Abstract
Coupling of G proteins to ligand-engaged chemokine receptors is the paramount event in G-protein-coupled receptor signal transduction. Previously, we have demonstrated that the human cytomegalovirus-encoded chemokine receptor US28 mediates human vascular smooth muscle cell (SMC) migration in response to either RANTES or monocyte chemoattractant protein 1. In this report, we identify the G proteins that couple with US28 to promote vascular SMC migration and identify other signaling molecules that play critical roles in this process. US28-mediated cellular migration was enhanced with the expression of the G-protein subunits Gα12 and Gα13, suggesting that US28 may functionally couple to these G proteins. In correlation with this observation, US28 was able to activate RhoA, a downstream effector of Gα12 and Gα13 in cell types with these G proteins but not in those without them and activation of RhoA was dependent on US28 stimulation with RANTES. In addition, inactivation of RhoA or the RhoA-associated kinase p160ROCK with a dominant-negative mutant of RhoA or the small molecule inhibitor Y27632, respectively, abrogated US28-induced SMC migration. The data presented here suggest that US28 functionally signals through Gα12 family G proteins and RhoA in a ligand-dependent manner and these signaling molecules are important for the ability of US28 to induce cellular migration.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus with an incidence of infection ranging between 40 and 100%, depending on age and socioeconomic status of the infected individual. Infection generally occurs during childhood, and after primary infection, HCMV establishes a lifelong persistence in the host. HCMV infection of healthy immunocompetent individuals is commonly asymptomatic. However, HCMV may cause life-threatening disease in immunocompromised patients (6), and HCMV is the leading cause of birth defects in virally infected neonates (11). HCMV has also been linked to long-term diseases, including atherosclerosis, arterial restenosis following angioplasty, and transplant vascular sclerosis associated with chronic allograft rejection (22-24, 26, 30, 31). A hallmark of all of these diseases is the migration of arterial smooth muscle cells (SMCs) from the vessel media to the intima, ultimately resulting in vessel occlusion.
HCMV infection of SMCs in vivo has been linked to a viral etiology of vascular disease. Previously, we have demonstrated that HCMV infection of primary arterial but not venous SMCs results in significant cellular migration (32). Similar to other herpesviruses, HCMV encodes chemokine receptor homologues, including UL33, UL78, US27, and US28. Ablation of one of these HCMV-encoded chemokine receptors, US28, within the virus, abrogates SMC migration, which is rescued only by expression of the viral homologue and not the cellular chemokine receptor CCR5 (32). US28 is structurally similar to the human chemokine receptor CCR1 (12) and binds chemokines of the β-class (CC), including monocyte chemoattractant protein-1 (MCP-1), MCP-3, RANTES, macrophage inflammatory polypeptides 1α (MIP-1α) and MIP-1β (5, 19, 29), and the CX3C chemokine fractalkine (15, 18). Expression of US28 in the presence of CC chemokines, including RANTES or MCP-1, was sufficient to promote SMC migration, which was inhibited by protein tyrosine kinase (PTK) inhibitors but not pertussis toxin (32). Recently, our group has demonstrated that US28 signals through the nonreceptor PTKs Src and focal adhesion kinase (FAK) and that this activity is necessary for US28-mediated SMC migration (33). US28-mediated SMC migration was inhibited by treatment with the Src inhibitor PP2, and through the expression of either of two dominant-negative (DN) inhibitors of FAK. US28 is the first viral G-protein-coupled receptor (GPCR) to be shown to mediate cellular movement, which is cell type specific and provides a molecular basis for the correlative evidence that links HCMV to the acceleration of vascular disease.
The first link between GPCRs and the intracellular signaling milieu are the heterotrimeric G proteins, consisting of a complex of α, β, and γ subunits. The Gα proteins are subdivided into one of four different subfamilies based on sequence similarities: Gαi, Gαq, Gα12, or Gαs. Upon ligand binding, GPCRs undergo conformational changes that promote interactions with α subunits of the heterotrimeric G-protein complex, which causes the exchange of GDP for GTP, initiating intracellular signaling cascades. The Gαi family (Gαi1, Gαi2, Gαi3, Gαo, and Gαz) has a wide tissue distribution and is pertussis toxin (PTX) sensitive. The only exceptions are Gαo and Gαz, which are PTX insensitive and limited to neuronal tissues (both Gαo and Gαz) and platelets (Gαz only) (10). Numerous hormones and inflammatory cytokines signal through Gαi pathways, as is evidenced by their PTX sensitivity (10, 25, 34). Signaling pathways activated by the ubiquitously expressed Gαs often stimulate the accumulation of cyclic AMP (cAMP) (10). Gαq family members activate a diverse array of signaling pathways that includes the activation of phospholipase C-β (PLC-β), producing the second messenger molecules inositol (1,4,5)-triphosphate and diacylglycerol. Gαq and Gα11 are ubiquitously expressed, while the other family members Gα14 (kidney, lung, spleen, and testis) and Gα15/16 (myeloid and lymphoid lineage cells) display a more limited tissue distribution (10). Gα12 family members Gα12 and Gα13 exhibit a ubiquitous tissue distribution (10) and interact with a number of different RhoGEFs stimulating robust RhoA signaling activity (7, 13, 14).
Cellular migration is an important process in a number of physiological responses, including leukocyte trafficking, the formation of secondary lymphoid organs, and remodeling of the vasculature. The most-well-characterized migratory cell type is leukocytes migrating in response to chemokines. The process of leukocyte migration and homing to secondary lymphoid tissues is highly complex, involving a multitude of chemokine receptors and chemokines from most of the chemokine families. In general, leukocyte migration induced by chemokine receptors is sensitive to PTX, suggesting a role for Gαi family G proteins in this process (2, 34). US28 has been shown to couple to a variety of different G proteins, including Gαi/o, Gα16, and Gαq/11, and different aspects of US28 signaling have been demonstrated to be either PTX sensitive or insensitive (4, 8). However, US28-mediated SMC migration has been shown to be insensitive to PTX, suggesting that Gαi is not involved in this event (32). Therefore, we examined the signaling components involved in US28-induced SMC migration. In this paper, we demonstrate that US28-induced SMC migration involves signaling through the Gα12 pathway.
