Abstract
We hypothesized that total body strength (S) and hypertrophic (H) resistance training (RT) protocols utilizing relatively short rest interval (RI) lengths between sets will elicit significant acute increases in total testosterone (TT) and cortisol (C) in healthy young men. 6 men, 26 (±2.4) years, completed 4 randomized RT sessions, following a control session (R). The S and H protocols were equated for volume-load (sets x repetitions x load); S: 8 sets x 3 repetitions at 85% 1-RM, H: 3 sets x 10 repetitions at 70% 1-RM, for all exercises. RI utilized: 60 seconds (S60, H60) and 90 seconds (S90, H90). Blood was drawn pre- (PRE), immediately post- (POST), 15 minutes post- (15 MIN), and 30 minutes post-exercise (30 MIN). H60 elicited significant increases in TT from PRE (7.32±1.85 ng/mL) to POST (8.87±1.83 ng/mL) (p<0.01), 15 MIN (8.58±2.15 ng/mL) (p<0.01), and 30 MIN (8.28±2.16 ng/mL) (p<0.05). H90 also elicited significant increases in TT from PRE (8.37±1.93 ng/mL) to POST (9.90±1.25 ng/mL) (p<0.01) and 15 MIN (9.46±1.27 ng/mL) (p<0.05). S60 elicited significant increases in TT from PRE (7.73±1.88 ng/mL) to 15 MIN (8.35±1.64 ng/mL) (p<0.05), and S90 showed a notable (p<0.10) difference in TT from PRE (7.96±2.29 ng/mL) to POST (8.75±2.45 ng/mL). All protocols did not significantly increase C (p>0.05). Utilizing relatively short RI between RT sets augment the acute TT response to hypertrophic and strength schemes. Shortening RI within high-intensity strength RT may lead to concomitant enhancements in muscle strength and size over a longer period of training.
Keywords: hypertrophy, maximal strength, resistance training, hormonal response, rest periods
INTRODUCTION
Resistance training robustly stimulates skeletal muscle growth (hypertrophy) (18), and although several physiological mechanisms contribute to the regulation of muscle growth, research demonstrates the importance of androgen signaling for mediating resistance training-induced hypertrophy (21) and long-term adaptations to resistance training (RT) (14, 22). Testosterone is a powerful anabolic hormone that stimulates muscle protein synthesis and intramuscular amino acid uptake, which result in enhanced net protein balance (36). Testosterone also increases androgen receptor (AR) content in muscle cells and associated myonuclei and satellite cells (9, 16, 34).
Elevated endogenous testosterone concentrations, specifically, transient resistance training-induced elevations in circulating testosterone, prevent catabolism of the muscle AR following acute bouts of resistance training (34) and potentiate gains in muscle strength following long-term RT (22). Therefore, maximizing the acute testosterone response to various types of RT (e.g., strength, hypertrophic, power), via evidence-based manipulation of acute program variables, such as intensity, volume, rest interval length between sets, and exercise selection and sequence, may ultimately promote greater tissue anabolism and enhanced recovery over a longer-term training period (34).
Evidence suggests that several RT program design-related factors influence the acute testosterone responses to resistance exercise. Regarding exercise selection and sequencing, large muscle-mass/multi-joint exercises should be performed prior to small muscle-mass/single-joint exercises (21). Intensity (i.e., load or %1-repetition maximum) and volume (i.e., sets x repetitions) have also been shown to affect the acute testosterone response (20, 21, 26, 33). Evidence demonstrates that protocols of sufficient intensity and volume produce substantial elevations in testosterone in men; the interaction between intensity and volume (i.e., volume-load, sets x repetitions x load) has yielded interesting results favoring training programs with a higher glycolytic component (moderate intensity [e.g., 65%–85% 1-repetition maximum], high volume [e.g., 3–5 sets x 10–15 repetitions], and relatively short rest interval lengths between sets (RI) [e.g., 60 seconds to as long as 2 minutes]), typically described as hypertrophic or muscular endurance resistance exercise (6, 26).
In contrast, strength RT has typically been prescribed using higher training intensities [e.g., ≥85% 1-repetition maximum], significantly less volume [e.g., 3–5 sets x ≤6 repetitions], and longer RI [e.g., 3–5 minutes] compared to hypertrophic/endurance RT (17, 19, 40). Importantly, traditionally prescribed higher intensity strength protocols have also been observed to be less effective at eliciting an increased acute anabolic hormonal response compared to moderate intensity hypertrophic protocols (33).
The differences in volume-load examined between strength and hypertrophic RT cannot be eliminated as a critical determinant of the blunted acute anabolic hormonal response observed following higher intensity strength RT (19, 28). Furthermore, many investigations have not controlled for resistance exercise volume-load when attempting to examine the influence of other acute program variables on acute hormonal response patterns (11, 19, 33).
The influence of rest interval length between sets on acute hormonal response patterns following strength RT has received little attention in the scientific literature, and very few investigations have examined the effects of total body hypertrophic and strength RT protocols when attempting to characterize acute hormonal response patterns in a population of healthy men (19). Additionally, no investigations have examined the effects of high intensity strength protocols performed with substantial volume (sets x reps) distributed over several sets (e.g., greater than 3–5 sets) and employing relatively short RI (e.g., less than 3 minutes). Therefore, the purpose of this study was to determine the acute hormonal response in healthy men to 4 RT protocols, in a volume-load controlled, randomized investigation. The 4 protocols examined include: 1) total body strength and 2) total body hypertrophic, each incorporating 2 different RI: 60 seconds and 90 seconds.
Specifically, this investigation aimed to determine if the use of relatively short rest interval lengths between sets in a strength-type protocol can augment the acute testosterone response. If the use of relatively short rest interval lengths significantly increases the acute testosterone response to a total body strength-type protocol, then, from a practical standpoint, repeated exposure to this type of acute strength (i.e., neuronal) training stimulus and consequent acute testosterone response may promote augmented neural and tissue adaptations over a longer period of higher intensity strength training, by increasing both the muscle strength and size. We hypothesized that volume-load equated total body hypertrophic RT protocols and strength RT protocols employing relatively short rest interval lengths between sets will elicit significant acute increases in total testosterone and cortisol.
