Abstract
Little is known about cellular determinants essential for human hepatitis B virus infection. Using the duck hepatitis B virus as a model, we first established a sensitive binding assay for both virions and subviral particles and subsequently elucidated the characteristics of the early viral entry steps. The infection itinerary was found to initiate with the attachment of viral particles to a low number of binding sites on hepatocytes (about 104 per cell). Virus internalization was fully accomplished in less than 3 h but was then followed by a period of unprecedented length, about 14 h, until completion of nuclear import of the viral genome. Steps subsequent to virus entry depended on both intact microtubules and their dynamic turnover but not on actin cytoskeleton. Notably, cytoplasmic trafficking of viral particles and emergence of nuclear covalently closed circular DNA requires microtubules during entry only at and for specific time periods. Taken together, these data disclose for the first time a series of steps and their kinetics that are essential for the entry of hepatitis B viruses into hepatocytes and are different from those of any other virus reported so far.
Infections by animal viruses begin with the attachment of the viruses to their respective host cells, followed by internalization, uncoating, and delivery of the viral genome to its cellular replication site. These early steps are poorly characterized for hepadnaviruses, pararetroviruses which almost exclusively infect hepatocytes, leading to both limited and persistent noncytotoxic infections (for a review, see reference 6). Studies addressing the early steps of the hepadnavirus life cycle were hampered mainly by the lack of permissive cell lines and low signal intensities. Despite these restrictions, a number of cellular proteins have been proposed to serve as receptors for human hepatitis B virus (HBV); however, none of these proteins renders infection-resistant cell lines or primary cells susceptible to HBV infection. Progress in this field has been greater in the surrogate model system of duck HBV (DHBV). A novel carboxypeptidase (CPD, also designated gp180) has been identified as a major pre-S binding protein and proposed to be the potential DHBV receptor (12, 13, 30, 31, 32). Consistent with this notion, CPD was shown to bind both viral particles and recombinant L protein with high affinity (33). More importantly, antibodies directed against CPD inhibit DHBV infection of primary duck hepatocytes (PDHs) (33), and heterologous expression of CPD leads to efficient internalization of viral particles in nonpermissive hepatoma cells but is not sufficient to confer susceptibility to DHBV infection (2, 11, 30). These studies suggest that additional or other factors are required for the establishment of a productive infection. Studies addressing the entry steps of the viral life cycle are further complicated by the fact that hepadnavirus infection leads to production of a huge excess of noninfectious, so-called subviral particles (SVPs) in addition to progeny virus, which makes the discrimination between SVPs and complete virions in protein-based assays nearly impossible. These SVPs, which are devoid of viral DNA and consist mainly of lipids and viral envelope proteins, presumably compete with virions for the same specific binding sites on hepatocytes (9). By using an indirect immunoautoradiographic assay, binding of surface antigen-containing DHBV particles to cells has been shown to be species and cell type specific (22). Furthermore, it was shown that attachment of viral particles to hepatocytes occurs in two steps, involving an unspecific nonsaturable one with low affinity followed by a second, saturable, high affinity binding one, as demonstrated by competition experiments (9). DHBV internalization has been shown to require energy (10). Since hepadnaviruses are enveloped viruses, a fusion of virus and host membranes must take place. Currently, there are only indirect evidences for this postulated fusion event, which presumably does not depend on low pH (10, 23). Regardless of which entry pathway is used, core particles have to deliver their cargo, the viral genome, into the nucleus, where the partially double-stranded, relaxed circular viral DNA (rcDNA) is repaired and converted into the so-called covalently closed circular DNA (cccDNA) (for a review, see reference 6). This step is essential and is a prerequisite for the establishment of a productive infection by hepadnaviruses, since cccDNA is the central template for viral transcription and replication (34).
To date, it is not known how the long distance between the cell surface and the nucleus is overcome by hepatitis B viruses. Upon entry, viruses often use the host cytoskeleton to move within the cell toward their respective replication center (reviewed in references 20 and 25).
In the present study, we first developed an attachment and postadsorption assay, after which we semiquantitatively investigated the timing and extent of infectious DHBV entry into primary hepatocytes together with the cellular factors involved. This approach revealed that DHBV entry and transport is unusual in several aspects and includes new strategies not reported for any other virus.
MATERIALS AND METHODS
Primary hepatocyte cultures.
Primary fetal duck hepatocytes were prepared and cultivated as described elsewhere (21). PDHs were seeded into 12-well plates at a density of about 5 × 105 liver cells per well.
Viruses, antibodies and drugs.
PDHs were infected with a DHBV-viremic goose serum containing about 1.1 × 1010 genome equivalents/ml, as determined by DNA-dot blot analysis of viral particles in fractions obtained by CsCl centrifugation using cloned DHBV DNA for calibration and containing about 1013 SVPs/ml. The approximate number of SVPs was calculated by measuring the number of L-protein molecules with an immunoblot calibrated with highly purified recombinant pre-S protein (kindly provided by S. Urban, Heidelberg, Germany) and based on the previously published educated guess that of the 100 envelope proteins of each viral particle about 20 are L-protein molecules (9, 24). Paclitaxel (Molecular Probes), nocodazole, cytochalasin D, and suramin (all from Sigma) were all reconstituted as 1,000-fold stocks in dimethyl sulfoxide and stored at −20°C. The concentrations used were determined by dose escalation experiments. Briefly, PDHs were pretreated with various concentrations of paclitaxel or nocodazole before and during inoculation with DHBV. Thereafter, the drugs and the virus were removed, and the cells were further cultivated for 3 days before harvest. The number of infected cells was determined by immunostaining for L protein. For immunoblot and fluorescence analysis, we used rabbit anti-DHBV-L-protein serum as previously described (21). Secondary goat anti-rabbit antibodies conjugated with Alexa 488 or HRPO were obtained from Molecular Probes or Dianova, respectively. Tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin was obtained from Molecular Probes. For attachment interference assays, we used three different rabbit anti-L-protein sera (KpnI, DPSI, and pre-S 1-41), unrelated rabbit immunoserum, or mouse anti-rabbit immunoglobulin G (IgG) (Sigma). The DPSI and KpnI antibodies were raised against viral L-protein spanning amino acids 1 to 131 and 44 to 185 of the L protein, respectively (28, 5). Pre-S 1-41 was raised against the N-terminal part of recombinant pre-S and was kindly provided by S. Urban.
Attachment assays.
To analyze the attachment of DHBV to cells with disrupted cytoskeletons, cultures were pretreated with paclitaxel (10 μM), nocodazole (16.5 μM), or cytochalasin D (20 μM) for 1 h at 37°C, after which virus inoculum was added and the cells were further incubated for 2 h at 4°C in the presence of the drugs. The control cells were left untreated. After being washed extensively with phosphate-buffered saline (PBS), the cells were lysed in 200 μl of PCR sample buffer, and PCR was performed (10).
To investigate the effect of neutralizing antibodies against L protein or suramin on the attachment of DHBV to PDHs, viremic serum was preincubated with 10 μl of the different antibodies for 30 min at 37°C or cells were pretreated with suramin (100 μg/ml) for 1 h. Then inoculum was added to the treated cells, or serum-antibody mixture was added to the untreated cells. Two hours after transfer to 4°C, the cells were washed with PBS and lysed in 200 μl of PCR sample buffer or Laemmli buffer.
To test whether the extent of virus binding depends on the multiplicity of genome equivalents (MGE) used, cells were exposed to different MGEs for 2 h at 4°C or the incubation period at 4°C was changed. The cells were harvested as described above. The amount of attached viral particles was extrapolated from the L-protein levels determined by immunoblotting. Serial dilutions of the known amount of recombinant pre-S protein were used for calibration. Signals were quantified after acquisition with a Fluor-S MultiImager (Bio-Rad) by using Quantity One software.