MATERIALS AND METHODS
Reagents.
Recombinant human RANTES and PDGF-BB were purchased from R&D Systems (Minneapolis, Minn.). The following antibodies specific for Gα proteins were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif.: Gαi1 (I-20), sc-391; Gα12 (S-20), sc-409; Gα13 (A-20), sc-410; Gαq (E-17), sc-393; Gα11 (D-17), sc-394; and Gαz (I-20), sc-388. Anti-RhoA rabbit polyclonal antibody sc-179 was also purchased from Santa Cruz Biotechnology. Anti-flag M2 monoclonal antibody (F-3165) was purchased from Sigma (St. Louis, Mo.). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibodies used for Western blotting (NA934V and NA931V, respectively) were purchased from Amersham, Piscataway, N.J. The p160ROCK inhibitor Y27632 was purchased from Calbiochem (San Diego, Calif.).
Cells.
Normal human dermal fibroblasts (NHDFs), U373MG (neuroglioblastoma cell line), and COS7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and penicillin-streptomycin-l-glutamine (PSG). Primary rat aortic SMCs (rat AoSMCs) were isolated from F344 rats and maintained in DMEM supplemented with 10% FCS and PSG. We used pulmonary artery SMCs (PASMCs) for experiments involving human arterial SMCs (Clonetics, Walkersville, Md.). These cells were life extended by genetically engineering them to constitutively express the human telomerase gene. The telomerase gene was introduced into these cells at passage 6 by using a retroviral vector system (pLXSN from BD Sciences-Clontech, Palo Alto, Calif.). Cells expressing the telomerase gene were selected for G418 resistance and then screened for telomerase activity by using the Trapeze telomerase activity kit (Intergen, Toronto, Canada) (data not shown). PASMCs were maintained in medium 199 supplemented with 20% FCS and PSG. These cells exhibited SMC characteristics and morphology when maintained in culture beyond passage 60 (data not shown). Carotid artery SMCs (CASMCs; Clonetics) were maintained in medium 199 supplemented with 20% FCS and PSG.
Adenovirus construction.
Adenoviruses expressing US28 were previously described (32). Human hemagglutinin (HA)-tagged RhoA cDNA constructs (dominant negative [DN] and wild type [WT]) in pCDNA3.1 (generously provided by P. Stork, Oregon Health Sciences University [OHSU], Portland) were subcloned into the EcoRI and XbaI sites within pAdTet7 (16). Gα subunit cDNA constructs in pCDNA3 were obtained from the Gutherie Institute (Sayre, Pa.) and subcloned into pAdTet7 under a constitutively expressed HCMV-MIEP or Tet-responsive promoter. pAdTet7, a plasmid that contains Tet-responsive enhancer sequences within a minimal CMV promoter also contains the simian virus 40 late poly(A) cassette, adenovirus E1A, and a single loxP site to increase recombination frequency. Recombinant adenoviruses were produced by pAdTet7-RhoA DN/WT, or cotransfection with pAdTet7-Gαi1, -q, -11, -12, -13, or -z of 293 cells expressing Cre recombinase (293-Cre) with adenovirus DNA (Ad5-ψ5) that contains an adenovirus genome with E1A/E3 deleted (16). Recombinant adenoviruses were expanded on 293-Cre cells, and the titers of the bulk stocks were determined on 293 cells by limiting dilution. Expression of US28, the Gα proteins, and RhoA (DN and WT) was driven by coinfection with Ad-Trans expressing the Tet-off transactivator as described previously (32). Cells were infected at a multiplicity of infection (MOI) of 10 or 30 PFU/cell for Ad-Trans and/or Ad-RhoA (WT or DN) and analyzed for protein expression and for their effects on US28-mediated migration.
RhoA activation assay.
The RhoA binding domain of Rhotekin was expressed as a fusion protein with glutathione S-transferase (GST-Rhotekin; kindly provided by J. Scott, OHSU) in Escherichia coli strain BL21 as previously described (9, 27). Bacteria were grown until an optical density at 600 nm (OD600) of 0.6 to 0.8 was achieved, at which time the bacteria were induced overnight with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After IPTG induction, the bacteria were lysed and the clarified supernatants were bound to glutathione-linked 4B-CL Sepharose beads (Amersham) overnight at 4°C. The beads were washed three times with phosphate-buffered saline (PBS) followed by three washes in RhoA wash buffer (50 mM Tris [pH 7.2], 1% Triton X-100, 150 mM sodium chloride, 10 mM magnesium chloride) with a final equilibration wash using RhoA lysis buffer (50 mM Tris [pH 7.2], 1% Triton X-100, 500 mM sodium chloride, 10 mM magnesium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]). For these assays, cells were plated onto 15-cm-diameter culture dishes, and at 75% confluence, the cells were serum starved for 24 h. The cells were infected with Ad-Trans and Ad-US28 at the following different MOIs, depending upon the ability of each cell type to be infected: NHDFs, 2,000; COS7 cells, 500; U373MG cells, 500; and rat AoSMCs, 1,000 (data not shown). After 16 h, the cells were stimulated with RANTES (ranging from 1 to 50 ng/ml) and then scraped in RhoA lysis buffer at 0 (unstimulated), 5, 10, or 30 min poststimulation. Other serum-starved cell cultures treated with 1% serum served as positive controls. Cell lysates were incubated with GST-Rhotekin beads (100 μl of a 1:1 slurry) for 45 min at 4°C and then washed four times with 1 ml of RhoA wash buffer. The final pellet was resuspended in 60 μl of 2× Laemmli's sample buffer, boiled, and then run on SDS-polyacrylamide gel electrophoresis (PAGE; 10% polyacrylamide). The gels were transferred to Immobilon-P membranes, and the blots were blocked overnight in 3% milk in PBS. All primary antibody incubations (1:1,000) were carried out in a mixture containing 1% bovine serum albumin, 0.01% polyvinylpyrrolidone, and 0.01% sodium azide for 3 h at room temperature. The blots were then washed 4 times with Tris-buffered saline-Tween (TBS-T) buffer (10 mM Tris [pH 7.2], 100 mM sodium chloride, 0.1% Tween 20). The secondary antibody (goat anti-rabbit conjugated to HRP) was added at 1:2,000 dilution in TBS-T buffer, and the incubation was carried out for 1 h at room temperature. After being washed three times with TBS-T buffer and once in TBS and after incubation with Western Lightning chemiluminescent reagents (Perkin-Elmer), the blots were visualized by autoradiography on Kodak MR or Biomax Light film.
