Abstract
Obstructive sleep apnea (OSA) is an underdiagnosed condition characterized by recurrent episodes of obstruction of the upper airway leading to sleep fragmentation and intermittent hypoxia during sleep. Obesity predisposes to OSA, and the prevalence of OSA is increasing worldwide because of the ongoing epidemic of obesity. Recent evidence has shown that surrogate markers of cardiovascular risk, including sympathetic activation, systemic inflammation, and endothelial dysfunction, are significantly increased in obese patients with OSA versus those without OSA, suggesting that OSA is not simply an epiphenomenon of obesity. Moreover, findings from animal models and patients with OSA show that intermittent hypoxia exacerbates the metabolic dysfunction of obesity, augmenting insulin resistance and nonalcoholic fatty liver disease. In patients with the metabolic syndrome, the prevalence of moderate to severe OSA is very high (∼60%). In this population, OSA is independently associated with increased glucose and triglyceride levels as well as markers of inflammation, arterial stiffness, and atherosclerosis. A recent randomized, controlled, crossover study showed that effective treatment of OSA with continuous positive airway pressure for 3 months significantly reduced several components of the metabolic syndrome, including blood pressure, triglyceride levels, and visceral fat. Finally, several cohort studies have consistently shown that OSA is associated with increased cardiovascular mortality, independent of obesity. Taken together, these results support the concept that OSA exacerbates the cardiometabolic risk attributed to obesity and the metabolic syndrome. Recognition and treatment of OSA may decrease the cardiovascular risk in obese patients.
Keywords: cardiovascular risk, metabolic syndrome, obesity, sleep apnea
Obstructive sleep apnea (OSA) is a clinical condition characterized by recurrent episodes of complete obstruction (apnea) or partial obstruction (hypopnea) of the upper airway, leading to increased negative intrathoracic pressure, sleep fragmentation, and intermittent hypoxia during sleep (1). Research on the current prevalence of OSA indicated that one-third of sleep studies showed some degree of OSA (apnea-hypopnea index ≥5 events per hour of sleep) (2). Among adults 30 to 70 years of age, approximately 13% of men and 6% of women have moderate to severe forms of OSA (apnea-hypopnea index ≥15 events per hour of sleep) (2). Comparing data from 2 decades ago (3), the current estimates represent an increase of millions of additional afflicted persons and are mainly explained by the ongoing obesity epidemic. Overall, it is estimated that 50% to 60% of people who are obese and patients with the metabolic syndrome have OSA (4,5). The prevalence of OSA is even higher in obese patients with diabetes mellitus (6) and morbid obesity (7). Despite such an overwhelming association, OSA remains unrecognized in the vast majority of these patients with high pre-test probability of OSA (8).
Why is OSA so common in patients with obesity and the metabolic syndrome? Several anatomic and functional factors predispose to OSA. Patients with OSA are able to compensate for the upper airway narrowing by increasing the activity of upper airway muscles that maintain airway patency during wakefulness (9). In contrast, this protective effect is lost during sleep, when relaxation of the muscles occurs and obstruction of the upper airways prevails. Obesity is a major risk factor for OSA because it promotes enlargement of soft tissue structures within and surrounding the airway, thereby contributing significantly to pharyngeal airway narrowing (10,11). An excess of fat deposition has also been observed under the mandible and in the tongue, soft palate, and uvula (9,12). Beyond the direct effects on the upper airway, obesity also contributes indirectly to upper airway narrowing during sleep. Lung volumes are markedly reduced by a combination of increased abdominal fat mass and recumbent posture. Reduction of lung volume may decrease longitudinal tracheal traction forces and pharyngeal wall tension, which predisposes to narrowing of the airway (Fig. 1) (13). In addition, obesity-related leptin resistance may impair the neuroanatomic interactions necessary for stable breathing, thereby contributing to the genesis of OSA (14).
Figure 1. Obesity Predisposes to Obstructive Sleep Apnea.
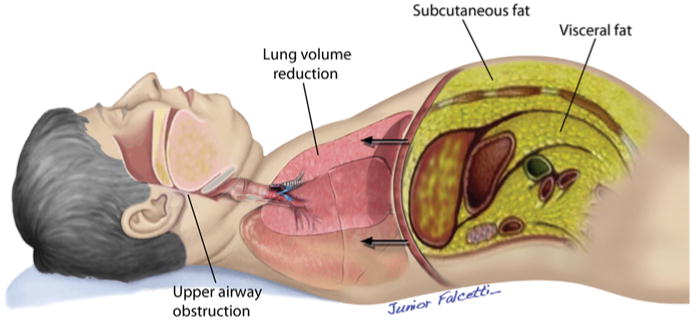
Anatomic factors that predispose obese patients to obstructive sleep apnea.
