 12% or 5% ± 3% of the levels in untreated cells after a 24- or 48-h cytokine treatment, respectively. Reduced CAR expression led to a decrease in adenovirus (Ad) binding of 80% ± 3% (compared with untreated endothelial cells), with a subsequent decrease in Ad-mediated gene transfer that was dependent on the dose and duration of cytokine treatment but not on the dose of Ad. A similar decrease in CAR protein level and susceptibility to Ad infection was observed in human microvascular endothelial cells, while CAR expression on normal human bronchial epithelial cells or A549 lung epithelial cells was less affected by cytokine treatments. Taken together, the data demonstrate that inflammatory cytokines decrease CAR mRNA and protein expression with a concomitant decrease in Ad binding, reflecting the impact of cell physiology on the function of CAR and the potential effect of inflammation on the ability of Ad to transfer genes to endothelial cells.
The biological mechanisms that regulate the availability of cell surface proteins for viral binding are central to an understanding of viral infections, with important implications for viral pathogenesis as well as for gene transfer with viral gene transfer vectors. In this context, the necessary first step in a viral infection involves an interaction of the viral capsid proteins with cell surface receptors. Conversely, the absence of a viral receptor can be correlated with protection against a viral infection, underscoring the importance of viral receptors during infections in vivo.
The coxsackievirus and adenovirus receptor (CAR), the high-affinity viral receptor for the majority of adenovirus (Ad) serotypes, is a 46-kDa cell surface glycoprotein which belongs to the immunoglobulin G (IgG) superfamily of proteins (3, 10, 30, 48). It consists of two IgG domains which make up the extracellular domain, a single membrane-spanning domain, and a palmitoylated cytoplasmic domain (3, 10, 13, 48, 49, 54). After the interaction of the Ad fiber with CAR, a secondary interaction occurs between the Ad penton base protein and cell surface integrins, leading to the internalization of the virus via receptor-mediated endocytosis (1, 14, 31, 31, 51-53, 55, 56). The cellular function of CAR has not yet been determined, but evidence points to a role for CAR in cell-cell contacts (14, 30, 31, 51, 52, 60). CAR localization on the basolateral surface of polarized epithelial cells is not random, but rather is highly concentrated near the apical end of the lateral plasma membrane, a site associated with tight junctions and adherence junctions (14, 51). CAR-expressing cells grown in suspension aggregate preferentially with other CAR-expressing cells, which suggests that CAR mediates cell adhesion through homotypic binding (14, 21). CAR also colocalizes with the cell adhesion molecules ZO-1 and β-catenin and can be coimmunoprecipitated by antibodies against these proteins (14, 51). Finally, the overexpression of CAR can lead to an increase in the electrical resistance of a cell monolayer, indicating that CAR contributes to a barrier function (14).
The cell surface expression of cell adhesion molecules on endothelial cells is highly regulated and is responsive to changing environmental conditions of cells, such as inflammation (9, 34). For example, the exposure of human umbilical vein endothelial cells (HUVEC) to inflammatory cytokines leads to a coordinated series of changes in the cell surface of endothelial cells that promotes endothelial cell-leukocyte interactions through intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule (VCAM), E-selectin, and P-selectin, while endothelial cell-endothelial cell interactions are diminished through the downregulation and/or redistribution of tight junction and adherens junction proteins, including platelet-endothelial cell adhesion molecule 1 (PECAM), VE-cadherin, and junctional adhesion molecule (JAM) (9, 20, 28, 33-35, 37, 38, 43, 45, 57). In the context of these considerations and given the characteristics of CAR and its expression in endothelial cells, we hypothesized that the surface expression of CAR by endothelial cells would be altered after a treatment with inflammatory cytokines and that the change in cell surface CAR expression would have consequences on the ability of Ad to infect these cells. To test this hypothesis, we utilized HUVEC treated with the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ), a common in vitro model system for the study of inflammation (46). The data demonstrated that after cytokine treatment, the CAR cell surface protein expression, total cellular CAR protein and mRNA levels, and ability of CAR to support an Ad infection were all diminished. Similar results were also observed for endothelial cells from a different source (human microvascular endothelial cells), whereas CAR expression was less affected by a cytokine treatment in cells of epithelial origin.
MATERIALS AND METHODS
Cell culture.
HUVEC were obtained from umbilical cords by a modification of the method described by Jaffe et al. (22). Briefly, umbilical cords were washed with cord buffer (0.14 M NaCl, 4 mM KCl, 11 mM glucose [Baker, Phillipsburg, N.J.], 10 mM HEPES [pH 7.4] [Sigma-Aldrich Corp., St. Louis, Mo.]) and reperfused with cord buffer containing 0.1% collagenase (type 1; Invitrogen, Carlsbad, Calif.). The cord was clamped and incubated with cord buffer at 37°C for 10 to 15 min. After incubation, the collagenase solution was eluted into a conical tube containing T199 medium (Biowhittaker, Walkersville, Md.), 18% fetal bovine serum (FBS; Life Technologies, Gaithersburg, Md.), 200 U of penicillin (Biowhittaker)/ml, 200 μg of streptomycin (Biowhittaker)/ml, 2 mM l-glutamine (Biowhittaker), 50 μg of heparin (Sigma-Aldrich Corp.)/ml, and 5 μg of endothelial mitogen (Biomedical Technologies, Stoughton, Mass.)/ml. The cells were centrifuged, washed, resuspended in endothelial cell medium, and finally plated onto four to six 35-mm-diameter petri dishes.
For all experiments, cells were maintained in EGM-2 medium supplemented with EGM-2 supplements (5% FBS and prepackaged SingleQuots containing hydrocortisone, basic human fibroblast growth factor with heparin, vascular endothelial growth factor, recombinant insulin-like growth factor, ascorbic acid, human epidermal growth factor, gentamicin, and amphotericin B [Fungizone] [all from Clonetics, Cambrex BioScience, Walkersville, Md.]) and were incubated in a humidified chamber at 37°C with 5% CO2. The supplemented EGM-2 medium was used for all experiments. Only cells from passages 2 to 5 were used for experiments, and the medium was changed every second day. For microscopy experiments, cells were cultured on poly-d-lysine-coated coverslips affixed to 35-mm-diameter tissue culture dishes as described previously (25). Transgene expression experiments were performed on cells grown to confluence in 96-well microplates (Falcon; Becton Dickinson, Franklin Lakes, N.J.).
Human microvascular endothelial cells (Clonetics) were cultured in an identical manner to HUVEC, as described above. Normal human bronchial epithelial cells (Clonetics) were maintained in BEBM medium supplemented with BEGM supplements (prepackaged SingleQuots containing retinoic acid, bovine pituitary extract, insulin, hydrocortisone, transferrin, triiodotyronine, epinephrine, human epidermal growth factor, gentamicin, and amphotericin B [all from Clonetics]) and were incubated in a humidified chamber at 37°C with 5% CO2. After cytokine treatment, the cells were grown to confluence and the medium was replaced with BEBM medium supplemented with BEGM supplements without retinoic acid. After the transfer to retinoic acid-free medium, the cells were cultured for at least 24 h prior to the cytokine treatment. Only cells from passages 2 to 5 were used for experiments, and the medium was changed every second day. A549 human lung epithelial carcinoma cells (clone CCL-185; American Type Culture Collection, Rockville, Md.) were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS (Life Technologies), 100 U of penicillin (Life Technologies)/ml, 100 μg of streptomycin (Life Technologies)/ml and 1% amphotericin B (Biofluids, Rockville, Md.).
Ad vectors.