SMC migration assay.
Cell migration assays were performed as previously described (32). Briefly, cells were added to the upper well of a Transwell (12-mm diameter, 3.0-μm pore size; Costar Corning, Cambridge, Mass.) at 105 cells per well. Cells were serum starved for 24 h and then incubated with HCMV at an MOI of 10 for 2 h. The inserts were washed and transferred to fresh 12-well plates. Cells migrating to the lower chamber were enumerated at 48 to 72 h postinfection with a Nikon TE300 microscope at a magnification of ×10. Experiments were done in at least triplicate wells, and 10 random readings of each well were made for each well. The average number of cells per well was determined by multiplying the average number of cells per ×10 field by the number of fields per well. Mean and standard deviation were calculated from at least triplicate wells.
For SMC migration studies involving RhoA and Gα proteins, SMCs were infected with HCMV (MOI of 10) for 2 h followed by coinfection of cells with Ad-Trans and Ad-RhoA DN (MOI of 100 to 200) or Ad-RhoA WT (MOI of 75 to 100) for an additional 2 h. Subsequently, the Transwells were transferred to fresh 12-well plates. Cellular migration was determined as described above. In migration experiments involving G proteins, cells were coinfected with HCMV and Ad-Trans and adenoviruses expressing either Gαi1 (MOI of 25 and 50), Gα12 (MOI of 50 and 100), Gα13 (MOI of 50 and 100), Gαq (MOI of 100 and 200), and Gα11 (MOI of 25 and 50) and the experiment was carried out as described above. Prior to their use in these experiments, the expression levels of all recombinant adenovirus-derived proteins were equalized in SMCs by adjusting the MOI as determined by Western blotting with antibodies directed against the various G-protein subunits.
For SMC migration experiments involving the p160ROCK inhibitor Y27632, SMCs were infected with HCMV as described above and treated with increasing concentrations of Y27632 or left untreated. Cellular migration was determined as described above.
Immunofluorescence.
PASMCs were grown in four-well chamber slides (Nalge-Nunc, Seattle, Wash.). Adenovirus vectors were used to express US28 as described above. Cells were untreated or treated with the p160ROCK inhibitor Y27362 at 2 h postinfection. At 20 h postinfection, the cells were washed with PBS and fixed in 1% paraformaldahyde for 10 min at room temperature. The samples were then permeabilized and blocked in 0.3% Triton X-100 in PBS with 10% FCS and 0.1% sodium azide. The cells were incubated with antibodies directed against US28-flag epitope in a 1:200 dilution for 1 h at room temperature. Cells were washed three times in PBS, and binding of the primary antibody was detected with a fluorescein-isocyanate-tetramethyl (FITC)-conjugated goat anti-mouse antibody for 1 h at room temperature. At this time, the cells were also stained for actin cytoskeleton with phalloidin (Molecular Probes, Eugene, Oreg.) to monitor alterations in the cellular actin cytoskeleton induced by US28. Fluorescence-positive cells were visualized on an inverted Nikon fluorescent microscope with a ×60 objective.
RESULTS
Gα12 is required for US28-induced SMC migration.
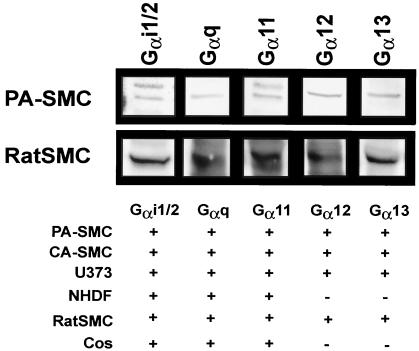
We have previously shown that US28 induces vascular SMC migration through a PTX-insensitive pathway (32). A number of studies have revealed that US28 binds multiple chemokines and activates signaling through several G-protein family members, including Gαi (4, 8, 12). Since the majority of cellular migration events are mediated by signaling pathways that involve PTX-sensitive Gαi/o, the signaling pathways involved in US28-induced migration are unknown. In this report, we sought to determine the G proteins that functionally couple to US28 to promote SMC migration. Since Gα-protein expression is tissue specific, the Gα-protein expression profiles were examined for a number of different cell types, including HCMV permissive cells (a neuroglioblastoma cell line, U373MG; PASMCs; CASMCs; and NHDFs) as well as cells commonly used in signaling studies, COS7 and rat AoSMCs). Cellular lysates were probed by Western blotting for the expression of a number of different Gα-protein subunits. Representative blots probed for Gαi1/2, Gαq, Gα11, Gα12, and Gα13 from PASMCs and rat AoSMCs are shown in Fig. 1. The ubiquitous Gαi1/2, Gαq/11 families of G proteins were detected in all of the cell types examined (Fig. 1). Interestingly, Gα12 and Gα13 expression was readily identifiable in rat AoSMCs, PASMCs, and U373MG but not in COS7 cells or NHDFs. Thus, G-protein expression profiles vary in a cell type-dependent manner.
FIG. 1.
Gα-protein detection in human vascular SMCs and rat AoSMCs. Shown are representative Western blots of Gα-protein expression in human PASMCs and rat AoSMCs and a table illustrating the presence of Gα subunits expressed in various HCMV-permissive cell types, including PASMCs, CASMCs, U373MG, and NHDFs as well as cell types commonly used in signal transduction studies, COS7 and rat AoSMCs.
