Abstract
Background
Chromosome 22q11.2 deletion syndrome (22q11DS) is a common genetic disorder with high rates of psychosis and other psychopathologies, but few studies discuss treatment. Our aim was to characterize the prevalence and treatment of major psychiatric illnesses in a well-characterized sample of individuals with 22q11DS.
Method
This was a cross-sectional study of 112 individuals aged 8 to 45 years with a confirmed diagnosis of 22q11DS. Each participant was administered a modified Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) and the Structured Interview for Prodromal Syndromes (SIPS). Phenotypes assessed were threshold and subthreshold psychosis, depression, mania, generalized and separation anxiety, obsessions/compulsions, inattention/hyperactivity and substance use. Histories of mental health care and current psychotropic treatment were obtained.
Results
Psychopathology was common, with 79% of individuals meeting diagnostic criteria for a disorder at the time of assessment. Diagnoses of psychosis were made in 11% of cases, attenuated positive symptom syndrome (APS) in 21%, and 47% experienced significant subthreshold symptoms. Peak occurrence of psychosis risk was during adolescence (62% of those aged 12–17 years). Criteria for a mood disorder were met by 14%, for anxiety disorder 34% and for attention deficit hyperactivity disorder (ADHD) 31%. Mental health care had been received by 63% of individuals in their lifetime, but only 40% continued therapy and 39% used psychotropics. Antipsychotics were used by 42% of participants with psychosis and none of the participants with APS. Half of those at risk for psychosis were receiving no mental health care.
Conclusions
Psychopathology is common in 22q11DS but is not adequately treated or clinically followed. Particular attention should be paid to subthreshold psychotic symptoms, especially in adolescents.
Keywords: 22q11.2 deletion syndrome, psychopathology, psychosis, treatment
Introduction
Serious mental illnesses often arise during childhood and adolescence when the brain undergoes a crucial stage of development; the underlying genetic and neurodevelopmental processes are currently the focus of intensive research. Chromosome 22q11.2 deletion syndrome (22q11DS) is a common genetic condition associated with increased risk of many psychiatric disorders including psychosis, mood disorders, anxiety disorders and attention deficit hyperactivity disorder (ADHD). The prevalence of 22q11DS is reported as 1:4000 births based on cases coming to clinical attention (Botto et al. 2003; Schreiner et al. 2013), with recent results from prenatal screening microarray analyses suggesting a possible higher frequency (Wapner et al. 2012). Given the increased incidence of psychiatric morbidity in 22q11DS, psychiatric investigation of these individuals provides a window into the development of mental illness and also helps to guide proper management for a relatively large at-risk population.
The genetic basis of 22q11DS is a hemizygous deletion on the 22nd chromosome that is usually de novo (90%) and typically 3 MB in size (88–92%); occasionally, the deletion is inherited (10%) or involves smaller portions of genome (8–12%) (Antshel et al. 2010; Bassett et al. 2011; Michaelovsky et al. 2012; Schreiner et al. 2013). Approximately 30 genes are affected. Deleted genes include those impacting neurotrans-mission (catechol-O-methyltransferase and proline dehydrogenase) and myelination (phosphatidylinositol 4-kinase) (Schreiner et al. 2013). Physical consequences are variable, with congenital heart defects in 50–75% of individuals, palate defects in 75%, hypoparathyroidism in 60%, immunologic abnormalities in 35–77%, and many other developmental abnormalities (Jawad et al. 2001; Bassett et al. 2011). Combinations of phenotypic characteristics were described previously as DiGeorge, velocardiofacial, Shprintzen and conotruncal anomaly face syndromes (McDonald-McGinn et al. 2001; Bassett & Chow, 2008).
22q11DS is also associated with significant neuro-psychiatric burden. Mild and borderline intellectual disability is common, with an average IQ of about 75 (Bassett et al. 2003; Vorstman et al. 2006; Antshel et al. 2010; Fabbro et al. 2012; Jolin et al. 2012). At least one psychiatric disorder is diagnosed in 73–90% of individuals with 22q11DS (Green et al. 2009; Stoddard et al. 2010; Jolin et al. 2012). ADHD and anxiety disorders are common, with 35–55% of individuals diagnosed with ADHD, 15–27% with generalized anxiety disorder (GAD), 3–10% with obsessive–compulsive disorder (OCD), 5–8% with separation anxiety and 17– 46% with specific phobias (Green et al. 2009; Jolin et al. 2009, 2012; Niklasson et al. 2009; Antshel et al. 2010; Stoddard et al. 2010; Fabbro et al. 2012). Mood disorders are present in 13–64%, predominantly represented by major depressive disorder (MDD) (Jolin et al. 2009; Antshel et al. 2010; Stoddard et al. 2010; Fabbro et al. 2012). Perhaps most remarkably, psychotic disorders are diagnosed in 23–32% of adults with 22q11DS (Murphy et al. 1999; Gothelf et al. 2007; Bassett & Chow, 2008; Green et al. 2009; Schreiner et al. 2013).
Despite significant interest in 22q11DS as a valuable model for psychiatric illness, few studies discuss treatment of these disorders and clinicians lack guidance on how psychiatric illnesses in 22q11DS are managed. In the current study we focused on psychosis and subthreshold psychotic symptoms. Our aim was to characterize the prevalence and treatment of major psychiatric illnesses in a well-characterized sample of individuals with 22q11DS, across developmental stages.
