Abstract
Objective
This prospective, post-marketing study collected sunitinib safety and efficacy data in Japanese patients with unresectable/metastatic renal cell carcinoma. Retrospective analysis investigated adverse events as potential sunitinib efficacy biomarkers.
Methods
Patients administered sunitinib, after its release, were registered until reaching a pre-specified number of cases. Primary starting dose was 50 mg/day orally on a 4-weeks-on and 2-weeks-off schedule. Physicians completed investigation forms at 6-week intervals for 24 weeks. Associations between baseline characteristics and adverse events were analyzed by Cox proportional hazards model and compared by χ2 test. The log-rank test compared survival in subpopulations based on selected factors.
Results
Of note, 1689 patients receiving sunitinib were registered between June 2008 and November 2009. Most of them were males (75%), aged <65 years (56%), and had Eastern Cooperative Oncology Group performance status 0/1 (90%), metastatic disease (88%) and previous systemic therapy (66%). Grade ≥3 adverse events occurred in 70%, with reduced platelet count the most common (34%). Characteristics significantly associated with Grade ≥3 adverse events were female sex, age ≥55 years, Eastern Cooperative Oncology Group performance status ≥2, history of several medical conditions and prior treatment. Objective response rate was 22%. Median progression-free survival was 22.7 weeks. Median overall survival was not reached; however, 24-week overall survival rate was 84%. Improved overall survival was associated with higher relative dose intensity during the first 6 weeks and specific adverse events: hypertension, hand–foot syndrome, hypothyroidism, leukopenia and thrombocytopenia.
Conclusions
Sunitinib demonstrated acceptable safety and useful efficacy in Japanese patients with unresectable/metastatic renal cell carcinoma. Potential biomarkers associated with greater efficacy were relative dose intensity and specific adverse events.
Keywords: renal cell carcinoma, Japanese, sunitinib, real world, biomarkers
Introduction
Sunitinib malate (Pfizer Inc., New York, NY, USA), an oral multitargeted inhibitor of vascular endothelial growth factor (VEGF) receptors and other receptor tyrosine kinases, was approved in Japan in April 2008 for the treatment of unresectable or metastatic renal cell carcinoma (RCC) and imatinib-resistant/intolerant gastrointestinal stromal tumor (GIST). Approval in RCC was based primarily on a global Phase III clinical registration trial in treatment-naïve patients with metastatic RCC (N = 750) in which sunitinib significantly improved progression-free survival (PFS) compared with interferon-α (IFN-α) (median PFS 11 vs. 5 months; P < 0.001), as well as objective response rate (ORR; 47 vs. 12%; P < 0.001) (1,2). In addition, median overall survival (OS) was 26.4 and 21.8 months with sunitinib and IFN-α, respectively (2). A Phase II clinical trial has also demonstrated the efficacy and tolerability of sunitinib in Japanese patients with metastatic RCC (N = 51) (3,4).
Post-marketing research to monitor possible adverse drug reactions has become a common requirement of regulatory approvals worldwide, particularly for oncology drugs, which often receive accelerated approval on the basis of a limited number of clinical trials. Post-marketing research allows evaluation of the use of a drug in much larger numbers of patients encompassing broader patient populations over longer durations and under less controlled conditions than do registrational clinical trials. A post-marketing activity that is particular to Japan is known as all-cases or all-patient surveillance, which entails registering and monitoring all patients who are prescribed a drug after marketing authorization until a pre-specified number of patients have been registered. This type of post-marketing study, which has become a requirement for most oncology drugs in Japan, facilitates faster and more accurate evaluation of adverse drug reactions than does standard post-marketing surveillance. Importantly, the Japanese system also allows assessment of drug efficacy in a ‘real-world’ non-trial setting.
An all-patient post-marketing survey was a regulatory requirement of the Pharmaceuticals and Medical Devices Agency (PMDA) at the time of Japanese approval of sunitinib and was conducted to expand the sunitinib safety database for the Japanese population and ensure appropriate usage of sunitinib after approval in Japan. The overall objective of the study was to collect and report sunitinib safety and efficacy data in Japanese patients with unresectable or metastatic RCC or imatinib-resistant/intolerant GIST. Results obtained in patients with RCC are reported here, while those obtained in patients with GIST will be reported elsewhere. In addition, based on prior studies that have identified treatment-associated adverse events (AEs) as potential biomarkers of sunitinib efficacy (5–9), a retrospective exploratory analysis was conducted using data from this study to investigate whether such AEs are also correlated with sunitinib efficacy in Japanese patients with advanced RCC. Given the increasing incidence of, and deaths due to, kidney cancer in Japan (10,11), an improved understanding of the clinical benefit with sunitinib, a reference standard of care, and its potential predictive biomarkers is critical to optimize therapy in this population.