The concept that OSA may be part of the metabolic syndrome is not new. In 1998, Wilcox et al. proposed the name “syndrome Z” for the combination of the metabolic syndrome (syndrome X) and OSA to reflect the close association of components of the metabolic syndrome with OSA (15). Since then, a major challenge to the field has been to understand whether OSA is a mere epiphenomenon or an additional burden that exacerbates the cardiometabolic risk of obesity and the metabolic syndrome. In the present review, we discuss recent evidence supporting the independent role of OSA in these scenarios. Because of space limitations, the cardiometabolic risk of obesity and the metabolic syndrome per se are not discussed in this review.
Can OSA Have Independent Effects on the Cardiometabolic Risk Attributed to Obesity and the Metabolic Syndrome?
Clinical and epidemiological studies have reported an independent association between OSA and cardiovascular events, suggesting that OSA may lead to cardiometabolic dysregulation (16–18). Studies in mice showed that a short-term regimen of intermittent hypoxia (a hallmark of OSA) caused large-amplitude oxygen swings in the liver, mirroring swings of SpO2, whereas fluctuations of oxygen levels were attenuated in muscle and nearly abolished in adipose tissue in both lean and obese mice (19). The data in the animal model suggest that OSA exacerbates hypoxia at the tissue level (19). The amplification of hypoxia in the adipose tissue may represent an important mechanism of cardiometabolic dysfunction in OSA because it is a major trigger of lipolysis, chronic inflammation, macrophage infiltration, reduction of adiponectin level, elevation of leptin level, adipocyte death, endoplasmic reticulum stress, and mitochondrial dysfunction (20). Despite clinical and experimental evidence, the vast majority of clinical studies that evaluated the impact of obesity and the metabolic syndrome on cardiovascular burden failed to consider OSA a potential confounding factor. Reflecting this gap, the American Heart Association Scientific Statement on metabolic syndrome referred to OSA in the section of “other fields of medicine,” with the same attention given to cholesterol gallstones and lipodystrophies (21). This may reflect a low awareness of OSA in the cardiovascular field associated with the perception that OSA is merely an epiphenomenon of obesity. For didactic purposes, we present the evidence linking OSA to cardiometabolic dysregulation in separate sections.
Metabolic dysregulation
An independent association between OSA, insulin resistance, and type 2 diabetes has been consistently demonstrated by a number of cross-sectional studies, observational studies, and large population-based studies (22–25). Moreover, the development of insulin resistance and pancreatic beta cell dysfunction has been linked to chronic intermittent hypoxemia observed in OSA (26). Even mild recurrent oxyhemoglobin desaturations (>2%) were independently associated with metabolic dysfunction (27). The causal role of intermittent hypoxia in insulin resistance has been further proven in a mouse model of OSA. Lean C57BL/6J mice developed insulin resistance acutely after several hours of hypoxic exposure (28). Chronic intermittent hypoxia exacerbated fasting hyperglycemia, glucose intolerance, and insulin resistance in both mice with diet-induced obesity and mice with genetic obesity (29,30). Thus, animal data suggest that intermittent hypoxia of OSA and obesity may interact to cause metabolic dysfunction. The role of intermittent hypoxia has also been shown in humans. Healthy volunteers were exposed to 6 h of intermittent hypoxia and developed insulin resistance and impaired beta cell function (31). Regarding lipid metabolism, consistent evidence from animal models of OSA has shown that chronic intermittent hypoxia induces fasting dyslipidemia in both lean and obese mice due to activation of the transcription factor sterol regulatory element-binding protein 1 and an important downstream enzyme of triglyceride and phospholipid biosynthesis, stearoyl-CoA desaturase-1 (32–34). Moreover, chronic intermittent hypoxia also impaired clearance of triglyceride-rich lipoproteins, inactivating adipose lipoprotein lipase (35,36). In humans, a randomized study showed that treatment of OSA with continuous positive airway pressure (CPAP) improves postprandial triglyceride and total cholesterol levels (37). Despite this evidence, there are no consistent data suggesting that OSA is a major risk factor for dyslipidemia (38).