Two Ad vectors were used: they were AdNull, an E1GE3G replication-deficient Ad containing a cytomegalovirus early-intermediate promoter-enhancer and a simian virus 40 polyadenylation site without a transgene in the expression cassette; and Adβgal, an identical vector that contained the β-galactosidase gene in the expression cassette (19). The Ad vectors were propagated in HEK293 cells and stored as previously described (39, 40). The virus concentration was determined based on the extinction coefficient for Ad (9.09 × 10−13 ml of particles−1 cm−1) (26). The carbocyanine dye Cy3 (Amersham Inc., Piscataway, N.J.) was covalently conjugated to capsid proteins of the Ad5 vector as previously described (27).
Cytokine treatment.
Confluent HUVEC were cultured for 24 or 48 h in the absence or presence of TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of these two cytokines (R&D Systems, Minneapolis, Minn.). To visualize the conversion of HUVEC from a cobblestone morphology to a spindle-shaped morphology after cytokine treatment, we imaged the cells by using an Olympus IX70 microscope equipped with a 20× NA 0.4 PhC achromatic objective lens. Confirmation of the effect of the cytokines was also evaluated by flow cytometry. After the cytokine treatment, the cells were washed with HEPES-buffered saline, pH 7.4 (Clonetics), removed from the plate with 0.025% trypsin, and suspended in phosphate-buffered saline, pH 7.4 (PBS), with 2% FBS (Life Technologies, Inc). Harvested cells were incubated with a fluorescein isothiocyanate (FITC)-conjugated antibody directed against PECAM or a phycoerythrin (PE)-conjugated antibody directed against ICAM-1 (Pharmingen, Franklin Heights, N.J.) for 30 min at 4°C, washed, and evaluated by flow cytometry in a FACSCalibur (Becton Dickinson) instrument. The background fluorescence was determined by the substitution of a control FITC-conjugated or PE-conjugated IgG1. Cytometric analysis was conducted with Flow Jo software (Tree Star, San Carlos, Calif.).
To evaluate cell viability, we cultured confluent HUVEC in the absence or presence of TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of the two for 24 or 48 h. Cell viability was assayed after cytokine treatment by use of a cell viability kit as described by the manufacturer (Live-Dead cytotoxicity kit for animal cells; Molecular Probes, Eugene, Oreg.). The data were quantified for three independent cultures per condition by using a random collection of images from four fields per culture generated with a Zeiss Axiovert 200 M epifluorescence microscope equipped with a 20× PlanFluor objective with automated stage position and focus control systems (Universal Imaging, Inc., Downingtown, Pa.). The numbers of live and dead cells per field were determined in an objective manner with the MetaMorph image analysis system (Universal Imaging, Inc.) by setting fixed image parameters and using digital image analysis to count the number of individual fluorescent signals per image.
Evaluation of CAR protein expression.
Confluent HUVEC were cultured for 48 h in the absence or presence of TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of these two cytokines. For analysis by flow cytometry, the cells were washed with HEPES-buffered saline and trypsinized, and CAR expression was evaluated with an anti-CAR antibody (RmcB; kindly provided by J. Bergelson, Children's Hospital of Philadelphia), with detection via the addition of a PE-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The background fluorescence was evaluated with a PE-conjugated isotype control. A549 cells were evaluated for CAR expression as a positive control. A cytometric analysis of 20,000 events per sample was conducted with Flow Jo software.
For Western blot analysis, the cells were washed twice in cold PBS and lysed in cold lysis buffer (Tropix, Bedford, Mass.) with 0.01 M dithiothreitol and proteinase inhibitors (Roche Applied Science, Indianapolis, Ind.). The proteins were denatured by heating at 95°C for 5 min in sample buffer (100 mM Tris-HCl [pH 8.8], 20% glycerol, 1% sodium dodecyl sulfate, 0.02% bromophenol blue, 2% β-mercaptoethanol, 6 M urea [Sigma-Aldrich Corp.]), separated by 10% polyacrylamide gel electrophoresis (Bio-Rad, Hercules, Calif.), and transferred to nitrocellulose (Bio-Rad). Equal loading of the proteins was confirmed by Coomassie blue (Bio-Rad) staining of an identically loaded gel. The membrane was exposed to blocking solution (5% dry milk and 0.1% Tween 20 in 1× PBS [Bio-Rad]) for 1 h and then probed with a primary rabbit anti-CAR peptide polyclonal antiserum (kindly provided by K. Sollerbrant, Ludwig Institute of Cancer Research, Stockholm, Sweden) (44) at a dilution of 1:1,000 in blocking solution for 20 h at 4°C. For visualization of the anti-CAR antibody binding, a secondary peroxidase-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc.) diluted in blocking solution was applied for 1 h at 23°C. Antibody binding was detected by exposure to a chemiluminescent peroxidase substrate (ECL reagent; Amersham Biosciences), and the blot was exposed to film and developed. The kinetics of the downregulation of cell surface expression were examined by incubating confluent HUVEC with both TNF-α and IFN-γ for 24 or 48 h before analysis. CAR expression in confluent cultures of human microvascular endothelial cells, normal human bronchial epithelial cells, or A549 cells was also evaluated by Western blot analysis 48 h after cytokine treatment.
CAR mRNA levels evaluated by quantitative RT-PCR.
The mRNA levels of the CAR transcript were analyzed by TaqMan quantitative reverse transcription-PCR (RT-PCR) (23). RNAs were isolated from untreated HUVEC or HUVEC treated with TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of the two for 24 or 48 h, as described above. The CAR transcript in each sample was reverse transcribed (binding of random hexamers, 10 min at 25°C; RT, 60 min at 42°C; inactivation, 5 min at 95°C). The resulting DNAs were amplified by 40 cycles of PCR (denaturation, 15 s at 95°C; extension, 1 min at 60°C) using the TaqMan real-time fluorescence PCR system with an ABI 7700 thermocycler (Applied Biosystems, Foster City, Calif.). Each cDNA sample was amplified in the presence of synthetic 24-mer oligonucleotide primers, beginning at position 419 at the 5′ end (AGCCTTCAGGTGCGAG ATGTTACG) and 761 at the 3′ end (TACGACAGCAAAAGATGATAAGAC) of the human CAR cDNA in the presence of SYBER Green (SYBER Green PCR master mix; Applied Biosystems). The PCR was also performed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers (TaqMan GAPDH control reagent; Applied Biosystems) to create an internal standard. Quantitation using SYBER Green (Applied Biosystems) was performed as described by the manufacturer. An initial validation experiment was performed to establish that the target (CAR) and the internal control (GAPDH) were amplified with the same efficiency at a range of template concentrations. Data were reported as ratios of CAR cDNAs in cytokine-treated cells and untreated cells.
Cy3-Ad binding evaluated by fluorescence microscopy.
Confluent HUVEC were grown on poly-d-lysine-coated coverslips and cultured in the absence or presence of TNF-α and IFN-γ for 48 h. The cells were washed one time with endothelial cell medium and infected with fluorophore-conjugated Ad (Cy3-Ad prepared as previously described) (25, 27). Untreated or cytokine-treated HUVEC were incubated with Cy3-Ad (1011 particles/ml in endothelial cell medium; approximately 6,000 PFU per cell) for 10 min at 37°C. The cells were washed three times in endothelial cell medium and fixed in 4% paraformaldehyde in PBS. The nuclei were stained blue with 4,6-diamidino-2-phenylindole (DAPI) (1 μM in PBS plus 0.1% Triton X-100; Molecular Probes), and Ad binding was evaluated by fluorescence microscopy. Eight fields from at least two coverslips per condition were imaged by collecting a series of images at different focal planes (Z-series) and combining them into a single image after deconvolution with MetaMorph imaging software. Automated counting of the Cy3 signal was performed as follows. A threshold was set at a grayness level that was 70% higher than the background intensity. The total number of objects was counted with MetaMorph image analysis software. For each field, the number of Cy3 signals was divided by the number of nuclei in the DAPI image to arrive at a number of Ad particles per nucleus.
Ad-mediated gene delivery evaluated by β-galactosidase expression in cytokine-treated endothelial cells.