Patients and methods
Study design and treatment
In this prospective post-marketing study, all patients treated with sunitinib in Japan after 13 June 2008 (the release date for sunitinib in Japan), were registered in a central system until a pre-specified number of cases accumulated. It was recommended that all patients begin treatment with sunitinib at a starting dose of 50 mg once-daily orally on Schedule 4/2 (4 weeks on treatment followed by 2 weeks off) in repeating 6-week cycles, although lower starting doses were used in some patients according to physician judgment. The safety and efficacy of sunitinib were observed in registered patients for 24 weeks from the start of treatment or until treatment was discontinued if sooner. Physicians were required to complete an investigation form at 6-week intervals during the 24-week observation period. Patients treated for 24 weeks were followed for up to 2 years. The study conformed to Good Post-Marketing Study Practice guidelines.
Specific objectives of this study included recording the incidences of AEs of Grade ≥3, according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, unexpected or unexpectedly severe AEs, and serious adverse events (SAEs), according to Japanese government regulations for reporting adverse drug reactions (12); investigating associations between baseline patient characteristics and AE incidences; and exploring the influence of several factors [baseline characteristics, relative dose intensity (RDI), and AE emergence] on sunitinib efficacy in Japanese patients with unresectable or metastatic RCC.
Analytical and statistical methods
Safety was assessed according to both the criteria indicated by the Japanese government and CTCAE version 3.0; however, only results according to CTCAE version 3.0 are reported here. The incidences (%) of all AEs observed were reported. Associations between baseline characteristics and AEs were analyzed using incidence rates (in person-years). The characteristics analyzed were sex, age, pregnancy status, compliance with proper use standards [i.e. patients must have had Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, resolution of acute toxicity of prior treatment, left ventricular ejection fraction of not less than the lower limit of normal, neutrophil count ≥1500/μl, platelet count ≥10 × 104/μl, hemoglobin ≥9.0 g/dl, transaminases ≤2.5 times the upper limit of normal (ULN), total bilirubin ≤1.5× ULN, serum creatinine ≤1.5× ULN, albumin ≥3.0 g/dl, serum calcium ≤12.0 mg/dl and thyroid stimulating hormone ≤ULN], prior experience of using the product, body surface area, target disease, presence/absence of metastases, disease stage, morbid period, hospitalization status, treatment history, medical history, complications, presence of hepatic dysfunction, severity of hepatic dysfunction, presence of renal impairment, severity of renal impairment, presence/absence of wound complications, concomitant drugs, use of CYP3A4 inhibitors, non-drug therapy, ECOG PS and starting dose. AE incidence rates were compared using the χ2 test. In addition, a multivariate Cox proportional hazards model was used to investigate associations between baseline characteristics and other factors and AE development.
Efficacy endpoints included ORR and rates of PFS and OS at 24 weeks. Tumor response was assessed by investigators using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0, although these assessments were not obligatory. Central review of investigator efficacy assessments was not performed. PFS was defined as the time from start of sunitinib treatment until either progressive disease or death. The Kaplan–Meier method was used to estimate PFS and OS, and the log-rank test was used to compare PFS and OS in subpopulations based on selected factors, including RDI and emergence of specific AEs. (For the determination of RDI during the first 6 weeks of sunitinib therapy, sunitinib at 50 mg/day on Schedule 4/2 was considered the planned dosing schedule based on the PMDA-approved dose/schedule in RCC.)
Results
Among 1689 patients who received sunitinib treatment and were registered in the survey between June 2008 and November 2009, 16 patients were not included in the analysis because their investigation forms had not been collected. Among the 1673 patients for whom investigation forms were collected, 2 were excluded from the safety analysis (due to a protocol violation in one case and absence of confirmed safety data in the other) and an additional 236 were excluded from the efficacy analyses (six cases involved off-label use of sunitinib and 230 were not evaluable for efficacy).
Baseline patient characteristics and details of sunitinib treatment and patient disposition for the 1671 patients in the safety analysis population are provided in Table 1. The majority of the patients were male (75%), were <65 years of age (56%), had an ECOG PS of 0 or 1 (90%), had metastatic disease (88%), and had received previous systemic therapy (66%). Seventy-eight percent of patients started sunitinib treatment at a dose of 50 mg/day, and the mean RDI of patients during the first 6 weeks of treatment was 73%. Sixty percent of patients discontinued treatment, among whom 40% (24% overall) discontinued due to AEs. At the end of the 24-week observation period, 48% of patients were still on treatment.