Protease protection assay.
After attachment, the inoculum was removed, and the cultures were washed with PBS and incubated with 1 ml of 0.25% trypsin-EDTA solution per well for 10 min at 37°C. The cells were resuspended in the trypsin solution, transferred to Eppendorf tubes, and pelleted by centrifugation. The pellet was lysed in 200 μl of PCR sample buffer.
Internalization assays.
To investigate the internalization of DHBV in microtubule (MT)-inactivated cells, the cultures were pretreated and incubated at 4°C as described above. After 2 h of binding, the cells were washed three times with PBS. New medium containing nocodazole or paclitaxel was added to the cells, and the plate was transferred to 37°C. After 1, 3, and 9 h of incubation at 37°C, the cells were rinsed with PBS and harvested as described in the protease protection assay. The control cells without treatment were handled identically.
cccDNA isolation and amplification after disruption of MTs.
The cells were pretreated for 1 h with nocodazole, while the control cells were left untreated. Then inoculum was added, and the cells were further incubated for 14 h. Virus bound to the cell surface was inactivated by low-pH treatment (10), and the cells were further cultivated in new medium containing no drug or nocodazole. The cells were harvested at the indicated time points by trypsin treatment. cccDNA was isolated according to a protocol described previously (27). The dried DNA pellet was dissolved in 10 μl of H2O, and 5 μl of the solution was subjected to PCR by using primer sets which allow preferential amplification of rcDNA and/or cccDNA (10).
Infection assays in cells treated with cytoskeletal inhibitors.
After a preparation step on day 3, the cells were preincubated with 10 μM paclitaxel, 16.5 μM nocodazole, or 20 μM cytochalasin D for 2 h, after which DHBV inoculum was added. Infection was allowed to proceed for 14 h in the presence of the drugs. Subsequently, the cells were washed with medium to remove unbound external virus as well as the drugs. Following low-pH treatment, the cells were cultivated in new medium for a further 3 days and harvested for immunoblot or immunofluorescence analysis of L protein levels.
Cytotoxicity assay.
Cells were treated with the indicated substances for 24 h, washed with PBS, and subsequently incubated with 0.4% trypan blue in PBS for 3 min. After being washed with PBS, the cultures were investigated for blue cells, and pictures were acquired. Parallel cultures were subjected to vitality assays using fluorescein diacetate (FDA) (dissolved in dimethyl sulfoxide). After incubation with FDA for 5 min at 37°C, the medium was changed, and pictures of fluorescent cells were acquired by using an epifluorescence microscope.
Wash-out assay.
The cells were exposed to DHBV at 4°C. After 2 h of binding, the cells were washed three times with PBS to remove unbound virus. New medium containing nocodazole was added, and the plate was transferred to 37°C to allow virus internalization. After 30 min and 1, 2, 4, 6, and 8 h of incubation, the cells were rinsed three times with PBS to remove unbound virus and the drug to allow recovery of the cytoskeleton. Three days later, the cells were harvested and stained or immunoblotted to determine L protein levels. The same procedure was performed in parallel on nontreated control cells.
Add-in assay.
Primary hepatocytes were treated with paclitaxel before or after exposure to DHBV at the indicated time points. After a further 16 h of incubation, the cells were washed to remove the drugs and unbound virus. Virus that was cell attached but still external was inactivated by a low-pH bath. Three days following infection, the cells were harvested and subjected to immunoblot analysis to determine L-protein levels.
Transfection of PDHs and fluorescence microscopy.
For visualization of the microtubules, 2 days after being seeded on coverslips, PDHs were transfected with 4 μg of plasmid encoding tubulin-green fluorescent protein (GFP) (Clontech) by using FuGene 6 according to the manufacturer's instructions (Roche). Germany Briefly, 80 μl of culture medium without any supplements and 20 μl of FuGene 6 were mixed before 4 μg of the plasmid was added. After being incubated at room temperature for 5 min, 100 μl of this mixture was added to the cells, which were then incubated overnight at 37°C. One day after transfection, the medium was removed and replaced with new medium. The transfection efficacy examined by epifluorescence live-cell microscopy was usually less than 1%. The cells transfected with tubulin-GFP were either not treated or treated for 1 h with nocodazole. Recovery of the cytoskeleton was allowed in some samples by washing with PBS and subsequent incubation for 1 h at 37°C. Following mounting and embedding in Mowiol (Calbiochem, LaJolla, Calif.), fluorescence was analyzed at room temperature with a Zeiss inverted epifluorescence microscope S100 (Axiovert, Zeiss, Germany). Images were acquired and processed with Openlab software.
For visualization of the actin fibers, PDHs were either treated or not treated for 1 h with cytochalasin D. Recovery of the network was allowed in some samples by washing with PBS and further incubation for 1 h without the drug. Then the cells were washed twice with PBS and fixed with 3.7% paraformaldehyde. After being washed, the fixed cells were treated with ice-cold acetone for 5 min and washed again. The coverslips were blocked with 1% bovine serum albumin in PBS for 30 min, after which TRITC-labeled phalloidin (stock, 50 μg/ml diluted 1:100 in PBS) was applied for 30 min. After being washed with PBS, the coverslips were incubated for a short time with 0.1% Triton X-100 in PBS. The coverslips were washed for a short time with water and mounted in Mowiol. Confocal images were acquired with the Zeiss confocal microscope LSM 510 Meta.
Immunofluorescence analysis.
The cultures were washed once with PBS and fixed with an ice-cold mixture of methanol and acetone (1:1) for 10 min at room temperature. The cells were rehydrated with PBS and incubated for 1 h at 37°C with rabbit anti-DHBV-L-protein serum KpnI diluted 1:800. The cells were then washed three times with PBS and incubated at 37°C with secondary goat anti-rabbit Alexa 488-labeled (green) antibody (diluted 1:800) for another 30 min. Nuclei were counterstained with Hoechst (final concentration, 4 μg/ml). The stained cells were washed with PBS and analyzed and photographed directly in the well. Pictures were taken with the same equipment as described above but with a 10-fold objective and a numerical aperture of 0.30. No L-protein signals were observed with cells incubated with a MGE of 220 for up to 36 h following removal of the inoculum by washing of the cells, indicating that positive staining in our system represents a reliable marker for de novo infection.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
The cells were washed with PBS and directly lysed in 200 μl of 4× Laemmli buffer per well. All samples were boiled for 5 min, and 20 μl of each sample was fractionated by denaturating sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently, proteins were transferred onto nitrocellulose membranes. Following blocking with 3% dried milk diluted in Tris-buffered saline (TBS; 50 mM Tris-HCl-150 mM NaCl), the membranes were incubated for 1 h at room temperature with rabbit anti-DHBV-L-protein serum KpnI (diluted 1:20,000). After several washings with TBS plus 0.1% Tween 20, the membranes were further incubated with horseradish peroxidase-coupled goat anti-rabbit IgG antibodies at a dilution of 1:20,000. Proteins were visualized by enhanced indirect chemiluminescence by using SuperSignal Pico West (Pierce) substrate. Signals were quantified after acquisition with a Fluor-S MultiImager by using Quantity One software.
RESULTS
Attachment and internalization kinetics of DHBV in primary hepatocytes.