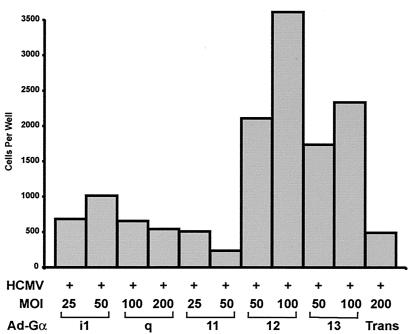
Overexpression of individual Gα proteins has been used to enhance or modulate GPCR-mediated signal transduction. An enhanced response resulting from overexpression of a single α subunit suggests the involvement of that Gα protein in signaling events initiated by the GPCR. In contrast, a reduced response may suggest that the particular Gα protein is not involved in the signaling pathway (20, 21). To identify individual Gα subunits that may enhance SMC migration induced by US28, a panel of adenoviruses expressing different individual Gα subunits was constructed. Adenoviruses expressing Gαq, Gα11, Gα12, Gα13, and Gαi1 were used to infect PASMCs to determine if any individual Gα proteins could enhance US28-mediated SMC migration. For these experiments, Gα-protein expression in PASMCs was normalized by altering the MOI according to Western blotting results from each specific adenovirus (data not shown). PASMCs were coinfected with adenoviruses expressing different individual Gα proteins and HCMVs, and then migration was assessed with a modified Boyden migration assay (32). As shown in Fig. 2, expression of Gα12 promotes US28-mediated SMC migration, and this migration is further enhanced with increasing expression levels of Gα12. Gα13, another member of the Gα12 family of G proteins, also promoted HCMV-mediated SMC migration, although to a lesser extent. Conversely, Gαi1, and Gαq/11 family G proteins failed to promote US28-mediated SMC migration. In the absence of HCMV infection, expression of these G proteins did not induce SMC migration (data not shown). These data suggest that Gα12 family G proteins functionally couple with US28 to promote SMC migration.
FIG. 2.
Gα12/13 enhances US28-mediated SMC migration. PASMCs were infected with HCMV and adenoviruses (Ad) expressing Gαq, Gα11, Gα12, Gα13, or Gαi1. Expression of the Gα proteins was normalized by Western blotting, and the MOI was adjusted accordingly such that equivalent amounts of protein were expressed. The total number of cells migrating from the upper to lower chamber was enumerated at 48 to 72 h postinfection.
US28-induced SMC migration is mediated through Gα12 activation of RhoA.
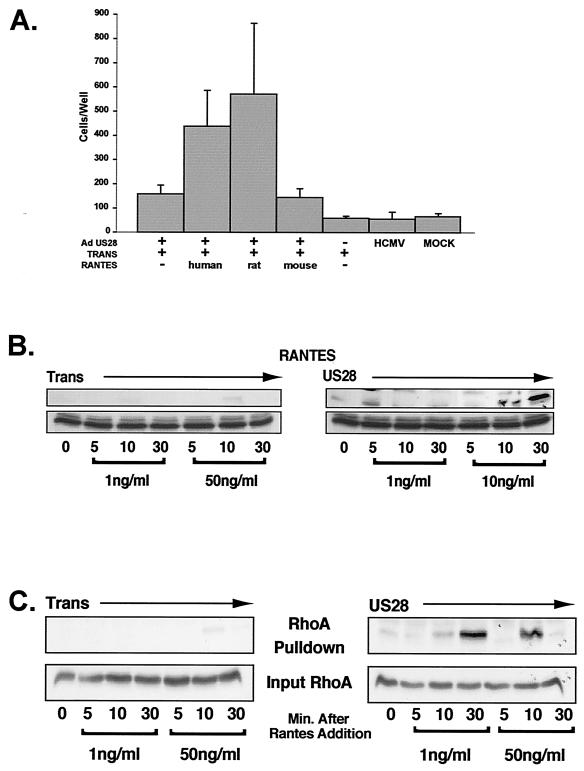
Signaling assays have a tendency to be very sensitive, and even small amounts of ligand can stimulate robust signaling activity. Previous observations have indicated that cells such as PASMCs cultured in vitro constitutively produce MCP-1 and addition of MCP-1-neutralizing antibodies to PASMCs expressing US28 abrogated migration (32). Therefore, the majority of our signaling studies were performed in rat AoSMCs and U373MG cells. Signaling molecules, including Gα proteins, small G proteins (i.e., RhoA, Rac, and CDC42), and PTKs (i.e., Src, FAK, and Pyk2), are highly conserved in mammals, and similar to human SMCs, US28 induces the migration of rat AoSMCs in response to recombinant human RANTES (Fig. 3A). Interestingly, rat RANTES also induced US28-mediated SMC migration; however, mouse RANTES failed to elicit the same effect.
FIG. 3.
US28 activates RhoA in a ligand-dependent manner. (A) The ability of US28 to induce migration of rat AoSMCs was determined by infecting cells with adenoviruses (Ad) expressing US28 in the presence of 10-ng/ml human, rat, or mouse RANTES. The number of cells migrating was determined by microscopic enumeration at 48 to 72 h postinfection. (B) Rat AoSMCs were serum starved for 16 to 18 h and subsequently infected with adenoviruses expressing US28. US28-expressing cells were either unstimulated or stimulated with 1- or 50-ng/ml RANTES at 16 to 18 h postinfection. At the indicated times, cells were harvested and lysates were applied to GST-Rhotekin-baited beads in order to precipitate active RhoA. The amount of bound/active RhoA was determined by Western blotting with an anti-RhoA polyclonal antibody. To ensure equal loading, lysates were also probed for total RhoA prior to being applied to GST-Rhotekin beads (lower panel). (C) US28-induced RhoA activity assay performed in U373MG cells as described above for rat AoSMCs.
Since US28-mediated SMC migration is enhanced by Gα12 and Gα13 and this family of G proteins are known to signal through RhoA, stimulating stress fiber formation as well contributing to migratory events, we examined the ability of US28 to activate RhoA (7, 14). To assess the ability of US28 to stimulate RhoA through the Gα12 family of G proteins, active RhoA pull-down assays using GST-tagged Rhotekin (GST-fused to the RhoA binding domain of Rhotekin) were performed. This fusion protein only binds to RhoA in the active GTP-bound state (27). For these experiments, serum-starved rat AoSMCs expressing US28 and/or Tet transactivator were stimulated with either 1- or 50-ng/ml RANTES for 0 (unstimulated), 5, 10, or 30 min. Cell lysates were administered to glutathione-linked Sepharose beads baited with GST-Rhotekin and then probed for RhoA. To ensure that equivalent amounts of proteins were used in each experiment, aliquots of cellular lysates were analyzed by Western blotting for input RhoA. Rat AoSMCs infected with adenoviruses expressing a Flag-tagged version of US28 and treated with RANTES display a dose-dependent and kinetic activation of RhoA (Fig. 3B). Control cells infected with adenoviruses expressing the Tet transactivator alone and treated with RANTES failed to activate RhoA. US28-mediated activation of RhoA was most pronounced at 10 and 30 min post-ligand stimulation (50-ng/ml RANTES).