Table 1.
Baseline patient characteristics and sunitinib treatment and disposition
| Sunitinib (N = 1671) | |
|---|---|
| Characteristic | |
| Male/female, n (%) | 1250/421 (75/25) |
| Median age (range), years | 62 (13–88) |
| Age <65/≥65 years, n (%)a | 939/712 (56/43) |
| ECOG performance status, n (%)b | |
| 0 | 938 (56) |
| 1 | 574 (34) |
| ≥2 | 154 (9) |
| Median body surface area (range), m2 | 1.6 (1.1–2.3) |
| Median height (range), m | 1.64 (1.34–1.86) |
| Median body weight (range), kg | 59 (29–112) |
| Metastatic disease, n (%) | 1476 (88) |
| Common sites of metastases, n (%) | |
| Lung | 1089 (65) |
| Bone | 520 (31) |
| Liver | 288 (17) |
| Previous radiotherapy, n (%) | 332 (20) |
| Previous systemic therapy, n (%) | 1101 (66) |
| IFN-α | 917 (55) |
| Sorafenib | 565 (34) |
| Interleukin-2 | 346 (21) |
| Treatment and disposition | |
| Patients with dose reductions, n (%)c | 973 (58) |
| Starting dose 50 mg (n = 1308) | 843 (64) |
| Starting dose 37.5 mg (n = 218) | 112 (51) |
| Starting dose 25 mg (n = 130) | 18 (14) |
| Other (n = 15) | 0 |
| Mean RDI by observation period, % | |
| Start through week 6 (n = 1618) | 73 |
| Weeks 7–12 (n = 1340) | 67 |
| Weeks 13–18 (n = 1110) | 63 |
| Weeks 19–24 (n = 951) | 60 |
| Patients who discontinued treatment, n (%) | 997 (60) |
| Reason for discontinuation, n (%)d | |
| AE | 394 (24) |
| Lack of efficacy | 368 (22) |
| Death | 184 (11) |
| Lost to follow-up | 59 (4) |
| Abnormal laboratory test value | 49 (3) |
| Other | 112 (7) |
ECOG, Eastern Cooperative Oncology Group; IFN-α, interferon-α; RDI, relative dose intensity.
aData missing for 20 patients (1%).
bData missing for 5 patients (<1%).
cRelative to the total number of patients at each dose level.
dRelative to the total number of patients who discontinued.
Safety
Treatment-related AEs were reported in 95% of patients (Table 2), with the most frequent by far being reduced platelet count (61%). Hand–foot syndrome, hypothyroidism, hypertension and reduced white blood cell count (33–37%) were also among the most commonly reported. Grade ≥3 AEs were reported in 70% of patients, with reduced platelet count again being the most frequent by far (34%). The next most common Grade ≥3 AEs were hypertension, reduced white blood cell count and reduced neutrophil count (10, 10 and 8%, respectively). Grade ≥3 AEs tended to occur early: within the first 4 weeks of treatment (Supplementary data, Fig. S1).
Table 2.
Most commonly reported treatment-related adverse events (AEs) of any grade or Grade ≥3 (N = 1671)
| No. of patients (%) |
||
|---|---|---|
| AE | Any grade | Grade ≥3 |
| Any AE | 1594 (95) | 1163 (70) |
| Platelet count decreaseda | 1019 (61) | 574 (34) |
| Hand–foot syndrome | 616 (37) | 93 (6) |
| Hypothyroidism | 593 (35) | 44 (3) |
| Hypertension | 584 (35) | 168 (10) |
| White blood cell count decreased | 547 (33) | 169 (10) |
| Stomatitis | 281 (17) | 21 (1) |
| Diarrhea | 271 (16) | 34 (2) |
| Pyrexia | 234 (14) | 20 (1) |
| Neutrophil count decreaseda | 224 (13) | 131 (8) |
| Decreased appetite | 211 (13) | 57 (3) |
| Anemiaa | 193 (12) | 76 (5) |
| Thrombocytopeniaa | 147 (9) | 83 (5) |
| Lipase increased | 195 (12) | 79 (5) |
| Malaise | 173 (10) | 53 (3) |
| Hemoglobin decreaseda | 91 (5) | 24 (1) |
| Neutropeniaa | 33 (2) | 24 (1) |
aRelated AE terms (e.g. platelet count decreased and thrombocytopenia) reflect identification by laboratory abnormality data or physician assessment, respectively.