Method
Sample
We evaluated a cohort of 112 participants aged 58 years who were diagnosed with 22q11DS. They were recruited primarily through the ‘22Q and You Center’ at the Children's Hospital of Philadelphia in addition to social networks. All participants had a confirmed deletion of the 22q11.2 region. Three mega-base deletions were characterized in 100 participants and 1.5–1.7 MB deletions in seven participants; deletion size was unknown in five participants. Exclusion criteria included the inability to provide assent or informed consent and moderate to severe intellectual disability based on clinical evaluation and IQ testing when available or estimated from the reading segment of the Wide Range Achievement Test 4 (WRAT4; estimated IQ<70) (Wilkinson & Robertson, 2006). To compare psychopathology across developmental stages, participants were divided into four age groups: children (8–11 years), adolescents (12–17 years), young adults (18–23 years) and adults (≥24 years).
Study procedures were conducted while the participants were medically stable and ambulatory. No changes were made in the participants’ usual medical and behavioral treatment. All procedures were approved by the Institutional Review Boards of the University of Pennsylvania and the Children's Hospital of Philadelphia. Informed consent/assent was obtained from each participant and accompanying parent, for children younger than 18. Twenty-one participants from a previously published pilot study are also included here (Goldenberg et al. 2012).
Procedures
Psychopathology was assessed with the modified Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS; Kaufman et al. 1997), the Structured Interview for Prodromal Syndromes (SIPS; Miller et al. 2003) and the psychotic and mood differential diagnoses modules of the Structured Clinical Interview for DSM-IV (SCID, modules C and D; First & Gibbon, 2004). Because of time considerations, only K-SADS sections for ADHD, mood disorders, generalized anxiety, separation anxiety, obsessive–compulsive behavior and substance-related disorders were included. To allow for in-depth assessment of subthreshold and threshold psychotic symptoms, portions of the K-SADS psychosis section were integrated into the SIPS (Miller et al. 2003). Positive, negative and disorganized symptoms are rated separately as typical (SIPS scores 0=‘absent’, 1=‘questionably present’ and 2=‘mild’), clinically significant but subthreshold (SIPS scores 3=‘moderate’, 4=‘moderately severe’ and 5=‘severe but not psychotic’) or fully psychotic (SIPS score 6=‘severe and psychotic’). The participants’ overall disease burden and function in expected roles was rated using the SIPS Global Assessment of Function (GAF; Miller et al. 2003). Excellent inter-rater reliability has been reported for these methods (Kaufman et al. 1997; Dreessen & Arntz, 1998; Miller et al. 2003). IQ was estimated for each participant using the WRAT4 reading segment, which serves as a reliable estimate of pre-morbid IQ (Wilkinson & Robertson, 2006; Goldenberg et al. 2012).
To facilitate efficient data collection and analysis, the instruments were integrated into one administrative tool, the Computer-Assisted Psychopathology Assessment (CAPA). Interviewers underwent formal training including lectures and mock interviews, followed by supervised administration and performance evaluation until competency was established. Laptop computers were used to record participants’ responses. The CAPA is adapted to be given separately to both the proband and collateral informants. Younger pro-bands (age 8–10 years) received clinical evaluations and collateral interviews were obtained from parents. The proband interview was administered to participants aged ≥11 years (n=96). The collateral version was administered independently to all parents of children under age 18 and also to parents of adult participants whenever possible. Participants and collateral informants were interviewed by experienced investigators and histories of psychiatric treatment and psychotropic medication usage within 6 months of assessment were obtained. The 6-month period was selected as reliable information could be obtained and this epoch was proximal to study assessment. Following each assessment, information from proband and collateral CAPA interviews and from available medical records were integrated into a narrative case summary and given ratings. These were presented at a case conference attended by two or more doctoral-level clinicians with expertise in psychosis and child psychopathology. The diagnosis of participants with clinically significant psychotic or subpsychotic symptoms underwent consensus review, and those without psychotic symptoms underwent review by the senior author (R.E.G.).
Diagnostic criteria
Participants were considered ‘psychosis prone’ if they met at least one of the following criteria: (1) one or more clinically significant positive symptoms rated 3–5 on the SIPS (unusual thought content, suspiciousness/persecutory ideas, grandiose ideas, perceptual abnormalities, disorganized communication); (2) two or more clinically significant negative or disorganized symptoms rated 3–5 on the SIPS (social anhedonia, avolition, expression of emotions, experience of emotions and self, ideational richness, occupational functioning, odd behavior or appearance, bizarre thinking, impaired attention, impairment in personal hygiene). In accordance with the Criteria of Prodromal Symptoms (COPS), attenuated positive symptom syndrome (APS) was applied when at least one positive subthreshold symptom occurred weekly and had increased in severity by one scale point within the past 12 months (Miller et al. 2003). The psychosis spectrum encompasses psychosis prone, APS and threshold-level psychotic disorders. All Axis I diagnoses were given in accordance with DSM-IV-TR criteria. ‘Not otherwise specified’ (NOS) diagnoses were given to participants who endorsed significant symptoms and experienced major change in functioning but did not meet specific DSM-IV criteria. For example, the designations of anxiety or depression NOS were given to participants who were seriously impaired by mood symptoms but did not meet frequency criteria (most of the day, nearly every day). Psychosis NOS was given when the timeline of mood and psychotic episodes was indeterminate, and a definitive diagnosis of schizo-affective disorder versus schizophrenia or psychotic mood disorder was not possible. Commensurately, the superimposed mood disorders were designated as depression NOS (depressive symptoms alone) or mood NOS (manic features).