In the metabolic syndrome scenario, occult OSA is common and independently associated with increased serum levels of triglycerides and glucose as well as with several metabolic and inflammatory parameters not included in the criteria for metabolic syndrome (cholesterol/high-density lipoprotein cholesterol ratio, uric acid, and C-reactive protein) (4).
Regarding the pathogenic adipose tissue endocrine responses, inappropriate secretion of several adipokines that have anti-inflammatory, antiatherogenic, and insulin-sensitizing properties have been described in OSA (39) but obesity per se is a potential confounding factor (40). Therefore, the relative role of OSA in adipokines remains to be established.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of progressive stages of liver disease ranging from simple steatosis to nonalcoholic steatohepatitis manifested by hepatic inflammation, necrosis, and fibrosis. NAFLD is strongly linked to obesity and independently associated with incident and prevalent cardiovascular disease (41).
Despite the well-known heterogeneous disease spectrum of NAFLD, the potential triggers for the progression of NAFLD are not fully understood. Consistent evidence from both humans and mice suggests that OSA is a potential candidate for exacerbation of NAFLD in obesity (42). Polotsky et al. (43) examined whether OSA and nocturnal hypoxemia predict the severity of steatohepatitis in severely obese individuals presenting for bariatric surgery. Taking advantage of the availability of liver biopsy specimens, the investigators found that patients with moderate to severe OSA and severe hypoxemia exhibited more severe hepatic lobular inflammation than patients with mild OSA (Fig. 2). Severe nocturnal hypoxemia was associated with ballooning of hepatocytes and pericellular fibrosis in the liver. Similar findings were described in patients with OSA by another group of investigators (44). Animal studies confirmed the role of chronic intermittent hypoxia in the progression of fatty liver in obesity (29).
Figure 2. Nonalcoholic Fatty Liver Disease in Severely Obese Individuals According to the Presence or Absence of OSA.
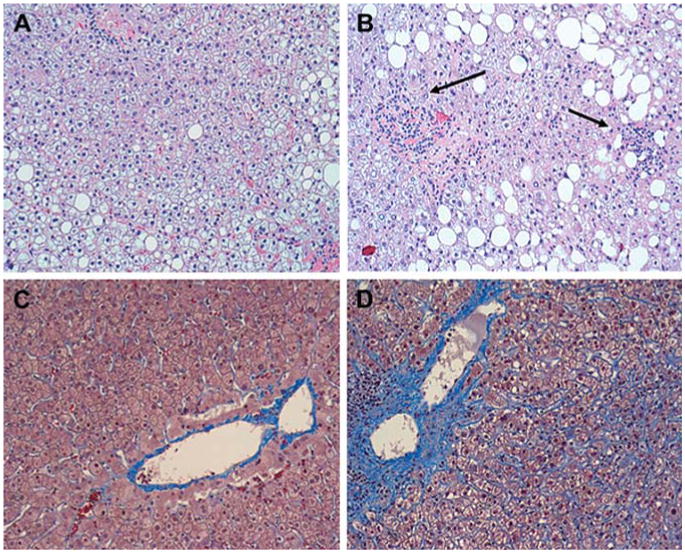
(A) Representative image of the liver without inflammation in a subject without OSA. Macrovesicular hepatic steatosis is evident, but inflammation is absent (hematoxylin-eosin; original magnification ×100). (B) Representative image of the liver in a subject with OSA and severe nocturnal oxyhemoglobin desaturation. Macrovesicular hepatic steatosis is evident, and lobular inflammation is present (arrows) (hematoxylin-eosin; original magnification ×100). (C) Representative image of liver without pericellular fibrosis in an individual without OSA (Masson trichrome; original magnification ×100). (D) Representative image of liver in a subject with OSA and severe nocturnal oxyhemoglobin desaturation. Prominent pericellular perisinusoidal fibrosis is present. Collagen depositions are stained blue and have a chicken-wire appearance (Masson trichrome; original magnification ×100). Reprinted with permission from Polotsky et al. (43). Abbreviation as in Figure 1.