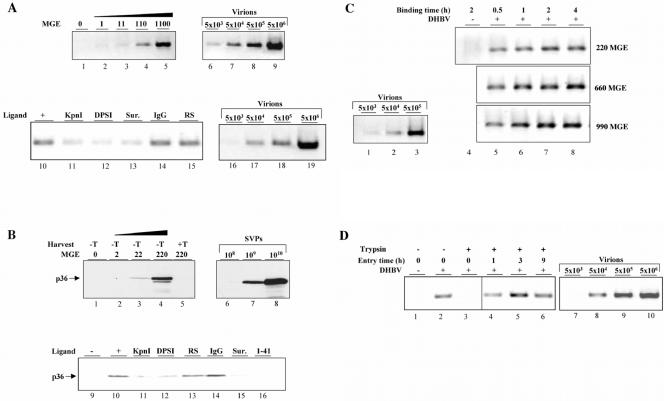
As a first step towards delineation of the nature and number of cellular factors restricting hepadnavirus infection in vitro, we analyzed viral attachment and entry kinetics by using DHBV-permissive PDHs. The cell cultures were exposed for 2 h at 4°C to DHBV at different MGEs ranging from 1 to 1,100. Subsequently, the cells were washed and harvested, and the amount of cell-associated viral DNA was semiquantitatively determined after agarose gel electrophoresis of amplified PCR products. Serial dilutions of a DHBV-viremic serum with known genome equivalents (GE) included in the PCR run revealed an assay sensitivity of about 103 GE and linearity between 103 to 106 GE (Fig. 1A, lanes 6 to 9). According to this assay, the amount of attached virions proportionally increased with the MGE (Fig. 1A, lanes 1 to 5). Based on comparative analysis of the PCR signals obtained from 5 × 105 hepatocytes inoculated with different MGEs together with those of the standard used, the average number of virions attached at 4°C during 2 h was estimated to be about 10 per cell (ranging from 1 to 10 in different experiments). Determination of the amount of viral particles in the inoculum before and after incubation with PDHs revealed very similar levels of L protein and viral DNA, as analyzed by immunoblotting and PCR, respectively (data not shown), indicating that only a minute fraction of the virus input (about 0.05%) bound to cells.
FIG. 1.
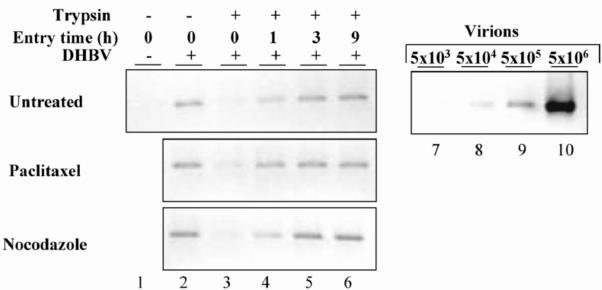
Binding and internalization kinetics of DHBV in PDHs. (A) To analyze binding of virions to hepatocytes, cells were incubated with DHBV at different MGEs for 2 h at 4°C, washed, harvested, and subjected to DHBV DNA-specific PCR. Serial dilutions of viremic serum were included as a standard. To test the effect of independent neutralizing antibodies (KpnI, DPSI, and 1-41) and suramin (Sur.) on virus attachment, inoculum containing 220 MGEs and cells were preincubated with the antiserum and suramin, respectively, for 30 min at 37°C prior to the attachment assay. Native rabbit serum (RS) and rabbit anti-mouse IgG (IgG) served as controls. The cells were preincubated with suramin for 1 h before the attachment assay was performed. (B) The MGE dependency and specificity of SVP binding to hepatocytes was investigated as described above. Serial dilutions of recombinant pre-S protein with a known protein concentration were included as a standard, and 20 molecules thereof were considered equivalent to one viral particle. The L protein in all immunoblots shown was detected with KpnI antiserum. −T and +T, harvest without and with trypsin, respectively. (C) Time course of DHBV binding to PDHs. Cultures were incubated with increasing MGEs for the indicated time points at 4°C. Subsequently, the cells were harvested, and the amount of cell-associated virions was determined by PCR. (D) Time kinetic of DHBV entry into PDHs. DHBV was allowed to attach to hepatocytes for 2 h at 4°C. Thereafter, the cells were immediately harvested by direct lysis after trypsin treatment or washed and shifted to 37°C in new medium to allow virus internalization. At the indicated time points, the cells were harvested by limited protease digestion to remove bound external virus and were analyzed for intracellular viral DNA by PCR. Serial dilutions of DHBV-viremic serum with 1.1 × 1010 GE served in every PCR as a standard.
A peculiarity of all hepadnavirus infections is the huge overproduction of SVPs lacking viral DNA and core protein. To answer the question of whether abundant natural SVPs present in DHBV-viremic serum are as binding-competent as the accompanying virions, we performed analogous experiments as described above and estimated the amount of bound SVPs by semiquantitative immunoblot analysis for L protein. Serial dilutions of a recombinant duck pre-S protein revealed a sensitivity of about 107 SVPs (data not shown) and a dose-dependent increase of between 108 and 1010 SVPs (Fig. 1B, lanes 6 to 8). This analysis indicated that the fraction of SVPs bound to hepatocytes was directly proportional to the amount of DHBV-positive serum applied to the cultures (Fig. 1B, lanes 1 to 4) and paralleled that of DNA-containing particles (Fig. 1A, lanes 1 to 5). Trypsin treatment of the cells prior to lysis virtually removed the signal originating from cell-bound SVPs (Fig. 1B, lane 5), excluding internalization of a significant fraction of viral particles under the conditions used. Based on the quantification of the immunoblot using standard dilutions of recombinant pre-S protein (Fig. 1B, lanes 6 to 8), we estimated the average number of SVPs which bind to hepatocytes during 2 h at 4°C when 5 × 105 cells are incubated with a MGE of 220 to be about 10,000. This calculation is based on the reported educated guess that one viral particle contains about 20 L-protein molecules (9, 24).
The specificity of virion and SVP binding was evaluated by the use of neutralizing antibodies or drugs in attachment interference experiments. Preincubation of the virus inoculum corresponding to a MGE of 220 with three independent L-protein antisera (KpnI, DPSI, and/or 1-41; see Material and Methods) strongly reduced binding of virions (Fig. 1A, lanes 10 to 12) and SVPs (Fig. 1B, lanes 9 to 12 and 16) to hepatocytes, while control rabbit serum or immunopurified mouse anti-rabbit IgG did not show any significant interference (Fig. 1A, lanes 14 and 15, and Fig. 1B, lanes 13 and 14). As the pre-S region of the L protein is known to mediate virus-hepatocyte interaction essential for productive infection (6), this finding implies that most of the virus binding under our experimental conditions is specific and relevant for productive infection. Suramin, a known inhibitor of DHBV infection (17), reduced attachment of both DNA-containing particles and SVPs to an extent similar to that of the neutralizing antibodies (Fig. 1A, lane 13, and Fig. 1B, lane 15), indicating that the well-known antiviral effect of suramin is due mainly to its interference with virus attachment. This finding further corroborates the physiological relevance of virus-binding sites for productive DHBV infection measured in our assay. In addition, the results suggest that under our experimental conditions both SVPs and virions bind to primary hepatocytes, with similar kinetics and efficiency.
We next examined the kinetics of virion binding to cells and, in particular, whether binding is saturable under our experimental conditions. To determine the time dependency of viral attachment to hepatocytes, the cells were incubated with increasing MGEs for different time periods at 4°C. Thereafter, unbound viral particles were washed off, the cells were lysed, and the amount of cell-associated viral DNA was determined by PCR. According to this assay, at 220 MGEs virus attachment was already detectable 30 min after virus inoculation and the signal increased proportionally with incubation time and reached a maximum between 2 and 4 h (Fig. 1C, lanes 4 to 8, MGE 220). These data indicate that at the MGE used, the majority of hepatocellular binding sites were occupied within 2 to 4 h of virus inoculation. However, the time course and extent of virion binding was dependent on the MGE used, as is evident from the further increase of signals when the MGE is tripled to 660. Under these conditions, the signals were already saturated 1 h after inoculation (Fig. 1C, lanes 4 to 8, MGE 660). A further increase in MGE had an effect on neither the time kinetics nor the extent of virus binding (Fig. 1C, lanes 4 to 8, MGE 990), which indicates that saturating conditions had been reached. Taken together, these data suggest that most of our experiments done at a MGE of 220 were performed close to saturating conditions and imply that the number of hepatocellular binding sites for viral particles is limited in the system used. Exposure of PDHs for up to 6 h to 4°C did not result in any detectable morphological alteration of hepatocytes, as determined by light microscopy. Moreover, no difference was observed in the number of trypan blue-stained cells in treated or untreated cultures. The viability of cells tested by vital staining with fluorescein diacetate was also not affected. These results clearly indicate that the cultures tolerate the incubation at 4°C for up to at least 6 h without gross morphological and functional impairment (data not shown).