A total of 253 patients (15%) died on study. Of these deaths, 216 were deemed to be unrelated to treatment, while the remaining 37 were considered to be possibly, probably or definitely related to treatment. These were most commonly due to disseminated intravascular coagulation, interstitial lung disease or reduced platelet count (three patients each; the last of these was also associated with disease progression in two cases and gastrointestinal hemorrhage in one case).
Association of patient baseline characteristics with incidences of adverse events
A wide variety of demographic and other baseline characteristics were evaluated to identify any associated with the development of AEs. Characteristics significantly associated with the development of Grade ≥3 AEs after adjusting for confounding factors in multivariate analysis were female sex, age ≥55 years, baseline ECOG PS ≥2, a history of several medical conditions [ischemic coronary artery disorder, depressive disorder, hepatic dysfunction or renal impairment (based on investigators’ assessment)], and prior treatment history (prior non-drug therapy [such as radiotherapy, oxygen supplementation or thoracic cavity drainage, among many others] or no prior sorafenib treatment; Table 3).
Table 3.
Baseline characteristics significantly associated with rate of development of Grade ≥3 AEs
| Characteristic | Grade ≥3 AE incidence rate, pts/1000 pd | Adjusted HRa,b (95% CI) | P valuec |
|---|---|---|---|
| Sex | |||
| Female | 14.4 | 1.24 (1.04–1.48) | 0.016 |
| Male | 10.1 | ||
| Age, years | |||
| ≥65 | 14.8 | 1.30 (1.08–1.57)d | 0.006d |
| 55–<65 | 9.4 | 1.81 (1.50–2.19)d | <0.001d |
| <55 | 8.2 | ||
| Baseline ECOG PS | |||
| ≥2 | 11.6 | 0.76 (0.58–0.98) | 0.035e |
| 1 | 12.0 | 1.00 (0.87–1.15) | 0.959e |
| 0 | 10.3 | ||
| Ischemic coronary artery disorders | |||
| Yes | 30.7 | 1.77 (1.08–2.88) | 0.022 |
| No | 10.8 | ||
| Depressive disorders | |||
| Yes | 26.5 | 1.50 (1.05–2.14) | 0.027 |
| No | 10.8 | ||
| Hepatic dysfunction | |||
| Yes | 12.3 | 1.25 (1.01–1.55) | 0.044 |
| No | 10.9 | ||
| Renal impairment | |||
| Yes | 12.2 | 1.19 (1.02–1.39) | 0.028 |
| No | 10.7 | ||
| Prior treatment with sorafenib | |||
| Yes | 9.3 | 0.84 (0.72–0.97) | 0.021 |
| No | 12.0 | ||
| Non-drug therapyf | |||
| Yes | 14.7 | 1.39 (1.20–1.61) | <0.001 |
| No | 10.1 | ||
CI, confidence interval; HR, hazard ratio; pts/1000 pd, number of patients/1000 person-days.
aHR <1 denotes risk reduction for the first category and HR >1 denotes risk reduction for the second category.
bObtained using a multivariate Cox proportional hazards model.
cχ2 test.
dVersus <55 years.
eVersus ECOG PS 0.
fSuch as radiotherapy, oxygen supplementation or thoracic cavity drainage, among many others.
Objective tumor response
Among the 1435 patients in the efficacy analysis population, 8 (1%) achieved complete responses, 304 (21%) partial responses and 578 (40%) stable disease, while 162 patients (11%) were not evaluable for response. The ORR was 22% [95% confidence interval (CI), 20–24]. Multivariate analysis showed that ORR differed significantly between patients with and without hepatic dysfunction (12 vs. 23%, respectively; P = 0.013). Similarly, ORR differed significantly between patients with an ECOG PS of 0 and ≥1 (26 vs. 15%; P = 0.010).