Sympathetic activity
Previous evidence showed that variations in sympathetic tone in obese patients are largely explained by their OSA status. In 1998, Narkiewicz et al. (45) reported provocative data on muscle sympathetic nerve activity in healthy normal-weight subjects and healthy sedentary obese subjects. Polysomnography revealed that 1 of 25 normal-weight subjects and 9 of 30 obese subjects had occult OSA. Interestingly, muscle sympathetic nerve activity was similar in normal-weight subjects and obese subjects without OSA. Muscle sympathetic nerve activity in obese subjects with OSA was significantly higher than that in obese subjects without OSA. The investigators concluded that obesity alone, in the absence of OSA, is not accompanied by increased sympathetic activity. The major limitation of this pivotal study was the small sample size and the lack of a control lean group. Seven years later, Grassi et al. evaluated muscle sympathetic nerve activity in 86 middle-aged normotensive subjects classified according to body mass index (BMI) and apnea/hypopnea index as lean and obese subjects with and without OSA (46). Subjects with and without OSA were matched for age, sex, and blood pressure values within the lean and obese groups. Compared with the non-OSA lean group, there were similar increases in muscle sympathetic nerve activity in the OSA lean and the non-OSA obese groups, whereas a further increase was detected in obese patients with OSA (Fig. 3). These results suggested that sympathetic activation in obesity occurs independently of OSA but OSA has an additive sympathostimulating effect (46).
Figure 3. Sympathetic Activity in Lean and Obese Subjects According to the Presence or Absence of OSA.
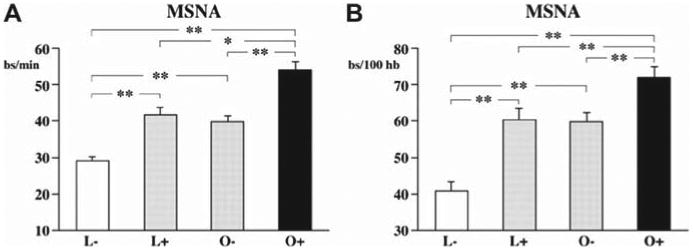
Muscle sympathetic nerve activity (MSNA) in lean (L) and obese (O) subjects according to the presence (+) or absence (−) of OSA. *p < 0.05; **p < 0.01. Reprinted with permission from Grassi et al. (46). Abbreviation as in Figure 1.
Two independent groups of investigators recently explored whether the sympathetic hyperactivity observed in the metabolic syndrome is further exacerbated by the presence of OSA (47,48). Both groups reported that patients with the metabolic syndrome and comorbid OSA have higher sympathetic drive and diminished baroreflex sensitivity compared with patients with the metabolic syndrome without OSA. CPAP therapy reduces muscle sympathetic nerve activity in patients with OSA (49).
Overall, current evidence implicates OSA in potentiating sympathetic activity in obese patients. It is conceivable that the sympathetic activation is induced by hypoxia via peripheral chemoreceptors (50).
Endothelial dysfunction, surrogate markers of atherosclerosis, and arterial stiffness
Several reports consistently showed that OSA is independently associated with endothelial dysfunction (51,52) and that treatment of OSA with CPAP significantly improved endothelial function (53,54). However, a systematic analysis of endothelial dysfunction in obese patients with and without OSA was performed only recently. Jelic et al. (55) measured brachial artery flow-mediated dilation, an indirect marker of endothelial nitric oxide (NO)-mediated reactivity, in 71 subjects with a BMI ranging from normal to obese who underwent polysomnography. Proteins that regulate basal NO production and inflammation and markers of oxidative stress were quantified in venous endothelial cells. The investigators reported that expression of endothelial NO synthase (eNOS), phosphorylated eNOS, and flow-mediated dilation were significantly lower (Fig. 4), whereas expression of nitrotyrosine (a marker of oxidative stress) was significantly greater in patients with OSA than in OSA-free subjects regardless of central adiposity. Expression of nuclear factor κB, a marker of inflammation, was greater in obese patients with OSA than in obese OSA-free subjects. Effective treatment of OSA with CPAP significantly increased flow-mediated dilation and expression of eNOS and phosphorylated eNOS, whereas expression of nitrotyrosine and nuclear factor κB significantly decreased (55). More recently, Namtvedt et al. also reported a positive association between severity of OSA and impaired endothelial function independently of obesity and other cardiovascular risk factors (56). In the metabolic syndrome scenario, Akishita et al. (57) found that patients with both the metabolic syndrome and OSA had lower flow-mediated dilation than patients with the metabolic syndrome alone or controls. Regarding surrogate markers of atherosclerosis and arterial stiffness, a previous investigation found that patients with both the metabolic syndrome and OSA had higher values of carotid intima-media thickness (Fig. 5), pulse wave velocity, and carotid diameter. The apnea-hypopnea index was independently associated with impairments in these 3 vascular parameters (58). Finally, in a preliminary single-arm study, Oyama et al. (59) reported that CPAP therapy improved endothelial dysfunction and decreased markers of oxidative stress in patients with the metabolic syndrome and OSA. Taken together, these findings suggest that OSA has an independent effect on endothelial dysfunction, surrogate markers of atherosclerosis, and arterial stiffness in obesity and the metabolic syndrome.
