Abstract
Chemokines and their receptors are essential for the development and organization of the hematopoietic/lymphopoietic system and have now been shown to be expressed by different types of cells in the nervous system. In mouse embryos, we observed expression of the chemokine (CXC motif) receptor 4 (CXCR4) by neural crest cells migrating from the dorsal neural tube and in the dorsal root ganglia (DRGs). Stromal cell-derived factor-1 (SDF-1), the unique agonist for CXCR4, was expressed along the path taken by crest cells to the DRGs, suggesting that SDF-1/CXCR4 signaling is needed for their migration. CXCR4 null mice exhibited small and malformed DRGs. Delayed migration to the DRGs was suggested by ectopic cells expressing tyrosine receptor kinase A (TrkA) and TrkC, neurotrophin receptors required by DRG sensory neuron development. In vitro, the CXCR4 chemokine receptor was upregulated by migratory progenitor cells just as they exited mouse neural tube explants, and SDF-1 acted as a chemoattractant for these cells. Most CXCR4-expressing progenitors differentiated to form sensory neurons with the properties of polymodal nociceptors. Furthermore, DRGs contained a population of progenitor cells that expressed CXCR4 receptors in vitro and differentiated into neurons with a similar phenotype. Our findings indicate an important role for SDF-1/CXCR4 signaling in directing the migration of sensory neuron progenitors to the DRG and potentially in other aspects of development once the DRGs have coalesced.
Keywords: CXCR4 receptors, SDF-1, neural crest, migration, sensory neurons, neurogenesis
Introduction
The neural crest (NC) is a population of cells that exists transiently in vertebrates and gives rise to a very large number of derivatives including neurons, glia, melanocytes, cartilage, muscle, bone, and connective tissue (Le Dourain and Kalcheim, 1999). Neural crest cells are induced early in embryogenesis at the dorsolateral edge of the neural plate, after which they delaminate and migrate along stereotyped routes to a large number of destinations in the developing embryo. Exactly how the diverse fates of these cells are determined and how their specific migration is directed are key questions, the answers to which are only partially known (Anderson, 2000).
One interesting and as yet unsolved problem concerns the development of the autonomic and sensory branches of the peripheral nervous system. The paths taken by progenitors migrating to the autonomic or dorsal root ganglion (DRG) overlap in many respects; sympathetic progenitors must migrate through a region in which sensory neurons subsequently develop, and yet it is clear that subpopulations of progenitors are selectively directed to these different destinations (Anderson, 2000). The factors responsible for directing the migration of these different populations of cells are unknown. One set of candidate molecules that we have now considered in this regard are the chemokines and their receptors. The chemokines are small secreted proteins that exert their effects by activating a family of G-protein-coupled receptors and that have been shown to play several fundamental roles in the control of leukocyte development and migration (Tran and Miller, 2003). Recently, however, it has become clear that chemokines also play an important part in the development of the CNS (Tran and Miller, 2003). In particular, several studies have determined that the chemokine stromal cell-derived factor-1 (SDF-1) and its unique receptor chemokine (CXC motif) receptor 4 (CXCR4) are important in this regard. For example, mice that lack either the CXCR4 receptor or SDF-1 show abnormal development of both the internal granule layer of the cerebellum (Zou et al., 1998) and the dentate gyrus of the hippocampus (Bagri et al., 2002; Lu et al., 2002). In both these cases, it appears that SDF-1 acts as important chemoattractant for granule cell progenitors, as well as increasing the proliferation of these cells (Bagri et al., 2002; Lu et al., 2002). Indeed, it is now clear that neural progenitors from the ventricular and subventricular zones of embryonic and adult mice express many types of chemokine receptors, including CXCR4 (Tran et al., 2004). In the peripheral nervous system, both McGrath et al. (1999) and Jazin et al. (1997) observed that CXCR4 was expressed in the developing DRGs. However, the expression of such receptors by migratory NC cells and their role in the development of postmigratory NC derivatives has not been determined. In the present series of experiments, we demonstrate that CXCR4 is expressed by migrating trunk NC cells and that SDF-1 acts as a chemoattractant for these cells. Disruption of SDF-1/CXCR4 signaling produces malformation of the DRGs, thereby establishing an important role for chemokine signaling in the development of these ganglia.
Materials and Methods
Dissection and cultures of mouse neural tube explants. Neural tubes were isolated from the trunk level of embryonic day 9.5 (E9.5)-E10.5 CD1 mice as described by Stemple and Anderson (1992). Briefly, trunk neural tubes were dissected in HBSS, treated with collagenase/dispase four times for 5 min at 0°C, and vigorously triturated until most of the neural tubes were clean and free of somites. The neural tube fragments were treated again with collagenase/dispase for 5 min at 37°C. They were washed in the culture media for 5 min at 0°C, then plated onto fibronectin (FN)-coated glass coverslips and incubated in high-glucose DMEM supplemented with 10% fetal bovine serum. The cultures were maintained for 24-48 h at 37°C with 5% CO2. To promote proliferation, 24-48 h migrating cells from neural tube explants were incubated in chemically defined medium (Stemple and Anderson, 1992) supplemented with basic fibroblast growth factor (bFGF; 20 ng/ml; R&D Systems, Minneapolis, MN), the recombinant human insulin growth factor (IGF; 20 ng/ml; R&D Systems), and chick embryo extract (CEE; 15%).
To induce differentiation, the cultures were switched to medium supplemented with bFGF (10 ng/ml), nerve growth factor (NGF; 100 ng/ml; Sigma, St. Louis, MO), the recombinant mouse leukemia inhibitory factor (LIF; 100 ng/ml; R&D Systems), and 1% CEE for another 7-10 d before Ca imaging or immunohistochemical analysis.
Generation of dorsal root ganglia dividing precursors and neurospheres. Dorsal root ganglia were dissected from E14.5 mouse embryos. The cells were dissociated by trypsinization for 5 min, triturated, and plated on poly-d-lysine/FN-coated coverslips in chemically defined media (Stemple and Anderson, 1992). After 3-4 d in cultures, a very small fraction of undifferentiated precursors proliferated and adhered to each other to form spherical clusters. These clusters lifted off the substrate and propagated in suspension as neurospheres with 20 ng/ml bFGF, 20 ng/ml IGF, and 15% CEE. After 7 d, the cultures were processed for Ca imaging or were subjected for differentiation, immunohistochemistry, in situ hybridization, or chemotaxis.
To promote differentiation of DRG dividing precursors (DRGDs), the cultures were switched to similar medium with 1% CEE and 10 ng/ml bFGF supplemented with NGF and LIF for another 7-10 d before Ca imaging or immunohistochemical analysis.