METHODS
Experimental Approach to the Problem
The central premise for this study is based on evidence suggesting that resistance training can induce significant protocol-dependent changes in acute hormonal response patterns lasting up to 30 minutes post-resistance training. Substantial evidence suggests different combinations of volume, intensity, and rest interval length between sets can influence the acute resistance training-induced anabolic hormone response (i.e., testosterone), but additional research is needed to fully elucidate if and how this response may have important implications for modulating short- and long-term adaptations to resistance training, such as muscle protein synthesis, AR content and half-life, and intramuscular amino acid uptake, leading to improved net protein balance, muscle growth, and strength gains (22, 34, 36).
Novel to this area of research, the comparisons in this investigation assisted in determining the acute testosterone and cortisol response patterns in healthy men that occur as a result of rest interval length manipulation within 2 different volume-load equated, total body protocols (strength-type and hypertrophic). Furthermore, this investigation assisted in determining if the use of relatively short rest interval lengths between sets enhanced the acute training stimulus by eliciting an elevated acute anabolic hormonal response to strength-type resistance exercise.
Subjects
Six men volunteered to participate in this study. The mean (SD) age, height, and mass of the participants were: 26 (2.4) years, 178.6 (5.9) cm, and 86.4 (1.2) kg, respectively (Table 1). All subjects were healthy and characterized as recreational resistance trainees, training at least 2 days per week (>2 years); none of the subjects were considered competitive weight lifters or engaged in any specific training/training cycle outside of this investigation. None of the subjects were taking any dietary or performance enhancing supplements. Each subject had the risks of the investigation explained to him and signed an informed consent form prior to participation in this study. The University of Southern California Health Sciences Campus Institutional Review Board approved all procedures involved in the study. Table 1 (about here)
Table 1.
| Age (y) | 26 ± 2.4 |
| Weight (kg) | 86.4 ± 1.2 |
| Height (cm) | 178.6 ± 5.9 |
| Percent body fat (%) | 12.1 ± 3.4 |
| Smith machine back squat 1RM (kg) | 151 ± 27 |
| Smith machine back squat 70% 1RM (kg) | 106 ± 19 |
| Smith machine back squat 85% 1RM (kg) | 128 ± 23 |
| Barbell bench press 1RM (kg) | 111 ± 8 |
| Barbell bench press 70% 1RM (kg) | 78 ± 6 |
| Barbell bench press 85% 1RM (kg) | 95 ± 6 |
| Narrow/neutral grip lat pulldown 3–8RM (kg) | 91 ± 0 |
| Narrow/neutral grip lat pulldown estimated 1RM (kg) | 116 ± 15 |
| Narrow/neutral grip lat pulldown 70% 1RM (kg) | 81 ± 11 |
| Narrow/neutral grip lat pulldown 85% 1RM (kg) | 98 ± 13 |
| Unilateral knee extension 3–8RM (kg) | 100 ± 0 |
| Unilateral knee extension estimated 1RM (kg) | 111 ± 7 |
| Unilateral knee extension 70% 1RM (kg) | 78 ± 5 |
| Unilateral knee extension 85% 1RM (kg) | 95 ± 6 |
1RM = 1-repetition maximum; 3–8RM = 3- to 8-repetition maximum.
Values are given as mean ± SD.
Procedures
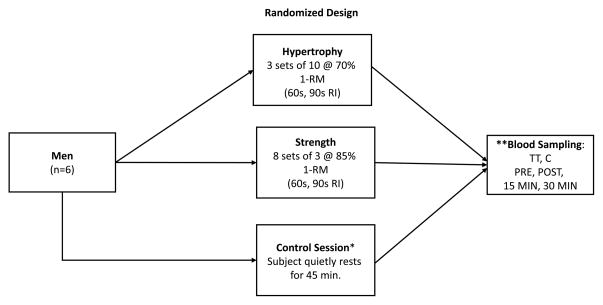
Following maximal strength testing in Visit 1, the subjects participated in a control session (R) during Visit 2 in order to account for the effects of the circadian rhythm on hormonal concentrations. Following Visits 1 and 2, all study subjects performed 4 experimental resistance training sessions in a randomized order (Visits 3–6), to determine hormonal response patterns among 2 resistance training protocols with 2 different rest interval lengths between sets (60 seconds and 90 seconds) (Figure 1). The 2 training protocols consisted of four exercises that activated large muscle masses, performed in the following sequence in every resistance training session: 1) smith machine barbell back squat (Life Fitness, Schiller Park, IL), 2) flat barbell bench press (Proformance Series by Tuff Stuff, Pomona, CA), 3) narrow/neutral grip lat pulldown (Proformance Series by Tuff Stuff), and 4) seated unilateral knee extension (Proformance Series by Tuff Stuff). The control session and acute resistance training sessions were performed on 5 separate occasions, separated by exactly 7 days. All study participants experienced no symptoms of dizziness and/or nausea during and/or after any of the 4 acute resistance training sessions
Figure 1.
A schematic of the experimental design for the study showing randomization of experimental acute resistance training sessions (*prior to experimental exercise sessions; **TT = Total Testosterone, C = Cortisol; RI = rest interval length between sets; 1-RM = 1-repetition maximum; PRE = pre-exercise; POST = immediately post-exercise; 15 MIN = 15 minutes post-exercise; 30 MIN = 30 minutes post-exercise).
Each subject completed Visits 2–6 on the same day, and at the same time, throughout the investigation. Each subject was also instructed to continue his normal activities of daily living and exercise regimen and to not engage in any strenuous activity or exercise 48 hours prior to all study visits.
Prior to undergoing any of the experimental resistance training sessions, all subjects had their blood pressure taken to ensure normal levels and completed a Physical Activity Readiness Questionnaire (PAR-Q). Participant characteristics were also obtained during Visit 1. These included: age, height, weight, and training history (years).