Progression-free survival
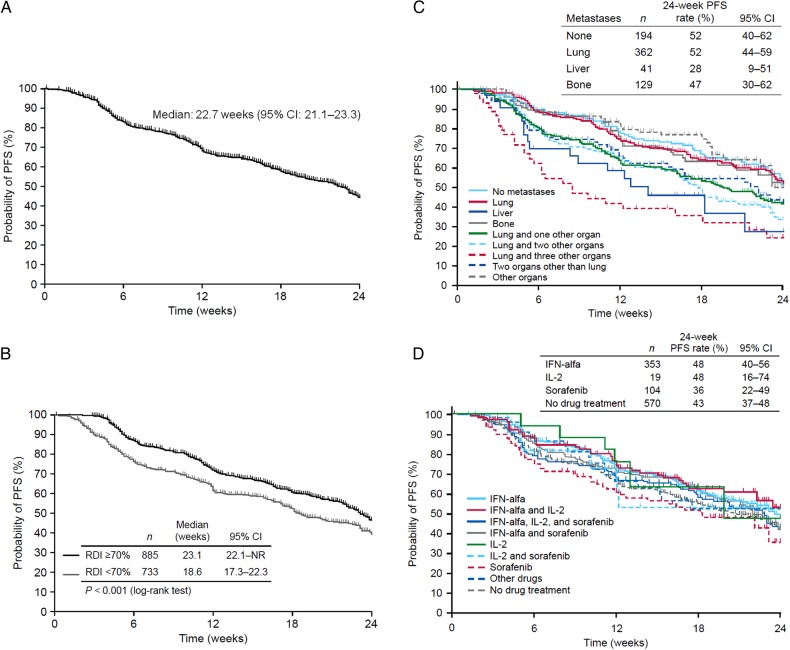
Median PFS in the study was 22.7 weeks (95% CI, 21.1–23.3; Fig. 1A); median duration of treatment was 22.1 weeks (95% CI, 21.1–24.3). Subgroup analysis showed that median PFS across the 24-week observation period was significantly longer among patients with an RDI of ≥70% than <70% during the first 6 weeks of treatment (23.1 vs. 18.6 weeks; P < 0.001; Fig. 1B). An analysis of PFS by metastatic site at baseline is shown in Fig. 1C. The PFS rate at 24 weeks in patients with lung metastases only (52%; 95% CI, 44–59) was nearly identical to that of patients with no metastases (52%; 95% CI, 40–62) and among the highest of all subgroups evaluated. PFS based on prior systemic treatment is shown in Fig. 1D. The 24-week PFS rate was numerically lower among patients who had received prior sorafenib (36%) or no prior systemic therapy (43%) than among those who had received IFN-α or Interleukin-2 (IL-2) (48% each); however, 95% CIs overlapped for all of these values.
Figure 1.
Progression-free survival through Week 24 from the start of sunitinib treatment. (A) Safety analysis population (N = 1671). (B) By relative dose intensity (RDI) during the first 6 weeks of treatment among 1618 patients with dose/duration data for this period. (C) By metastatic site at baseline. (D) By prior systemic drug treatment.
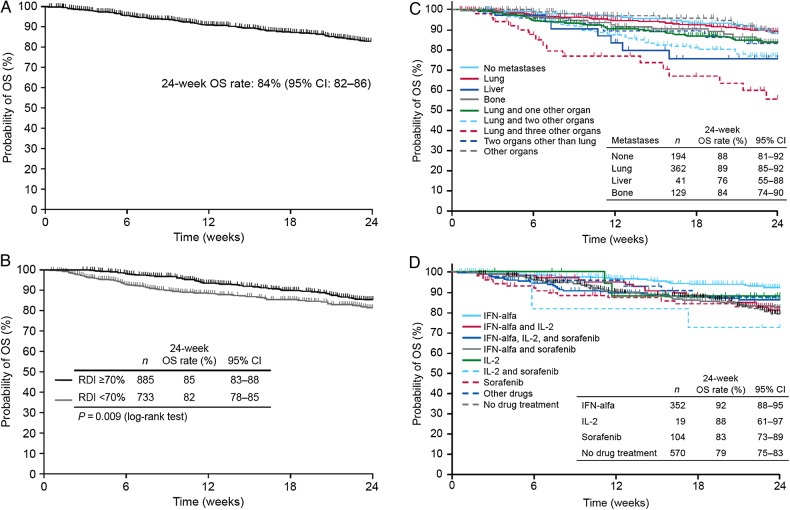
Overall survival
Median OS was not reached by the end of the 24-week observation period (Fig. 2A). The rate of OS at 24 weeks was 84% (95% CI, 82–86). In subgroup analysis, the 24-week OS rate was nominally higher among patients with an RDI ≥70% than <70% during the first 6 weeks of treatment (85 vs. 82%, respectively), although this difference was statistically significant (P = 0.009; Fig. 2B); consistent with this, the 24-week OS rate was also higher in patients with an RDI ≥70% than <70% during the first 12 weeks of treatment (data not shown). Twenty-four-week OS rates among patients with lung metastases at baseline (89%) were similar to those of patients without metastases (88%) and were numerically among the highest of the subgroups analyzed (Fig. 2C). Rates among patients with liver or bone metastases were 76 and 84%, respectively. Ninety-five percent CIs for all of these values overlapped. OS based on prior systemic treatment is shown in Fig. 2D. The 24-week OS rate was highest among patients who had received prior IFN-α (92%; 95% CI, 88–95), while that of patients who had received no prior systemic therapy was significantly lower (79%; 95% CI, 75–83). Rates among patients who had received prior IL-2 or sorafenib were intermediate (88 and 83%, respectively, with overlapping 95% CIs).