Figure 4. Endothelial Dysfunction in Normal, Overweight, and Obese Subjects According to the Presence or Absence of OSA.
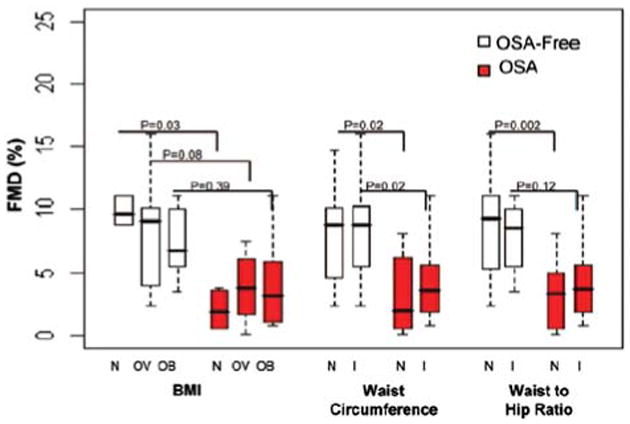
Flow-mediated dilation (FMD) in normal (N), overweight (OV), and obese (O) stratified by body mass index (BMI) and as having central obesity by increased (I) vs normal (N) waist circumference or waist-to-hip ratio. Reprinted with permission from Jelic et al. (55). Abbreviation as in Figure 1.
Figure 5. Carotid Intima-Media Thickness in Patients With Metabolic Syndrome With and Without OSA.
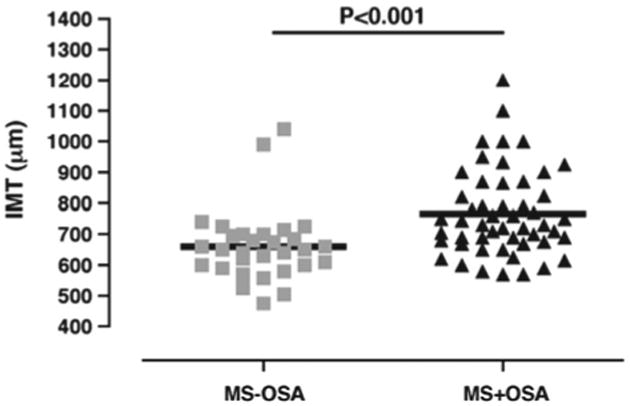
Carotid intima-media thickness (IMT) in patients with metabolic syndrome (MS) according to the presence or absence of OSA. Reprinted with permission from Drager et al. (58). Abbreviation as in Figure 1.
High blood pressure
The relationship between OSA and high blood pressure is supported by a large body of evidence and has been extensively discussed in previous reviews (60,61). Increased blood pressure in patients with OSA is multifactorial in origin and may depend on sympathetic overactivity, systemic inflammation, oxidative stress, endogenous vasoactive factors, and endothelial dysfunction (60,61).
Regarding the impact of OSA treatment on blood pressure, a previous meta-analysis not involving drug-resistant hypertension suggested a very modest impact of CPAP on blood pressure (approximately 2.5 mm Hg for systolic blood pressure and 2 mm Hg for diastolic blood pressure) (62) but very heterogeneous populations (including normotensive patients) were combined, which potentially may mitigate the effects. The results of recent randomized studies suggest that the effect of CPAP is more significant in the subgroup of patients with uncontrolled and drug-resistant hypertension (63,64). More recently, Drager et al. (65) showed that 3 months of CPAP therapy normalized blood pressure in patients with OSA who had pre-hypertension and masked hypertension. This result suggests that treatment of OSA may prevent the development of sustained hypertension.
Despite the aforementioned evidence, there is a paucity of data that specifically address high blood pressure in patients with OSA according to the presence or absence of obesity and the metabolic syndrome. Previous investigations showed inconsistent results. For instance, studies of the adrenergic overdrive in obesity and the metabolic syndrome did not show further increases in blood pressure in patients with OSA (46,47). In contrast, Trombetta et al. (48) found that patients with the metabolic syndrome and OSA had higher blood pressure than patients with the metabolic syndrome alone. Further studies with a higher sample size and analysis of 24-h ambulatory blood pressure monitoring should clarify this issue.