During Visit 1, maximum strength 1-repetition maximum (1-RM) for the smith machine barbell back squat and flat barbell bench press exercises were measured with a 1-RM method and the narrow/neutral grip lat pulldown and seated unilateral knee extension exercises with a 3–8-RM indirect method (to estimate 1-RM).
1-RM was assessed as previously described by Matuszak et al. (24). The indirect method involved 1 to 2 warm-up sets, followed by a working set that allowed completion of (no less than) 3 repetitions to (no more than) 8 repetitions. From the indirect method, the repetition maximum was used to estimate 1-RM based off of an estimated repetition maximum/%1-RM relationship chart (4)
All subjects participated in a control session (R) in order to account for the effects of the circadian rhythm on hormonal concentrations. The control session was performed during Visit 2, prior to all experimental exercise sessions. During the control session, subjects sat quietly for 45 minutes in lieu of training.
Maximum Strength Protocol
In the total body maximum strength protocol (S), the intensity of training was 85% 1-RM for all resistance training exercises; 3 repetitions were performed each set for 8 sets. Subjects performed two experimental S protocols, each with different rest interval lengths between sets (RI). The RI incorporated in this protocol: 60 seconds (S60) and 90 seconds (S90).
Muscular Hypertrophy Protocol
In the total body muscular hypertrophy protocol (H), the intensity of training was 70% 1-RM for all resistance training exercises; 10 repetitions were performed each set for 3 sets. Subjects performed two experimental H protocols, each with different RI. The RI incorporated in this protocol: 60 seconds (H60) and 90 seconds (H90).
Volume Load
All exercise techniques were structured according to anatomical characteristics of each subject, with grip widths and positions marked and kept constant for each exercise throughout the study. Volume load was equated for the S and H protocols for each subject; the only acute program variable manipulated throughout the study was rest interval length between sets.
Minimal assistance (i.e., spotting) was given to study participants only occasionally, when necessary to ensure maximum safety, and not systematically during the study. This was done in order to ensure all repetitions were completed during every set of exercise. The hydration status of the study participants entering each study visit was not controlled for in this investigation. However, during the training workouts and the recovery periods, subjects were allowed to drink water ad libitum.
Dietary Re-Call Questionnaire
Subjects completed a diet re-call questionnaire for the day prior to and the day of the control session and all 4 experimental acute resistance training sessions (i.e., 5 2-day diet re-calls throughout the duration of the study). These questionnaires were qualitative in nature and utilized to ensure that each subject’s individualized dietary intake and nutrient timing remained similar throughout the study. Specifically, each subject re-called the types of foods, approximate quantities of those foods, and at what time of day those foods were consumed. All subjects were instructed to repeat their pattern of eating for the day prior to and the day of the control session and all 4 experimental acute resistance training sessions, as well as to have their last light meal no less than two hours prior to the control session and all 4 experimental acute resistance training sessions.
Blood Draws
To avoid the effects of the circadian rhythm on hormonal concentrations, each subject performed the control and experimental acute resistance training sessions on the same day of the week and at the same time of day. Before each experimental resistance exercise session, the subjects rested for ~15 minutes in a seated position, and then a pre-exercise (PRE) blood sample (~10mL) was drawn with a single needle stick. Blood samples were also drawn immediately post- (POST; within 2 minutes post-exercise), 15 minutes post- (15 MIN), and 30 minutes post-exercise (30 MIN) via an indwelling venous catheter placed into an antecubital vein for the determination of total testosterone (TT) and cortisol (C) concentrations. In the control session (R; Visit 2), the same blood sampling procedures were followed. The subjects did not perform a resistance training protocol, however, and sat passively for 45 minutes after which blood samples were obtained at the same time points as in the experimental acute resistance training sessions.
All blood samples were drawn with the subjects in a seated position, collected in 10mL serum tubes and allowed to coagulate, and centrifuged at 3,000 rpm for ~10 minutes at room temperature. The serum was then separated from the blood cells and stored at −80°C until analyzed. Receptors in the tissues targeted in training are exposed to the specific serum levels of hormone concentrations, therefore, hormone concentrations were not adjusted due to changes in plasma blood volume (29). Serum samples for hormone analyses were only thawed once prior to analyses. Serum concentrations of TT and C were determined in duplicate using enzyme linked immunosorbent assay (ELISA) kits from DRG Diagnostics International (Berlin, Germany). All assays were carried out as advised by manufacturer’s directions. To determine the results/hormone concentrations, we calculated the average absorbance values for each set of standards and participant samples. Then, we constructed a standard curve by plotting the mean absorbance obtained from each standard against its concentration with the absorbance value on the vertical (Y) axis and concentration on the horizontal (X) axis. Lastly, using the mean absorbance value for each sample, we determined the corresponding concentration from the standard curve. All samples for each subject were assayed in the same assay for each hormone to avoid inter-assay variation (1). The intra-assay coefficients of variation were 4.5 and 5.6 for TT and C, respectively.
Statistical Analyses
Descriptive statistics were performed on all baseline strength and anthropometric variables. To determine whether there was an effect of exercise protocol across each experiment, longitudinal linear mixed effects modeling was used to assess whether there was a difference across exercise protocol (Control, H60, H90, S60, and S90). Because each individual was his own control, and experimental conditions were closely controlled across visits, comparison of the different protocols was made without adjusting for covariates. Variances across conditions were constrained to be equal. Because this was a pilot study, and therefore not fully powered, all p < .10 were examined for effect size. All analyses were performed using SPSS (V.20).