Figure 2.
Overall survival through Week 24 from the start of sunitinib treatment. (A) Safety analysis population (N = 1671). (B) By RDI during the first 6 weeks of treatment among 1618 patients with dose/duration data for this period. (C) By metastatic site at baseline. (D) By prior systemic drug treatment.
Association of adverse events with overall survival
The possible association of the development of specific AEs with prolonged survival was evaluated (Table 4). The 24-week OS rate was significantly higher among patients who had the following AEs than among those who did not: hand–foot syndrome (94 vs. 77%; P<0.001), hypertension (87 vs. 76%; P < 0.001), hypothyroidism (87 vs. 82%; P = 0.003), leukopenia (91 vs. 78%; P < 0.001) or thrombocytopenia (87 vs. 76%; P < 0.001). Among AEs analyzed, only anemia did not appear to be associated with higher 24-week OS rates.
Table 4.
Association of the development of AEs with 24-week OS rates
| AE (n) | 24-week OS rate, % | 95% CI | P valuea |
|---|---|---|---|
| Anemia | |||
| Present (362) | 84 | 79–88 | 0.733 |
| Absent (1308) | 84 | 82–86 | |
| Hand–foot syndrome | |||
| Present (607) | 94 | 92–96 | <0.001 |
| Absent (1063) | 77 | 73–80 | |
| Hypertension | |||
| Present (774) | 87 | 84–89 | <0.001 |
| Absent (337) | 76 | 70–81 | |
| Hypothyroidism | |||
| Present (653) | 87 | 84–90 | 0.003 |
| Absent (1017) | 82 | 79–85 | |
| Leukopenia | |||
| Present (747) | 91 | 88–93 | <0.001 |
| Absent (923) | 78 | 74–81 | |
| Thrombocytopenia | |||
| Present (1165) | 87 | 85–89 | <0.001 |
| Absent (505) | 76 | 71–80 | |
OS, overall survival.
aLog-rank test.
Results were similar for analysis of the associations between these AEs and PFS, with the exception that the presence of anemia was also significantly associated with an improved 24-week PFS rate (data not shown).
Discussion
In this post-marketing all-cases survey, sunitinib demonstrated acceptable safety and useful efficacy in Japanese patients with unresectable or metastatic RCC. Grade ≥3 AEs tended to occur early, within the first 4 weeks of treatment, with few occurring after this time point. This may have been the result of successful management of AEs through dose reductions. In addition, no new or unexpected AEs emerged during this study. With median OS not reached, comparisons with previous efficacy results are difficult; however, the 24-week OS rate of 84% compared favorably with previously published 1-year OS rates (68%) (13,14). In addition, results from exploratory biomarker analyses were consistent with prior studies, identifying potential AE biomarkers for improved survival with sunitinib, including hypertension, hand–foot syndrome, hypothyroidism and cytopenias.
The safety profile observed in the present study was broadly similar to that previously reported in Japanese or Asian patients (4,14–16). For example, the frequency of discontinuations due to AEs (24%) was comparable to that in most studies that specifically assessed Asian patients (22–25%) (4,14,16), as well as that of the international Phase III study in treatment-naïve RCC (19%) (2). As given in previous reports, myelosuppression, hand–foot syndrome and hypertension were among the most commonly reported AEs, including Grade ≥3 AEs. However, in comparison with earlier studies, fatigue, skin discoloration, anorexia and gastrointestinal disorders were not reported as frequently. Additionally, previous reports did not identify hypothyroidism as a very common AE among patients with RCC. Such discrepancies may be due to differences in clinical priority assigned to AEs by physicians in a ‘real-world’ setting as part of a post-marketing survey or increased familiarity with use of sunitinib and subsequent improvement in AE management compared with earlier studies. Finally, the incidences of Grade ≥3 AEs were affected by several demographic and baseline characteristics, including gender, age, performance status and renal impairment.