Data analysis
Clinical data were analyzed with Stata SE (StataCorp LP, USA). Trends in categorical variables were evaluated using Fisher's exact test and continuous variables were evaluated using ANOVA. Odds ratios (ORs) of diagnosis with psychopathology were calculated using logistic regression, with age and sex as covariates. Significance threshold was two-tailed and set at α ≤0.05.
Results
Study population
Of the 112 participants included in this study, 26 were children aged 8–11 years, 45 were adolescents aged 12–17 years, 22 were young adults aged 18–23 years, and 19 were adults aged 524 years. Overall, 53% of the participants were male and 47% were female. Caucasian race dominated the sample at 91%. IQ estimated by the WRAT4 was an average of 87.1±12.2. There were no significant differences in sex or race distributions or mean IQ estimates across the age groups. Demographic information is detailed in Table 1.
Table 1.
Demographics of participants with 22q11.2 deletion syndrome (22q11DS)
| Age group (years) |
||||||
|---|---|---|---|---|---|---|
| Parameter | All ages | 8–11 | 12–17 | 18–23 | ≥24 | p value |
| n | 112 | 26 | 45 | 22 | 19 | |
| Age (years), mean±S.D. | 18.1±8.1 | 10.2±1.2 | 15.0±1.8 | 20.8±1.9 | 32.9±6.3 | ≤0.001 |
| Sex, n (%) | ||||||
| Male | 59 (53) | 16 (62) | 27 (60) | 11 (50) | 5 (26) | N.S. |
| Female | 53 (47) | 10 (38) | 18 (40) | 11 (50) | 14 (74) | N.S. |
| Race, n (%) | ||||||
| Caucasian | 102 (91) | 24 (92) | 41 (91) | 20 (91) | 17 (89) | N.S. |
| African American | 6 (5) | 1 (4) | 2 (4) | 1 (5) | 2 (11) | N.S. |
| Other/Mixed | 4 (4) | 1 (4) | 2 (4) | 1 (5) | 0 (0) | N.S. |
| Estimated IQ±S.D. | 87.1±12.2 | 87.2±10.5 | 88.8±13.1 | 83.6±9.87 | 86.8±14.6 | N.S. |
S.D., Standard deviation; N.S., not significant (p>0.05).
p values are given for age group by demographic parameter interactions determined by ANOVA for age and estimated IQ; Fisher's exact test for sex and race.
Overall burden of psychopathology
The prevalence of psychopathology was high in our sample of individuals with 22q11DS. Table 2 details the current prevalence of assessed psychopathologies for the overall sample. At least one diagnosis was given in 79% of the sample (including ‘psychosis prone’). Two or more co-morbidities were recorded in 42% of cases, and 16% had at least three. Mood and anxiety disorders were prevalent at 14% and 34% respectively. The most common mood disorder was major depression, at 9% of the total sample, followed by depression NOS at 3%. One participant was diagnosed with each of mood disorder NOS, dysthymia and bipolar I disorder. GAD was the most common anxiety disorder at 15%, followed by anxiety NOS at 12%, OCD at 8% and separation anxiety at 6%. Additionally, ADHD was diagnosed in 31% of the total sample. Substance-related disorders were rare, with no one meeting criteria for current substance or alcohol abuse or dependence.
Table 2.