In similar experiments performed with U373MG cells, the ligand-dependent activation of RhoA was observed in cells expressing US28. In these experiments, US28-mediated activation of RhoA peaked at 30-min post-RANTES addition (1 ng/ml) (Fig. 3C). When higher concentrations of RANTES (50 ng/ml) were administered to US28-expressing cells, peak RhoA stimulation occurred earlier, reaching maximal levels at 10 min post-ligand stimulation. The slight differences in the timing of US28-mediated activation of RhoA in U373MG cells versus rat AoSMCs may be due to cell-type-specific differences in the ability to respond or surface expression of US28. Regardless of the kinetics, it is clear that US28 activates RhoA in two very distinct cell types (rat AoSMCs and U373MG) and this activation is ligand dependent.
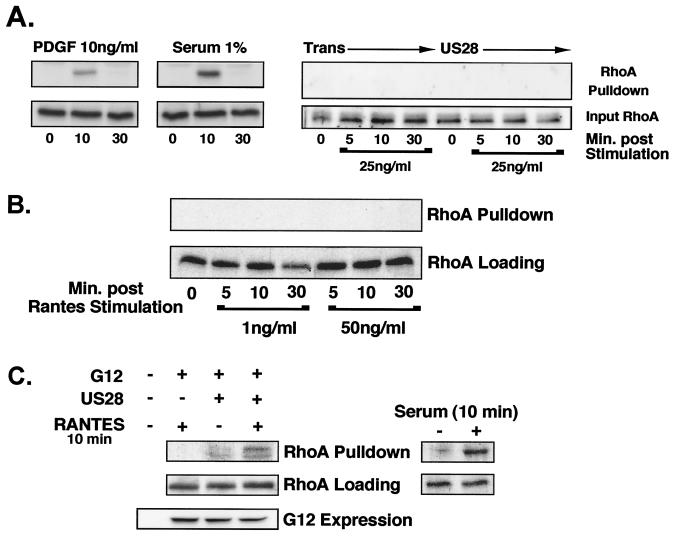
Since COS7 cells and NHDFs lack detectable Gα12 and Gα13 protein expression, these two cell types provide the ideal background to test the necessity of Gα12 family G proteins in US28's ability to activate RhoA. Therefore, active RhoA pull-down assays were performed in these two different cell types. In both COS7 cells (Fig. 4A) and NHDFs (Fig. 4B), US28 does not display any activation of RhoA either in the presence or absence of ligand. Although US28 does not induce the activation of RhoA in either of these cell types, both COS7 cells and NHDFs are fully capable of activating RhoA, as serum-starved NHDFs and COS7 cells are able to stimulate RhoA activity when treated with 1% serum (Fig. 4A and C).
FIG. 4.
Reconstitution of NHDFs with Gα12 restores US28-induced RhoA activation. (A) COS7 cells were serum starved for 18 h and then infected with adenoviruses expressing US28 and/or Ad-trans. Cells were stimulated at 18 h postinfection with 25-ng/ml RANTES for 0 (unstimulated), 5, 10, or 30 min. Alternatively, serum-starved COS7 cells were stimulated with 10-ng/ml platelet-derived growth factor (PDGF)-BB or 1% serum for 10 or 30 min. Active RhoA assays were performed, and RhoA was visualized by SDS-PAGE and Western blotting. The amount of total input RhoA was also assessed by Western blotting (lower lanes). (B) NHDFs were serum starved and infected as described above. Infected cells were stimulated with 1- to 50-ng/ml RANTES for 0, 5, 10, or 30 min or with 1% serum for 10 min (positive control), and RhoA activity was determined as described above. (C) NHDFs were infected with adenoviruses expressing either Gα12 and/or US28 and then treated with 10-ng/ml RANTES for 10 min.
Since US28 was unable to stimulate RhoA activity in cell types that lack detectable Gα12 and Gα13 expression, experiments were performed to determine whether reconstituting NHDFs with Gα12 would restore US28's ability to activate RhoA. The titers of Gα12 expression levels were factored in to reflect normal Gα12 expression levels observed in PASMCs (data not shown). NHDFs do not display detectable Gα12 (Fig. 4C), and upon expression of Gα12 in the absence of US28 or in the presence of US28 without ligand, there was no observable RhoA stimulation. However, 10 min post-ligand stimulation (RANTES), cells coinfected with adenoviruses expressing both US28 and Gα12 induced >10-fold activation of RhoA compared to untreated Gα12/US28-expressing cells. Therefore, the US28-RhoA signaling pathway can be functionally restored in NHDFs by reconstituting these cells with Gα12. These data suggest that US28 activation of RhoA is cell type specific and dependent upon endogenous G-protein expression.
RhoA activity is required for US28-mediated SMC migration.
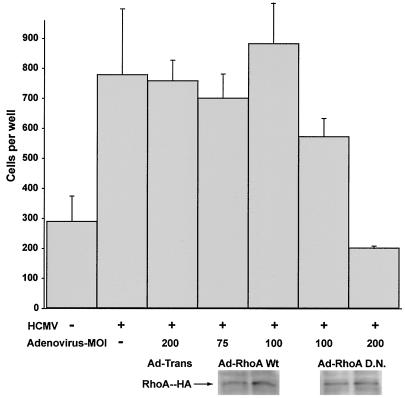
Since US28-induced SMC migration is enhanced by Gα12 and Gα13 and Gα12 is required for US28 to activate RhoA, the necessity of RhoA in US28-mediated migration was examined. Adenoviruses expressing either WT or DN RhoA (RhoA mutant that is unable to exchange GDP for GTP) were assessed in migration assays. Protein expression (which was determined by Western blotting for HA-tagged RhoA DN and WT) was normalized in SMC by adjusting the MOI accordingly (data not shown). Expression of WT RhoA consistently enhanced HCMV-mediated SMC migration (Fig. 5), whereas expression of DN RhoA abrogated US28-induced SMC migration. Since DN RhoA blocked US28-mediated SMC migration, this observation indicates that RhoA activity is required for US28-mediated SMC migration. Thus, US28 coupling with Gα12 family G proteins activates RhoA, which is functionally important for the ability of US28 to induce cellular migration.