Ca imaging. The intracellular free calcium concentration was measured using digital video microfluorimetry as described previously by Meucci et al. (1998). Briefly, migrating neural crest cells (MNCs), DRGDs, or DRG neurospheres were plated on FN-coated glass coverslips, rinsed briefly with HEPES buffer [containing the following (in mm): 120 NaCl, 5.4 KCl, 1.6 MgCl2, 1.8 CaCl2, 11 glucose, and 25 HEPES, pH 7.4 at 37°C], and loaded with 2 m fura-2 AM (Molecular Probes, Eugene, OR) in HEPES buffer for 30 min at room temperature. Cultures were then rinsed and kept in the dark in HEPES at room temperature for an additional 30 min to allow for complete dye deesterification. Glass coverslips were then mounted on the stage of a Nikon (Tokyo, Japan) Diaphot inverted epifluorescence microscope equipped for digital fluorescence microscopy. Fluorescence was digitally monitored at 520 nm after excitation at 340 nm (bound Ca2+) and 380 nm (free Ca2+) (20× water immersion lens). Ratios of F340/F380 were collected before and during treatment with chemokines (e.g., SDF-1, 10-20 nm) and other agents [bradykinin (100 μm), capsaicin (2 μm), icilin (2 μm)] using MetaFluor software from Universal Imaging Corporation (West Chester, PA).
Reverse transcription-PCR. Total RNA was prepared from freshly dissociated neural tube (NT) migrating cells or DRG neurospheres using Trizol reagent (Invitrogen, San Diego, CA). Reverse transcription (RT) was performed using the SuperScript first-strand synthesis system to form cDNA that was primed with 50 ng of oligo (dT) oligonucleotide [PCR primers for chemokine were as described by Tran et al. (2004)]. After heating at 96°C for 5 min, PCR amplification was performed for 35 cycles as follows: 96°C for 30 s, 56°C for 1 min, and 72°C for 1 min, and PCR was performed using Taq polymerase (Invitrogen). Products were analyzed using a 1.2% agarose gel and sequenced automatically (Applied Biosystems, Foster City, CA).
For immunolabeling of the sensory neurons, mouse embryos at E12.5 to E14.5 were collected and genotyped by PCR by using primers derived from the second exon of the CXCR4 gene: forward primer, 5′-CTG GTG CTT TAC GGT ATC GC-3′; reverse primer, 5′-CCT AGA CGC TCA TTC ACA TG-3′.
CXCR4 knock-out (KO) embryos and wild-type (wt) littermates were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer at least overnight, subsequently were cryoprotected in 30% sucrose, and then were processed for the expression of tyrosine receptor kinase A (TrkA) and TrkC by in situ hybridization as described by Ma et al. (1999).
In situ hybridization. Cultures were fixed in 4% paraformaldehyde for 1 h and washed in PBS. Probes for CXCR4 and SDF-1/CXCL12 were generated as described by Lu et al. (2002). The probe for SOX10 was a kind gift from Drs. M. E. De Bellard and M. Bronner-Fraser (California Institute of Technology, Pasadena, CA) (Kuhlbrodt et al., 1998; De Bellard et al., 2002). In situ hybridization was conducted for 20 h at 70°C using digoxigenin (DIG)-labeled riboprobes complementary to the coding region of CXCR4 or SDF-1 [50% formamide, 5× SSC, 0.1% Tween 20, 500 g/ml tRNA, 200 g/ml acetylated bovine serum albumin (BSA), and 50 g/ml heparin]. Cells were washed four times with 50% formamide, 2× SSC, and 0.1% Tween at 70°C for 20 min and then washed three times using Tris-buffered saline and Tween 20 (TBST; 25 mm Tris-HCl, pH 7.5, 136 mm NaCl, 2.68 mm KCl, and 1% Tween 20). Finally, cells were incubated with 10% lamb serum in TBST buffer for 1 h and then treated with anti-DIG antibody followed by antibody detection according to the manufacturer's protocol (Roche Products, Welwyn Garden City, UK). Whole-mount in situ hybridization of mouse embryos was performed as described previously by Wilkinson (1992), Grove et al. (1998), and Agarwala et al. (2001). Basically, mouse embryo were fixed in 4% paraformaldehyde (PFA), bleached in H2O2/methanol (MeOH), rehydrated through methanol/TBST (75% MeOH, 50% MeOH, 25% MeOH, TBST), and blocked by using a prehybridization solution [50% formamide, 5× SSC, 5× Denhardt's, 50 μg/ml bakers yeast RNA (Sigma), 100 μg/ml heparin]. This procedure was followed by hybridization of the denatured DIG-labeled RNA probes to cRNA molecules at 65°C overnight. Embryos were then washed to remove unhybridized probes and blocked with 10% heat-inactivated sheep serum for 2-3 h at room temperature. Immunological detection of DIG-labeled hybrids was achieved by using an anti-DIG antibody conjugated with alkaline phosphatase (anti-digoxigenin-Ap Fab fragments; Roche Products). This strategy catalyzed a color reaction requiring 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (Roche Products) as substrates, with labeled probes appearing as a dark blue color. Fluorescent in situ hybridization was performed as by Agarwala et al. (2001) and by R. Parniak and C. Ragsdale (unpublished observations).
Immunohistochemistry. Fluorescence immunohistochemistry was performed as described by Tran and Miller (2003) and Tran et al. (2004). For NT migrating cells, DRG dividing cells, or differentiated cell cultures, the cells were fixed with 4% paraformaldehyde/2% sucrose in PBS for 15 min. After three washes in PBS, the cells were blocked in 4% BSA and 0.1% Triton X-100 for 1 h. The cultures were immunolabeled separately with the following antibodies: goat anti-CXCR4 (1:300; Novus Biological, Littleton, CO), mouse anti-nestin (1:300; BD Biosciences, San Jose, CA), anti-p75 (1:250), goat anti-Brn3a (1:500; Dr. E. Turner, Department of Psychiatry, University of California, San Diego, CA), or goat anti-phox2b (1:500; gift from C. Goridis, Centre National de la Recherche Scientifique UMR8542, Paris, France) antibody in 4% BSA and 0.1% Tween 20 overnight at 4°C, followed by three washes in blocking solution. They then were incubated with the appropriate secondary goat anti-rabbit or anti-mouse antibodies conjugated with FITC (Alexa Fluor 488; A 11001; Molecular Probes) or Alexa Fluor 633 (1:500) (T 6391; Molecular Probes) for 1 h at room temperature at 1:200 and 1:500. Finally, the cells were treated with mounting solution, Prolong Antifade kit (Molecular Probes), on a microscope slide and examined by fluorescence microscopy.