Current psychopathologies of participants with 22q11.2 deletion syndrome (22q11DS)
| Age group (years) |
||||||
|---|---|---|---|---|---|---|
| Parameter | All ages | 8–11 | 12–17 | 18–23 | ≥24 | p value |
| Any psychopathology | 89 (79) | 22 (85) | 37 (82) | 18 (82) | 12 (63) | N.S. |
| Co-morbidities ≥2 | 47 (42) | 8 (31) | 23 (51) | 11 (50) | 5 (26) | N.S. |
| Co-morbidities ≥3 | 18 (16) | 2 (8) | 7 (16) | 7 (32) | 2 (11) | N.S. |
| Mood disorders | 16 (14) | 0 (0) | 5 (11) | 7 (32) | 4 (21) | ≤0.01 |
| MDD | 10 (9) | 0 (0) | 5 (11) | 4 (18) | 1 (5) | |
| Depression NOS | 3 (3) | 0 (0) | 0 (0) | 2 (9) | 1 (5) | |
| Mood NOS | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | |
| Dysthymia | 1 (1) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | |
| Bipolar I disorder | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | |
| Anxiety disorders | 38 (34) | 6 (23) | 16 (36) | 10 (45) | 6 (32) | N.S. |
| GAD | 17 (15) | 3 (12) | 7 (16) | 3 (14) | 4 (21) | |
| Anxiety NOS | 13 (12) | 3 (12) | 5 (11) | 4 (18) | 1 (5) | |
| Separation anxiety | 7 (6) | 2 (8) | 2 (4) | 2 (9) | 1 (5) | |
| OCD | 9 (8) | 1 (4) | 4 (9) | 3 (14) | 1 (5) | |
| ADHD | 35 (31) | 10 (38) | 15 (33) | 6 (27) | 4 (21) | N.S. |
| Substance/alcohol abuse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | N.S. |
| Psychosis prone | 53 (47) | 13 (50) | 28 (62) | 10 (45) | 2 (11) | ≤0.001 |
| APS | 24 (21) | 5 (19) | 13 (29) | 5 (23) | 1 (5) | N.S. |
| Psychotic | 12 (11) | 2 (8) | 2 (4) | 4(18) | 4 (21) | N.S. |
| Schizophrenia | 6 (5) | 0 (0) | 0 (0) | 3 (14) | 3 (16) | ≤0.01 |
| Psychosis NOS | 4 (4) | 2 (8) | 1 (2) | 0 (0) | 1 (5) | |
| Delusional disorder | 2 (2) | 0 (0) | 1 (2) | 1 (5) | 0 (0) | |
MDD, Major depressive disorder; NOS, not otherwise specified; GAD, generalized anxiety disorder; OCD, obsessive–compulsive disorder; ADHD, attention deficit hyperactivity disorder; APS, attenuated positive symptom syndrome; N.S., not significant (p>0.05).
Values given as n (%).
p values are given for age group by diagnosis interactions determined by Fisher's exact test.
Several participants met criteria for psycho-pathology in the past, but are now in full or partial remission (not detailed in Table 2). These include two participants with past MDD in partial remission, two participants with past MDD in full remission, one participant with depression NOS in full remission, two participants with past separation anxiety in full remission, three participants with past ADHD in full remission, one participant with past ADHD in partial remission, one participant with alcohol abuse in partial remission, and one participant with past sedative and opioid abuse in full remission.
A high proportion of our sample experienced clinically significant psychotic symptoms. Criteria for a psychotic disorder were met by 11%, including 5% schizophrenia, 4% psychosis NOS and 2% delusional disorder. Additionally, 47% were diagnosed as psychosis prone based on subthreshold symptoms, nearly half of whom had APS (21% of total sample). Of the 53 participants who were psychosis prone, the great majority (77%) experienced subthreshold positive symptoms whereas 12 were considered psychosis prone on the basis of only negative or disorganized symptoms. There were no cases of full or partial remission of subthreshold symptoms or psychotic disorders. Adjusting for age and sex, participants who were psychosis prone or psychotic were more likely than non-psychotic individuals to have a lifetime diagnosis of a mood disorder (OR 5.6, p ≤ 0.05) or anxiety disorder (OR 3.2, p ≤ 0.05). All participants diagnosed with OCD were either psychosis prone (n = 6) or psychotic (n = 3).
Psychopathology across developmental stages
To investigate patterns of psychopathology across developmental stages, prevalence is described in four cross-sectional age groups (Table 2). Significant age group×prevalence interactions were present for mood disorders (p ≤ 0.01), psychosis prone (p ≤ 0.001) and schizophrenia (p ≤ 0.01). Mood disorders and schizophrenia were more prevalent in older participants whereas adolescents were most likely to experience significant subthreshold psychotic symptoms. Mood disorders increased from 0% in children and 16% in adolescents to 36% in young adulthood and 37% in adulthood. Schizophrenia was absent in younger age groups but affected 14% of young adults and 16% of adults. However, the proportion of psychosis-prone individuals peaked in adolescence at 62% and then decreased to 45% in young adulthood and 11% in adulthood. APS also peaked in adolescence at 29%, but this trend was not statistically significant. There were no significant differences in the age-related prevalence of anxiety disorders, ADHD or psychotic disorders other than schizophrenia.
Sex differences in psychopathology
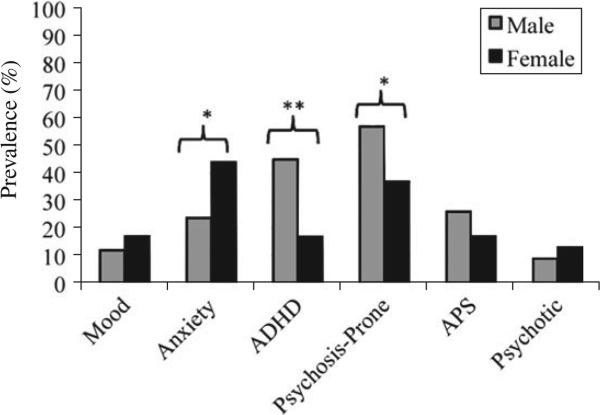
There were significant sex differences in psycho-pathology rates. Specifically, anxiety disorders were significantly more common in females (44%) than males (24%, p ≤ 0.05). Conversely, ADHD was more common in males (45%) than females (17%, p ≤ 0.01). Males were also more likely to be psychosis prone (57% v. 37%, p ≤ 0.05). There were no significant sex differences in the prevalence of mood disorders or threshold level psychosis. These effects are displayed in Fig. 1. Sex differences were heterogeneous among the different age groups. Female risk for anxiety disorders was significantly higher only in childhood (50% v. 6%, p40.05), as was male risk for ADHD (56% v. 10%, p ≤ 0.05), whereas male risk for being psychosis prone was significantly higher only in adolescence (78% v. 39%, p ≤ 0.05).