FIG. 5.
RhoA activity is essential for US28-induced SMC migration. PASMCs were infected with HCMV along with adenoviruses (Ad) expressing either WT or DN RhoA at the indicated MOI. Cellular migration was determined by microscopy at 48 to 72 h postinfection. Cellular migration is expressed as a percentage when compared to the number of cells migrating when coinfected with HCMV and Ad-Trans.
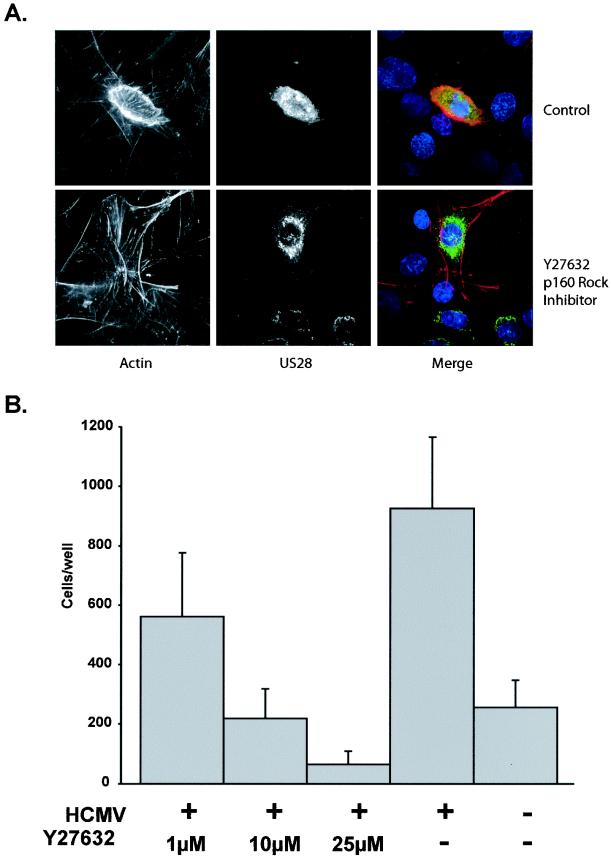
The small G protein RhoA is capable of activating a number of factors that may contribute to migratory processes. Dia and Rho-associated kinases (ROCKs), including p160ROCK, are downstream effectors of active RhoA (28). ROCK activation leads to the phosphorylation of myosin light chain (MLC) kinase, by both inhibiting MLC phosphatases and phosphorylating MLC, leading to reorganization of the actin cytoskeleton (1, 17). Expression of US28 in PASMCs resulted in actin cytoskeletal rearrangements and altered cellular morphology (Fig. 6A). To examine the contribution of RhoA-activated ROCKs in this process, PASMCs were infected with adenoviruses expressing US28 and then treated with a p160ROCK inhibitor, Y27632. When PASMCs expressing US28 were treated with Y27632, US28-induced actin cytoskeletal rearrangements were inhibited compared to those in untreated control cells (Fig. 6A). These results suggest that RhoA-activated ROCKs may also play an essential role in US28-induced migratory processes. To assess the importance of RhoA-activated ROCKs in US28-mediated cellular migration, cellular migration experiments were performed in the presence or absence of Y27632. This inhibitor effectively blocked US28-induced cellular migration, suggesting that ROCK activity is essential in US28-mediated cellular migration (Fig. 6B). Therefore, US28-induced SMC migration is ligand induced and dependent upon Gα12-mediated activation of RhoA, which subsequently activates the Rho-associated kinase p160ROCK.
FIG. 6.
p160ROCK activity is important for US28-mediated actin cytoskeleton reorganization and cellular migration. (A) PASMCs expressing US28 were left untreated or were treated with the p160ROCK inhibitor Y27632. RANTES-treated cells were fixed 4 h post-ligand addition. Cells were stained for actin with phalloidin (red), the US28 flag epitope, and for nuclei with Hoechst DNA staining dye. (B) PASMCs were infected with HCMV and then treated with increasing concentrations of the p160ROCK inhibitor Y27632. The number of cells migrating from the upper to lower chamber was enumerated by microscopy and is expressed as a total number of cells migrating from the upper to lower chamber.
DISCUSSION
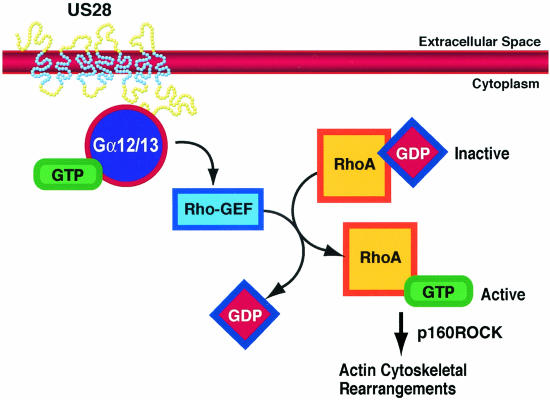
In the present report, we demonstrate that US28 signaling through Gα12 family G proteins is important for the induction of vascular SMC migration. Migration was enhanced in the presence of Gα12 family G proteins but not in the presence of G proteins from either the Gαi or Gαq/11 family. This finding supports our previous findings that US28-induced SMC migration occurs in a PTX-insensitive manner. Consistent with activation of the Gα12 family members was our finding that US28 promoted RhoA activation. RhoA is a small GTPase with critical importance in the signaling events that mediate the cytoskeletal rearrangements associated with cellular migration. Importantly, US28 failed to activate RhoA in fibroblasts or COS cells that do not display Gα12 protein expression by Western blotting. However, US28's RhoA-stimulating potential can be restored in fibroblasts by introducing Gα12 into these cells by using adenovirus vectors, indicating that activation of this pathway is Gα12 dependent. US28 signaling in SMC induced cytoskeletal rearrangements, and these effects were prevented by treating the cells with an inhibitor to the RhoA effector, p160ROCK, thus identifying this molecule as playing an essential role downstream of RhoA in US28-induced SMC migration (pathway summarized in Fig. 7). In this report, we provide the first evidence that US28 signals in a ligand-dependent manner through Gα12 family G proteins.
