Abstract
Hepatitis C virus (HCV) is a major health problem. However, the mechanism of hepatocyte infection is largely unknown. We demonstrate that the dendritic cell (DC)-specific C-type lectin DC-SIGN and its liver-expressed homologue L-SIGN/DC-SIGNR are important receptors for HCV envelope glycoproteins E1 and E2. Mutagenesis analyses demonstrated that both HCV E1 and E2 bind the same binding site on DC-SIGN as the pathogens human immunodeficiency virus type 1 (HIV-1) and mycobacteria, which is distinct from the cellular ligand ICAM-3. HCV virus-like particles are efficiently captured and internalized by DCs through binding of DC-SIGN. Antibodies against DC-SIGN specifically block HCV capture by both immature and mature DCs, demonstrating that DC-SIGN is the major receptor on DCs. Interestingly, internalized HCV virus-like particles were targeted to nonlysosomal compartments within immature DCs, where they are protected from lysosomal degradation in a manner similar to that demonstrated for HIV-1. Lewis X antigen, another ligand of DC-SIGN, was internalized to lysosomes, demonstrating that the internalization pathway of DC-SIGN-captured ligands may depend on the structure of the ligand. Our results suggest that HCV may target DC-SIGN to “hide” within DCs and facilitate viral dissemination. L-SIGN, expressed by THP-1 cells, internalized HCV particles into similar nonlysosomal compartments, suggesting that L-SIGN on liver sinusoidal endothelial cells may capture HCV from blood and transmit it to hepatocytes, the primary target for HCV. We therefore conclude that both DCs and liver sinusoidal endothelial cells may act as reservoirs for HCV and that the C-type lectins DC-SIGN and L-SIGN, as important HCV receptors, may represent a molecular target for clinical intervention in HCV infection.
Hepatitis C virus (HCV) is the causal agent of hepatitis C, which is a major health problem affecting 170 million people worldwide (1). Approximately 90% of patients develop chronic hepatitis (11), of which 20 to 30% progress to liver cirrhosis and end-stage liver disease (20, 43). HCV is an enveloped positive-stranded RNA virus (8) that belongs to the Flaviviridae family. The genome encodes a single polyprotein (24, 44), and a combination of host and viral peptidases process the polyprotein into at least nine different structural and nonstructural proteins (21, 23, 29). The HCV envelope is formed by two heavily N-glycosylated type I transmembrane envelope glycoproteins E1 (31 kDa) and E2 (70 kDa) (28, 33, 34), which are expressed as heterodimers on the virus membrane (34).
A characteristic feature of HCV is the high incidence of persistent infection and chronic hepatitis with a strong risk for the development of hepatocellular carcinoma, although some patients exhibit acute self-limited infection (10). This high incidence of chronicity suggests that the virus has developed efficient mechanisms to escape host immune responses. Indeed, cellular immune responses are weak in chronically infected patients (7, 32, 39), although the reason for this poor reaction remains unclear.
HCV infects mainly hepatocytes but also peripheral blood mononuclear cells. However, the precise mechanisms of early HCV infection are largely unknown, especially how HCV infects hepatocytes in the liver. Attempts to elucidate these early events have been hampered by the difficulty in obtaining sufficient amounts of free virions from either the plasma of infected individuals or in vitro systems for virus propagation. Nevertheless, it is generally accepted that HCV envelope glycoproteins E1 and E2, as in other enveloped viruses, may play a major role in virus binding and entry into target cells. Indeed, several putative HCV receptors that interact with the HCV envelope glycoproteins, such as CD81 (36), the scavenger receptor class B type I (42), and the asialoglycoprotein receptor (41), have been identified. Recently, it was demonstrated that the C-type lectins DC-SIGN and L-SIGN/DC-SIGNR may be involved in HCV binding through their interaction with HCV envelope glycoprotein E2 (14, 31, 38).
DC-SIGN is specifically expressed on dendritic cells (DCs) (16, 25), and plays a key role in the dissemination of human immunodeficiency virus type 1 (HIV-1) by DCs through HIV-1 gp120 binding (15). Recent studies have demonstrated that DC-SIGN also functions as a receptor for other pathogens, including cytomegalovirus (22), Ebola virus (2), and Mycobacterium tuberculosis (19). It is becoming clear that other pathogens besides HIV-1 target DC-SIGN to promote their survival, and similarly, HCV binding to DC-SIGN may not only promote HCV dissemination but also modulate DC function necessary for establishing chronic infections. Indeed, it has been shown that chronic HCV infection impairs DC maturation as well as their immune stimulatory function (3, 4). Thus, DCs may be a target for HCV to escape immune surveillance, and knowledge about the interaction of DCs with HCV is essential to fully understand and combat HCV infections.