Fig. 1.
Differences in psychopathology by sex. ADHD, Attention deficit hyperactivity disorder; APS, attenuated positive symptom syndrome. * p<0.05, ** p<0.01.
Mental health treatment
Many participants had been treated by a mental health specialist in their lifetime (n = 71, 63%). However, fewer individuals with 22q11DS maintained therapy at the time of the assessment (n = 45, 40%) or took psycho-tropic medication within 6 months (n = 44, 39%). Even fewer participants had ever been hospitalized for psychiatric reasons (n = 9, 8%). Table 3 details mental health behavioral treatment (e.g. by a psychiatrist, psychologist, social worker or school counselor), psychotropic usage and psychiatric hospitalization for the total sample and also for individuals with various psychopathologies meeting criteria at the time of assessment.
Table 3.
Treatment modalities experienced by participants with 22q11.2 deletion syndrome (22q11DS)
| Current psychopathology |
||||||
|---|---|---|---|---|---|---|
| Treatment mode | All participants | Mood/anxiety disorder | ADHD | Psychosis prone | APS | Psychotic |
| Count | 112 (100) | 47 (42) | 35 (31) | 53 (47) | 24 (21) | 12 (11) |
| Lifetime mental health care | 71 (63) | 37 (79)** | 20 (57) | 37 (70) | 20 (83) | 11 (92) |
| Current | 45 (40) | 25 (53)* | 13 (37) | 28 (53)* | 13 (54) | 8 (67) |
| Any psychotropic | 44 (39) | 23 (49) | 19 (54)* | 24 (45) | 10 (42) | 9 (75)* |
| Antidepressants and anxiolytics | 26 (23) | 17 (36)** | 8 (23) | 17 (32)* | 7 (29) | 6 (50)* |
| Mood stabilizers | 7 (6) | 6 (13)* | 2 (6) | 3 (6) | 1 (4) | 2 (17) |
| Stimulants and alpha-2 agonists | 19 (17) | 8 (17) | 12 (34)** | 11 (21) | 5 (21) | 2 (17) |
| Antipsychotics | 8 (7) | 7 (15)** | 2 (6) | 3 (6) | 0 (0) | 5 (42)*** |
| Psychiatric hospitalization | 9 (8) | 6 (13) | 2 (6) | 4 (8) | 2 (8) | 4 (33)** |
ADHD, Attention deficit hyperactivity disorder; APS, attenuated positive symptom syndrome.
Current mental health care describes any care from a mental health specialist, such as a psychiatrist, psychologist, social worker or school counselor. The designation of ‘lifetime’ refers to whether the event has ever occurred whereas ‘current’ refers to care at the time of the assessment. There were no significant differences in the proportion of participants receiving mental health care, psychotropic treatment or psychiatric hospitalization among the age groups or between the sexes. Statistical significance is given for psychopathology and treatment modality interactions comparing those currently meeting criteria for the specified disorders with those who do not, regardless of other co-morbidities, as determined by Fisher's exact test:
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001.
All values given as n (%).
Rates of psychotropic treatment are low. Anti-psychotic medications were taken by less than half of individuals with psychosis (42%), 6% of the psychosis-prone individuals and none of those with APS. A third of participants diagnosed with ADHD were on a stimulant or an alpha-2 agonist. Similarly, 36% of participants diagnosed with anxiety or mood disorders were treated with an antidepressant or anxiolytic, and 13% were treated with a mood stabilizer. Regardless of other co-morbidities, participants with a mood or anxiety diagnosis were more likely than those without to receive current (p ≤ 0.05) and lifetime mental health care (p ≤ 0.01), in addition to treatment with mood stabilizers (p ≤ 0.05), anxiety/antidepressant (p ≤ 0.01) and antipsychotic medication (p ≤ 0.01). ADHD diagnosis was associated with more treatment with stimulants and non-stimulant ADHD medication (p ≤ 0.01). Psychosis-prone individuals were more likely to be under mental health care at the time of the assessment (p ≤ 0.05), but were not more likely to be treated with psychotropics. Individuals with APS were neither more likely to be under mental health care nor to be treated with psychotropics. However, individuals with psychotic diagnoses were more likely to be treated with an antipsychotic (p ≤ 0.001) and to have a history of psychiatric hospitalization (p ≤ 0.01). There were no significant differences in the proportions of participants receiving mental health care, psychotropic medication or psychiatric hospitalization among the age groups or between the sexes.
There were 73 separate medications used by 44 participants within 6 months of the assessment. The most commonly used classes of medication were selective serotonin reuptake inhibitors (SSRIs) and stimulants, each prescribed 19 times. Table 4 lists each class of psychotropic medication and the corresponding individual medications along with the frequency of their usage.
Table 4.