FIG. 7.
US28 signaling through Gα12 family G proteins. Upon ligand binding, US28 couples with Gα12/13 in vascular SMCs. Gα12 family G proteins stimulate RhoGEFs, which activate RhoA through promoting the exchange of GDP for GTP. Active RhoA plays a critical role in the actin cytoskeleton rearrangements that are necessary for US28-induced SMC migration. RANTES stimulation of US28 also leads to the activation of the RhoA effector p160ROCK, which is essential in US28-mediated SMC migration.
US28-G-protein coupling and usage.
Why has US28 acquired the ability to bind multiple G proteins? Under physiological conditions GPCR-G-protein coupling is restricted and highly regulated. This regulation occurs at multiple levels, including the ability of the receptor to specifically couple to different G-protein family members and at the level of differential G-protein expression within tissues and cell types. While most, but not all, host chemokine receptors are expressed in cells of hematopoietic origin, US28 can be expressed in a wide variety of cell types because HCMV productively infects hematopoietic cells, endothelial cells, epithelial cells, neuronally derived cells, and vascular SMCs. All of these cells are important in HCMV pathogenesis, but we do not yet know in which of these cell types US28 is functionally required in vivo. However, since each of these cell types expresses various profiles of G proteins, the virus-encoded chemokine receptor must be able to function in each cell and must acquire the ability to couple to multiple G proteins. In general, hematopoietic cell migration induced by the chemokine receptors CCR1, CCR2, CCR5, and CXCR4 is PTX sensitive, suggesting the involvement of Gαi family Gα proteins, which are highly expressed in these cells (2, 34). Interestingly, CCR2 can couple to multiple G proteins, including Gαi, Gαq, and Gα16, although in hematopoietic cells Gαi coupling is dominant since CCR2-mediated migration is sensitive to PTX (3). However, vascular SMC migration induced by the angiotensin II type 1 receptor is mediated by receptor coupling to Gαq and Gα12, which are highly expressed in these cells (35). Interestingly, CCR2 expression can be induced in SMCs, which may be important under inflammatory conditions and may explain why CCR2 can functionally couple to more than one G protein. Together, these cell type differences in the receptor-G-protein coupling used to mediate migration suggest that G-protein expression is an important determinant in GPCR signaling. Thus, the ability of US28 to bind multiple G proteins may have been acquired through selection in order to allow US28 to signal in the presence of different G-protein environments.
Signaling through US28.
Previously, US28 has been shown to stimulate intracellular signal transduction through a number of different Gα proteins. US28 coupling to Gαi and the Gαq family member Gα16 promotes ERK2 activation and calcium flux (4). Other Gαq family members Gαq and Gα11 also couple with US28, promoting PLC-β and NF-κB signaling activity (8). However, a biological outcome resulting from signaling through these G proteins has not been established. There exists a clear relationship between Gαi and hematopoietic cell migration, which when taken with the ability of US28 to signal through these pathways may indicate that this viral GPCR has the inherent capacity to induce migration of cell types other than SMCs such as monocytes. Enhancing or altering the migration potential of these cells would be important for virus escape and dissemination.
In this report, we demonstrate for the first time that US28 couples with G proteins from the Gα12 family, which is important to mediate SMC migration. Expression of other G proteins (Gαi and Gαq/11 family members) in vascular SMCs did not enhance US28-mediated migration, which is consistent with our earlier findings showing that this migration event operates through PTX-insensitive G proteins (32). This finding is also suggestive that even in the presence of other G proteins such as Gαi or Gαq that couple to US28, Gα12 coupling is dominant in SMCs. We also demonstrate that US28 activates RhoA in a Gα12-dependent manner, since reconstituting fibroblasts with Gα12 restored the capacity of US28 to activate RhoA. Activation of Gα12 family members can directly stimulate RhoGEFs that then promote RhoA activity by stimulating the release of bound GDP, which promotes the GDP/GTP exchange. However, recently FAK, a non-receptor PTK, has been shown to bind and phosphorylate p190RhoGEF upon stimulation through growth factor receptors or integrins (36). We have recently reported that US28 activates FAK in a ligand-dependent manner, suggesting that US28 may promote RhoA activation through either direct activation of Gα12/13 G-protein family members or through FAK (33). Regardless of the mechanism, RhoA activity is critical for US28-induced SMC migration, since expression of a DN inhibitor of RhoA prevented vascular SMC migration. In addition, treatment of cells with an inhibitor to a downstream effector of RhoA, p160ROCK, also blocked migration, which further supports our findings, demonstrating the importance of RhoA in US28-induced migration.
SMCs, endothelial cells, epithelial cells, fibroblasts, and monocytes/macrophages are all important cell types in the pathogenesis of HCMV, and defining the signaling characteristics of US28 in these cell types is critical to a better understanding of how the virus interacts with and persists in the host. In this report, we further characterize the signaling pathway used by US28 in vascular SMCs that is important for the induction of cellular migration. The intracellular signaling cascade initiated by US28 binding to RANTES leading to SMC migration is dependent upon the G protein Gα12 and the activation of RhoA. Importantly, our results demonstrate that ligand-dependent US28 signaling occurs in a cell-type-specific manner, which minimally depends on the cellular G-protein environment.
Acknowledgments
We would like to thank Andrew Townsend for assistance in graphic design. We would also like to thank Phil Stork, Dario Diviani, and John Scott from Oregon Health Sciences University for insight and helpful discussions regarding RhoA and G-protein signaling studies.
This work was supported by grants from the National Institutes of Health to J. Nelson (HL65754 and HL71695).