To determine how fast and how much of the attached virus enters the cells, we performed a protease protection assay. This assay is based on the assumption that, once inside the cell, a virus is protected from degradation by an extracellularly added protease. In our experiments, cells were exposed to DHBV for 2 h at 4°C and nonbound virus was removed by washing. Then the cells were harvested either directly or after 10 min of trypsin treatment. Parallel cultures were shifted to 37°C for 1, 3, and 9 h to allow for internalization, trypsin-treated subsequently to remove cell surface-bound virus, and harvested, and the amount of cell-associated viral DNA was determined by PCR. In these experiments, >90% of attached, but not yet internalized, virions could be removed by trypsin (Fig. 1D, lanes 2 and 3), indicating that at 4°C virtually all virions were cell surface located. Interestingly, after incubation of the cells at 37°C for only 1 h following attachment about 70% of initially attached DHBV became protease resistant (Fig. 1D, lane 4); this fraction increased to 100% after incubation for a further 2 h (Fig. 1D, lane 5). A more-detailed kinetic analysis revealed that DHBV entry began after the shift to 37°C, with a delay of about 30 min (unpublished data). From these experiments, we conclude that almost all virions which attached to the cell surface were internalized and that attachment was fully accomplished in less than 3 h.
In summary, our data demonstrate that only a very small percentage of total virions added to the cell culture medium binds to and enters into hepatocytes within 3 h, but incoming viral rcDNA is repaired in the nucleus and can be detected as cccDNA only after a time period of unprecedented length, 20 h after infection (11, 22).
Disruption of the cytoskeleton increases viral attachment to hepatocytes.
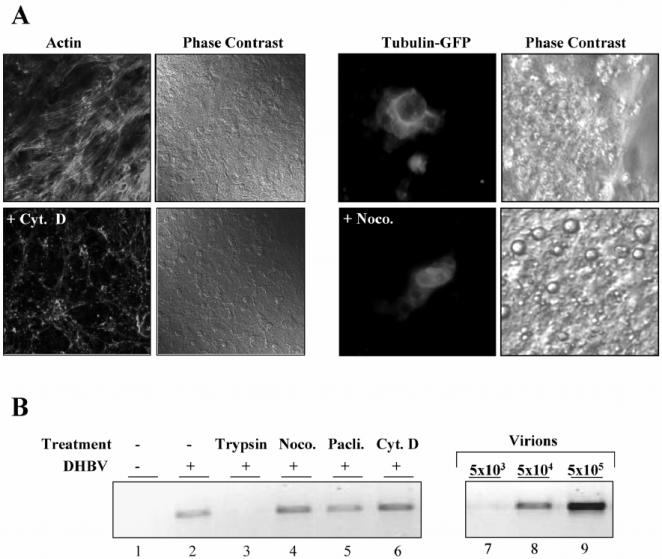
In light of the previous findings, one wonders if DHBV fails to take advantage of energy- and cytoskeleton-dependent pathways for its intracellular transport, unlike what is reported for many other viruses. To examine whether the cytoskeleton is exploited during viral entry into duck hepatocytes, we first determined the effects of three drugs that interfere with cytoskeleton structure and function on DHBV attachment: cytochalasin D and nocodazole, which specifically depolymerize actin and tubulin filaments, respectively, and paclitaxel, which prevents dynamic turnover of MTs by stabilizing them. Treatment of PDHs with cytochalasin D and nocodazole for 1 h completely depolymerized actin and tubulin filaments, respectively, as was evident from drastic changes in the cytoskeleton network visualized by fluorescence microscopy of TRITC-phalloidin-stained actin (Fig. 2A, left panel) and ectopically expressed tubulin-GFP (Fig. 2A, right panel) in PDHs. In marked contrast to untreated cells, which had arrays of actin microfilaments, cytochalasin D-treated cells exhibited a granular pattern of actin, indicating efficient fragmentation of the actin network (Fig. 2A, panels labeled Actin). Tubulin-GFP-transfected PDHs showed strong fluorescence at the nuclear rim, from which fine bundles of MTs were emanating, a pattern diverging for unknown reasons from that seen in hepatoma cell lines. Following treatment with nocodazole, the filamentous MT pattern collapsed and became predominantly diffuse (Fig. 2A, panels labeled Tubulin-GFP). Careful examination by light microscopy revealed no gross morphological changes in drug-treated PDHs (Fig. 2A, panels labeled Phase Contrast). Removal of the drugs resulted in reorganization of the cytoskeleton after about 30 min, indicating that their effects are reversible (unpublished data).
FIG. 2.
(A) Visualization of the organization of actin and the tubulin network and their disruption in PDHs. Cells were treated for 1 h with cytochalasin D (Cyt. D), and subsequently, the actin fibers were stained with TRITC-labeled phalloidin. Primary hepatocytes expressing GFP-conjugated tubulin were treated for 1 h with nocodazole (Noco.). Fluorescence and corresponding phase contrast images of the same field are shown, as well as pictures from untreated control cultures. (B) The effect of cytoskeleton-disrupting drugs on the binding of DHBV to hepatocytes. Cells were pretreated with cytochalasin D, nocodazole, or paclitaxel (Pacli.) for 1 h before DHBV was added to the cells for 2 h at 4°C. Subsequently, the cells were harvested, and bound viral DNA was determined by PCR. In one sample (lane 3), the external virus was removed with trypsin before harvest.
After successful demonstration of drug-induced cytoskeleton inactivation, we determined potential effects of the drugs on DHBV attachment. Cells were pretreated for 1 h with cytochalasin D, nocodazole, or paclitaxel before exposure to DHBV for 2 h at 4°C. The cultures were then washed and harvested by direct lysis, and the amount of bound DHBV was determined by PCR. Disruption of the actin and tubulin network by cytochalasin D or nocodazole prior to adsorption had no negative effect on virus attachment but strongly increased the attachment of virions compared to that for untreated cells (Fig. 2B, lanes 2, 4, and 6). The MT-stabilizing drug paclitaxel had no influence on DHBV binding (Fig. 2B, lanes 2 and 5). Taken together, our data indicate that an intact cytoskeleton is dispensable for attachment of DHBV to hepatocytes and suggest the existence of some cryptic virus binding sites, which become accessible after treatment of cells with cytoskeleton-disrupting, but not MT-stabilizing, drugs.
To exclude the possibility that the effects seen in our experiments result from unspecific cytotoxic side effects of the drugs, we treated persistently infected PDHs with the substances for 24 h and then tested viability with trypan-blue dye exclusion, by FDA staining, and morphologically by light microscopy. None of these assays showed any signs of cytotoxicity which could account for the interference with DHBV infection (data not shown). Moreover, none of the drugs affected ongoing intracellular viral gene expression in chronically infected PDHs, as tested by immunoblotting of cell lysates for the viral and cellular proteins L and actin, respectively (data not shown).
Actin cytoskeleton is dispensable for productive viral infection.