Results of this study, like previous studies, suggested that while the safety profile of sunitinib in Asian patients is broadly similar to that in non-Asian patients, there may be some differences (4,14–16). Myelosuppressive AEs and hand–foot syndrome, in particular, have been observed at relatively high frequencies and with greater severity in Asian patients than in non-Asian patients in this and other studies, particularly in those studies that have compared these populations directly (4,14–17). For example, Grade ≥3 reduced platelet count occurred in 34% of the patients in the present study compared with 9% of patients receiving sunitinib in the Western Phase III study (2).
The ORR obtained in the present study (22%) was lower than those previously reported in several international studies or those involving Asian patients (35–53%) (2,4,13,14). However, a large international expanded-access trial with 3464 evaluable patients reported an ORR with single-agent sunitinib that was comparable to that of the present study (17%) (18). The conduct of this global Phase IIIb/4 study was similar in a number of respects to the present postmarking study; the ORRs may therefore reflect a lack of specific requirements for evaluation of response in both studies (e.g. the frequency of computed tomography imaging is not strictly specified). The median PFS was shorter in the present study (22.7 weeks or 5.2 months) than was reported in a number of other studies (9–12 months) (2,4,13,14,18). This may be due to differences in everyday clinical practice, as compared with clinical trials, in which patients and physicians may be more likely to discontinue treatment for various reasons.
A number of factors were found to be associated with efficacy in this study. Consistent with previously reported prognostic models (19,20), patients with a baseline ECOG PS of 0 achieved greater ORR, as did patients without hepatic dysfunction. In addition, prolonged PFS and OS were associated with higher RDI after the first 6 weeks of treatment, which is consistent with a previously reported pharmacokinetic–pharmacodynamic meta-analysis of several studies in predominately non-Asian patients with a variety of solid tumors that showed that sunitinib efficacy correlated with increased drug exposure (21). Analysis of RDI during the first 12 weeks of treatment produced a similar finding (data not shown). In addition, consistent with previous studies involving predominately non-Asian patients (5–9), the emergence of certain AEs (hypertension, hand–foot syndrome, hypothyroidism, leukopenia and thrombocytopenia) was associated with improved survival.
Of note, in patients with lung metastases only at baseline, clinical outcomes (PFS and OS) were found to be little different than those of patients without metastases, suggesting that sunitinib is an appropriate treatment for these patients. A recent retrospective analysis of the global Phase III and Japanese Phase II RCC studies also demonstrated sunitinib efficacy in patients in whom lung was the sole site of metastasis (22).
Limitations of this study include the lack of outcomes data for prior therapies, lack of a comparator arm, independent review of tumor response and standardized safety and efficacy assessment (e.g. not all physicians may have strictly applied RECIST), as well as a relatively high level of missing efficacy data (14% of patients). Moreover, interpretation of OS data was made more difficult due to the short follow-up period in this study, especially since the observed OS was longer than had been expected from previously published data. In addition, there are limitations specific to the AE biomarker analyses that may have confounded the results, including variability in AE assessment among physicians and lack of pharmacokinetic data coinciding with the occurrence of each AE biomarker, precluding analysis of the potential impact of drug exposure (i.e. the relationship between each AE biomarker and RDI). Furthermore, given the potential confounding influence of post-sunitinib treatment, predictive value for OS alone may not be evidence of specific biomarkers for sunitinib; instead, these AEs may be predictors of survival for a generalized class of therapies (e.g. VEGF inhibitors) or may be more prognostic for outcome based on patient and/or disease characteristics.
The standard dosing regimen for sunitinib is 50 mg/day orally on a 4-weeks-on and 2-weeks-off schedule; however, the difficulty of strict adherence to this schedule is now widely accepted. In this study, we did not collect individual patient data on precise dosing schedules, which were likely modified by the treating physicians. Thus, the optimal dosing schedule for sunitinib remains an open question for further research (23–25).
In summary, the results of this study confirm the acceptable safety and useful efficacy of sunitinib in a large robust population of patients with unresectable or metastatic RCC who reflect the broader patient population found in the ‘real-world’ setting in Japan. Such a post-marketing study also better reflects everyday clinical practices than those used in randomized controlled trials. Finally, in addition to confirming the clinical benefit with sunitinib in this population, this study has provided important hypothesis-generating and supporting information on predictive markers for efficacy and prognosis, which may help individualize and optimize use of sunitinib in this RCC patient population.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Funding
This work was supported by Pfizer Inc. Funding to pay the Open Access publication charges for this article was provided by Pfizer.