Dunn chamber and chemotaxis. To assess the chemotaxis of SDF-1 (10-20 nm), we used neural tube migrating cells with Dunn chambers from Hawksley Technology (West Sussex, UK). The Dunn Chambers contain one inner ring and one outer ring separated each other by a 2 μm gap air space that allow for the generation of a stable chemotactic gradient and the observation of cell migration in the context of the gradient (Zicha et al., 1997). After 48 h of cultures on FN-coated coverslips, migrating neural crest were dissociated and then placed into the inner well of the Dunn chamber. A fibronectin-treated coverslip was then placed on the Dunn chamber. Using a small needle, media alone or media (70 μl) containing SDF-1 was dispersed into the outer well, making sure that no bubbles were formed. The coverslip was sealed in place using vacuum grease. The dunn chambers were then inverted (FN-coated coverslips upside down) and placed in an incubator set at 37°C for 30 min to allow cells to adhere to the FN-coated coverslips. Subsequently, chambers were removed from the incubator and placed in an upright microscope, whereupon cell counts were made for eight nonoverlapping fields in the inner and outer wells using 20× objective.
Quantification of neural crest migration. To elucidate the possible role of SDF-1/CXCR4 signaling in MNC migration, an MNC outgrowth culture system was used to examine migration of neural crest cells derived from CXCR4 KO embryos and from nontransgenic outgrowth cultures treated with SDF-1 blocking antibody or AMD3100 (AIDS Reagent Program, National Institutes of Health, Bethesda, MD), a CXCR4 antagonist. Digital images of the outgrowth cultures at 24-48 h were acquired by confocal microscopy, and MetaMorph software (Universal Imaging Corporation) was used to measure the area of the outgrowth. This was obtained by taking the area of the entire explant and subtracting from it the area of the dense central neural tube mass. This resulted in a number referred to as the migration index and provides an estimate of the migration rate of the neural crest cells.
Statistical analysis. Results from at least three experiments were analyzed by ANOVA with Bonferroni post hoc tests. p values <0.05 were considered significant.
Results
DRG formation in the absence of CXCR4 receptors
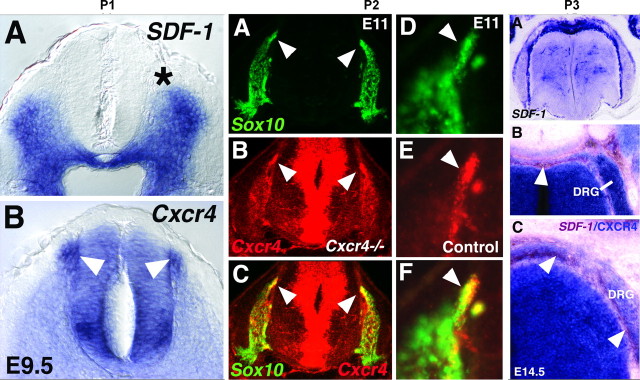
To investigate the involvement of SDF-1/CXCR4 signaling in the formation of DRGs, we examined the expression of SDF-1 and CXCR4 mRNA in the developing spinal cord and DRGs. From at least as early as E9.5 (Fig. 1P1,A), expression of SDF-1 is wide-spread in tissue surrounding the ventral spinal cord, including tissue lining the path of cell migration to the growing DRGs (Fig. 1P1,A, asterisk marks the approximate position of crest cell exit from the neural tube). Prominent CXCR4 expression appears in a group of cells that, by their position, are likely to be crest cells emerging from the neural tube (Fig. 1P1,B, white arrowheads point to the site of migrating crest cells). Using fluorescence in situ hybridization for SOX10, a neural crest cell marker (Southard-Smith et al., 1998), we confirmed that migrating neural crest cells also expressed CXCR4 (Fig. 1P2,A-F).
Figure 1.
The expression patterns of SDF-1, CXCR4, and Sox10 within the spinal cords of mouse embryos. P1, A, B, Coronal sections through the wild-type mouse mid-spinal cord at E9.5, processed with in situ hybridization to show mRNA expression of SDF-1 and CXCR4. SDF-1 expression extends close to the site at which neural crest cells emerge from the neural tube (A, asterisk). The latter cells are marked by prominent expression of CXCR4 (B, arrowheads). Marked expression also appears in a distinct region of the ventral spinal cord, which may approximately correspond to the position of developing motor neurons. P2, A-F, Migrating DRG neural crest cells are CXCR4 positive. Sections through the spinal cord of a CXCR4 mutant (A-C) and a littermate control (D-F) at E11. Sections were processed for double-fluorescence in situ hybridization (Sox10, green, encodes a transcription factor expressed in neural crest cells; CXCR4, red). A-C, In a CXCR4 homozygous mutant, single fluorescence for Sox10 marks neural crest cells in and migrating to the DRGs on either side of the spinal cord (A). Arrowheads point to a patch of migrating cells also labeled for CXCR4 fluorescence (B, C). C is an overlay of A and B. D-F, Higher magnification of a Sox10- D, F; arrowhead) and CXCR4- (E, F) expressing cell group migrating toward the DRG (F is an overlay of D, E). P3, A-C, Coronal sections through wild-type E14.5 spinal cord processed with one- or two-color in situ hybridization for SDF-1 (blue in A) or both SDF-1 (brown in B, C) and CXCR4 (blue in B, C). SDF-1 expression is strong over the dorsal neural tube and runs ventrally in two streams that appear to surround the DRGs (B, C; arrowheads). Note that at this later age, E14.5, neural crest migration to the DRGs is near completion.
To test whether SDF-1/CXCR4 signaling regulates DRG development, we examined DRG formation in embryos lacking functional CXCR4 receptors (Zou et al., 1998). The migration and consolidation of neural crest cells into DRGs were assessed by expression of TrkA and TrkC in CXCR4 homozygote mutants and wild-type or heterozygote littermates. Neurotrophin receptors are expressed by different subtypes of DRG sensory neurons and their precursors; more specifically, TrkA, the NGF receptor, is implicated in development of mechanoreceptors (stretch-sensing), thermoreceptors, and nociceptors (pain-sensing) (Kirstein et al., 2002), whereas TrkC, the neurotrophin-3 receptor, appears associated with development of proprioceptors (limb position-sensing) (Kirstein et al., 2002). At E12.5, sensory neurons labeled with TrkA (Fig. 2A-C′,D,E) riboprobe showed that DRGs are evenly spaced and compact in control embryos (Fig. 2A,A′) but disorganized in CXCR4 mutants (Fig. 2B,B′, C,C′). Individual DRGs were irregularly shaped, broken up, or small (Fig. 2, compare A′ to B′,C′), and dorsal to the DRGs, ectopic satellites of TrkA-expressing cells were evident (Fig. 2, white arrowheads indicate ectopic cells in E, compare with D), presumably representing cells that had slowed or stalled in their migration. Similarly, DRGs marked by expression of TrkC are regularly shaped in control mice (Fig. 2F) but split or malformed in CXCR4 mutant mice (Fig. 2G).