Psychotropic medications used in participants with 22q11.2 deletion syndrome (22q11DS)
| Category/medication name | Frequency | Category/medication name | Frequency |
|---|---|---|---|
| SSRI | 19 | Alpha-2 agonist | 6 |
| Sertraline | 8 | Clonidine | 3 |
| Fluoxetine | 7 | Guanfacine | 2 |
| Citalopram | 2 | Methyldopa | 1 |
| Escitalopram | 2 | Other antidepressant | 5 |
| Stimulant | 19 | Venlafaxine | 2 |
| Methylphenidate | 8 | Trazodone | 1 |
| Dexmethylphenidate | 7 | Amitriptyline | 1 |
| Dextroamphetamine | 3 | Clomipramine | 1 |
| Lisdexamfetamine | 1 | Benzodiazepine | 4 |
| Anticonvulsant | 8 | Lorazepam | 2 |
| Valproate | 3 | Alprazolam | 1 |
| Carbamazepine | 2 | Others | |
| Levetiracetam | 1 | Benztropine | 3 |
| Lamotrigine | 1 | Thorazine | 1 |
| Gabapentin | 1 | Lithium | 1 |
| Atypical antipsychotic | 7 | Buspirone | 1 |
| Risperidone | 5 | Hydroxyzine | 1 |
| Quetiapine | 1 | ||
| Aripiprazole | 1 |
SSRI, Selective serotonin reuptake inhibitor.
Medication usage rates correspond to regular use within 6 months of the assessment.
Discussion
Mental illness in 22q11DS represents a growing field of interest in psychiatry. However, to our knowledge, this is the first large-scale study to assess and report both subthreshold psychosis and detailed treatment data in a well-characterized sample of individuals with 22q11DS. Administration of the CAPA allowed efficient, thorough and accurate assessment of psychiatric symptoms and other useful information, such as a history of mental health care. Conducting separate collateral and proband interviews for participants aged 511 years was particularly important, in our experience. Many children revealed subthreshold psychotic symptoms that were unknown to their parents. Conversely, parents were better able to identify the time course and ADHD symptoms. The exclusion of individuals with more severe intellectual disability restricts the generalizability of our findings but improves the internal validity of our measures by minimizing possible false positive responses due to misunderstanding of subtle questions. Indeed, despite its sensitivity for subthreshold symptoms, the SIPS was not validated for developmentally delayed populations such as those of 22q11DS. We are undertaking a refinement of the SIPS to better adapt it for this context.
Overall, individuals with 22q11DS are consistently at increased risk for psychiatric illness compared to the general population, with substance abuse as an exception (Merikangas et al. 2010). In our sample, the majority of individuals were diagnosed with at least one psychiatric disorder (79%). More individuals with 22q11DS were diagnosed as psychosis prone (47%) than with any other class of psychiatric illness. Psychotic disorders affected 11% overall and one in five participants aged ≥18. These rates are largely consistent with published reports and support the validity of our methods. Two large-scale studies of individuals with 22q11DS identified 15–20% mood disorder, 11–17% GAD, 4–16% OCD, 5–8% separation anxiety and 36–43% ADHD (Green et al. 2009; Antshel et al. 2010). Substance abuse and dependence seem to be uniformly uncommon (Bassett et al. 2003; Antshel et al. 2010). Reports on the prevalence of psychosis and psychosis-prone disorders are more varied. Two studies found psychotic disorders to be present at similar rates of 10–12% (Vorstman et al. 2006; Green et al. 2009). By contrast, Antshel et al. (2010) reported prodromal symptoms in 20% but no psychosis whereas Shapiro et al. (2011) noted APS to be present in 57% of adolescents with 22q11DS. This variability is probably due largely to differences in methods and the developmental stages being studied. Features of psychosis may emerge in childhood and adolescence, then remit, remain or progress with age. Thus, rates of subthreshold and threshold psychotic symptoms might be highly variable depending on the phase in which they are captured. We also found that those with psychotic features were more likely to have a lifetime diagnosis of mood or anxiety disorder. Most markedly, all participants with OCD were either psychosis prone or psychotic. This finding is consistent with the results of a longitudinal study that found the presence of an anxiety disorder at baseline to be predictive of the development of psychotic symptoms at follow-up (Gothelf et al. 2007). Perhaps individuals with significant anxiety are at even higher risk than the 22q11DS population at large.
The rate of ADHD and anxiety disorders remained constant across the age groups whereas mood disorders and schizophrenia became more prevalent in the older groups. A similar pattern was described by Green et al. (2009), who also reported anxiety as constant and psychosis as highest in adults; in their sample, ADHD peaked in childhood and mood disorders in young adulthood but these trends were not observed here. Importantly, we found adolescents to be at highest risk for psychosis, with 62% psychosis prone and 29% with APS. This suggests that individuals with 22q11DS should receive careful psychiatric evaluations during adolescence when major psycho-pathology may emerge.
Some sex-related differences present in the general population were reflected in our sample, with anxiety being more common in females and ADHD more common in males. However, the usual female predominance in mood disorders was not found in our sample of participants with 22q11DS. Within the 22q11DS literature, sex differences in psychopathology prevalence are rarely discussed. One study of 84 children found no sex differences in MDD or ADHD (Antshel et al. 2006), whereas another study with 172 participants across developmental stages reported consistent findings in ADHD (more prevalent in males) and anxiety (more prevalent in females) (Green et al. 2009). Notably, adolescent males were particularly likely to be psychosis prone (78%); this effect may reflect unique developmental processes and vulnerabilities in the adolescent male brain. Further replication is needed to corroborate our findings regarding sex differences, which have been reported in non-deleted individuals with schizophrenia (Hafner et al. 1998; Gur et al. 2004).