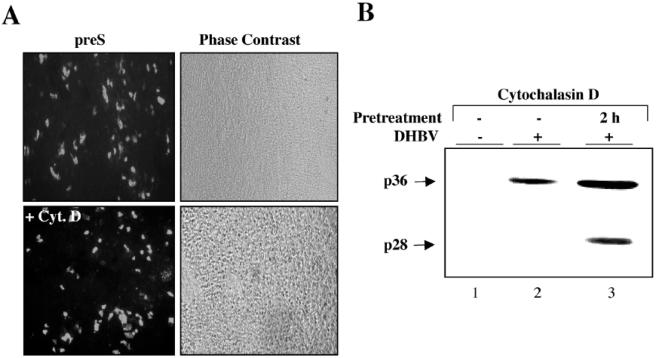
Next, we asked whether virus entry or intracellular trafficking depends on the actin network. To answer this question, PDHs were pretreated with cytochalasin D for 2 h at 37°C and subsequently exposed to DHBV with the drug still present. After 14 h of incubation, the drug and unbound virus were removed by washing the cells with PBS. In addition, surface-attached, but not yet internalized, virus was inactivated by a brief shift to low pH, and then medium without inhibitor was added. Three days after treatment and infection, the cells were harvested, and the efficiency of infection was estimated by analysis of the number of infected cells and the level of intracellular viral protein expression by indirect immunofluorescence staining and immunoblotting for the L-protein, respectively. Cytochalasin D treatment had no negative effect on infection and even slightly increased the number of infected cells compared to untreated cells (Fig. 3A). Immunoblot analysis of cell extracts showed elevated L-protein signals in the treated cells compared to those of the nontreated cells (Fig. 3B, lanes 2 and 3) and thus corroborated this finding. These data are consistent with the increase in virus attachment to cytochalasin-treated cells shown above (Fig. 2B, lanes 2 and 6) and indicate that the actin cortex and filaments are not essential for infection and may even pose a hindrance. Taken together, our data show that strictly actin-dependent processes are not required for viral entry or trafficking to initiate a productive infection in primary hepatocytes.
FIG. 3.
The actin cytoskeleton is dispensable for the infectious entry of DHBV in primary hepatocytes. The actin cytoskeleton was disrupted by cytochalasin D treatment for 2 h before virus inoculation for 16 h with the drug still present. After being washed and receiving pH shock, cells were cultivated in new medium for a further 3 days before harvest. Cultures were analyzed for L-protein expression by immunostaining (A) and -blotting (B). Fluorescence and phase contrast images of the same field of treated and untreated cells are shown. The corresponding immunoblot analysis is shown on the right.
MTs are essential for productive viral infection of hepatocytes.
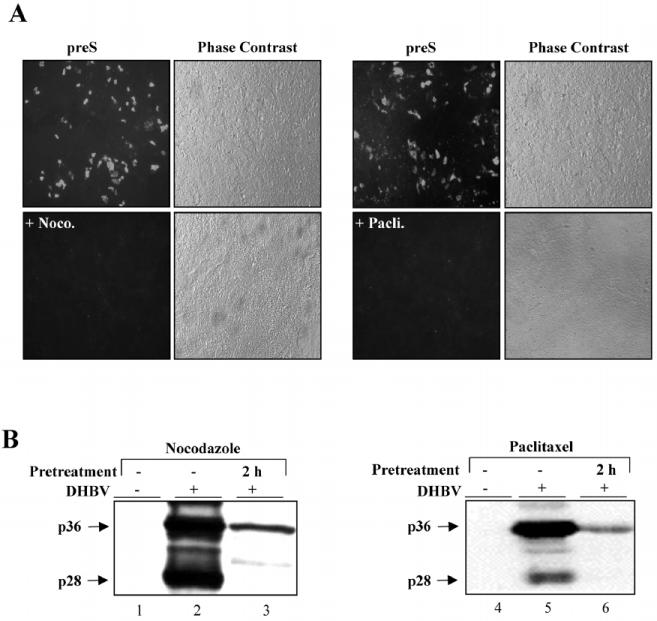
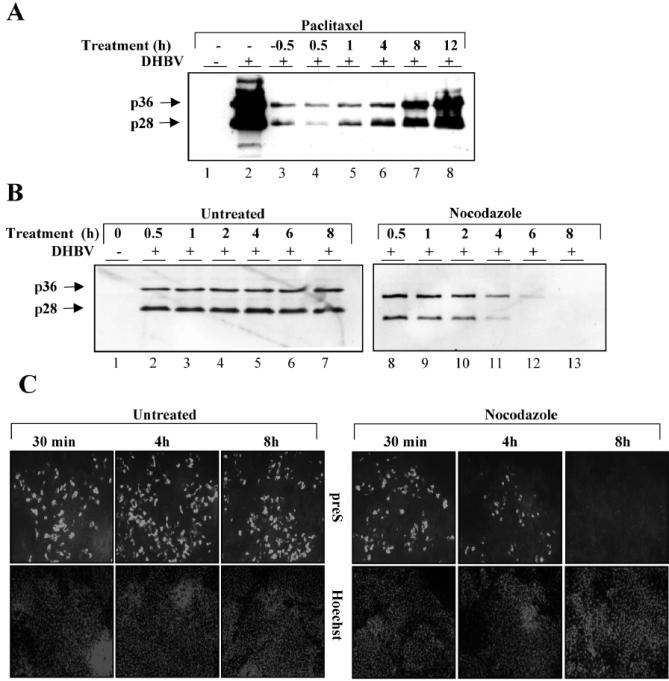
Since actin filaments are dispensable for DHBV infection, we next asked whether the same applies to MTs. For inactivation of MTs, cells were pretreated with nocodazole or paclitaxel for 2 h before virus inoculation and then further incubated in the presence of the drug and the virus for 14 h at 37°C. Nonbound virus and drugs were then washed away and cell-bound, noninternalized virus was inactivated by low-pH treatment for 2 min. The cells were further incubated at 37°C for 3 days, harvested, and tested for indicators of ongoing viral replication by indirect immunofluorescence staining and immunoblotting. Immunostaining for L protein revealed no or very few positive hepatocytes in nocodazole- and paclitaxel-treated cultures compared to about 104 L-protein-positive cells in the nontreated control well (Fig. 4A), indicating that infection was almost completely inhibited by both drugs. Drastically lower levels of intracellular L protein detected by immunoblotting in treated cells (Fig. 4B, lanes 1 to 6) support this conclusion. The antiviral effects were observed with doses as low as 100 nM paclitaxel and 1.65 μM nocodazole, which are nontoxic concentrations for PDHs, as analyzed by various vitality assays mentioned above and in the Materials and Methods section (data not shown). These findings indicate that without functional MTs during the first 14 h of virus entry and trafficking, establishment of a productive infection is extremely inefficient. Furthermore, our data clearly demonstrate that DHBV needs both intact and dynamic MTs. Their inactivation for 16 h early in viral infection interferes irreversibly with the establishment of infection.
FIG. 4.
MTs are essential for the establishment of DHBV infection in primary hepatocytes. MTs were disrupted or stabilized by nocodazole (Noco.) or paclitaxel (Pacli.) treatment, respectively, for 2 hours before exposure to virus for 16 h in the continuous presence of the drugs. After the virus and drugs were removed by washing and pH shock, the cells were further cultivated in new medium for 3 days. Afterward, they were fixed for immunostaining (A) or harvested for detection of L protein by immunoblotting (B).
MTs are not required for internalization of DHBV in PDHs.