Figure 2.
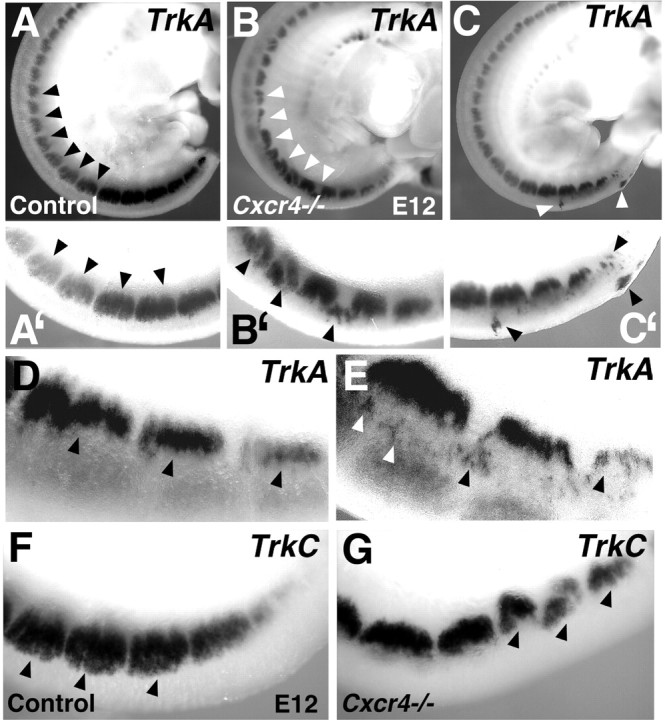
Abnormal DRG development in the absence of CXCR4 receptors. Whole-mount E12 embryos processed to show DRG expression of TrkA (A-C′, D, E) and TrkC (F, G). A′-C′ are high-magnification views of the bottom third of A-C. In contrast with the regularly shaped DRGs forming in control embryos (A, A′), DRGs in CXCR4 null mutants are misshapen, disorganized, misshapen, or small (B, B′, C, C′). Arrowheads indicate normal DRGs (A, A′), compared with split, misshapen DRGs and cell islands in B, B′, C, and C′. Ectopic cells dorsal to the DRGs are indicated in E but are absent from the more regular DRGs in a control mouse (D). Similarly, DRGs marked by expression of TrkC are regularly shaped in a control mouse (F) but split or malformed in a CXCR4 null mouse (G).
The spatial relationship of SDF-1 and CXCR4 expression was further examined using two-color in situ hybridization (Fig. 1P3,A-C). SDF-1 expression at E14.5 lined the developing DRGs. The DRGs themselves expressed CXCR4, as did a stream of cells leading from the dorsal neural tube to the DRGs (Fig. 1P3, white arrowheads in C indicate this cell stream with immediately adjacent expression of SDF-1), suggesting a role of SDF-1/CXCR4 signaling in neural crest cell migration and DRG formation.
Expression of CXCR4 by MNCs in vitro
To determine the role of SDF-1/CXCR4 signaling in neural crest development in more detail, we examined the behavior of MNCs exiting neural tube explants. We found that E9.5 mouse trunk neural tube explants, cultured for 24 h on FN-coated coverslips, gave rise to MNCs (Fig. 3A) with two basic morphologies (De Bellard et al., 2003). Using immunohistochemistry, MNCs were shown to express p75 (Fig. 3C), a marker associated with the embryonic neural crest (Rao and Anderson, 1997), as well as the neuroepithelial stem cell marker nestin (Fig. 3D), indicating that they consisted of undifferentiated neural crest-derived cells. In some experiments, the neural tube was removed, and cells were trypsinized and replated on FN-coated coverslips. These cells proliferated, adhered to each other, and formed spherical clusters after 3-4 d in culture. After 7 d, the clusters lifted off the substrate, floated in suspension, and could be propagated as a nonadherent neurospheres (Fig. 3B) resembling those obtained using embryonic or adult cells from the CNS (Tran et al., 2004). These cells stained for the same markers and responded the same way as MNCs (data not shown).
Figure 3.

Characterization of MNCs. A shows a trunk neural tube explant (Ne) at E9.5 with migrating cells exiting neural tube fragments. B, After replating, these cells formed nonadherent neurospheres. Scale bar, 150 μm. Also illustrated are immunostaining of migrating neural crest cells for p75 (C), nestin (D), CXCR4 (E, F), Brn3a (G), and Phox2b (H).
Immunohistochemistry revealed that the CXCR4 receptor was not generally expressed by cells in the neural tube. In contrast, cells at the edge of the neural tube that were preparing to delaminate and cells that were actively migrating away from the neural tube strongly expressed the receptor (Fig. 3E,F). Virtually all migrating cells stained positively for CXCR4 (Fig. 3F). MNCs generally stained for the transcription factor Brn3a (Fig. 3G), whereas only a small number of cells expressed the transcription factor Phox2b (Fig. 3H), suggesting that under these culture conditions (20 ng/ml FGF2, 20 ng/ml IGF, and 15% CEE), most of the cells were of the sensory lineage (Fedtsova and Turner, 1995).