REFERENCES
- 1.Amano, M., Y. Fukata, and K. Kaibuchi. 2000. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261:44-51. [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., and I. F. Charo. 1996. Differential regulation of G-protein-mediated signaling by chemokine receptors. J. Biol. Chem. 271:21814-21819. [DOI] [PubMed] [Google Scholar]
- 3.Arai, H., C. L. Tsou, and I. F. Charo. 1997. Chemotaxis in a lymphocyte cell line transfected with C-C chemokine receptor 2B: evidence that directed migration is mediated by betagamma dimers released by activation of Galphai-coupled receptors. Proc. Natl. Acad. Sci. USA 94:14495-14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billstrom, M. A., G. L. Johnson, N. J. Avdi, and G. S. Worthen. 1998. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 72:5535-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodaghi, B., T. R. Jones, D. Zipeto, C. Vita, L. Sun, L. Laurent, F. Arenzana-Seisdedos, J. L. Virelizier, and S. Michelson. 1998. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J. Exp. Med. 188:855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In P. M. Howley (ed.), Fields virology, 3 ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 7.Buhl, A. M., N. L. Johnson, N. Dhanasekaran, and G. L. Johnson. 1995. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 270:24631-24634. [DOI] [PubMed] [Google Scholar]
- 8.Casarosa, P., R. A. Bakker, D. Verzijl, M. Navis, H. Timmerman, R. Leurs, and M. J. Smit. 2001. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J. Biol. Chem. 276:1133-1137. [DOI] [PubMed] [Google Scholar]
- 9.Diviani, D., J. Soderling, and J. D. Scott. 2001. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J. Biol. Chem. 276:44247-44257. [DOI] [PubMed] [Google Scholar]
- 10.Fields, T. A., and P. J. Casey. 1997. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem. J. 321:561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler, K. B., F. P. McCollister, A. J. Dahle, S. Boppana, W. J. Britt, and R. F. Pass. 1997. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J. Pediatr. 130:624-630. [DOI] [PubMed] [Google Scholar]
- 12.Gao, J. L., and P. M. Murphy. 1994. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J. Biol. Chem. 269:28539-28542. [PubMed] [Google Scholar]
- 13.Gohla, A., R. Harhammer, and G. Schultz. 1998. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J. Biol. Chem. 273:4653-4659. [DOI] [PubMed] [Google Scholar]
- 14.Gohla, A., S. Offermanns, T. M. Wilkie, and G. Schultz. 1999. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J. Biol. Chem. 274:17901-17907. [DOI] [PubMed] [Google Scholar]
- 15.Haskell, C. A., M. D. Cleary, and I. F. Charo. 2000. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J. Biol. Chem. 275:34183-34189. [DOI] [PubMed] [Google Scholar]
- 16.Hsia, D. A., S. K. Mitra, C. R. Hauck, D. N. Streblow, J. A. Nelson, D. Ilic, S. Huang, E. Li, G. R. Nemerow, J. Leng, K. Spencer, D. A. Cheresh, and D. D. Schlaepfer. 2003. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160:753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 18.Kledal, T. N., M. M. Rosenkilde, and T. W. Schwartz. 1998. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 441:209-214. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn, D. E., C. J. Beall, and P. E. Kolattukudy. 1995. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem. Biophys. Res. Commun. 211:325-330. [DOI] [PubMed] [Google Scholar]
- 20.Lee, C. H., D. Park, D. Wu, S. G. Rhee, and M. I. Simon. 1992. Members of the Gq alpha subunit gene family activate phospholipase C beta isozymes. J. Biol. Chem. 267:16044-16047. [PubMed] [Google Scholar]
- 21.Lee, C. H., I. C. Shin, J. S. Kang, H. C. Koh, J. H. Ha, and C. K. Min. 1998. Differential coupling of G alpha q family of G-protein to muscarinic M1 receptor and neurokinin-2 receptor. Arch. Pharm. Res. 21:423-428. [DOI] [PubMed] [Google Scholar]
- 22.Margulies, B. J., H. Browne, and W. Gibson. 1996. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology 225:111-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melnick, J. L., E. Adam, and M. E. DeBakey. 1998. The link between CMV and atherosclerosis. Infect. Med. 1998:479-486. [Google Scholar]
- 24.Melnick, J. L., B. L. Petrie, G. R. Dreesman, J. Burek, C. H. McCollum, and M. E. DeBakey. 1983. Cytomegalovirus antigen within human arterial smooth msucle cells. Lancet ii:644-647. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 26.Peterson, P. K., H. H. Balfour, S. C. Marker, D. S. Fryd, R. J. Howard, and R. L. Simmons. 1980. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine (Baltimore) 59:283-300. [PubMed] [Google Scholar]
- 27.Ren, X. D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridley, A. J. 2001. Rho GTPases and cell migration. J. Cell Sci. 114:2713-2722. [DOI] [PubMed] [Google Scholar]
- 29.Schall, T. J., B. Stein, G. Gorgone, and K. B. Bacon. 1994. Cytomegalovirus encodes a functional receptor for C-C chemokines, p. 201-213. In G. McFadden (ed.), Viroceptors, virokines and related immune modulators encoded by DNA viruses. R. G. Landes Company, Austin, Tex.
- 30.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 31.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. The HCMV chemokine receptor US28 is a potential target in vascular disease. Curr. Drug Targets Infect. Disord. 1:151-158. [DOI] [PubMed] [Google Scholar]
- 32.Streblow, D. N., C. Söderberg-Nauclér, J. Vieira, P. Smith, E. Wakabayashi, F. Rutchi, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 33.Streblow, D. N., J. Vomaske, P. Smith, R. Melnychuk, L. A. Hall, D. Pancheva, M. Smit, P. Casarosa, D. D. Schlaepfer, and J. A. Nelson. 2003. Human cytomegalovirus chemokine US28 induced SMC migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 278:50456-50465. [DOI] [PubMed] [Google Scholar]
- 34.Thelen, M. 2001. Dancing to the tune of chemokines. Nat. Immunol. 2:129-134. [DOI] [PubMed] [Google Scholar]
- 35.Touyz, R. M., and E. L. Schiffrin. 2000. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 52:639-672. [PubMed] [Google Scholar]
- 36.Zhai, J., H. Lin, Z. Nie, J. Wu, R. Canete-Soler, W. W. Schlaepfer, and D. D. Schlaepfer. 2003. Direct interaction of focal adhesion kinase with p190RhoGEF. J. Biol. Chem. 278:24865-24873. [DOI] [PubMed] [Google Scholar]