Confluent HUVEC were cultured in the absence of cytokines or in the presence of TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of these two cytokines for 48 h. The cells were washed one time, infected with Adβgal at 1011 particles/ml (approximately 6,000 PFU per cell) in endothelial cell medium for 10 min at 37°C, and incubated for 24 h to allow for expression. Cell lysates were prepared by washing the cells two times and rinsing them with lysis buffer (Tropix). β-Galactosidase activity in the cell lysates was measured by a chemiluminescence assay (Tropix) using a Monolight 3010 luminometer (Pharmingen). The total protein levels were determined by the Bradford colorimetric assay (Pierce Biotechnology, Rockford, Ill.), with bovine serum albumin used to generate a standard curve of protein concentrations. β-Galactosidase activity was expressed as β-galactosidase units per milligram of protein per cell, using a standard curve for purified β-galactosidase (Sigma). Comparable experiments were performed with confluent cultures of human microvascular endothelial cells, normal human bronchial epithelial cells, or A549 cells 48 h after cytokine treatment. Ad-mediated gene transfer to HUVEC was further evaluated under a number of distinct conditions. To determine the dose dependence of the cytokine treatment, we treated confluent HUVEC with various doses of TNF-α (0.06 to 2,000 ng/ml) or IFN-γ (0.03 to 1,000 ng/ml) in endothelial cell culture medium for 48 h. To determine the dose response of cytokine combinations, we treated confluent endothelial cells with a constant dose of TNF-α (20 ng/ml) or IFN-γ (10 ng/ml) and various doses of IFN-γ (0.03 to 1,000 ng/ml) or TNF-α (0.06 to 2,000 ng/ml), respectively. To determine the kinetics of the cytokine effect on HUVEC, we incubated confluent cultures with TNF-α (20 ng/ml) and IFN-γ (10 ng/ml) for 0 to 24 h in endothelial cell medium. To evaluate the effect of the Adβgal dose on gene transfer after cytokine treatment, we treated confluent HUVEC with TNF-α (20 ng/ml) and IFN-γ (10 ng/ml) in endothelial cell medium for 48 h and then washed out the cytokine. Untreated and cytokine-treated cells were then infected with graded doses of Adβgal (108 to 1011 particles/ml, corresponding to a range from approximately 6 to 6,000 PFU per cell). In all cases, the cells were lysed 24 h after infection and the β-galactosidase activity per milligram of protein was determined.
Statistical analysis.
All data are presented as means ± standard errors of the means; statistical evaluations were performed by using the two-tailed Student t test.
RESULTS
Characterization of cytokine-treated endothelial cells.
Given the wealth of data documenting changes in the endothelial cell surface after an exposure to inflammatory cytokines and the evidence implicating CAR as a mediator of cell-cell contact in other cell types, we wanted to test the hypothesis that the cell surface expression of CAR on endothelial cells is modulated in response to inflammatory cytokines. As a model of inflammatory cytokine treatment, HUVEC were cultured in the presence of TNF-α, IFN-γ, or a combination of the two. Confluent HUVEC were treated with cytokines and evaluated by phase-contrast microscopy. Untreated confluent monolayers of HUVEC showed a typical cobblestone morphology. IFN-γ-treated cells exhibited a mild elongation, while cells treated with either TNF-α or a combination of the two cytokines adopted a spindle-shaped morphology that is typical of cytokine-treated endothelial cells (Fig. 1A). The state of the cytokine response was also evaluated by a flow cytometric analysis of cell surface markers of inflammation (Fig. 1B). In HUVEC treated with TNF-α, IFN-γ, or a combination of the two, ICAM expression was elevated relative to untreated cells both 24 and 48 h after cytokine treatment in a manner consistent with previous descriptions of the cytokine-mediated activation of HUVEC (4, 5). PECAM expression remained high throughout the cytokine treatment. The viability of cytokine-treated HUVEC was evaluated by a fluorescence-based assay. Compared to untreated cells, the cytokine-treated cells did not show a significant increase in the proportion of dead cells 24 or 48 h after cytokine treatment under these experimental conditions (not shown), indicating that any apparent changes in cell surface receptor expression were not due to cell death.
FIG. 1.
Characterization of cytokine-treated and nontreated HUVEC. Confluent HUVEC were treated with TNF-α (20 ng/ml) and/or IFN-γ (10 ng/ml) for either 24 or 48 h. (A) Cell morphology. Untreated cells had a cobblestone morphology, while cells treated with individual cytokines or a combination of cytokines showed varying degrees of spindle-shaped morphology. (B) Flow cytometry. Cells were collected 24 and 48 h after cytokine treatment, labeled with a FITC-labeled anti-PECAM or PE-labeled anti-ICAM-1 antibody, and evaluated by flow cytometry. Histograms show the distributions of fluorescence intensities due to the cell surface staining of PECAM or ICAM-1 in cytokine-treated versus untreated cells. Nonspecific background fluorescence was evaluated by using a control IgG.
Cell surface CAR expression and total cellular CAR protein levels are reduced in HUVEC after treatment with inflammatory cytokines.
Cell surface CAR expression, indicated by the fluorescence intensity, was relatively low in untreated HUVEC in comparison to A549 epithelial cells, which yielded a 10-fold higher fluorescence signal (not shown). Exposing confluent HUVEC to TNF-α or IFN-γ for 48 h led to a reduction in the cell surface expression of CAR, as analyzed by flow cytometry (Fig. 2A). Individual treatments with either TNF-α or IFN-γ led to a reduction in the mean CAR-specific fluorescence intensity compared to that in untreated cells. Cell surface CAR expression decreased 72% after a TNF-α treatment (P < 0.001) and 58% after a IFN-γ treatment (P < 0.001). The combination of the two cytokines led to a 95% decrease in cell surface CAR expression (P < 0.001 compared to the control or the TNF-α or IFN-γ treatment).
FIG. 2.
Analysis of CAR expression in response to cytokine treatment. Confluent HUVEC were cultured in the absence or presence of TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of the two cytokines for 48 h. (A) Cell surface expression of CAR evaluated by flow cytometry. Cell surface CAR expression was detected by using an anti-CAR monoclonal antibody and was analyzed by flow cytometry. The distributions of fluorescence intensities due to the cell surface staining of CAR in cytokine-treated versus untreated cells are shown. Nonspecific background fluorescence was evaluated by using a control IgG. CAR expression is shown for TNF-α-treated cells (left), IFN-γ-treated cells (center), and cells treated with a combination of the two cytokines (right). (B) CAR protein level evaluated by Western blot analysis. Lysates of HUVEC were prepared after a 48-h exposure to cytokines. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with a polyclonal antibody raised against the intracellular domain of the CAR protein, with detection via a peroxidase-conjugated secondary antibody and chemiluminescence reagents. The lanes, from left to right, contain untreated HUVEC, TNF-α-treated HUVEC, IFN-γ-treated HUVEC, HUVEC treated with a combination of the two cytokines, and A549 cells (positive control for CAR expression). (C) Comparative quantitative analysis of cell surface CAR and total CAR protein. To determine the effect of cytokines on the cell surface expression of CAR, we averaged the mean fluorescence intensity measurements of flow cytometry histograms for three experiments per condition, and the results are shown with the standard errors of the means. For each condition, the level of cell surface CAR, as determined by flow cytometry, was plotted as a percentage of the level of cell surface CAR expression in untreated cells (black bars). To determine the effect of cytokines on the total cellular level of CAR protein, we evaluated digital images of the Western blot analyses (white bars). For each condition, the level of CAR was plotted as a percentage of the level of total cellular CAR expression in untreated cells.