Since virus attachment to hepatocytes was not dependent on MTs, we next asked whether inhibition of DHBV infection by nocodazole and paclitaxel was due to failure in virus internalization. If true, no virus inoculum or only a small amount of virus inoculum should be internalized in drug-treated cells compared to that for nontreated cells. This difference should be detectable in the protease protection assay used above (Fig. 1D) and was therefore experimentally tested. After being pretreated and incubated for 2 h with DHBV and drugs at 4°C, the cells were washed and harvested by lysis, and the amount of bound virus was determined by PCR (Fig. 5, lane 2). Trypsin treatment of the cells prior to lysis reduced the amount of cell-bound virus to about 10% compared to that for nontreated cells (Fig. 5, lanes 2 and 3), consistent with results shown above (Fig. 1D). In order to determine how much of the attached virus enters drug-treated and nontreated cells under these conditions, parallel cultures were transferred to 37°C and further incubated for 1, 3, and 9 h in new medium with or without the drugs. Cell surface-bound, but not yet internalized, virus was then removed by trypsin treatment. Determination of the fraction of protease-protected, intracellular viral DNA showed no difference between that for drug-treated and nontreated cells (Fig. 5, lanes 1 to 6). These data indicate that neither disruption of MTs by nocodazole nor prevention of their dynamic turnover by paclitaxel has any detectable influence on the efficiency and kinetics of DHBV internalization. Therefore, the block of DHBV infection by both drugs is presumably due to a postentry event.
FIG. 5.
MTs are required for a postentry step of DHBV other than attachment and internalization. PDHs were treated with nocodazole or paclitaxel for 1 h at 37°C before being inoculated with DHBV at 4°C for 2 h. The cells were harvested immediately or after trypsin digestion or shifted to 37°C to allow internalization of bound viral particles. At the indicated time points, cells were harvested by limited protease digestion and analyzed for intracellular viral DNA by PCR.
MT-dependent virus trafficking is vulnerable only at specific, early entry time points.
The data obtained so far indicate that MTs play a critical role in a postentry step, which raises several interesting questions. For instance, from which time point on after virus uptake and for how long thereafter are MTs required for the establishment of infection? Moreover, is there a critical time period when MT inactivation leads to an irreversible block of virus infection, and if so, what are the underlying mechanisms? To answer these emerging questions, we first determined at which time point upon viral entry MTs become dispensable for productive infection by employing an add-in experiment. Cells were treated either 0.5 h prior to or at different time points (0.5 to 12 h) after exposure of cultures to virus with paclitaxel or nocodazole. Non-cell-associated and noninternalized viral particles were washed off after 16 h of further incubation and inactivated by low-H treatment. The cells were thereafter maintained for a further 3 days in medium devoid of any drug or virus. As an indirect measurement of the level of productive infection that had occurred, steady-state levels of intracellular L protein were determined. This add-in assay revealed very low infection efficiency when cells were treated prior to infection and up to 4 h postinfection compared to that for nontreated cells (Fig. 6A, lanes 2 to 6). Paralyzing MTs 8 to 12 h after virus inoculation led to increasingly higher levels of L-protein expression, indicative of a parallel increase in productive DHBV infection compared to that for cells treated with drugs at earlier time points (Fig. 6A, lanes 2 to 8). Similar results were obtained with nocodazole (unpublished data). These findings demonstrate that successful intracellular trafficking of DHBV depends on a MT-sensitive step very early in infection (0 to about 4 h after viral entry), while MTs become increasingly dispensable thereafter. Thus, disturbance of viral trafficking at very early stages led to almost complete elimination of the infection. Unexpectedly, this was irreversible because infection did not occur despite the removal of MT-inactivating drugs 22 h after infection, which predictably led to fast reformation of MTs for about 3 days before the cells were harvested. A possible explanation for this irreversible infection block is the assumption that internalized, but arrested, virus particles are unstable and are rendered noninfectious, which implies that cellular enzymes cannot degrade viral or subviral particles efficiently after entry as long as they are residing in certain cellular compartments for only limited time periods. This seems most likely to be the case during the first 3 to 4 h after infection and, if correct, predicts that destruction and reformation of MTs during this period should not pose any risk for the infectivity of intracellular viral particles. Therefore, reconstitution of the MT network up to about 4 h after virus uptake should lead to productive infection, whereas reformation at later time points should not lead to productively infected cells. We have tested this hypothesis experimentally in a wash-out assay by allowing DHBV to bind to cells for 2 h at 4°C. The nonbound virus was subsequently removed, and new medium containing nocodazole or no drug was added to the cells before shifting them to 37°C to initiate a nearly synchronous internalization. Nocodazole was removed at different time points (0.5 to 8 h) after shifting the cultures to 37°C to allow reformation of MTs. Thereafter, the cells were further incubated for 3 days, harvested, and analyzed for signs of productive infection. Detection of L protein in cell lysates by immunoblotting revealed no or little negative effect on PDH infection after treatment with nocodazole for up to 2 h (Fig. 6B, lanes 8 to 10). Increasing inhibition of infection was observed in this assay when the drug was washed out after 4 to 8 h (Fig. 6B, lanes 11 to 13). Immunostaining of cells for L-protein expression corroborated these results and, moreover, demonstrated that inhibition of infection is predominantly due to reduced numbers of infected cells rather than to lower L-protein expression levels in infected individual cells (Fig. 6C). Taken together, these data are in perfect agreement with our prediction that destruction of MTs within the first 4 h after virus uptake does not interfere with infection when MTs are allowed to reform thereafter. However, destruction of MTs for longer time periods leads to an irreversible abortion of viral infection.
FIG. 6.
Disruption of microtubules induces an irreversible postentry block leading to abortion of DHBV infection. (A) Primary hepatocytes were treated with paclitaxel before or after exposure to DHBV at the indicated time points. After a further 16 h of incubation, the cells were washed, and external virus was inactivated by a low-pH wash. Three days following inoculation, the cells were harvested and subjected to immunoblot analysis for L protein. (B and C) The inverse wash-out experiment. Cells were exposed to the virus for 2 h at 4°C. The cultures were then washed with PBS, and new medium containing nocodazole or no drug was added. After the cultures were shifted to 37°C, the drug was removed at the indicated time points by washing and the addition of new medium. Three days later, the cells were harvested and immunoblotted (B) or -stained (C) for L protein. Nuclei were counterstained with Hoechst.
Lack of cccDNA formation without intact MTs.
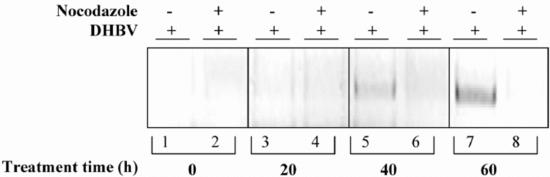
The data outlined above indicate that MTs are indispensable for an early step subsequent to virus internalization. In order to elucidate whether this step occurs before or after nuclear transport of the viral genome, we determined the amount of intranuclear viral cccDNA produced from incoming partially double-stranded genomes by repair in MT-disrupted cells. This repair reaction, converting incoming rcDNA into cccDNA, is completed after about 20 h following viral entry, after which a cccDNA pool that indicates successful viral entry is established (11, 22). To test the effect of nocodazole on nuclear viral genome transport, PDHs were pretreated with nocodazole for 1 h. Afterwards, virus inoculum was added, and the cells were incubated for 20 h at 37°C with the drug still present. Virus attached to cells was inactivated by treatment with low pH. New medium containing nocodazole was added, and 20, 40, and 60 h later, cells were harvested for PCR analysis that preferentially detects cccDNA. In this assay, there was no cccDNA detectable in the absence of functional MTs (Fig. 7, lanes 2, 4, 6, and 8). Since nocodazole does not affect virus binding to, and internalization into, hepatocytes, we conclude from this experiment that MTs are essential for a postentry step prior to viral genome delivery into the nucleus. Our data indicate that MTs play a critical role in a transport step occurring after virus attachment and internalization and prior to arrival of incoming viral genomes in the nucleus.