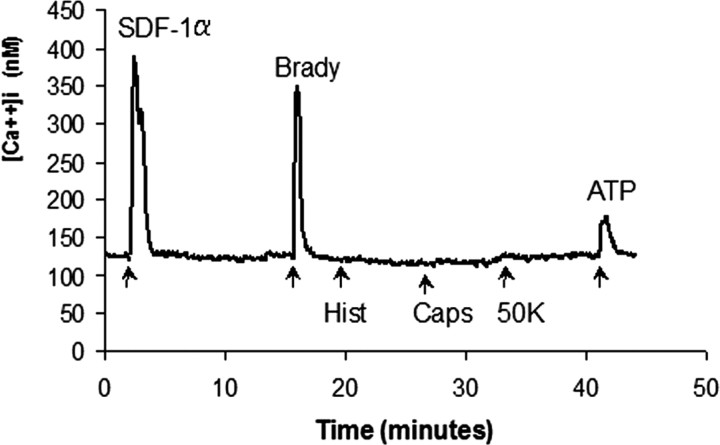
To demonstrate that the CXCR4 receptors were functional, we performed calcium (Ca2+) imaging studies on single MNCs (Fig. 4). We observed that cells responded robustly to the addition of SDF-1 (2340 of 3465; 68%), although they did not respond to a number of other chemokines including TECK (thymus expressed chemokine), CTACK (cutaneous T cell-attracting chemokine), monocyte chemotactic protein 1 (MCP-1), MCP-2, RANTES (Regulated upon Activation, Normal T cell Expressed and Secreted), Fractalkine, macrophage inflammatory protein 1, IP-10 (interferon-γ-inducible 10 kDa protein), or macrophage-derived chemokine. Cells also did not respond to a number of stimuli that would be expected to produce Ca2+ signals in sensory neurons. For example, no response was observed to 50 mm K depolarization, indicating a general absence of voltage-dependent Ca2+ channels, or to capsaicin or icilin (Fig. 4), indicating a lack of the transient receptor potential vanilloid receptor subtype 1 or transient receptor potential melastatin 8 channels, which are normally expressed by populations of polymodal nociceptors (Jordt et al., 2003). However, even at this stage, we did observe that many cells responded to bradykinin, which is typical of sensory and autonomic neurons (Fig. 4 and data not shown). All cells responded robustly to ATP, which was used as a positive control at the end of each experiment.
Figure 4.
SDF-1 induces [Ca2+]i changes in MNCs. Trunk MNCs responded to SDF-1α, bradykinin (Brady), and ATP but not to capsaicin (Caps), high K [50 mm (50K)], or histamine (Hist). ATP-induced Ca responses were present in all cells tested. A total of 2340 cells of 3465 (67.5%) showed [Ca2+]i increase when stimulated by SDF-1α and 3255 of 3735 (87%) by bradykinin.
We directly addressed the possibility that SDF-1 might act as a chemoattactant migratory cue for MNCs using a microchemotaxis assay. This assay allowed us to assess the migration of cells in response to a gradient of a chemoattractant (Zicha et al., 1997). We found that MNCs migrated toward a source of SDF-1. The effects of SDF-1 were inhibited by the selective CXCR4 antagonist AMD3100, indicating that they were mediated by the CXCR4 receptor (Fig. 5a). We also used an MNC outgrowth culture system to examine migration of neural crest cells derived from wt and CXCR4 KO embryos. These explants were cultured for a period of 24-48 h. After 24 h, a ring of MNCs was observed around the neural tube explant, and this had expanded in size after 48 h (Fig. 5b,c). We used an anti-SDF-1 blocking antibody or AMD3100 to block SDF-1/CXCR4 function in wt outgrowth cultures. Treatment with either reagent resulted in a decrease in the extent of cell migration measured over 48 h (Table 1). In addition, when we cultured explants from CXCR4 KO mice, they also exhibited a reduction in the extent of MNC migration (Table 2). These data suggest that there is a cellular source of SDF-1 in the cultures, which we confirmed using in situ hybridization for SDF-1 (Fig. 5d).
Figure 5.
SDF-1 is a chemoattractant for migrating neural crest cells. a, Trunk MNCs migrated toward an SDF-1α (20 nm) gradient (90 min). Migration was inhibited by AMD3100 (AMD; 20 μm) (n = 4). Error bars represent SEM. *p < 0.05, significantly different from the control group; **p < 0.05, significantly different from the SDF-1-treated group. b, c, Neural crest cell migration from neural tube explant cultures. Neural crest cells rapidly emerged from explanted neural tube fragments and migrated out as a monolayer of cells surrounding the central dense mass of neuroepithelial tissue. Note the increase in the area of the neural crest outgrowth from 24 h (b) to 48 h (c) in culture. d, Insitu hybridization using SDF-1 mRNA probe showing expression of SDF-1 by a population of MNCs. e, Negative control for SDF-1 sense riboprobe.
Table 1.
Rate of migration of MNCs after SDF-1/CXCR4 signaling
|
|
Migration index |
||
|---|---|---|---|
| Strain |
18 h |
48 h |
|
| CXCR4 genotype | |||
| −/− | 0.376 ± 0.028 (3)* | 0.609 ± 0.0725 (3)* | |
| +/− | 0.585 ± 0.053 (14) | 2.122 ± 0.512 (14) | |
| +/+ |
0.679 ± 0.028 (17) |
2.493 ± 0.425 (17) |
|
Neural tube explants were incubated for 24-48 h, and the migration index in each outgrowth culture was determined. Note that MNC migration was less extensive when SDF-1/CXCR4 is perturbed. The migration indices of neural crest cells are derived from CXCR4 KO mice compared with wt. Data are the mean ± SEM. The numbers in parentheses represent the number of outgrowth cultures analyzed.
Significantly different compared with wild-type and heterozygous KO values; p < 0.05.
Table 2.
Effects of treating MNCs with an SDF-1 blocking antibody or AMD3100 on migration indices in wild-type outgrowth cultures
|
|
Migration index |
||
|---|---|---|---|
| Treatment |
18 h |
48 h |
|
| Control | 0.367 ± 0.054 (10) | 1.192 ± 0.122 (10) | |
| SDF-1 antibody | 0.288 ± 0.076(4)** | 1.016 ± 0.098 (4) | |
| AMD3100 |
0.191 ± 0.028 (4)*
|
0.549 ± 0.108 (4)*
|
|
Data are the mean ± SEM. The numbers in parentheses represent the number of outgrowth cultures analyzed.
Significantly different compared with control values; p < 0.05.
Significantly different compared with control values; p < 0.05.
When MNCs were incubated in media containing NGF (100 ng/ml) and LIF (100 ng/ml) for 7-10 d, many cells assumed a neuronal morphology (Fig. 6a). We observed that differentiated cells still uniformly expressed CXCR4 receptors (Fig. 6b). The majority of the cells also exhibited Brn3a immunoreactivity (Fig. 6c), although a few cells expressed Phox2b (Fig. 6d). Cells exhibited Ca2+ responses to SDF-1α (280 of 540; 54%), bradykinin (520 of 540; 96%), and ATP (Fig. 6e). However, whereas MNCs did not respond to depolarizing concentrations of K (Fig. 4a), differentiated cells exhibited a robust Ca2+ response to 50 mm K (Fig. 6e). Furthermore, whereas MNCs did not respond to capsaicin (Fig. 4), populations of differentiated cells exhibited strong responses to capsaicin (380 of 540; 71%) (Fig. 6e). This profile suggests that MNCs had differentiated into neurons with properties similar to those of small polymodal nociceptors (Fedtsova and Turner, 1995). These data support previous studies of neural crest development, in which NGF and LIF regulate generation of nociceptive neurons by MNCs (Murphy et al., 1994; Corness et al., 1998).