In order to investigate the amount of total CAR protein in endothelial cells after a 48-h treatment with TNF-α or IFN-γ, we analyzed HUVEC lysates for their protein content by Western blot analysis. Anti-CAR staining identified a 46-kDa band in HUVEC, as was previously reported (Fig. 2B) (11, 44). CAR expression in cells treated with either TNF-α or IFN-γ decreased 86 or 87%, respectively, compared to untreated HUVEC (P < 0.001) (Fig. 2B and C). Consistent with the flow cytometry data, the combination of TNF-α and IFN-γ caused a 99% decrease in the total cellular CAR compared with untreated HUVEC (P < 0.001).
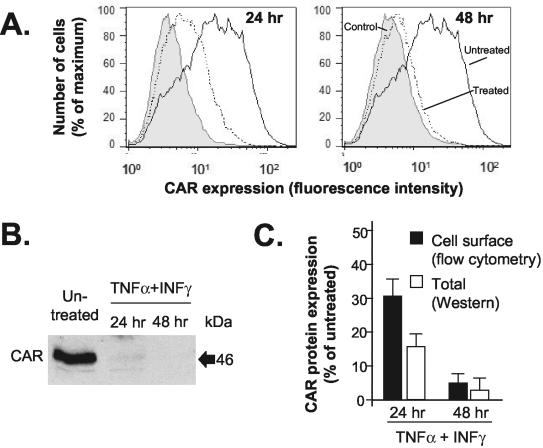
Time course for the decrease in cell surface CAR expression and total cellular CAR protein in HUVEC after treatment with inflammatory cytokines.
To evaluate the kinetics of the downregulation of cell surface CAR expression, we treated HUVEC with TNF-α and IFN-γ for 24 or 48 h. A progressive decrease in the amount of cell surface CAR was observed by flow cytometry 24 and 48 h after the addition of TNF-α and IFN-γ (Fig. 3A). The amount of cell surface CAR on treated versus untreated HUVEC decreased 71 and 95% at 24 and 48 h, respectively (P < 0.001 for each time point compared with untreated HUVEC and for a comparison of the two time points). In order to investigate the time course of the reduction in the total CAR protein level, we evaluated HUVEC lysates by Western blot analysis after a 24- or 48-h treatment with a combination of TNF-α and IFN-γ (Fig. 3B). Consistent with the flow cytometry data, the 46-kDa signal for cytokine-treated HUVEC compared to untreated HUVEC decreased 84 and 99% 24 and 48 h, respectively, after treatment with TNF-α and IFN-γ (P < 0.001 for each time point compared with untreated HUVEC; P < 0.05 for a comparison of the two time points) (Fig. 3C).
FIG. 3.
Time course for downregulation of CAR after treatment with TNF-α and IFN-γ in HUVEC. Confluent HUVEC were cultured with TNF-α (20 ng/ml) and IFN-γ (10 ng/ml) for 24 or 48 h. (A) Time course for decrease in cell surface CAR. Cell surface CAR expression was detected by using an anti-CAR monoclonal antibody and was analyzed by flow cytometry as described in the legend to Fig. 2. The panels show anti-CAR staining of populations of untreated HUVEC or TNF-α/IFN-γ-treated HUVEC 24 and 48 h after the addition of cytokines. Nonspecific cell fluorescence was assessed by binding of a control IgG. (B) Time course for decrease in CAR protein level, as evaluated by Western blot analysis. Lysates of HUVEC were assayed after either 24 or 48 h of exposure to cytokines. (C) Comparative quantitative analysis of time courses for decreases in cell surface CAR and in total CAR protein. The graph presents data from flow cytometry (mean fluorescence intensities for CAR cell surface expression in cytokine-treated HUVEC as percentages of CAR expression on untreated HUVEC [black bars]) and Western blot analysis (digital image analysis of CAR band intensities for cytokine-treated HUVEC as percentages of the CAR level in untreated HUVEC [white bars]).
CAR mRNA levels decrease after cytokine treatment.
The amount of CAR mRNA, quantified relative to the amount of GAPDH mRNA in the sample, demonstrated that after 48 h, CAR expression showed an 83 or 74% reduction in TNF-α-treated or IFN-γ-treated cells, respectively (Fig. 4A). The combination of TNF-α and IFN-γ decreased CAR expression at the mRNA level 95% compared to that in untreated HUVEC (P < 0.01 compared to untreated cells or IFN-γ-treated cells). Although the combination treatment caused a larger decrease in the amount of CAR mRNA than the TNF-α treatment alone, the result was not statistically different (P > 0.1), suggesting that the effect on mRNA levels was largely controlled by the TNF-α treatment. The effect of the combination of TNF-α and IFN-γ on CAR mRNA expression increased over time, with decreases of 79% at 24 h and 95% at 48 h (Fig. 4B). While the average level of CAR mRNA was lower at 48 h than at 24 h, consistent with the decrease in the amount of CAR protein, the difference in the CAR mRNA levels at 24 and 48 h was not significantly different (P > 0.1).
FIG. 4.
Analysis of CAR mRNA levels after cytokine treatment. HUVEC were grown to confluence and treated with individual cytokines or a combination of cytokines, and RNAs were isolated 24 or 48 h after treatment. For each condition, the data presented are percentages of CAR mRNA remaining compared to that from untreated HUVEC. (A) CAR mRNA levels in HUVEC treated for 48 h with TNF-α, IFN-γ, or a combination of both cytokines. (B) CAR mRNA levels in HUVEC treated with a combination of TNF-α and IFN-γ for 24 or 48 h.
Ad binding and reporter gene expression are reduced after cytokine treatment.
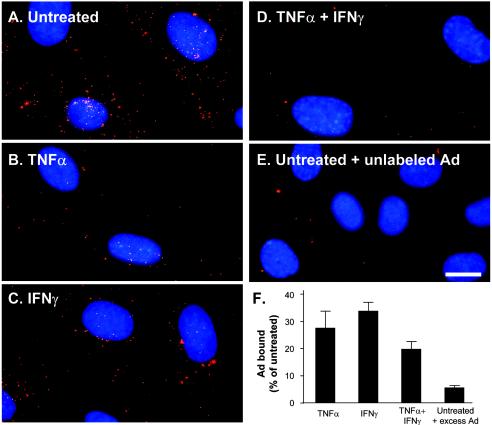
To assess the functional expression of CAR at the cell surface, we incubated HUVEC grown on coverslips with TNF-α, IFN-γ, or a combination of the two for 48 h. After the cytokine treatment, the cultures were infected with a fluorophore-labeled Ad vector (Cy3Ad). The cytokine-treated HUVEC bound less Ad than the untreated HUVEC, as indicated by a reduced red fluorescence signal associated with the cells (Fig. 5). To demonstrate the specificity of the binding reaction, we infected untreated HUVEC with Cy3Ad in the presence of a 100-fold excess of unlabeled Ad, a technique which was previously shown to produce competitive inhibition of the Cy3Ad binding (27). Cy3Ad binding in the presence of excess Ad was reduced 95% ± 1% in untreated cells. Binding was also reduced in TNF-α-treated cells (72% ± 6% decrease), IFN-γ-treated cells (64% ± 4% decrease), and TNF-α- and IFN-γ-treated cells (80% ± 3% decrease) compared to that in untreated cells. Binding in each cytokine treatment group was significantly lower than binding to untreated cells (P < 0.001) and significantly higher than binding of the negative control (P < 0.001). Among the cytokine-treated groups, the only significant difference was found between the IFN-γ treatment and the combination treatment (P < 0.01).
FIG. 5.