FIG. 7.
Lack of cccDNA in MT-disrupted cells. PDHs were pretreated with nocodazole for 1 h prior to virus exposure and further incubated for 20 h in the presence of the drug. Afterward, bound but not internalized virus was inactivated by a low-pH wash. New medium containing no drug or nocodazole was added, and cells were harvested 20, 40, and 60 h later, after trypsin treatment. The cccDNA was detected by PCR by using gap region-specific primers.
DISCUSSION
The molecular characterization of viral and cellular components acting at early stages of host-virus interaction is of key importance, since these factors mainly determine the host range, tissue tropism, and pathogenicity of viruses. For the hepatitis B virus, one of the major human viral pathogens, it has been difficult to study authentic interaction of the virus with hepatocytes.
To address this issue, we established a sensitive assay, allowing for the first time the quantification of the time dependence of both virion and subviral particle binding to primary liver cell cultures. This binding assay revealed a specific and dose-dependent increase of virion and SVP binding to hepatocytes. The results are strongly supported by the elegant experiments of Pugh et al., showing that the cellular binding of surface antigen-containing DHBV particles is cell type and species specific (22). Our attachment experiments with a viremic serum containing both virions and natural SVPs demonstrate that the number of binding sites on hepatocytes is limited and relatively low, with an estimated number of about 104 per cell. This number was calculated from the amount of L-protein molecules bound in the form of viral particles to hepatocytes, as measured by semiquantitative immunoblotting standardized with known amounts of recombinant pre-S protein and by taking into account the reported educated guess of 20 L-protein molecules per viral particle (9, 24). The kinetics and efficiency of SVP binding to cells were comparable to and paralleled that of accompanying virions, suggesting that they are similar in their attachment capability and probably compete for the same cellular attachment sites as previously suggested (9). The latter conclusion was further substantiated by our data showing that neutralizing antibodies and suramin interfere with attachment of both virions and SVPs. However, our data do not exclude possible differences in binding kinetics and efficacy between virions and SVPs when tested separately, because putative cooperative effects would not be visible. This speculation is compatible with the enhancement of infection by SVPs described previously (3). Determination of the time course of virion binding at different MGEs revealed that virus attachment is time dependent and can be saturated. Together with the fact that the saturation can be achieved earlier with higher MGEs indicates that the number of hepatocellular binding sites is indeed limited.
Using a protease protection assay to specifically follow the timing of DHBV entry, we found that bound viral particles are taken up with rather slow kinetics compared to those for Semliki Forest virus and adenovirus (20, 25), which both enter host cells by clathrin-coated, pit-mediated endocytosis within about 10 min instead of the more than 1 h needed by DHBV. In this respect, DHBV is similar to simian virus 40 (SV40), which is internalized within 2 h (1). The relatively slow entry kinetics of DHBV might be a consequence of slow two-step kinetics of receptor binding and/or subsequent coreceptor recruitment, probably similar to that described for SV40 (1).
The time period required until the first copy of hepadnavirus cccDNA is formed after infection was reported to be about 20 h (11, 22). Taking this and our evidence for a maximal time requirement of 3 h for virion entry into consideration, there are at least 14 h left for cytoplasmic trafficking of viral particles, nuclear delivery, and conversion of the viral genome from rcDNA into cccDNA. This long period may be due to an unusual restrictive process during cytoplasmic and nuclear trafficking or conversion from rcDNA to cccDNA that is unique to hepadnaviruses. In any case, our results suggest a rate-limiting postentry step for hepadnaviruses so far not reported for any other virus, and a detailed understanding of this step may reveal strategies also exploited by other viruses for which intracellular trafficking has so far not been analyzed.
The cytoskeleton is likely to be involved in virus attachment and may critically contribute to this step by, for example, maintenance of plasma membrane structures. However, our data indicate that both intact actin filaments and microtubules are dispensable for adsorption of DHBV to hepatocytes and suggest the existence of some cryptic virus binding sites which become accessible after treatment of cells with cytoskeleton-disrupting, but not -stabilizing, drugs. Cell-cell junctions, microvilli, and invaginations may harbor such cryptic sites (14). Since formation of these structures depends at least in part on the cytoskeleton, the diffusion-controlled access of virus to such binding sites may be facilitated by drug-induced destruction of the cytoskeleton. According to our data, disruption of the submembranous, dense actin cortex has no negative impact on the efficiency of DHBV entry, cytoplasmic trafficking, and nuclear genome transfer. This finding is remarkable, given the fact that human immunodeficiency virus, adenovirus, SV40 virus, and vaccinia virus all require actin for uptake and establishment of infection (for reviews, see references 20 and 25 and references therein) and thus points to another distinct feature in the early stage of the hepadnavirus life cycle.
Once inside the hepatocyte, incoming DHBV virions have to deliver their cargo, the viral genome, to the nucleus to launch viral replication. Directional transport of incoming viral particles, free or membrane bound, most likely involves the host cytoskeleton. The data outlined in this study suggest that in contrast to actin filaments, MTs play an essential role early in DHBV infection. Furthermore, successful trafficking of incoming viral particles requires both intact and dynamic MTs. DHBV diverges in this requirement from all other viruses, according to our knowledge (7). For instance, the intracellular transport of papovaviruses depends on the intactness (which can be disrupted by nocodazole) but not on the dynamic turnover (which can be prevented by paclitaxel) of MTs (15). Our data are consistent with the notion that viruses depend on cytoskeletal elements for their intracellular motility and provide a novel example of the variation in strategies employed by different viruses.
The inhibition of DHBV infection in cells without functional MTs was strong but not complete. The residual low level of infection observed in treated cells may be due to incomplete drug-mediated MT inactivation or the existence of a minor, MT-independent infection pathway which may include cytoplasmic trafficking by diffusion. Alternatively, after reformation of MTs, a few intracellular viruses may be able to reach the nucleus and manage to establish a low level of infection. In addition, Glu-tubulin, a nocodazole-resistant minor variant of alpha-tubulin (29, 8), might be able to rescue the transport of DHBV to a small extent after nocodazole treatment.
Our data show that MTs are essential for a postentry step in the viral life cycle, since there was no negative interference with the kinetics or extent of viral uptake in hepatocytes with nonfunctional microtubules. This notion together with the absence of any cccDNA formation in cells with disturbed MTs strongly suggests that MTs play a pivotal role in an entry step occurring after virus binding and uptake and prior to the nuclear delivery of incoming viral genomes. However, two questions remain: which of the several distinct early steps is dependent on microtubules, and which mechanisms are involved? The correlative kinetic analysis of MT disturbance and DHBV infectivity revealed that the entry-limiting microtubule-sensitive step occurs between 4 and 8 h postinfection. Depolymerization and reformation of the MTs at 4 h after viral entry or earlier did not interfere significantly with productive infection. Disruption followed by reformation of MTs after this time period led to almost complete elimination of infection. These findings indicate that the infectivity loss of intracellular viral particles by arrest of MT-mediated motility is irreversible during a certain early stage in the viral life cycle. The basis for this unusual finding is unclear at present, and we know of no precedent in the animal viruses to explain this peculiar irreversible abortion of viral entry (16, 18, 26). Intracellular viral particles, free or in vesicles, seem to maintain their infectivity as long as they are arrested for only limited time periods and reside in certain cellular compartments independent of their connection to MTs. Extension of this transport block results in rapid loss of viral infectivity pointing to a MT-dependent and strictly regulated key event in the viral life cycle. In the case of SV40, it has been reported that the MT-dependent step occurs between 3 and 6 h after infection and is required for transport of SV40 between the caveosome and smooth endoplasmic reticulum-derived perinuclear tubules (19). Although endocytosis has been suggested as an entry route for DHBV (10), its mode and the subsequent uncoating steps are unknown. Therefore, it is currently impossible to link our data to certain stages of hepadnavirus entry. However, the MT-sensitive step in the infectious entry pathway may reflect an analogous transport step and likely involves MT-mediated core and genome transport. This speculative assumption is compatible with our finding that, in hepatocytes lacking functional MTs, the viral genome does not arrive in the nucleus and is not converted to cccDNA and amplified. Not mutually exclusive to that finding, hepadnavirus genome transport may depend on an uncoating and core maturation process facilitated by association of the viral particles with MTs. Indeed, MTs serve as a favored site for maturation of viral particles upon entry, as previously reported for human immunodeficiency virus (4).