Conflict of interest statement
Hideyuki Akaza has received consulting fees from Pfizer, Bayer and GlaxoSmithKline. Seiji Naito has received consulting fees from Pfizer, Novartis and GlaxoSmithKline. Naomi Ueno, Kouji Aoki, Hiroyuki Houzawa and Sang-Yoon Lee are full-time Pfizer employees and own Pfizer stock. Susan Pitman Lowenthal was an employee of Pfizer; when these analyses were conducted, Susan Pitman Lowenthal owns Pfizer stock.
Supplementary Material
Acknowledgements
We thank all of the patients and their families, and the investigators, research nurses, study coordinators and operations staff. Medical writing support was provided by Andy Gannon and Wendy Sacks at ACUMED®, part of the KnowledgePoint360 Group, an Ashfield company (New York, NY, USA) with funding from Pfizer Inc.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 2010;40:194–202. [DOI] [PubMed] [Google Scholar]
- 4.Tomita HY, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010;40:1166–72. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaelson MD, Cohen DP, Li S, et al. Hand-foot syndrome (HFS) as a potential biomarker of efficacy in patients (pts) with metastatic renal cell carcinoma (mRCC) treated with sunitinib (SU). J Clin Oncol 2011;29(Suppl. 7; abstract 320). [Google Scholar]
- 7.Dienstmann R, Braña I, Rodon J, Tabernero J. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist 2011;16:1729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donskov F, Carus A, Barrios CH, et al. Neutropenia and thrombocytopenia during treatment as biomarkers of sunitinib efficacy in patients with metastatic renal cell carcinoma. Poster presented at the 2011 European Multidisciplinary Cancer Congress, Stockholm, Sweden, September 23–27, 2011. (Abstract 1141). [Google Scholar]
- 9.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer 2011;117:534–44. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. GLOBOCAN 2008. Available at: http://globocan.iarc.fr/.
- 11.World Health Organization. GLOBOCAN 2012. Available at: http://globocan.iarc.fr/.
- 12.Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare of Japan. Revision of the Standard Operating Procedure for Adverse Drug Reaction, Infection and Defect Reports for Medicines or Medical Devices from Medical Institutions. Notification No. 0729-2. July 29, 2010. Available at: http://www.info.pmda.go.jp/iyaku/file/h220729-001.pdf.
- 13.Barrios CH, Hernandez-Barajas D, Brown MP, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer 2012;118:1252–9. [DOI] [PubMed] [Google Scholar]
- 14.Yoo C, Kim JE, Lee JL, et al. The efficacy and safety of sunitinib in Korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol 2010;40:980–5. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Chung HC, Mainwaring P, et al. An Asian subpopulation analysis of the safety and efficacy of sunitinib in metastatic renal cell carcinoma. Poster presentation at the joint 15th Congress of the European Cancer Organisation and 34th Congress of the European Society for Medical Oncology, Berlin, Germany, September 20–24, 2009. (abstract 7118). [Google Scholar]
- 16.Barrios CH, Hernandez-Barajas D, Brown MP, et al. Clinical outcome of Asian vs. non-Asian patients with metastatic renal cell carcinoma with continuous once-daily dosing of sunitinib as first-line therapy. Poster presentation at the European Society for Medical Oncology 35th (ESMO) Congress, Milan, Italy, October 8–12, 2010. (abstract 913P). [Google Scholar]
- 17.Li XS, Song Y, Gong K, et al. Clinical study of sunitinib in the treatment of metastatic renal clear cell carcinoma: a single center 23 cases experience. Zhonghua Wai Ke Za Zhi 2010;48:375–7. [PubMed] [Google Scholar]
- 18.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 2009;10:757–63. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96. [DOI] [PubMed] [Google Scholar]
- 20.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 21.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357–71. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara N, Akaza H, Tomita Y, et al. Efficacy and safety of sunitinib (SU) in patients (pts) with metastatic renal cell carcinoma (mRCC) and lung metastases (mets) only at baseline. J Clin Oncol 2012;30(suppl) (abstract e15098). [Google Scholar]
- 23.Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer 2014;50:1084–9. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnason GA, Khalil B, Hudson JM, et al. Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol 2014;32:480–7. [DOI] [PubMed] [Google Scholar]
- 25.Tan HS, Li H, Hong YW, et al. Efficacy and safety of an attenuated-dose sunitinib regimen in metastatic renal cell carcinoma: results from a prospective registry in Singapore. Clin Genitourin Cancer 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.