RESULTS
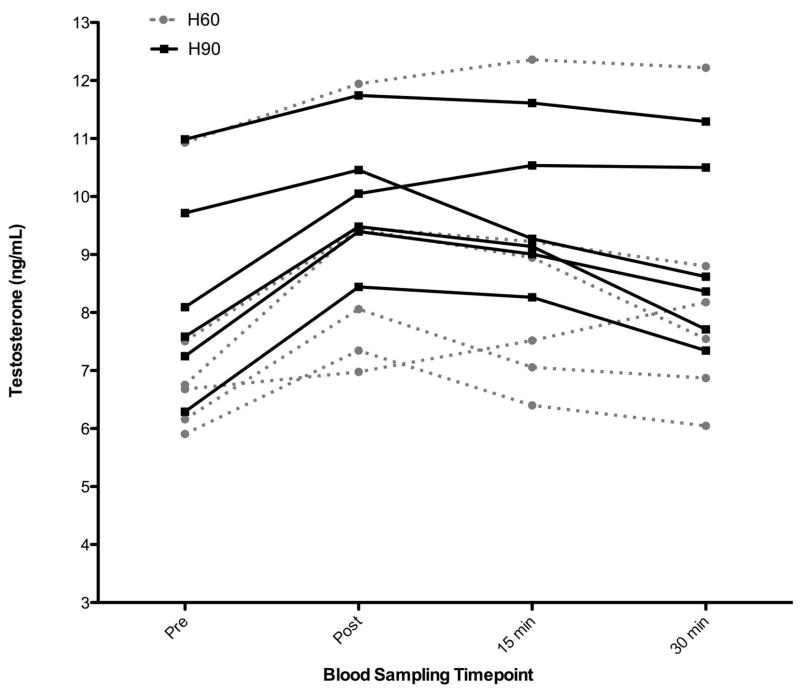
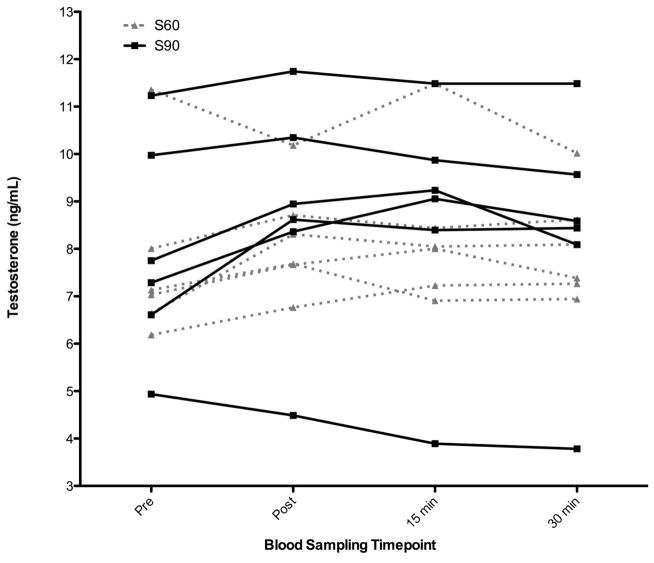
Baseline anthropometric data is presented in Table 1. There were increases in testosterone from pre-test in most conditions. Across conditions, there was a significant effect of condition (p = 0.06). Post-hoc analysis showed that this was due to the difference between H90 and the Control condition (p = 0.02). This represented a very large effect size (Cohen’s d = 1.2). While not statistically significant, the effect sizes (Cohen’s d) between many of the conditions were moderate to large: Control vs S90 = 0.6, H90 vs H60, S60, and S90 = 0.7, 0.9, 0.6, respectively. Analyses of the raw hormone data revealed (Figure 2): the H60 protocol elicited significant increases in TT concentrations from PRE (7.32±1.85 ng/mL) to POST (8.87±1.83 ng/mL) (p<0.01), 15 MIN (8.58±2.15 ng/mL) (p<0.01), and 30 MIN (8.28±2.16 ng/mL) (p<0.05). The H90 protocol also elicited significant increases in TT concentrations from PRE (8.37±1.93 ng/mL) to POST (9.90±1.25 ng/mL) (p<0.01) and 15 MIN (9.46±1.27 ng/mL) (p<0.05). Additionally (Figure 3): the S60 elicited significant increases in TT concentrations from PRE (7.73±1.88 ng/mL) to 15 MIN (8.35±1.64 ng/mL) (p<0.05), and S90 showed a notable (p<0.10) difference in TT concentration from PRE (7.96±2.29 ng/mL) to POST (8.75±2.45 ng/mL). All four acute exercise protocols did not significantly increase C concentrations from PRE to any time point post-exercise (p>0.05).
Figure 2.
Spaghetti graph of total serum testosterone concentration mean (SD) from rest (PRE) to immediately post-exercise (POST), Pre to 15 minutes post-exercise (15 MIN), and Pre to 30 minutes post-exercise (30 MIN) for the each of the two resistance training protocols: hypertrophic with 60 second rest interval length (H60 protocol; closed circles/dashed line), hypertrophic with 90 second rest interval length (H90 protocol; closed squares/solid line). The numbers represent the absolute hormone concentration (ng/mL) from pre-exercise (PRE) to the three different post-exercise time points.
Figure 3.
Spaghetti graph of total serum testosterone concentration mean (SD) from rest (PRE) to immediately post-exercise (POST), Pre to 15 minutes post-exercise (15 MIN), and Pre to 30 minutes post-exercise (30 MIN) for the each of the two resistance training protocols: strength-type with 60 second rest interval length (S60 protocol; closed triangles/dashed line), and strength-type with 90 second rest interval length (S90 protocol; closed squares/solid line). The numbers represent the absolute hormone concentration (ng/mL) from pre-exercise (PRE) to the three different post-exercise time points.