Evaluation of Ad capsid binding to cytokine-treated or untreated HUVEC. HUVEC were grown to confluence on poly-d-lysine-coated coverslips and were left untreated or were treated with TNF-α (20 ng/ml) and/or IFN-γ (10 ng/ml) for 48 h. The cells were then infected with Cy3-Ad (1011 particles/ml for 10 min), fixed, and evaluated by fluorescence microscopy. A composite image of the total Cy3-Ad content of HUVEC was created by collecting images at different focal planes (Z-series, shown in red). Nuclei are shown by staining with DAPI (blue). (A) Cy3-Ad binding to untreated HUVEC. (B) Cy3-Ad binding to TNF-α-treated HUVEC. (C) Cy3-Ad binding to IFN-γ-treated HUVEC. (D) Cy3-Ad binding to TNF-α/IFN-γ-treated HUVEC. (E) Cy3-Ad binding to untreated HUVEC in the presence of a 100-fold excess of unlabeled Ad capsid. (F) Quantitation of Cy3-Ad binding to HUVEC. Bar = 10 μm.
As a further functional demonstration of the effects of the inflammatory cytokine-induced decrease in cell surface CAR expression, the effect of cytokine treatment on Ad-mediated gene transfer was assessed. HUVEC were treated with TNF-α, IFN-γ, or a combination of the two. After 48 h, the cytokines were removed and HUVEC were infected with an Ad expressing β-galactosidase (Adβgal), and the β-galactosidase enzyme activity was measured 24 h later. Treatment with either TNF-α or IFN-γ led to reduced transgene expression (P < 0.001 compared to untreated cells) (Fig. 6A). The exposure of HUVEC to a combination of TNF-α and IFN-γ led to a 30-fold decrease in the resulting β-galactosidase expression level compared to that in untreated cells (P < 0.001). A comparison of Ad-mediated gene transfer after a treatment with either a single cytokine or a combination of the two cytokines indicated the presence of a synergistic response to the cytokine combination, with significantly less β-galactosidase expression after the treatment with the combination of cytokines (P < 0.001). The difference in β-galactosidase expression was not due to a direct effect of the cytokines on transcription from the cytomegalovirus (CMV) promoter in the Ad vector expression cassette, as verified by the observation that a cytokine treatment of pre-Adβgal-infected HUVEC resulted in an increase or decrease in β-galactosidase activity of less than twofold (not shown), which is in agreement with a previous study of endothelial cells (36).
FIG. 6.
Ad-mediated gene expression in cytokine-treated HUVEC. Confluent HUVEC were cultured for 48 h in the absence or presence of TNF-α, IFN-γ, or a combination of the two cytokines. The cultures were subsequently infected with Adβgal, an Ad expressing the β-galactosidase marker transgene (1011 particles/ml for 10 min). After 24 h, the β-galactosidase activity was quantified and expressed relative to the total protein. β-Galactosidase data in all graphs were plotted as percentages of the expression level in untreated HUVEC. For all graphs, data points represent the means ± standard errors of 12 to 20 observations. (A) Synergism of cytokines. The data compare Ad-mediated gene transfer to untreated HUVEC or HUVEC treated with TNF-α (20 ng/ml), IFN-γ (10 ng/ml), or a combination of the two cytokines for 48 h prior to infection with Adβgal. (B) Cytokine dose response. Confluent HUVEC were cultured with various doses of TNF-α (0.06 to 2,000 ng/ml) or IFN-γ (0.03 to 1,000 ng/ml) for 48 h prior to infection with Adβgal. Synergistic effects of the cytokine treatments were investigated by culturing cells with various doses of IFN-γ or TNF-α and constant levels of IFN-γ (20 ng/ml) or TNF-α (20 ng/ml), respectively, for 48 h prior to infection with Adβgal. (C) Time dependence. The cytokine effect is shown as a function of the time of cytokine treatment. Cultures of confluent HUVEC were grown in the absence or presence of TNF-α (20 ng/ml) and IFN-γ (10 ng/ml) for 0 to 24 h and then infected with an Adβgal vector. (D) Ad vector dose response. Confluent HUVEC were cultured and treated with TNF-α and IFN-γ or were left untreated. Untreated and cytokine-treated cells were then infected with graded doses (1011 to 108 particles/ml for 10 min) of Adβgal. β-Galactosidase expression in cytokine-treated HUVEC was significantly less than that in untreated HUVEC at each Ad concentration (P < 0.03 for comparisons at all Ad doses).
HUVEC were incubated with a range of doses of TNF-α or IFN-γ 48 h prior to infection with Adβgal to establish a dose response for the individual cytokines. To further characterize the synergy between the two cytokines, we treated endothelial cells with constant levels of TNF-α or IFN-γ in the presence of a range of doses of IFN-γ or TNF-α, respectively. A decreased susceptibility to Ad-mediated gene transfer correlated with an increased cytokine dose for both TNF-α and IFN-γ (Fig. 6B). A marked shift in the dose-response curve occurred when the second cytokine was present. TNF-α concentrations as low as 6 pg/ml and IFN-γ concentrations as low as 30 pg/ml caused a significant reduction in Ad-mediated gene transfer when the Ad infection was performed in the presence of a constant concentration of the other cytokine (P < 0.001 for each).
Based on the observation that the cytokine-mediated downregulation of CAR expression was time dependent, the kinetics of the decreased susceptibility of Ad-mediated gene transfer were examined after the cytokine treatment. HUVEC were incubated with TNF-α and IFN-γ for 0 to 24 h prior to Adβgal infection. The resulting β-galactosidase expression data indicated that no significant difference in Ad-mediated gene transfer occurred within 3 h after the cytokine treatment (P > 0.2 compared to untreated cells), while a decrease of >50% was observed after a 6-h exposure to cytokines (P < 0.001 compared to untreated cells). The decrease in gene transfer reached a plateau approximately 24 h after the cytokine exposure (Fig. 6C).
To evaluate the effect of the Ad dose during infection, we used a range of vector concentrations during the 10-min infection. Untreated and cytokine-treated cells were infected with graded doses of Adβgal. The reduction in Ad-mediated gene transfer was observed at all Ad doses tested (108 to 1011 particles/ml) (Fig. 6D).
Effect of cytokine treatment on CAR expression in microvascular endothelial cells and epithelial cells.
To determine whether the cytokine-mediated decrease in CAR levels and sensitivity to Ad infection was restricted to endothelial cells, we evaluated the effect of cytokine treatment in endothelial cells from an alternative source (human microvascular endothelial cells) or in two types of epithelial cells (normal human bronchial epithelial cells and the lung epithelial A549 cell line).
The cytokine treatment of microvascular endothelial cells resulted in the same characteristic morphological changes as those observed for HUVEC (not shown). A Western blot analysis of TNF-α-, IFN-γ-, or TNF-α/IFN-γ-treated microvascular endothelial cells showed a dramatic decrease in CAR levels, similar to the results observed in HUVEC (Fig. 7A). The results obtained with epithelial cells were quite different. In bronchial epithelial cells, the TNF-α treatment had no significant effect on CAR expression, while IFN-γ alone or in combination with TNF-α caused a clear reduction in the CAR level. In contrast, cytokine treatment of A549 cells had no apparent effect on CAR expression.
FIG. 7.
CAR expression and Ad-mediated gene expression in human microvascular endothelial cells and epithelial cells after cytokine treatment. Confluent cultures of human microvascular endothelial cells, normal human bronchial epithelial cells, or A549 lung epithelial carcinoma cells were cultured in the absence or presence of TNF-α (20 ng/ml) and/or IFN-γ (10 ng/ml) for 48 h. (A) CAR protein levels evaluated by Western blot analysis. Cell lysates were prepared, and equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with a polyclonal antibody raised against the intracellular domain of the CAR protein, with detection via a peroxidase-conjugated secondary antibody and chemiluminescence reagents. The lanes, from left to right, contain untreated cells, TNF-α-treated cells, IFN-γ-treated cells, and cells treated with a combination of the two cytokines. (B) Ad-mediated gene expression in cytokine-treated cells. Cell cultures were infected with Adβgal and evaluated for β-galactosidase activity as described in the legend to Fig. 6.