Because the overwhelming majority of our participants were Caucasian, we were unable to assess differences in psychopathology related to race. Such analyses in the 22q11DS population seems to be absent from the literature as most studies include predominately Caucasian samples. This disparity does not seem to be explained by failure to recruit a racially representative sample. In our own institution, Caucasians are disproportionately diagnosed with 22q11DS and predominate in the clinical care center; one proposed explanation is that the most canonical phenotypes of 22q11DS are masked in African Americans, thus leading to underdiagnosis (McDonald-McGinn et al. 2005; Veerapandiyan et al. 2011). This possibility is supported by one population-based study of laboratory-confirmed diagnoses of 22q11DS that found similar rates among Caucasians, African Americans and Asians, whereas the rate was nearly twofold in Hispanics (Botto et al. 2003). However, this result has neither been replicated nor contradicted, so we cannot determine whether 22q11DS is underdiagnosed in non-Caucasian races or simply less prevalent. A large-scale screening study would be helpful for determining the true incidence of 22q11DS and its rate in different races.
Across demographic characteristics, individuals with 22q11DS seem to be undertreated for their psychiatric illnesses. Although many individuals receive some type of mental health care in their lifetime (63%), fewer continued to be followed by a mental health specialist (40%) or treated with psychotropic medication (39%). Remarkably, antipsychotic treatment was used in less than half of participants with unremitted psychosis and none of the participants with APS. Only half of those with APS or psychosis were receiving any mental health care at all. These results are consistent with a study of 73 children aged 9–18 that found lifetime psychotherapy treatment in 64% and current psychotropic treatment in 20% (Fabbro et al. 2012). To our knowledge, no previous study has reported more detailed information on the treatment of psychiatric disorders in 22q11DS. When psychotropics are used, they tend to be medications commonly prescribed in the general population. A possible exception is the common use of anticonvulsants such as valproate; these may serve doubly as mood stabilizers and anticonvulsants in this population at higher risk for seizures.
These results underscore the need for greater awareness and more consistent treatment of mental illness in 22q11DS. With the majority of individuals affected by at least one psychiatric disorder, symptoms of major mental illnesses should be assessed frequently and carefully. Although psychotropic medication is not always appropriate, individuals who are psychosis prone or psychotic should, at minimum, be followed by a mental health specialist. Indeed, the greater scrutiny and awareness already present in the 22q11DS community may be benefiting this population. The gap between psychiatric diagnosis and treatment in individuals with 22q11DS is in fact smaller than in the general population. Only 14% of adolescents with psychopathology in a large population-based sample were treated with psychotropic medication; 14% of those with mood disorders were treated with anti-depressants and 20% of those with ADHD were treated with stimulants (Merikangas et al. 2013). The general population, however, is not at a similarly elevated risk for psychotic illness.
It is tempting to apply insights broadly from studying the abundant psychopathology present in 22q11DS, but we must be cautious and realistic with our generalizations. Non-deleted populations may not experience similar trends, such as the tendency for adolescents to be most abundantly psychosis prone. Our definition of ‘psychosis prone’ is based on the hypothesis that subthreshold symptoms, including negative and disorganized symptoms, increase an individual's risk for developing a threshold psychotic disorder. However, it is possible that these symptoms may also comprise an unrelated aspect of the 22q11DS phenotype, such as developmental delay. We hope to evaluate our definition of ‘psychosis prone’ with a longitudinal follow-up. Other limitations include the infeasibility of determining the intended clinical indication of prescribed psychotropic medications, and thus the proportion of seemingly appropriate medication usage may be falsely elevated due to co-morbidity and coincidence. We cannot report the effectiveness of treatments when they are given, although this information that would be of high relevance clinically. Furthermore, the sample included individuals who volunteered to participate in a study of brain and behavior and might be enriched with patients and families who seek help. Our rates are, however, comparable to those reported in the literature. Indeed, because we did not assess for specific phobia or oppositional defiant disorder, we may be presenting an underestimation of overall psycho-pathology among our participants.
We conclude that psychiatric illness and especially subthreshold psychosis are highly common in the 22q11DS population, across developmental stages and for both sexes. Awareness needs to be promoted because these disorders are not sufficiently treated. Further efforts should be made to provide adequate family support and establish treatment guidelines for psychiatric illness in this population.
Acknowledgments
This study was funded by National Institutes of Health (NIH) grants MH087626 and MH087636. Additional support came from the Doris Duke Charitable Foundation Clinical Research Fellowship (S.X.T.) and K08 MH 079364 (M.E.C.). We thank E. Wilkins, C. Conroy, O. Abbas, A. Cassidy and A. Savitt of Neuropsychiatry at the University of Pennsylvania, A. Bailey of the ‘22q and You’ Center, C. Franconi of the Human Genetics Department and K. Borgmann-Winter of the Department of Child and Adolescent Psychiatry, the Children's Hospital of Philadelphia.
Footnotes
Declaration of Interest
None.