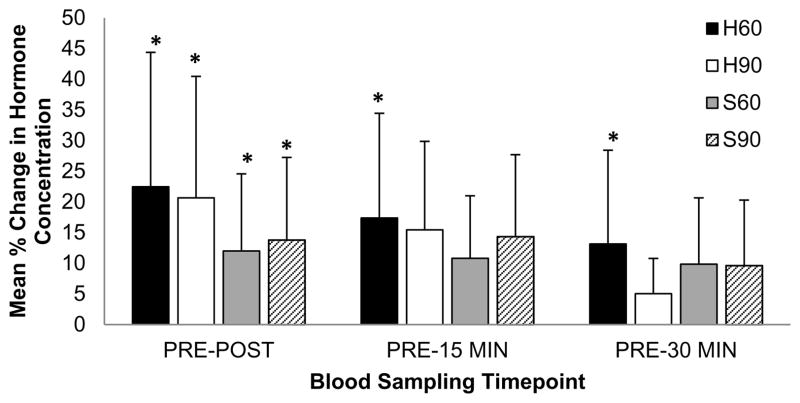
Analyses of relative hormone data revealed (Figure 4): the H60 protocol elicited a significantly different percent change in TT concentrations from PRE to POST (22.5±13.4%), 15 MIN (17.4±10.0%), and 30 MIN (13.2±11.5%) in comparison with the R condition (p<0.05). The H90 protocol elicited a significantly different percent change in TT concentrations from PRE to POST (20.7±12.7%; p<0.05), and a notable (p=0.077) percent change from PRE to 15 MIN (15.4±14.6%) in comparison with the R condition. The S60 protocol elicited a significantly different percent change in TT concentrations from PRE to POST (12.0±7.3%; p<0.05), a notable (p=0.068) percent change from PRE to 15 MIN (10.8±9.7%), and a notable (p=0.088) percent change from PRE to 30 MIN (9.8±9.8%) in comparison with the R condition. The S90 protocol elicited a significantly different percent change in TT concentrations from PRE to POST (13.8±10.8%; p<0.05), and a notable (p=0.068) percent change from PRE to 15 MIN (14.3±12.9%) in comparison with the R condition. All four acute exercise protocols did not elicit significantly different percent changes in C concentrations from PRE to any time point post-exercise in comparison to the R condition (p>0.05).
Figure 4.
Comparison of total serum testosterone concentration mean (SD) from rest (PRE) to immediately post-exercise (POST), Pre to 15 minutes post-exercise (15 MIN), and Pre to 30 minutes post-exercise (30 MIN) for the each of the four resistance training protocols: hypertrophic with 60 second rest interval length (H60 protocol; black bars), hypertrophic with 90 second rest interval length (H90 protocol; open bars), strength-type with 60 second rest interval length (S60 protocol; light grey bars), and strength-type with 90 second rest interval length (S90 protocol; hatched bars). The numbers represent the percent change from pre-exercise (PRE) to the three different post-exercise time points. *Significant (p<0.05) difference from Pre value in comparison with the control (R) condition.
DISCUSSION
The primary findings from this investigation suggest that utilizing relatively short rest interval lengths (60 seconds and 90 seconds) within volume load-equated total body hypertrophic and strength-type protocols lead to significant enhancements in the acute anabolic (serum total testosterone) hormonal response, while eliciting non-significant changes in the acute catabolic (serum cortisol) hormonal response. This investigation also suggests that traditional hypertrophic protocols augment the acute serum total testosterone response to a greater absolute and relative magnitude, when compared to strength-type protocols equal in volume-load to the hypertrophic protocols and employing relatively short rest interval lengths between sets. This is the first study to examine volume load-equated total body protocols aimed at promoting different training adaptations, specifically, muscle hypertrophy versus muscle strength, and the influence of rest interval length manipulation on acute hormonal responses.
Previous research suggests that testosterone increases protein synthesis, augmenting muscle mass and strength in young and older men (35). Part of the adaptive response to testosterone may be mediated through the growth hormone-insulin-like growth factor-I (GH-IGF-I) system (10, 35). Testosterone administration can stimulate both the peripheral GH-IGF-I system (15) and intramuscular IGF-I system in men (35). Increases in local IGF-I concentrations could increase the rate of muscle protein turnover via effects on either synthesis or degradation. In vitro, IGF-I stimulates myoblasts to express myogenin, which mediates the differentiation of myoblasts to myotubes (10). IGF-I may also reduce muscle protein degradation, which could potentially influence net muscle protein synthesis.
Furthermore, evidence indicates testosterone-induced muscle fiber hypertrophy is associated with increases in myonuclei and satellite cells (32). Muscle hypertrophy involves the addition of newly formed myonuclei via the fusion of myogenic precursor cells to adult myofibers (2). Therefore, it is possible that testosterone-induced increases in satellite cell number and fusion of satellite cells with muscle fibers precede muscle fiber hypertrophy and the increases in myonuclear number.
To date, only one study by Kraemer et al. (19) has examined the influence of rest interval length manipulation within total body strength-type protocols (i.e., ≥85% 1-RM, lower training volume relative hypertrophic) on acute testosterone responses. The investigators utilized a strength scheme that incorporated 5 upper body exercises (4 multi-joint movements: bench press, lat pulldown, seated row, military press; 1 single-joint movement: arm curl), 2 lower body exercises (1 multi-joint movement: leg press; 1 single-joint movement: bilateral knee extension), and 1 trunk exercise (bent leg, incline sit-ups). All exercises were performed on a Universal weight machine, except for the arm curl and trunk exercises. This study examined the influence of employing a 1 versus 3 minute rest interval length within a strength-type protocol that utilized 3 to 5 sets of a 5RM for all 8 exercises. Utilizing a 1 or 3 minute rest interval length, they showed significant acute increases in testosterone concentrations at 0, 5, and 15 minutes post-exercise; acute cortisol responses were not investigated in this study. Interestingly, the acute testosterone response patterns to the strength protocol with either rest interval length (1 or 3 minutes) increased similarly; this is in contrast with more recent investigations suggesting blunted acute hormonal responses to strength-type schemes with relatively long rest interval lengths (i.e., 3 to 5 minutes) (5–7, 26).
McCaulley et al. (26) examined a strength protocol consisting of 11 sets of 3 repetitions at 90% 1-RM, employing a 5 minute rest interval length between sets, and utilizing the back squat exercise only. This protocol elicited a non-significant acute increase in serum total testosterone concentration from pre- to immediately post-exercise, and a non-significant acute decrease in serum cortisol concentration from pre- to immediately post-exercise. Crewther et al. (6) examined acute salivary testosterone and cortisol responses to a maximal strength scheme prescribed as 6 sets of 4 repetitions at 88% 1-RM, using a 4-minute rest interval length between sets; 3 sets were performed on only two different lower body exercises: an isoinertial supine squat machine and modified Smith machine. Crewther et al. showed little or no acute changes in testosterone or cortisol concentrations in response to the maximal strength scheme. Lastly, Raastad et al. (27) examined a strength protocol of 3 sets of a 3RM for back squats and front squats and 3 sets of a 6RM for bilateral knee extensions, while utilizing a 4- to 6-minute rest interval length between sets. This study also found no significant changes in serum testosterone or cortisol concentrations from pre- to immediately post-exercise. All of these studies appear to be limited by the exercise selection within their scheme design, as they did not examine total body training protocols, which are commonly prescribed in strength and conditioning practices.