As described above, decreased CAR expression was accompanied by a decrease in Ad-mediated gene transfer to HUVEC. To determine whether the decrease in Ad-mediated gene transfer would also be observed in microvascular endothelial cells, we measured Ad-mediated β-galactosidase expression after 48 h of cytokine treatment (Fig. 7B). Both cytokines resulted in a strong reduction in β-galactosidase expression (74% ± 4% decrease for TNF-α and 75% ± 2% decrease for IFN-γ compared to untreated cells; P < 0.001 for a comparison of each condition to untreated cells). The combined treatment gave a synergistic effect, reducing β-galactosidase expression by 99% ± 1% (P < 0.001 compared to untreated cells). Ad infections of epithelial cells were less sensitive to cytokine treatment. In human bronchial epithelial cells, β-galactosidase expression was unaffected by TNF-α (6% ± 4% increase). In contrast, IFN-γ resulted in a clear reduction in enzyme activity (53% ± 10% reduction; P < 0.01 compared to untreated cells), an effect that was potentiated by the combined treatment with TNF-α (69% ± 10% reduction; P < 0.01 compared to untreated cells). These results are in good agreement with the CAR levels observed by Western blot analysis (Fig. 7A). Like bronchial epithelial cells, A549 cells treated with TNF-α did not show a significant effect on Ad-mediated gene transfer (11% ± 15% increase). In contrast to normal human bronchial epithelial cells, A549 cells also failed to respond to IFN-γ alone (7% ± 7% increase), and Ad-mediated gene transfer increased modestly in the presence of a combination of the two cytokines (52% ± 12% compared to untreated cells; P < 0.05).
DISCUSSION
Throughout the course of evolution, viruses have acquired the ability to use as their receptors cell surface proteins that play integral roles in the biology of the target cell (2, 29, 58). In particular, cell surface proteins that play a role in cell adhesion have been utilized as viral receptors, including such cell adhesion molecules as ICAM, which acts as a receptor for rhinoviruses, JAM, which acts as a receptor for reoviruses, and integrins, which are receptors for many viruses, including Ad, adeno-associated virus, coxsackievirus, echovirus, and Sindbis virus (2, 29, 58). Since the use of cell surface proteins is one principal mechanism by which a cell responds to changes in its environment, it is not surprising that viral receptor protein expression can be modulated in response to the environment of the target cell. Endothelial cells are exquisitely capable of responding to changes in their environment such as tissue damage and infection (24). Inflammatory cytokines released in response to these insults act on endothelial cells to promote the recruitment of circulating leukocytes (9, 34). Therefore, we tested the hypothesis that the cell surface expression of CAR would be altered in endothelial cells after cytokine exposure and examined the effects of the cytokines on synergism as well as the time dependence of these effects.
Using a well established in vitro model of inflammation (HUVEC grown in the presence of inflammatory cytokines TNF-α and/or IFN-γ [46]), we assayed CAR expression by measuring the cell surface level of CAR (by flow cytometry), the total cellular CAR protein (by Western blot analysis), the CAR mRNA level (by quantitative RT-PCR), the amount of Ad binding (by digital image analysis), and Ad-mediated gene expression (by a β-galactosidase transgene assay). At the level of CAR protein, the cytokines acted synergistically, with the combination of cytokines causing a larger decrease in CAR protein and CAR function as a viral receptor than either cytokine alone. At the level of CAR mRNA, the majority of the decrease could be attributed to the exposure to TNF-α. The CAR protein level showed time dependence, with a progressive decrease observed from 24 to 48 h after initiation of the exposure to cytokines. After 48 h, CAR mRNA and protein levels had both decreased approximately 20-fold (95% decrease in cell surface CAR by flow cytometry, 99% decrease in total cellular CAR by Western blot analysis, and 95% decrease in CAR message by quantitative RT-PCR). The close agreement for the rates of decrease in CAR mRNA and protein levels suggests that the regulation of the CAR protein level is principally controlled at the level of transcription of CAR mRNA. A dramatic decrease in CAR protein levels was also observed after the treatment of human microvascular endothelial cells with TNF-α and IFN-γ, but the effect of cytokine treatment was less dramatic in normal human bronchial epithelial cells and was absent from A549 lung epithelial carcinoma cells, suggesting that CAR regulation in endothelial cells is particularly sensitive to inflammatory cytokines.
The fact that CAR protein levels are synergistically affected by the presence of both TNF-α and IFN-γ while CAR mRNA fails to show the same extent of synergism leaves open the possibility of posttranslational control of the CAR protein levels that may be regulated through intracellular signaling pathways activated by cytokines. Interestingly, the extent of the decrease in Ad-mediated gene transfer was more dramatic and occurred more rapidly than the decrease in CAR protein, likely indicating that the decrease in the amount of CAR protein was only one factor in the decrease in Ad-mediated gene transfer. One obvious candidate for regulation (transcription of the transgene mRNA from the CMV promoter) does not explain the extent of decreased gene expression, since our observations and those of others (36) show that if the CMV-driven β-galactosidase transgene is introduced into endothelial cells before cytokine treatment, then the cytokine effect on β-galactosidase gene expression is minor compared to the suppression of CAR expression.
Understanding the biology of CAR.
The effects of a cytokine treatment on CAR show both similarities to and differences from the effects of a cytokine treatment on other endothelial cell surface proteins. Inflammatory cytokines such as TNF-α and IFN-γ are known to increase the levels of cell surface receptors, including ICAM-1, VCAM, E-selectin, and P-selectin (9, 20, 28, 33-35, 37, 38, 43, 45, 57). The increase in these proteins reflects a shift in endothelial cell physiology that enables enhanced interactions with leukocytes. Since CAR is thought to form homotypic interactions between adjacent endothelial cells (11), the decreased level of cell surface CAR may decrease the adhesion of adjacent endothelial cells, thereby facilitating the transmigration of leukocytes after the leukocytes bind to the luminal surfaces of the endothelial cells. The plethora of changes that occur in endothelial cells after treatment with TNF-α and IFN-γ impact the barrier function of endothelial cells, as measured by a decreased electrical resistance and an increased permeability (7). However, since the levels of many cell surface proteins are altered by cytokine treatment, the relative contribution of CAR to the barrier function of endothelial cells cannot be determined in the present experimental system.
In support of this model of an inverse coordination of endothelial cell-endothelial cell contact versus endothelial cell-leukocyte contact, TNF-α and IFN-γ affect the cell surface expression of other proteins that form endothelial cell-endothelial cell interactions, including JAM, VE-cadherin, and PECAM, by the redistribution of these proteins away from the sites of endothelial cell-endothelial cell contact, by decreased cell surface expression, and/or by decreased transcription (9, 20, 28, 34, 37, 38, 43, 45, 57). Therefore, the observation of cytokine-regulated cell surface expression with longer term effects on transcription and the total cell protein level of CAR is consistent with the proposed role of CAR as a mediator of cell adhesion.
Previous descriptions of the pharmacologic modulation of CAR expression have focused on CAR in epithelial cells, including transformed cell lines. Cytostatic agents have been observed to upregulate CAR expression (6, 18, 42). Interestingly, inflammatory cytokines also upregulated CAR expression in HeLa ovarian carcinoma cells (6). In this report, observations in A549 cells, a transformed epithelial cell line, supported previous studies that showed a modest increase in transgene expression with comparable levels of CAR expression. To ensure that the difference did not reflect an artifact of transformation, we evaluated primary human bronchial epithelial cells. CAR protein levels in normal human bronchial epithelial cells decreased moderately after treatment with IFN-γ or a combination of the two cytokines, but not after treatment with TNF-α alone. This observation contrasts with the substantial effect of cytokine treatment observed in endothelial cells and thus indicates that the dramatic downregulation of CAR is endothelial cell specific and a general phenomenon observed in endothelial cells derived from both large vessels and microvessels from diverse tissues (HUVEC and microvascular endothelial cells from the lung).