Heart remodeling and arrhythmias
Recent evidence indicates that OSA is independently associated with incident heart failure (66). There are multiple mechanisms by which OSA could lead to heart remodeling, including exacerbation of nighttime and daytime hypertension, increased sympathetic tone, increased left ventricular afterload due to increased arterial stiffness, and exaggerated swings in intrathoracic pressure during obstructive episodes, among others (67–69). The impact of OSA on heart remodeling has recently been explored in obese patients. Avelar et al. (70) performed echocardiography and laboratory testing in 455 severely obese subjects and 59 nonobese reference subjects and showed that more severe nocturnal hypoxemia, increased systolic blood pressure, and BMI are independently associated with increased left ventricular mass. Otto et al. compared the cardiac structural and functional changes in obese and otherwise healthy adults with and without OSA (71). Obese patients with OSA had an increased left atrial volume index and altered diastolic function compared with similarly obese patients without OSA, which implicates OSA in early subclinical impairment of cardiac function (71). Taken together, the early cardiac changes induced by OSA may contribute to heart remodeling, heart failure, and arrhythmias reported in obese patients. Indeed, in a retrospective cohort study of 3,542 adults in Olmsted County, Minnesota, obesity and the magnitude of nocturnal oxygen desaturation were independent risk factors for incident atrial fibrillation in individuals <65 years of age (72). It is conceivable that structural changes in the left atrium may contribute to an increased risk of atrial fibrillation in patients with OSA (68). Recent detailed electrophysiological and electroanatomic mapping found an abnormal atrial substrate in patients with OSA compared to age- and BMI-matched controls without OSA (73). The patients with OSA showed both structural changes, including increased atrial size and extensive areas of low voltage, and regions of electrical silence and conduction abnormalities (73). Regarding the impact of treatment of OSA on atrial fibrillation, appropriate treatment with CPAP in patients with OSA is associated with lower recurrence of atrial fibrillation at 12 months compared with untreated patients (74).
Reversing metabolic syndrome components in the treatment of patients with OSA
Recent studies were designed to explore the impact of OSA on the metabolic syndrome and its components (75–78). In a nonrandomized study, Dorkova et al. showed that CPAP therapy reduced several components of the metabolic syndrome in patients who used CPAP for ≥4 h/night for 8 weeks, including blood pressure, triglyceride levels, and glucose levels, compared with patients with low adherence to CPAP (<4 h/night) (75). More recently, Sharma et al. performed a crossover, double-blind, randomized study exploring the impact of treatment with CPAP for 3 months on components of the metabolic syndrome (76). In this study, treatment with CPAP (vs. sham CPAP [i.e., a placebo]) was associated with significant mean decreases in systolic blood pressure, diastolic blood pressure, serum total cholesterol levels, low-density lipoprotein cholesterol levels, triglyceride levels, and glycated hemoglobin. The prevalence of the metabolic syndrome was significantly reduced after CPAP therapy (reversal found in 11 of 86 patients [13%] undergoing CPAP therapy vs. 1 of 86 [1%] undergoing sham CPAP) (76). Surprisingly, treatment with CPAP also reduced BMI and visceral adiposity, which may partially explain the metabolic improvement (76). The reduction in BMI and visceral adiposity after the use of CPAP for the treatment of patients with OSA is in contrast to other reports (77,78). However, an important caveat is that none of the studies discussed in the preceding text fully controlled for physical activity and diet pre-intervention and post-intervention. Further studies are necessary to clarify these important issues.