However, other studies have examined acute hormonal response patterns to total body strength-type schemes with relatively long rest interval lengths, and have found similar results. Beaven et al. (5) also examined acute salivary testosterone responses to various total body resistance training protocols, including hypertrophic, strength, power, and endurance, and utilized the bench press, leg press, seated row, and back squat exercises. Of the four protocols examined, the acute testosterone response to the strength protocol, consisting of 3 sets of 5 repetitions at 85% 1-RM with a 3-minute rest interval length between sets, was significantly blunted compared to the hypertrophic and endurance schemes (not the power scheme, utilizing 3 sets of 5 repetitions at 40% 1-RM with a 3-minute rest interval length between sets). Smilios et al. (33) examined 3 different total body strength protocols utilizing 2, 4, or 6 sets of 5 repetitions at 88% 1-RM for the bench press, latissimus pulldown, and back squat exercises, 80% 1-RM for the overhead press exercise, and a 3-minute rest interval length between sets. This study found no significant acute changes in testosterone or cortisol concentrations from pre- to immediately post-exercise, even though total work was doubled and tripled, suggesting that an increase in total work does not appear to be a stimulus for enhanced acute anabolic/catabolic hormonal concentrations.
While several studies have concluded that hypertrophic resistance exercise prescriptions (e.g., multiple sets, 8–12 repetitions, 70–80% 1-RM, and relatively short rest interval lengths between sets of 1–2 minutes) elicit acute increases in anabolic and catabolic hormones (5–7, 19–21, 26, 27, 33), possibly due to differences in volume-load when compared to strength-type resistance exercise prescriptions, this investigation suggests that rest interval length between sets, rather than volume-load, may be an acute program variable critical to eliciting an enhanced acute anabolic hormonal response. Specifically, the utilization of relatively short rest interval lengths between sets within total body strength-type protocols may provide a stimulus for enhanced acute anabolic hormonal responses from pre- to post-resistance exercise. Thus, the results of this investigation also indicate that volume-load may not be the only factor related to the enhanced acute anabolic hormonal responses to hypertrophic resistance exercise protocols; rather, the combination of volume-load, utilization of relatively short rest interval lengths between sets, and selection/sequencing of exercises that activate large muscle masses may trigger the drastic acute increases in anabolic hormonal concentrations.
The scheme designs used in our study were total body in nature and incorporated exercises that challenged large muscle masses, such as the quadriceps, pectoralis major, and latissimus dorsi. Thus, exercise selection and sequencing may also be key determinants of the acute hormonal responses elicited by any given training stimulus. Repeated exposure to this type of strength (i.e., neuronal) training stimulus and consequent acute hormonal response may promote augmented neural and tissue adaptations over a longer-term period of higher intensity strength training, by increasing both the muscle strength and size; however, this hypothesis warrants additional investigation. Traditionally, strength programs incorporate relatively long rest interval lengths between sets (e.g., 3 to 5 minutes between sets), which, in combination with higher intensities and lower volumes of training, do not elicit significant acute increases in anabolic hormones (5–7, 13, 19–21, 26, 27, 33) or significant size gains over a longer-term period of training (12).
The participants in this study were not competitive weight lifters, but did have several years of experience resistance training. Therefore, given sufficient training experience (e.g., >2 years) and a trained state, maintenance of repetition performance over repeated sets of higher intensity resistance exercise is attainable, even when utilizing 60 second or 90 second rest interval lengths. This recommendation is in contrast to the current body of literature regarding optimizing rest interval lengths between sets, which suggests 3- to 5-minute rest interval lengths are needed for sufficient recovery between “strength training” sets. However, a notable limitation to this research may be that the general suggestion to rest 3 to 5 minutes between training sets to maximize/maintain repetition performance over repeated sets is derived exclusively from hypertrophic and/or muscular endurance protocols, none of which are prescribed as <8RM (8, 37–40). This study provides evidence to suggest that repetition performance can be maintained throughout a higher intensity strength protocol, even when employing rest interval lengths typically prescribed for hypertrophic resistance exercise.
The biochemical mechanisms responsible for the observed increases in blood concentrations of testosterone are not fully understood and are largely beyond the scope of the current paper. However, previous studies propose that significant acute increases in lactate during resistance exercise may decrease blood pH and markedly increase catecholamine levels; this is hypothesized to be an important mechanism responsible for increased testosterone concentrations (20, 23). Although blood concentrations of lactate were not measured in the current investigation, Kraemer et al. (19) reported significant increases in blood lactate from pre- to mid-, immediately post-, 5 minutes post-, and 15 minutes post-training, in response to a total body, heavy resistance exercise protocol utilizing 5-RM loads with a 60-second rest interval length in between sets. Therefore, through performance of total body strength protocols, such as those examined in the present study, which utilize higher intensities (i.e., >85% 1-RM or ≤5-RM loads), relatively short rest interval lengths (i.e., ≤90 seconds), and several multi-joint/compound movements, it is possible that this combination of acute program variables results in large increases in blood concentrations of lactate that lead to a significant rise in blood concentrations of testosterone.