The coordinated regulation of a series of cell surface proteins suggests the involvement of a common intracellular signaling pathway that acts at the transcriptional level. The common sensitivity to TNF-α suggests that downstream signaling may act through the NF-κB pathway, and the sensitivity to IFN-γ suggests that downstream signaling may also involve the Janus kinase signal transducers and activators of transcription (JAK-STAT) pathway (8, 32). NF-κB is known to regulate the transcription of other cell adhesion molecules, such as ICAM, VCAM, and E-selectin (15). Interestingly, the CAR gene contains several NF-κB and STAT binding sites in the 5′ untranslated region, suggesting that the CAR promoter may be sensitive to these transcription factors (GenBank accession no. AF242862).
Implications for viral tropism.
Viral tropism is dependent to a large degree on the expression of high-affinity receptors. The success of an Ad infection in vivo depends not only on the presence of CAR, but also on the access of Ad capsids to the CAR receptor. The importance of CAR localization for Ad infections was initially highlighted by studies of Ad infections in a differentiated epithelium. Despite that fact that they express CAR, polarized airway epithelial cells are resistant to Ad infection when the Ad is applied from the apical surface (31, 52, 60). The lack of Ad infection was traced to the localization of CAR on the basolateral surface, and in particular, to cell-cell junctions. Endothelial cells represent another CAR-expressing polarized cell type, and CAR expression levels have been reported to increase as endothelial monolayers reach confluence (11).
This study and previous characterizations of the effects of inflammatory cytokines on the cell surface expression of membrane proteins in endothelial cells suggest that the tropism of viruses can be modified during the response to inflammation. Harari et al. (17) created an Ad capsid with a genetically engineered fiber that incorporated a single-chain antibody specific for E-selectin, a marker of inflamed endothelial cells. They showed that the E-selectin-targeted vector preferentially infected cytokine-treated endothelial cells. Therefore, it is reasonable to hypothesize that endothelial cells at sites of inflammation express smaller amounts of CAR and therefore may be resistant to Ad infection. In contrast, normal epithelial cells, as shown in this study, are not likely to be affected by cytokines as dramatically as endothelial cells, and there is potential for a cytokine-mediated upregulation of CAR in transformed epithelial cells, indicating that some tumors may exhibit elevated susceptibility to Ad infection during periods of inflammation, as reported previously (6).
The prediction from the in vitro system reported here has an in vivo correlate. An in vivo animal study using a model of liver injury showed that Ad-mediated gene transfer to endothelial cells was decreased in an inflammatory milieu (59). The data presented here provide a potential mechanism for decreased endothelial infection during inflammation and support the principle that the physiology of the target cell and the host organism can play a significant role in the initial stages of viral infection. Cytokine levels that occur in patients can approach the levels used throughout this study (TNF-α, 20 ng/ml; IFN-γ, 10 ng/ml) and often exceed the minimum cytokine concentrations that were observed to have an effect on Ad-mediated gene transfer, as shown in Fig. 6B (TNF-α, 60 pg/ml; IFN-γ, 30 pg/ml). For example, a variety of patients with afflictions including rheumatoid arthritis, adult respiratory distress syndrome, and skin or gastrointestinal ulcers have been documented to have locally elevated levels of TNF-α and/or IFN-γ ranging from 0.5 to 10 ng/ml (12, 16, 41, 47, 50). Based on the present study, the levels of cytokine expression in these clinical settings may be sufficient to alter gene transfer to endothelial cells at the site of inflammation. It is also interesting to consider the possibility that the inflammatory response at the site of a wild-type Ad infection may reduce the propensity of the Ad to spread to nearby endothelial and epithelial cells, thus limiting the spread of the infection through the tissue and bloodstream.
Acknowledgments
We thank G. Lam and R. McKinney for assistance with endothelial cell cultures, A. Cieciuch for assistance with TaqMan RT-PCR, L. Cohen-Gould in the Optical Microscopy Core Facility for assistance with automated imaging and analysis, B. De and D. Bonnyay for helpful discussions, and N. Mohamed and T. Virgin-Bryan for assistance with preparation of the manuscript.
This work was supported in part by grant P01 HL59312; by the Will Rogers Memorial Fund, Los Angeles, Calif.; and by GenVec, Inc., Gaithersburg, Md. T.V. was supported in part by the BLANCEFLOR Boncompagni-Ludovisi, née Bildt, and Lisa och Johan Grönbergs Stiftelse.
REFERENCES
- 1.Bai, M., B. Harfe, and P. Freimuth. 1993. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 67:5198-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bevilacqua, M. P. 1993. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 11:767-804. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, J. R., and J. S. Pober. 1996. Prolonged cytokine exposure causes a dynamic redistribution of endothelial cell adhesion molecules to intercellular junctions. Lab. Investig. 75:463-472. [PubMed] [Google Scholar]
- 6.Bruning, A., and I. B. Runnebaum. 2003. CAR is a cell-cell adhesion protein in human cancer cells and is expressionally modulated by dexamethasone, TNFalpha, and TGFbeta. Gene Ther. 10:198-205. [DOI] [PubMed] [Google Scholar]
- 7.Burke-Gaffney, A., and A. K. Keenan. 1993. Modulation of human endothelial cell permeability by combinations of the cytokines interleukin-1 alpha/beta, tumor necrosis factor-alpha and interferon-gamma. Immunopharmacology 25:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Caamano, J., and C. A. Hunter. 2002. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15:414-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlos, T. M., and J. M. Harlan. 1994. Leukocyte-endothelial adhesion molecules. Blood 84:2068-2101. [PubMed] [Google Scholar]
- 10.Carson, S. D. 2001. Receptor for the group B coxsackieviruses and adenoviruses: CAR. Rev. Med. Virol. 11:219-226. [DOI] [PubMed] [Google Scholar]
- 11.Carson, S. D., J. T. Hobbs, S. M. Tracy, and N. M. Chapman. 1999. Expression of the coxsackievirus and adenovirus receptor in cultured human umbilical vein endothelial cells: regulation in response to cell density. J. Virol. 73:7077-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carty, E., M. De Brabander, R. M. Feakins, and D. S. Rampton. 2000. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 46:487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, C. J., J. Gaetz, T. Ohman, and J. M. Bergelson. 2001. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J. Biol. Chem. 276:25392-25398. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins, T., M. A. Read, A. S. Neish, M. Z. Whitley, D. Thanos, and T. Maniatis. 1995. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 9:899-909. [PubMed] [Google Scholar]
- 16.Grayson, L. S., J. F. Hansbrough, R. L. Zapata-Sirvent, C. A. Dore, J. L. Morgan, and M. A. Nicolson. 1993. Quantitation of cytokine levels in skin graft donor site wound fluid. Burns 19:401-405. [DOI] [PubMed] [Google Scholar]
- 17.Harari, O. A., T. J. Wickham, C. J. Stocker, I. Kovesdi, D. M. Segal, T. Y. Huehns, C. Sarraf, and D. O. Haskard. 1999. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther. 6:801-807. [DOI] [PubMed] [Google Scholar]