Perspectives
The current literature clearly shows that OSA is an emerging risk factor for modulating the cardiometabolic consequences of obesity. There is consistent evidence from animal models and clinical studies suggesting that OSA augments metabolic, inflammatory, autonomic, vascular, and cardiac dysfunction of obesity and exacerbates the metabolic syndrome. However, OSA is commonly underdiagnosed. Several challenges and research priorities should be mentioned. First, we need to call attention to the need for more screening for OSA in patients with obesity and the metabolic syndrome. We should keep in mind that traditional risk factors for OSA such as excessive daytime somnolence may not be present in a significant proportion of patients (4). It would be important to actively pursue other symptoms and clinical characteristics such as frequent snoring and difficult-to-control hypertension, nocturnal blood pressure abnormalities, atrial fibrillation, or left ventricular hypertrophy. Second, there is an urgent need to provide a cost-effective way to appropriately screen for and diagnose OSA in millions of patients with obesity and/or the metabolic syndrome. Portable monitoring (79) and new technologies for diagnosing OSA (80) are promising options in high-risk groups, given that full polysomnography may not be readily available. Third, despite the tight link between obesity and OSA, it is not clear why a significant proportion of obese patients, including those with severe forms of obesity, do not develop OSA. Comprehensive genetic studies with anatomic and functional upper airway assessment should be undertaken to elucidate protective mechanisms (81). Fourth, there is a clear reciprocal interaction between obesity and OSA, but there are several gaps in knowledge (82). Therefore, there is a clear demand for additional research at both the basic science and clinical levels to understand the mechanisms by which OSA and its components exacerbate metabolic dysfunction and vascular impairment in obesity and the metabolic syndrome. Finally, large randomized clinical studies will be necessary to define the impact of treatment of OSA on metabolic and cardiovascular outcomes.
Acknowledgments
The authors thank Junior Falcetti for the medical illustration.
Abbreviations and Acronyms
- BMI
body mass index
- CPAP
continuous positive airway pressure
- eNOS
endothelial nitric oxide synthase
- NAFLD
nonalcoholic fatty liver disease
- NO
nitric oxide
- OSA
obstructive sleep apnea
Footnotes
The authors have reported that they have no relationships relevant to the content of this paper to disclose.
References
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 Apr 14; doi: 10.1093/aje/kws342. [Epub ahead of print]; http://dx.doi.org/10.1093/aje/kws343. [DOI] [PMC free article] [PubMed]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 5.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster GD, Sanders MH, Millman R, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valencia-Flores M, Orea A, Castano VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–9. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 8.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 9.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 10.Horner RL, Shea SA, McIvor J, Guz A. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med. 1989;72:719–35. [PubMed] [Google Scholar]
- 11.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–6. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 12.Stauffer JL, Buick MK, Bixler EO, et al. Morphology of the uvula in obstructive sleep apnea. Am Rev Respir Dis. 1989;140:724–8. doi: 10.1164/ajrccm/140.3.724. [DOI] [PubMed] [Google Scholar]
- 13.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 14.Polotsky M, Elsayed-Ahmed AS, Pichard L, et al. Effects of leptin and obesity on the upper airway function. J Appl Physiol. 2012;112:1637–43. doi: 10.1152/japplphysiol.01222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. “Syndrome Z”: the interaction of sleep apnoea, vascular risk factors and heart disease. Thorax. 1998;53(Suppl 3):S25–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 19.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–90. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. Erratum in: Circulation 2005;112:e297. [DOI] [PubMed] [Google Scholar]
- 22.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 23.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a population-based survey. Obesity (Silver Spring) 2013;21:847–51. doi: 10.1002/oby.20288. [DOI] [PubMed] [Google Scholar]
- 26.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep. 2008;31:1018–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19:2167–74. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–44. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698–706. doi: 10.1161/01.RES.0000183879.60089.a9. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Grigoryev DN, Ye SQ, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643–8. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 34.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–80. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drager LF, Li J, Shin MK, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783–90. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013 Jan 17; doi: 10.1164/rccm.201209-1688OC. [E-pub ahead of print]; http://dx.doi.org/10.1164/rcm.201209-1688OC. [DOI] [PMC free article] [PubMed]
- 37.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184:355–61. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 38.Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2010;17:161–5. doi: 10.1097/MED.0b013e3283373624. Erratum in: Curr Opin Endocrinol Diabetes Obes 2010;17:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carneiro G, Togeiro SM, Ribeiro-Filho FF, et al. Continuous positive airway pressure therapy improves hypoadiponectinemia in severe obese men with obstructive sleep apnea without changes in insulin resistance. Metab Syndr Relat Disord. 2009;7:537–42. doi: 10.1089/met.2009.0019. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-de-la-Torre M, Mediano O, Barceló A, et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2012;16:649–56. doi: 10.1007/s11325-011-0552-7. [DOI] [PubMed] [Google Scholar]