The phenomenon of increased muscle activity and decreased force output has been termed neuromuscular inefficiency and may be indicative of peripheral fatigue (3). McCaulley et al. (26) reported slight elevations in agonist muscle activity in response to a hypertrophic protocol, while a strength protocol with extended rest interval lengths in between sets (i.e., 5 minutes) resulted in decreased neuromuscular performance (i.e., decreased peak force, rate of force development, and muscle activity) (26). This study indicates that the difference in agonist muscle activity reported between hypertrophic protocols and strength protocols with extended rest interval lengths in between sets is representative of the variations in acute neuromuscular responses that can be elicited by varying the metabolic demand of resistance exercise through manipulation of acute program variables. Specifically, previous research shows that shorter rest interval lengths in between sets may increase motor unit recruitment, in spite of attenuated force outputs (30); this may explain the increased muscle activity observed following hypertrophic protocols (26). As motor unit recruitment increases, recruitment of a greater number of muscle fibers enables greater hormone-tissue interaction within the region of a larger percentage of total muscle mass; therefore, muscle tissue activation may be a precursor to anabolism (21, 31). Even though a causative link between increased muscle activity and the acute increases in testosterone in response to the total body strength protocols examined in the present investigation cannot be made, it is possible that this type of strength training stimulus may elicit enhanced metabolic demands through neuromuscular inefficiency, greater peripheral fatigue, and/or increased motor unit recruitment, more closely resembling hypertrophic training stimuli and resulting in improved neuromuscular performance, increased blood testosterone concentrations, and potentially greater chronic hypertrophic adaptations.
The present study was limited by a small number of study participants (n=6), and this small sample size did not allow for sufficient statistical power to utilize alternative statistical procedures, such as repeated measures ANOVA. However, post-hoc t-test power analyses of the mean relative hormone data from the 6 study participants indicated a high average power of 82%. Furthermore, the present study only examined young, resistance trained men, so future research in this area should include investigations of older men, as well as untrained study participants. Lastly, the acute resistance training protocols in the present study did not utilize “RM loads,” which are commonly prescribed in addition to “%1-RM loads,” and this study did not include a determination of the acute hormonal responses to (traditional) strength-type resistance training protocols employing relatively long rest interval lengths between sets; within a single investigation, using the same study sample, future research should aim to compare acute hormonal responses to strength-type resistance training protocols with very dissimilar rest interval lengths between sets.
The impact of repeatedly elevated acute hormone concentrations following longer-term resistance training periods (i.e., strength-type resistance training) on chronic adaptations, such as maximal strength and/or changes in lean mass, warrants additional investigation. Evidence supports the important role of testosterone in the maintenance of muscle mass and function in men (25), but more research is needed to elucidate the exact role of testosterone in the context of acute resistance training-induced response patterns and the influence, if any, these acute response patterns have on chronic changes in muscle size and strength following longer-term periods of resistance training.
While the mechanism(s) underlying acute bout hormonal responses leading to chronic improvements in muscle size and strength remain to be fully clarified, recent evidence suggests AR in muscle cells are up-regulated 3 hours post-resistance training only when an acute resistance training bout elicits an increase in circulating testosterone (34). Moreover, testosterone increases the AR in muscle cells and associated myonuclei and satellite cells (16). Nevertheless, the precise mechanism for this up-regulation are not fully understood and may also be influenced by nutrient timing (i.e., pre- and/or post-training consumption of carbohydrate- and/or protein-containing nutrition) (36). Therefore, future research should attempt to more clearly understand the link between acute hormonal responses (i.e., testosterone) and the chronic muscular and functional performance adaptations elicited by variations in resistance training scheme designs, especially strength-type schemes, in elite athletic, recreationally athletic, pathological, and older populations.
PRACTICAL APPLICATIONS
Traditionally, higher intensity training protocols designed to enhance muscular strength (i.e., neuronal schemes utilizing intensities ≥85% 1-RM) have employed relatively long rest interval lengths between training sets (e.g., 3–5 minutes), which lead to no significant acute increases in anabolic hormones. The results of the present study demonstrate that the utilization of relatively short rest interval lengths (i.e., 60 seconds and 90 seconds) between repeated high intensity training sets elicit significant acute changes in testosterone concentrations from pre- to post-exercise. Strength-power athletes capable of training at higher intensities and with volume-loads equal to traditional hypertrophic resistance exercise prescriptions may enhance their adaptations to strength training via the use of shortened rest interval lengths between sets, if utilization of relatively short rest interval lengths during a longer-term strength phase of training results in simultaneous hypertrophic adaptations and increases in strength, due to the enhanced anabolic hormonal environment induced by rest interval length manipulation (i.e., greater acute increases in testosterone leading to enhanced protein synthesis and recovery from training). Lastly, the hypertrophic protocols examined in the present study elicited greater absolute and relative acute changes in testosterone concentrations compared to the strength protocols, and this was most likely due to a combination of training variables, such as volume-load, rest interval length, and exercise selection/sequence.
Acknowledgments
The authors would like to thank the study participants, University of Southern California Clinical Trials Unit nursing staff, and Adriana Del Padilla for their contribution to the successful completion of the experimental protocol.