Figure 6.

Characteristics of differentiated trunk MNCs. a, Trunk MNCs were differentiated with NGF plus LIF (100 ng/ml) for 2 weeks. Immunostaining for CXCR4 (b), Brn3a (c), and Phox2b (d). Nuclear counterstaining with Hoechst 33342 (hoechst; blue) in b and d. e, Changes in [Ca2+]i in response to chemokines. Note that neurons exhibited responses to capsaicin (Caps) and to 50 mm K (50K; compare with Fig. 3). A total of 280 cells of 540 (52%) showed [Ca2+]i increase when stimulated by SDF-1α, 520 of 540 (96%) by bradykinin (Brady), and 380 of 540 (71%) by capsaicin.
Expression of CXCR4 by cells from embryonic DRG
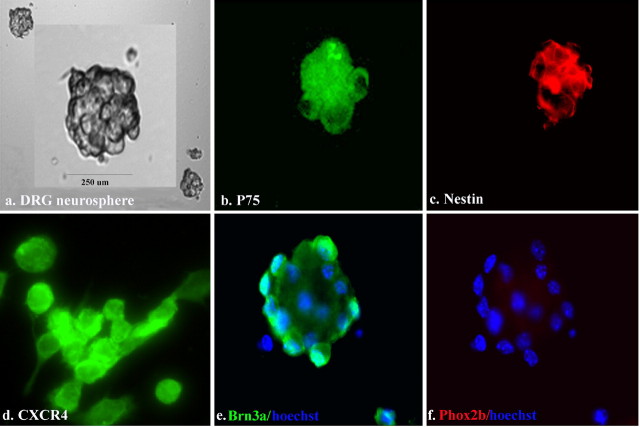
It is known that after formation of the DRGs, these ganglia contain a population of dividing cells that continues to contribute to their maturation (Namaka et al., 2001; An et al., 2002). We therefore attempted to culture such a population from the DRGs of E14 mice, a time at which ganglion formation is nearly complete. We found that mouse E14.5 DRGs contained a population of dividing cells that could proliferate as nonadherent neurospheres when cultured in defined media supplemented with FGF plus IGF (Fig. 7a). These neurospheres had an appearance similar to those prepared using neural cells from the CNS (Tran et al., 2004) or the peripheral enteric nervous system (Schafer et al., 2003). Characterization using immunohistochemistry indicated that DRGDs stained positively for CXCR4 receptors (Fig. 7d). DRGDs also expressed p75 (Fig. 7b), nestin (Fig. 7c), and Brn3a (Fig. 7e), but no cells expressed Phox2b (Fig. 7f). Thus, DRGDs (appropriately) seemed to follow only the sensory lineage.
Figure 7.
Characterization of dividing DRG neurospheres. a, Phase-contrast illustration of a DRG neurosphere from a 7-d-old culture of an E14.5 mouse DRG. Anti-p75 staining (b), anti-nestin (c), anti-CXCR4 (d), anti-Brn3a with Hoechst 33342 (hoechst) counterstain in blue (e), and same neurosphere shown in e assessed for Phox2b staining with Hoescht 33342 counterstain in blue (f).
Interestingly, and in contrast to the results with migrating MNCs, DRGDs responded to many different chemokines (Fig. 8B) in addition to SDF-1. This is characteristic of differentiated polymodal nociceptors that are also sensitive to multiple chemokines (Oh et al., 2001). A survey of chemokine receptors using RT-PCR confirmed the expression of most of the known chemokine receptors by these cells (Fig. 8A). Of the known chemokine receptors, only chemokine (CC motif) receptor 4 (CCR4), CCR6, CXCR1, and CXCR5 appeared to be absent. As was the case for MNCs, DRGDs did not respond to capsaicin, icilin (data not shown), or 50 mm K+ (Fig. 8B). This pattern of responsiveness suggest that DRGDs have not differentiated to a sensory neuron phenotype.
Figure 8.
DRG neurospheres express a variety of chemokine receptors. A, RT-PCR products indicating that different chemokine receptors are expressed by E14.5 DRG neurospheres. pb, Pair base. B, [Ca2+]i changes in response to chemokines in dividing DRG neurospheres. A total of 170 cells of 540 (32%) showed [Ca2+]i increases in response to SDF-1α. Note that there were no responses to either 50 mm K (50K) or to capsaicin (Caps). Rantes, Regulated upon Activation, Normal T cell Expressed and Secreted; Teck, thymus expressed chemokine; Fract, Fractalkine; Ctack, cutaneous T cell-attracting chemokine; Hist, histamine.
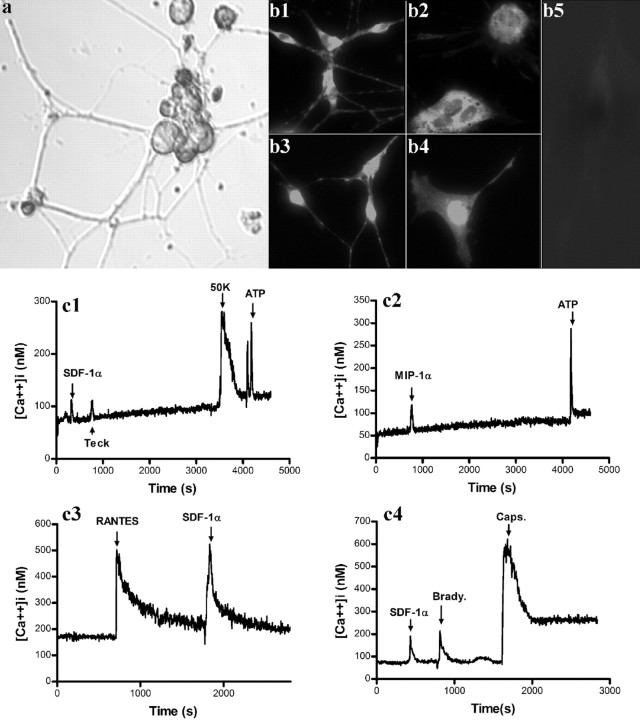
We wondered whether DRGDs could also give rise to nociceptive neurons. When cultured in defined media with NGF plus LIF, DRGDs stopped dividing, and many cells assumed a neuronal morphology (Fig. 9a). Differentiated cells were sensitive to SDF-1α and to other chemokines (Fig. 9c1-c4), but unlike dividing DRGDs, they also responded to 50 mm K and capsaicin (Fig. 9c1,c4). Differentiated neurons stained for CXCR4 receptors (Fig. 9b1,b2) and were also positive for the transcription factor Brn3a (Fig. 9b3,b4), but no cells derived from DRG were positive for Phox2b (Fig. 9b5) (Greenwood et al., 1999). Thus, under these culture conditions, DRGDs differentiate into populations of neurons with properties similar to nociceptors, consistent with previously published findings (Murphy et al., 1994; Corness et al., 1998).