- 41.Gastaldelli A, Kozakova M, Højlund K, et al. RISC Investigators. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–44. doi: 10.1002/hep.22845. [DOI] [PubMed] [Google Scholar]
- 42.Mirrakhimov AE, Polotsky VY. Obstructive sleep apnea and nonalcoholic Fatty liver disease: is the liver another target? Front Neurol. 2012;3:149. doi: 10.3389/fneur.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–34. doi: 10.1164/rccm.200804-608OC. Erratum in: Am J Respir Crit Care Med 2009;180:910–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aron-Wisnewsky J, Minville C, Tordjman J, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–33. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 46.Grassi G, Facchini A, Trevano FQ, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46:321–5. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 47.Grassi G, Seravalle G, Quarti-Trevano F, et al. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens. 2010;28:1313–20. doi: 10.1097/HJH.0b013e328337a9fd. [DOI] [PubMed] [Google Scholar]
- 48.Trombetta IC, Somers VK, Maki-Nunes C, et al. Consequences of comorbid sleep apnea in the metabolic syndrome-implications for cardiovascular risk. Sleep. 2010;33:1193–9. doi: 10.1093/sleep/33.9.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–5. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 50.Trombetta IC, Maki-Nunes C, Toschi-Dias E, et al. Obstructive sleep apnea is associated with increased chemoreflex sensitivity in patients with metabolic syndrome. Sleep. 2013;36:41–9. doi: 10.5665/sleep.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 52.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–91. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 53.Imadojemu VA, Gleeson K, Quraishi SA, Kunselman AR, Sinoway LI, Leuenberger UA. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2002;165:950–3. doi: 10.1164/ajrccm.165.7.2102003. [DOI] [PubMed] [Google Scholar]
- 54.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 55.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namtvedt SK, Hisdal J, Randby A, et al. Impaired endothelial function in persons with obstructive sleep apnoea: impact of obesity. Heart. 2013;99:30–4. doi: 10.1136/heartjnl-2012-303009. [DOI] [PubMed] [Google Scholar]
- 57.Akishita M, Ohike Y, Yamaguchi Y, Iijima K, Eto M, Ouchi Y. Obstructive sleep apnea exacerbates endothelial dysfunction in people with metabolic syndrome. J Am Geriatr Soc. 2011;59:1565–6. doi: 10.1111/j.1532-5415.2011.03526.x. [DOI] [PubMed] [Google Scholar]
- 58.Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208:490–5. doi: 10.1016/j.atherosclerosis.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35:231–6. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedrosa RP, Krieger EM, Lorenzi-Filho G, Drager LF. Recent advances of the impact of obstructive sleep apnea on systemic hypertension. Arq Bras Cardiol. 2011;97:e40–7. doi: 10.1590/s0066-782x2011005000017. [DOI] [PubMed] [Google Scholar]
- 61.Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: interactions and implications for management. Hypertension. 2008;51:605–8. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 62.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 63.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 64.Pedrosa RP, Drager LF, de Paula L, Amaro A, Bortolotto LA, Lorenzi-Filho G. Effects of obstructive sleep apnea treatment on blood pressure in patients with resistant hypertension: a randomized trial. Chest. 2013 Apr 18; doi: 10.1378/chest.13-0085. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–55. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 66.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 68.Drager LF, Bortolotto LA, Pedrosa RP, Krieger EM, Lorenzi-Filho G. Left atrial diameter is independently associated with arterial stiffness in patients with obstructive sleep apnea: potential implications for atrial fibrillation. Int J Cardiol. 2010;144:257–9. doi: 10.1016/j.ijcard.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Koshino Y, Villarraga HR, Orban M, et al. Changes in left and right ventricular mechanics during the Mueller maneuver in healthy adults: a possible mechanism for abnormal cardiac function in patients with obstructive sleep apnea. Circ Cardiovasc Imaging. 2010;3:282–9. doi: 10.1161/CIRCIMAGING.109.901561. [DOI] [PubMed] [Google Scholar]
- 70.Avelar E, Cloward TV, Walker JM, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49:34–9. doi: 10.1161/01.HYP.0000251711.92482.14. [DOI] [PubMed] [Google Scholar]
- 71.Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 72.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 73.Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–7. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 75.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 76.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–86. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 77.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67:1081–9. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 78.Sivam S, Phillips CL, Trenell MI, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40:913–8. doi: 10.1183/09031936.00177011. [DOI] [PubMed] [Google Scholar]
- 79.Collop NA, Anderson WM, Boehlecke B, et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 80.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel SR, Larkin EK, Redline S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int J Obes (Lond) 2008;32:795–800. doi: 10.1038/sj.ijo.0803803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ong CW, O'Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17:123–31. doi: 10.1016/j.smrv.2012.05.002. [DOI] [PubMed] [Google Scholar]