References
- 1.Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Hakkinen K. Short vs. long rest period between the sets in hypertrophic resistance training: influence on muscle strength, size, and hormonal adaptations in trained men. J Strength Cond Res. 2005;19:572–582. doi: 10.1519/15604.1. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Babault N, Desbrosses K, Fabre MS, Michaut A, Pousson M. Neuromuscular fatigue development during maximal concentric and isometric knee extensions. J Appl Physiol. 2006;100:780–785. doi: 10.1152/japplphysiol.00737.2005. [DOI] [PubMed] [Google Scholar]
- 4.Baechle TR, Earle RW, editors. Essentials of Strength Training and Conditioning. Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 5.Beaven CM, Gill ND, Cook CJ. Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. Journal of Strength & Conditioning Research. 2008;22:426–432. doi: 10.1519/JSC.0b013e3181635843. [DOI] [PubMed] [Google Scholar]
- 6.Crewther B, Cronin J, Keogh J, Cook C. The salivary testosterone and cortisol response to three loading schemes. Journal of Strength & Conditioning Research. 2008;22:250–255. doi: 10.1519/JSC.0b013e31815f5f91. [DOI] [PubMed] [Google Scholar]
- 7.Crewther B, Keogh J, Cronin J, Cook C. Possible stimuli for strength and power adaptation: acute hormonal responses. Sports Medicine. 2006;36:215–238. doi: 10.2165/00007256-200636030-00004. [DOI] [PubMed] [Google Scholar]
- 8.de Salles BF, Simao R, Miranda F, da Novaes JS, Lemos A, Willardson JM. Rest interval between sets in strength training. Sports Med. 2009;39:765–777. doi: 10.2165/11315230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 10.Florini JR, Ewton DZ, Roof SL. Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol Endocrinol. 1991;5:718–724. doi: 10.1210/mend-5-5-718. [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Ishii N, Kizuka T, Takamatsu K. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37:955–963. [PubMed] [Google Scholar]
- 12.Goto K, Nagasawa M, Yanagisawa O, Kizuka T, Ishii N, Takamatsu K. Muscular adaptations to combinations of high- and low-intensity resistance exercises. J Strength Cond Res. 2004;18:730–737. doi: 10.1519/R-13603.1. [DOI] [PubMed] [Google Scholar]
- 13.Goto K, Sato K, Takamatsu K. A single set of low intensity resistance exercise immediately following high intensity resistance exercise stimulates growth hormone secretion in men. J Sports Med Phys Fitness. 2003;43:243–249. [PubMed] [Google Scholar]
- 14.Hansen S, Kvorning T, Kjaer M, Sjogaard G. The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports. 2001;11:347–354. doi: 10.1034/j.1600-0838.2001.110606.x. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs CJ, Plymate SR, Rosen CJ, Adler RA. Testosterone administration increases insulin-like growth factor-I levels in normal men. J Clin Endocrinol Metab. 1993;77:776–779. doi: 10.1210/jcem.77.3.7690364. [DOI] [PubMed] [Google Scholar]
- 16.Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–1534. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer WJ, Aguilera BA, Terada M, Newton RU, Lynch JM, Rosendaal G, McBride JM, Gordon SE, Hakkinen K. Responses of IGF-I to endogenous increases in growth hormone after heavy-resistance exercise. J Appl Physiol. 1995;79:1310–1315. doi: 10.1152/jappl.1995.79.4.1310. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer WJ, Hakkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87:982–992. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. Journal of Applied Physiology. 1990;69:1442–1450. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- 20.Kraemer WJ, Noble BJ, Clark MJ, Culver BW. Physiologic responses to heavy-resistance exercise with very short rest periods. Int J Sports Med. 1987;8:247–252. doi: 10.1055/s-2008-1025663. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kvorning T, Andersen M, Brixen K, Madsen K. Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol Endocrinol Metab. 2006;291:E1325–1332. doi: 10.1152/ajpendo.00143.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lu SS, Lau CP, Tung YF, Huang SW, Chen YH, Shih HC, Tsai SC, Lu CC, Wang SW, Chen JJ, Chien EJ, Chien CH, Wang PS. Lactate and the effects of exercise on testosterone secretion: evidence for the involvement of a cAMP-mediated mechanism. Med Sci Sports Exerc. 1997;29:1048–1054. doi: 10.1097/00005768-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Matuszak ME, Fry AC, Weiss LW, Ireland TR, McKnight MM. Effect of rest interval length on repeated 1 repetition maximum back squats. Journal of Strength and Conditioning Research. 2003;17:634–637. doi: 10.1519/1533-4287(2003)017<0634:eorilo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 26.McCaulley GO, McBride JM, Cormie P, Hudson MB, Nuzzo JL, Quindry JC, Travis Triplett N. Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur J Appl Physiol. 2009;105:695–704. doi: 10.1007/s00421-008-0951-z. [DOI] [PubMed] [Google Scholar]
- 27.Raastad T, Bjoro T, Hallen J. Hormonal responses to high- and moderate-intensity strength exercise. Eur J Appl Physiol. 2000;82:121–128. doi: 10.1007/s004210050661. [DOI] [PubMed] [Google Scholar]
- 28.Ratamess NA, Kraemer WJ, Volek JS, Maresh CM, Vanheest JL, Sharman MJ, Rubin MR, French DN, Vescovi JD, Silvestre R, Hatfield DL, Fleck SJ, Deschenes MR. Androgen receptor content following heavy resistance exercise in men. J Steroid Biochem Mol Biol. 2005;93:35–42. doi: 10.1016/j.jsbmb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Rubin MR, Kraemer WJ, Maresh CM, Volek JS, Ratamess NA, Vanheest JL, Silvestre R, French DN, Sharman MJ, Judelson DA, Gomez AL, Vescovi JD, Hymer WC. High-affinity growth hormone binding protein and acute heavy resistance exercise. Med Sci Sports Exerc. 2005;37:395–403. doi: 10.1249/01.mss.0000155402.93987.c0. [DOI] [PubMed] [Google Scholar]
- 30.Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev. 1987;15:95–151. [PubMed] [Google Scholar]
- 31.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 33.Smilios I, Pilianidis T, Karamouzis M, Tokmakidis SP. Hormonal responses after various resistance exercise protocols. Medicine & Science in Sports & Exercise. 2003;35:644–654. doi: 10.1249/01.MSS.0000058366.04460.5F. [DOI] [PubMed] [Google Scholar]
- 34.Spiering BA, Kraemer WJ, Vingren JL, Ratamess NA, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Hakkinen K, Maresh CM. Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol. 2009;114:195–199. doi: 10.1016/j.jsbmb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 36.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40:1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Willardson JM. A brief review: factors affecting the length of the rest interval between resistance exercise sets. J Strength Cond Res. 2006;20:978–984. [PubMed] [Google Scholar]
- 38.Willardson JM, Burkett LN. A comparison of 3 different rest intervals on the exercise volume completed during a workout. J Strength Cond Res. 2005;19:23–26. doi: 10.1519/R-13853.1. [DOI] [PubMed] [Google Scholar]
- 39.Willardson JM, Burkett LN. The effect of rest interval length on bench press performance with heavy vs. light loads. J Strength Cond Res. 2006;20:396–399. doi: 10.1519/R-17735.1. [DOI] [PubMed] [Google Scholar]
- 40.Willardson JM, Burkett LN. The effect of rest interval length on the sustainability of squat and bench press repetitions. J Strength Cond Res. 2006;20:400–403. doi: 10.1519/R-16314.1. [DOI] [PubMed] [Google Scholar]