Figure 9.
Characteristics of differentiated DRG neurospheres. a, Differentiated DRG neurosphere cells after culture with NGF and LIF (100 ng/ml) for 7-10 d. Immunostaining of differentiated DRG cells for CXCR4 (b1, b2), Brn3a (b3, b4), and Phox2b (b5). DRG cells gave rise to both neuronal cells (b1, b3) and flat cells (b2, b4) that were positive for CXCR4 and Brn3a. b5 indicates lack of staining for Phox2b. Changes in [Ca2+]i in response to 50 mm K (50K), chemokines, and other agonists in differentiated DRG cells (c1-c4). A total of 130 cells of 420 (31%) showed [Ca2+]i increase when stimulated by SDF-1α, 110 of 160 (69%) by bradykinin (Brady.), and 260 of 400 (65%) by capsaicin (Caps.).
It is interesting to note that differentiation of DRG cells also yielded a small number of cells with non-neuronal morphologies (Fig. 9b2,b4). These cells were large and flat and also expressed CXCR4 chemokine receptors. We have not pursued the identity of these cells further, but they are likely to be glia or other types of satellite cells known to occur in the DRG (Heblich et al., 2001).
Discussion
Previous studies have demonstrated that SDF-1/CXCR4 signaling is important in regulating the migration and proliferation of neural cells that form the dentate gyrus and internal granule layer of the cerebellum (Zou et al., 1998; Bagri et al., 2002). However, CXCR4 and SDF-1 are expressed in several other areas of the developing nervous system, including the developing DRGs (Jazin et al., 1997; McGrath et al., 1999; Chalasani et al., 2003). Indeed, it has been shown that SDF-1 is involved in regulating path finding by the TrkA-positive axons of developing sensory neurons (Chalasani et al., 2003). Because axon guidance cues frequently also guide cell migration, these observations further stimulated us to examine a possible role for SDF-1/CXCR4 in the migration of neural crest cells destined for the DRGs.
The experiments reported here support such a role. SDF-1 and CXCR4 are expressed in close juxtaposition in and around the developing DRGs, SDF-1 produces chemoattractant effects on neural crest cells in culture, and disruption of the gene coding for CXCR4 results in a predictable phenotype characterized by delayed migration of TrkA- and TrkC-expressing neurons to the DRG. A defect in condensation of the DRG may also be present.
Previous findings suggest that DRG neurons coexpress more than one neurotrophin receptor at early stages of development (such as E11 in mouse) (Snider and Wright, 1996). Subsequently, TrkA is needed, in particular, for the development of complete populations of nociceptive DRG neurons, and TrkC is needed for proprioceptive neurons (Kirstein and Farinas, 2002). Our findings in CXCR4 null mice therefore suggest that several types of sensory neuron and their precursors are affected in the absence of SDF-1/CXCR4 signaling. Indeed, it is known that several waves of migration are important for DRG development (Ma et al., 1999; Maro et al., 2004).
In NC cultures, we show, more specifically, that small polymodal nociceptors and/or their precursors are among the SDF-1-responsive cell population. NC cells migrating away from the E9.5-E10.5 mouse neural tube express functional CXCR4 receptors, and the chemokine SDF-1 acts as a chemoattractant for these cells. Second, the same cells can be shown to differentiate in culture into a population of neurons that express markers that are selectively expressed by small polymodal nociceptors, including the hot and cold receptors for capsaicin and icilin, respectively (Jordt et al., 2003).
It is known that the size of the DRGs continues to increase after their initial formation, even continuing postnatally to some extent (Namaka et al., 2001; An et al., 2002; Le Dourain et al., 2004). We identified a population of dividing cells from embryonic DRGS and observed that, as with trunk MNCs, they could differentiate into a population of neurons with properties similar to those of polymodal nociceptors. Both DRGDs and their resulting neuronal progeny were sensitive to SDF-1. However, unlike MNCs, which were only sensitive to SDF-1, DRGDs and their daughter neurons were sensitive in vitro to a large variety of chemokines and expressed numerous types of chemokine receptor. Mature DRG nociceptors in vivo express a similarly wide range of chemokine receptors, which have been shown to participate in the perception of pain, particularly in the context of inflammation (Oh et al., 2001). These observations indicate that several aspects of in vivo development were strikingly conserved in our in vitro experiments. Moreover, our findings suggest that chemokine signaling plays additional roles in DRG development after migration is complete.
Although the loss of SDF-1/CXCR4 signaling disrupts DRG development, the DRG clearly form, suggesting that SDF-1 signaling is coordinated with the activity of other guidance molecules. In the early stages of trunk NC cell migration, extracellular matrix components (Krull, 2001) and transmembrane receptor/ligands of the ephrin family (Krull, 2001; Maschoff and Baldwin, 2001; Santiago and Erickson, 2002) are thought to be important for cells to delaminate from the neural tube and migrate through the rostral portion of the somatic sclerotome. However, during subsequent stages of migration, the signals that determine specific routes taken by different cell populations are generally unknown. De Bellard et al. (2002) reported that in mice deficient in the Notch ligand Delta-1 displayed severely disrupted NC cell migration resulting in partially fused and malformed DRGs containing diminished numbers of neurons. This was associated with downregulation of ephrin B2 and its receptor eph B2 by the somites and NC cells, respectively. Additional clues regarding the role of SDF-1 in neural crest migration may come from a study of axon guidance (Chalasani et al., 2003), showing that SDF-1 contributes to the guidance of DRG sensory axons by reducing the repellent activity of semaphorins. Additionally, SDF-1 reduced the repellent effect of slit-2 on retinal ganglion cell axons (Chalasani et al., 2003). It is therefore worth identifying repellant migratory cues along the pathway taken by neural crest cells toward the forming DRGs and determining whether SDF-1 modifies their effects. Notably, in the study cited above, SDF-1, by itself, had neither attractive nor repellent effects on retinal or DRG axons (Chalasani et al., 2003). Our present findings therefore differ in an important respect. Results from in vitro experiments strongly suggest that SDF-1 is in itself a migratory chemoattractant for neural crest cells.