Continuous block of the timely coordinated, intracellular trafficking of viral particles may finally increase their susceptibility toward proteolytic processing and destruction. Conformational changes in structural proteins, particularly the envelope proteins, which are thought to occur upon attachment, entry, and uncoating of DHBV, might explain their enhanced susceptibility for degradation. Incoming viral particles and proteins may already be sensed and attacked in the cytoplasm by the proteolytic machinery of the host cell. In line with this notion, we found that one of the most critical components of the viral envelope, the L protein, is very unstable and rapidly cleared by cellular proteases, predominantly but not exclusively involving the proteasome (unpublished data).
The infectious entry pathway of hepadnaviruses clearly involves a series of highly coordinated and vulnerable steps prior to arrival of the viral genome in the nucleus, initiating productive infection. The persistent failure to propagate HBVs in several replication-competent hepatoma cells is mainly due to disturbance in one or more of the early steps during viral entry. The data and methods presented here may help to analyze and overcome refractory cell lines by reconstitution of their respective defective entry steps and further pave the way toward the establishment of permissive cell lines. Such convenient cell lines or animal models are prerequisites for the development of novel antiviral strategies that are apparently needed. It is also obvious that drugs affecting viral binding, intracellular trafficking, and nuclear import would be potent inhibitors of viral infection. However, a more-complete understanding of how hepadnaviruses exploit host factors for their entry in hepatocytes is required to accomplish this aim.
Acknowledgments
We thank Stephan Urban and Nicole Schmut, Heidelberg, Germany, for recombinant pre-S protein and rabbit anti-pre-S-protein (1-41) serum. We also thank Cova Lucyna, Lyon, France, for the DPSI antibody.
This work was supported by grants from the DFG, the BMBF, the NGFN, and Hepnet. The Heinrich-Pette-Institute is supported by the Freie und Hansestadt Hamburg and the Bundesministerium für Gesundheit und Soziale Sicherheit. Li Lin is a recipient of a DAAD scholarship.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breiner, K. M., S. Urban, and H. Schaller. 1998. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J. Virol. 72:8098-8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruns, M., S. Miska, S. Chassot, and H. Will. 1998. Enhancement of hepatitis B virus infection by noninfectious subviral particles. J. Virol. 72:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinskaya, A., B. Brichacek, A. Mann, and M. Stevenson. 1998. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 188:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernholz, D., G. Wildner, and H. Will. 1993. Minor envelope proteins of duck hepatitis B virus are initiated at internal pre-S AUG codons but are not essential for infectivity. Virology 197:64-73. [DOI] [PubMed] [Google Scholar]
- 6.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus. (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 7.Gilbert, J. M., I. G. Goldberg, and T. L. Benjamin. 2003. Cell penetration and trafficking of polyomavirus. J. Virol. 77:2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khawaja, S., G. G. Gundersen, and J. C. Bulinski. 1988. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J. Cell Biol. 106:141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klingmuller, U., and H. Schaller. 1993. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J. Virol. 67:7414-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kock, J., E. M. Borst, and H. J. Schlicht. 1996. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J. Virol. 70:5827-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kock, J., and H. J. Schlicht. 1993. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J. Virol. 67:4867-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroki, K., R. Cheung, P. L. Marion, and D. Ganem. 1994. A cell surface protein that binds avian hepatitis B virus particles. J. Virol. 68:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroki, K., F. Eng, T. Ishikawa, C. Turck, F. Harada, and D. Ganem. 1995. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J. Biol. Chem. 270:15022-15028. [DOI] [PubMed] [Google Scholar]
- 14.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, W. J., Y. M. Qi, K. N. Zhao, Y. H. Liu, X. S. Liu, and I. H. Frazer. 2001. Association of bovine papillomavirus type 1 with microtubules. Virology 282:237-244. [DOI] [PubMed] [Google Scholar]
- 16.Mabit, H., M. Y. Nakano, U. Prank, B. Saam, K. Dohner, B. Sodeik, and U. F. Greber. 2002. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 76:9962-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offensperger, W. B., S. Offensperger, E. Walter, H. E. Blum, and W. Gerok. 1993. Suramin prevents duck hepatitis B virus infection in vivo. Antimicrob. Agents Chemother. 37:1539-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa-Goto, K., K. Tanaka, W. Gibson, E. Moriishi, Y. Miura, T. Kurata, S. Irie, and T. Sata. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 77:8541-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 20.Ploubidou, A., and M. Way. 2001. Viral transport and the cytoskeleton. Curr. Opin. Cell Biol. 13:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prassolov, A., H. Hohenberg, T. Kalinina, C. Schneider, L. Cova, O. Krone, K. Frolich, H. Will, and H. Sirma. 2003. New hepatitis B virus of cranes that has an unexpected broad host range. J. Virol. 77:1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh, J. C., Q. Di, W. S. Mason, and H. Simmons. 1995. Susceptibility to duck hepatitis B virus infection is associated with the presence of cell surface receptor sites that efficiently bind viral particles. J. Virol. 69:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigg, R. J., and H. Schaller. 1992. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J. Virol. 66:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlicht, H. J., C. Kuhn, B. Guhr, R. J. Mattaliano, and H. Schaller. 1987. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J. Virol. 61:2280-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 26.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summers, J., P. M. Smith, and A. L. Horwich. 1990. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J. Virol. 64:2819-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunyach, C., C. Rollier, M. Robaczewska, C. Borel, L. Barraud, A. Kay, C. Trepo, H. Will, and L. Cova. 1999. Residues critical for duck hepatitis B virus neutralization are involved in host cell interaction. J. Virol. 73:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thyberg, J., and S. Moskalewski. 1989. Subpopulations of microtubules with differential sensitivity to nocodazole: role in the structural organization of the Golgi complex and the lysosomal system. J. Submicrosc. Cytol. Pathol. 21:259-274. [PubMed] [Google Scholar]
- 30.Tong, S., J. Li, and J. R. Wands. 1999. Carboxypeptidase D is an avian hepatitis B virus receptor. J. Virol. 73:8696-8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong, S., J. Li, and J. R. Wands. 1995. Interaction between duck hepatitis B virus and a 170-kilodalton cellular protein is mediated through a neutralizing epitope of the pre-S region and occurs during viral infection. J. Virol. 69:7106-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban, S., K. M. Breiner, F. Fehler, U. Klingmuller, and H. Schaller. 1998. Avian hepatitis B virus infection is initiated by the interaction of a distinct pre-S subdomain with the cellular receptor gp180. J. Virol. 72:8089-8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urban, S., C. Schwarz, U. C. Marx, H. Zentgraf, H. Schaller, and G. Multhaup. 2000. Receptor recognition by a hepatitis B virus reveals a novel mode of high affinity virus-receptor interaction. EMBO J. 19:1217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiser, B., D. Ganem, C. Seeger, and H. E. Varmus. 1983. Closed circular viral DNA and asymmetrical heterogeneous forms in livers from animals infected with ground squirrel hepatitis virus. J. Virol. 48:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]