- 18.Hemminki, A., A. Kanerva, B. Liu, M. Wang, R. D. Alvarez, G. P. Siegal, and D. T. Curiel. 2003. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 63:847-853. [PubMed] [Google Scholar]
- 19.Hersh, J., R. G. Crystal, and B. Bewig. 1995. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 2:124-131. [PubMed] [Google Scholar]
- 20.Hofmann, S., H. Grasberger, P. Jung, M. Bidlingmaier, J. Vlotides, O. E. Janssen, and R. Landgraf. 2002. The tumour necrosis factor-alpha induced vascular permeability is associated with a reduction of VE-cadherin expression. Eur. J. Med. Res. 7:171-176. [PubMed] [Google Scholar]
- 21.Honda, T., H. Saitoh, M. Masuko, T. Katagiri-Abe, K. Tominaga, I. Kozakai, K. Kobayashi, T. Kumanishi, Y. G. Watanabe, S. Odani, and R. Kuwano. 2000. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res. Mol. Brain Res. 77:19-28. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaner, R. J., S. Worgall, P. L. Leopold, E. Stolze, E. Milano, C. Hidaka, R. Ramalingam, N. R. Hackett, R. Singh, J. Bergelson, R. Finberg, E. Falck-Pedersen, and R. G. Crystal. 1999. Modification of the genetic program of human alveolar macrophages by adenovirus vectors in vitro is feasible but inefficient, limited in part by the low level of expression of the coxsackie/adenovirus receptor. Am. J. Respir. Cell Mol. Biol. 20:361-370. [DOI] [PubMed] [Google Scholar]
- 24.Leopold, P. L. 2003. Cell physiology as a variable in gene transfer to endothelium. Curr. Atheroscler. Rep. 5:171-177. [DOI] [PubMed] [Google Scholar]
- 25.Leopold, P. L., B. Ferris, I. Grinberg, S. Worgall, N. R. Hackett, and R. G. Crystal. 1998. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum. Gene Ther. 9:367-378. [DOI] [PubMed] [Google Scholar]
- 26.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozaki, H., K. Ishii, H. Horiuchi, H. Arai, T. Kawamoto, K. Okawa, A. Iwamatsu, and T. Kita. 1999. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J. Immunol. 163:553-557. [PubMed] [Google Scholar]
- 29.Parkes, R. J., and S. L. Hart. 2000. Adhesion molecules and gene transfer. Adv. Drug Deliv. Rev. 44:135-152. [DOI] [PubMed] [Google Scholar]
- 30.Philipson, L., and R. F. Pettersson. 2004. The coxsackie-adenovirus receptor—a new receptor in the immunoglobulin family involved in cell adhesion. Curr. Top. Microbiol. Immunol. 273:87-111. [DOI] [PubMed] [Google Scholar]
- 31.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platanias, L. C. 2003. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol. Ther. 98:129-142. [DOI] [PubMed] [Google Scholar]
- 33.Pober, J. S., M. P. Bevilacqua, D. L. Mendrick, L. A. Lapierre, W. Fiers, and M. A. Gimbrone. 1986. Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J. Immunol. 136:1680-1687. [PubMed] [Google Scholar]
- 34.Pober, J. S., and R. S. Cotran. 1990. Cytokines and endothelial cell biology. Physiol. Rev. 70:427-451. [DOI] [PubMed] [Google Scholar]
- 35.Pober, J. S., M. A. Gimbrone, L. A. Lapierre, D. L. Mendrick, W. Fiers, R. Rothlein, and T. A. Springer. 1986. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J. Immunol. 137:1893-1896. [PubMed] [Google Scholar]
- 36.Ritter, T., C. Brandt, S. Prosch, A. Vergopoulos, K. Vogt, J. Kolls, and H. D. Volk. 2000. Stimulatory and inhibitory action of cytokines on the regulation of hCMV-IE promoter activity in human endothelial cells. Cytokine 12:1163-1170. [DOI] [PubMed] [Google Scholar]
- 37.Rival, Y., A. Del Maschio, M. J. Rabiet, E. Dejana, and A. Duperray. 1996. Inhibition of platelet endothelial cell adhesion molecule-1 synthesis and leukocyte transmigration in endothelial cells by the combined action of TNF-alpha and IFN-gamma. J. Immunol. 157:1233-1241. [PubMed] [Google Scholar]
- 38.Romer, L. H., N. V. McLean, H. C. Yan, M. Daise, J. Sun, and H. M. De Lisser. 1995. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J. Immunol. 154:6582-6592. [PubMed] [Google Scholar]
- 39.Rosenfeld, M. A., W. Siegfried, K. Yoshimura, K. Yoneyama, M. Fukayama, L. E. Stier, P. K. Paakko, P. Gilardi, L. D. Stratford-Perricaudet, and M. Perricaudet. 1991. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 252:431-434. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld, M. A., K. Yoshimura, B. C. Trapnell, K. Yoneyama, E. R. Rosenthal, W. Dalemans, M. Fukayama, J. Bargon, L. E. Stier, and L. Stratford-Perricaudet. 1992. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 68:143-155. [DOI] [PubMed] [Google Scholar]
- 41.Saxne, T., M. A. Palladino, D. Heinegard, N. Talal, and F. A. Wollheim. 1988. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 31:1041-1045. [DOI] [PubMed] [Google Scholar]
- 42.Seidman, M. A., S. M. Hogan, R. L. Wendland, S. Worgall, R. G. Crystal, and P. L. Leopold. 2001. Variation in adenovirus receptor expression and adenovirus vector-mediated transgene expression at defined stages of the cell cycle. Mol. Ther. 4:13-21. [DOI] [PubMed] [Google Scholar]
- 43.Shaw, S. K., B. N. Perkins, Y. C. Lim, Y. Liu, A. Nusrat, F. J. Schnell, C. A. Parkos, and F. W. Luscinskas. 2001. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-alpha plus interferon-gamma does not reduce leukocyte transmigration under flow. Am. J. Pathol. 159:2281-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sollerbrant, K., E. Raschperger, M. Mirza, U. Engstrom, L. Philipson, P. O. Ljungdahl, and R. F. Pettersson. 2003. The coxsackievirus and adenovirus receptor (CAR) forms a complex with the PDZ domain-containing protein ligand-of-numb protein-X (LNX). J. Biol. Chem. 278:7439-7444. [DOI] [PubMed] [Google Scholar]
- 45.Stewart, R. J., T. S. Kashour, and P. A. Marsden. 1996. Vascular endothelial platelet endothelial adhesion molecule-1 (PECAM-1) expression is decreased by TNF-alpha and IFN-gamma. Evidence for cytokine-induced destabilization of messenger ribonucleic acid transcripts in bovine endothelial cells. J. Immunol. 156:1221-1228. [PubMed] [Google Scholar]
- 46.Stolpen, A. H., E. C. Guinan, W. Fiers, and J. S. Pober. 1986. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am. J. Pathol. 123:16-24. [PMC free article] [PubMed] [Google Scholar]
- 47.Suter, P. M., S. Suter, E. Girardin, P. Roux-Lombard, G. E. Grau, and J. M. Dayer. 1992. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am. Rev. Respir. Dis. 145:1016-1022. [DOI] [PubMed] [Google Scholar]
- 48.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van't Hof, W., and R. G. Crystal. 2002. Fatty acid modification of the coxsackievirus and adenovirus receptor. J. Virol. 76:6382-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace, H. J., and M. C. Stacey. 1998. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers—correlations to healing status. J. Investig. Dermatol. 110:292-296. [DOI] [PubMed] [Google Scholar]
- 51.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789-799. [DOI] [PubMed] [Google Scholar]
- 52.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 53.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wickham, T. J., E. J. Filardo, D. A. Cheresh, and G. R. Nemerow. 1994. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 57.Wojciak-Stothard, B., A. Entwistle, R. Garg, and A. J. Ridley. 1998. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell Physiol. 176:150-165. [DOI] [PubMed] [Google Scholar]
- 58.Young, J. A. T. 2001. Virus entry and uncoating, p. 87-91. In D. M. Knipe et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 59.Yu, Q., L. G. Que, and D. C. Rockey. 2002. Adenovirus-mediated gene transfer to nonparenchymal cells in normal and injured liver. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G565-G572. [DOI] [PubMed] [Google Scholar]
- 60.Zabner, J., P. Freimuth, A. Puga, A. Fabrega, and M. J. Welsh. 1997. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J. Clin. Investig. 100:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]