Overall, the patterns of expression of CXCR4 and SDF-1 in the developing embryo support the suggestion that CXCR4 mediated signaling is important in the development of the DRG. Indeed, CXCR4 signaling may play multiple roles in DRG development including the guidance of migration, as described here, and of sensory neuron axons, as described previously (Chalasani et al., 2003). Clearly, interaction of CXCR4 signaling with other key regulators of neural crest development needs to be further defined (Lee et al., 2004). It should also be noted that expression of chemokine receptors by sensory neurons continues in the mature animals, where they may play an important role in aspects of inflammatory pain (Krull, 2001), the development of sensory neuropathies (Keswani et al., 2003; Bodner et al., 2004), and other related phenomena.
Footnotes
This work was supported by National Institutes of Health Grants 5R01DA013141-04, 4R37MH040165-19, 5R01NS043095-02, and 5R01HD042330-02. We thank Dr. Daniel R. Littman (Skirball Institute of Molecular Medicine, New York University Medical Center, New York, NY) for CXCR4 KO mice. We also thank Drs. C. Ragsdale and R. Parniak for their advice on in situ hybridization techniques.
Correspondence should be addressed to Prof. Richard J. Miller, Department of Molecular Pharmacology and Biological Chemistry, Northwestern University, 303 East Chicago Avenue, Ward 8-296, Chicago, IL 60611. E-mail: r-miller10@northwestern.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/253995-09$15.00/0
References
- Agarwala S, Sanders TA, Ragsdale CW (2001) Sonic hedgehog control of size and shape in midbrain pattern formation. Science 291: 2147-2150. [DOI] [PubMed] [Google Scholar]
- An M, Luo R, Henion PD (2002) Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J Comp Neurol 446: 267-275. [DOI] [PubMed] [Google Scholar]
- Anderson DJ (2000) Genes, lineages and the neural crest: a speculative review. Philos Trans R Soc Lond B Biol Sci 355: 953-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ (2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249-4260. [DOI] [PubMed] [Google Scholar]
- Bodner A, Toth PT, Miller RJ (2004) Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol 118: 246-253. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA (2003) A chemokine, SDF-1 reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci 23: 1360-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corness J, Stevens B, Fields RD, Hokfelt T (1998) NGF and LIF both regulate galanin gene expression in primary DRG cultures. NeuroReport 9: 1533-1536. [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Ching W, Gossler A, Bronner-Fraser M (2002) Disruption of segmental neural crest migration and ephrin expression in delta-1 null mice. Dev Biol 249: 121-130. [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Rao Y, Bronner-Fraser M (2003) Dual function of Slit 2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J Cell Biol 162: 269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE (1995) Brn 3.0 expression identified early post mitotic CNS neurons and sensory neural precursors. Mech Dev 53: 291-304. [DOI] [PubMed] [Google Scholar]
- Greenwood AL, Turner EE, and Anderson DJ (1999) Identification of dividing determined sensory neurons precursors in the mammalian neural crest. Development 126: 3545-3559. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW (1998) The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125: 2315-2325. [DOI] [PubMed] [Google Scholar]
- Heblich F, England S, Docherty RJ (2001) Indirect actions of bradykinin on neonatal rat dorsal root ganglion neurones: a role for non-neuronal cells as nociceptors. J Physiol (Lond) 536: 111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazin EE, Soderstrom S, Ebendal T, Larhammar D (1997) Embryonic expression of the mRNA for the rat homologue of the fusin/CXCR4 HIV-1 coreceptor. J Neuroimmunol 79: 148-154. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Mc Kerny DD, Julius D (2003) Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol 13: 487-492. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A (2003) Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol 54: 287-296. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Farinas I (2002) Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci 59: 1787-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull CE (2001) Segmental organization of neural crest migration. Mech Dev 105: 37-45. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M (1998) Sox 10, a novel transcriptional modulator in glial cells. J Neurosci 18: 237-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dourain NM, Kalcheim C (1999) From the neural crest to the ganglia of the peripheral nervous system: the sensory ganglia. In: The neural crest, Ed 2, pp 153-194. Cambridge, UK: Cambridge UP.
- Le Dourain NM, Creuzet S, Couly G, Dupin E (2004) Neural crest cell plasticity and its limits. Development 131 4637-4649. [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L (2004) Instructive role of Wnt/b-catenin in sensory fate specification in neural crest stem cells. Science 303: 1020-1023. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ (2002) Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA 99: 7090-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ (1999) Neurogenin1 and neurogenin 2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev 13: 1717-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P (2004) Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci 7: 930-938. [DOI] [PubMed] [Google Scholar]
- Maschoff KL, Baldwin HS (2001) Molecular determinants of neural crest migration. Am J Med Genet 97: 280-288. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J (1999) Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 213: 442-456. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ (1998) Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA 95: 14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Reid K, Ford M, Furness JB, Bartlett PF (1994) FGF2 regulates proliferation of neural crest cells, with subsequent neuronal differentiation regulated by LIF or related factors. Development 120: 3519-3528. [DOI] [PubMed] [Google Scholar]
- Namaka MP, Sawchuk M, MacDonald SC, Jordan LM, Hochman S (2001) Neurogenesis in postnatal mouse dorsal root ganglia. Exp Neurol 172: 60-69. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21: 5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Anderson DJ (1997) Immortalization and controlled in vitro differentiation of murine multipotent neural crest stem cells. J Neurobiol 32: 722-746. [DOI] [PubMed] [Google Scholar]
- Santiago A, Erickson CA (2002) Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development 129: 3621-3623. [DOI] [PubMed] [Google Scholar]
- Schafer KH, Hagi CI, Rauch U (2003) Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int 19: 340-344. [DOI] [PubMed] [Google Scholar]
- Snider WD, Wright DE (1996) Neurotrophins cause a new sensation. Neuron 16: 229-232. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ (1998) SOX10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18: 60-64. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ (1992) Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71: 973-985. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ (2003) Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci 4: 444-455. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren DJ, Veldhouse T, Miller RJ (2004) Chemokine receptors are widely expressed by embryonic and adult neural cell cells. J Neurosci Res 76: 20-34. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG (1992) Whole mount in situ hybridization of vertebrate embryos. In: In situ hybridization: a practical approach (Wilkinson DG, ed), pp 75-83. Oxford: IRL.
- Zicha D, Dunn GA, Jones GE (1997) Analyzing chemotaxis using the Dunn direct viewing chamber. In: Basic cell culture protocols (Pollard JW, Walker JM, eds), pp 769-775. Totowa, NJ: Humana. [DOI] [PubMed]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR (1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595-599. [DOI] [PubMed] [Google Scholar]