References
- Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, Kates WR. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:333–344. [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Current Psychiatry Reports. 2008;10:148–157. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. American Journal of Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A, Vorstman J, International 22q11.2 Deletion Syndrome Consortium Practical guidelines for managing patients with 22q11.2 deletion syndrome. Journal of Pediatrics. 2011;159:332–9.e1. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O'Leary LA, Wong L-Y, Elixson EM. A population-based study of the 22q11. 2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Dreessen L, Arntz A. Short-interval test-retest interrater reliability of the Structured Clinical Interview for DSM-III-R personality disorders (SCID-II) in outpatients. Journal of Personality Disorders. 1998;12:138–148. doi: 10.1521/pedi.1998.12.2.138. [DOI] [PubMed] [Google Scholar]
- Fabbro A, Rizzi E, Schneider M, Debbane M, Eliez S. Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS). European Child and Adolescent Psychiatry. 2012;21:379–385. doi: 10.1007/s00787-012-0273-x. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Hilsenroth MJ, Segal DL, editors. Comprehensive Handbook of Psychological Assessment. Vol. 2, Personality Assessment. John Wiley & Sons, Inc.; Hoboken, NJ.: 2004. pp. 134–143. [Google Scholar]
- Goldenberg PC, Calkins ME, Richard J, McDonald-McGinn D, Zackai E, Mitra N, Emanuel B, Devoto M, Borgmann-Winter K, Kohler C, Conroy CG, Gur RC, Gur RE. Computerized neurocognitive profile in young people with 22q11.2 deletion syndrome compared to youths with schizophrenia and at-risk for psychosis. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2012;159B:87–93. doi: 10.1002/ajmg.b.32005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. American Journal of Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biological Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hafner H, an der Heiden W, Behrens S, Gattaz WF, Hambrecht M, Loffler W, Maurer K, Munk-Jorgensen P, Nowotny B, Riecher-Rossler A, Stein A. Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophrenia Bulletin. 1998;24:99–113. doi: 10.1093/oxfordjournals.schbul.a033317. [DOI] [PubMed] [Google Scholar]
- Jawad AF, McDonald-McGinn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Journal of Pediatrics. 2001;139:715–723. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- Jolin EM, Weller RA, Jessani NR, Zackai EH, McDonald-McGinn DM, Weller EB. Affective disorders and other psychiatric diagnoses in children and adolescents with 22q11.2 deletion syndrome. Journal of Affective Disorders. 2009;119:177–180. doi: 10.1016/j.jad.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Jolin EM, Weller RA, Weller EB. Occurrence of affective disorders compared to other psychiatric disorders in children and adolescents with 22q11.2 deletion syndrome. Journal of Affective Disorders. 2012;136:222–228. doi: 10.1016/j.jad.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Minugh-Purvis N, Kirschner RE, Jawad A, Tonnesen MK, Catanzaro JR, Goldmuntz E, Driscoll D, Larossa D, Emanuel BS, Zackai EH. The 22q11.2 deletion in African-American patients: an underdiagnosed population? American Journal of Medical Genetics. Part A. 2005;134:242–246. doi: 10.1002/ajmg.a.30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, Zackai EH. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genetics in Medicine. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Rapoport J, Vitiello B, Olfson M. Medication use in US youth with mental disorders. JAMA Pediatrics. 2013;167:141–148. doi: 10.1001/jamapediatrics.2013.431. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He MJ-P, Burstein M, Swanson MSA, Avenevoli S, Cui ML, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Study – Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelovsky E, Frisch A, Carmel M, Patya M, Zarchi O, Green T, Basel-Vanagaite L, Weizman A, Gothelf D. Genotype-phenotype correlation in 22q11.2 deletion syndrome. BMC Medical Genetics. 2012;13:122. doi: 10.1186/1471-2350-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Archives of General Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30:763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Schreiner MJ, Lazaro MT, Jalbrzikowski M, Bearden CE. Converging levels of analysis on a genomic hotspot for psychosis: insights from 22q11.2 deletion syndrome. Neuropharmacology. 2013;68:157–173. doi: 10.1016/j.neuropharm.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophrenia Research. 2011;129:20–28. doi: 10.1016/j.schres.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophrenia Research. 2010;118:118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerapandiyan A, Abdul-Rahman OA, Adam MP, Lyons MJ, Manning M, Coleman K, Kobrynski L, Taneja D, Schoch K, Zimmerman HH, Shashi V. Chromosome 22q11.2 deletion syndrome in African-American patients: a diagnostic challenge. American Journal of Medical Genetics. Part A. 2011;155A:2186–2195. doi: 10.1002/ajmg.a.34226. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, Savage M, Platt LD, Saltzman D, Grobman WA, Klugman S, Scholl T, Simpson JL, McCall K, Aggarwal VS, Bunke B, Nahum O, Patel A, Lamb AN, Thom EA, Beaudet AL, Ledbetter DH, Shaffer LG, Jackson L. Chromosomal microarray versus karyotyping for prenatal diagnosis. New England Journal of Medicine. 2012;367:2175–2184. doi: 10.1056/NEJMoa1203382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test, Fourth Edition (WRAT4) Psychological Assessment Resources; Lutz, FL.: 2006. [Google Scholar]