L-SIGN, the liver homologue of DC-SIGN, is specifically expressed by liver sinusoidal endothelial cells (LSECs) (5, 37), a specialized endothelial cell type with antigen-presenting cell function (26). L-SIGN, like DC-SIGN, binds HIV-1 gp120 and may be involved in HIV-1 transmission to T cells (5). Similarly, L-SIGN expressed by LSECs may capture HCV from blood and mediate infection of adjacent hepatocytes, the main target cells for HCV.
In order to unravel the interaction of HCV with both C-type lectins DC-SIGN and L-SIGN and their roles in virus dissemination, we investigated the interaction of both C-type lectins with virus-like particles (VLPs) consisting of either HCV glycoprotein E1 or E2 alone or an E1/E2 heterocomplex as a suitable surrogate for native HCV particles. We demonstrate that the C-type lectins DC-SIGN and L-SIGN interact similarly with both glycoproteins E1 and E2 and that the HCV glycoproteins occupy the same binding pocket in DC-SIGN as HIV-1 gp120 and mycobacterial mannosylated lipoarabinomannan (ManLAM). Both immature and mature DCs strongly bind the VLPs of E1 and E2, and this interaction is primarily mediated by DC-SIGN. Moreover, VLPs are rapidly internalized by DC-SIGN on immature DCs and targeted to EEA-1-positive early endosomes, where the VLPs are protected from degradation. In contrast, Lewis X (Lex) antigens are internalized by DC-SIGN and targeted to lysosomes for destruction. Furthermore, we demonstrate that LSECs efficiently capture HCV particles in situ through L-SIGN. L-SIGN rapidly internalizes HCV into early endosomes. Our data suggest that HCV may target DC-SIGN and L-SIGN to hide within DCs and LSECs, respectively.
MATERIALS AND METHODS
Antibodies and proteins.
The following antibodies were used: mouse anti-MR (clone 19; BD Pharmingen, San Diego, Calif.), DC-SIGN-specific mouse antibody AZN-D1 (16), DC-SIGN- and L-SIGN-specific antibody AZN-D2 (22), mouse anti-L-SIGN (R&D Systems, Minneapolis, Minn.), mouse anti-LAMP-1 (H4A3; BD Pharmingen, San Diego, Calif.), mouse anti-EEA-1 (BD Pharmingen), human and mouse anti-HCV E1 (1C4 and 23C12, respectively; Innogenetics, Ghent, Belgium) and mouse anti-E2 antibody (4H6B2; Innogenetics), goat anti-human immunoglobulin G1 conjugated with fluorescein isothiocyanate (FITC) and peroxidase (Jackson Immunoresearch, West Grove, Pa.), goat anti-mouse conjugated with FITC (Zymed Laboratories Inc., South San Fransisco, Calif.) goat anti-mouse conjugated with Alexa fLuor 488 and 594 and streptavidin Alexa Fluor 488 (Molecular Probes, Eugene, Oreg.).
HIV-1 gp120 was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS (National Institutes of Health; HIV-1 IIIB gp120).
HCV envelope glycoproteins.
HCV envelope glycoproteins E1 and E2 were expressed separately either in Vero cells by infection with recombinant vaccinia virus or in the yeast Hansenula polymorpha. Both glycoproteins were derived from the structural region of a genotype 1b isolate and lacked the C-terminal membrane anchor (E1, amino acids 192 to 326; and E2, amino acids 384 to 673). A C-terminal His tag was added for purification purposes except for the E1 protein produced in Vero cells.
Despite the truncation of the membrane anchor, all proteins were retained intracellularly and were thus purified from cell lysates. In brief, the envelope proteins as expressed in Vero cells were extracted by means of Triton X-100 and purified by lentil affinity chromatography, resulting in an E1- or E2-enriched protein fraction consisting mainly of aggregated material. These aggregates were reduced to monomeric proteins by incubation with dithiothreitol and Empigen BB. After blocking of the free cysteines with iodoacetamide, the monomeric E1 protein was recovered by size exclusion chromatography in the presence of Empigen BB. For the E2 protein, both size exclusion chromatography and nickel metal affinity chromatography were required to obtain pure E2 monomers. In case of expression in yeast cells, the cells were lysed by means of guanidinium HCl and sulfonation. The recovered proteins were purified by nickel metal affinity chromatography in the presence of Empigen BB. Finally, the proteins were reduced by incubation with dithiothreitol and free cysteines were blocked with iodoacetamide.
Vero cell- and yeast cell-derived monomeric E1, E2, and equimolar mixtures of E1 and E2 were subjected to an additional size exclusion chromatography in the presence of betaine. This step allowed exchange of the detergent Empigen, resulting in the formation of particles ranging in size from 10 to 100 nm.
Cells.
Immature DCs were cultured as described before (40). In short, human blood monocytes were isolated from buffy coats by a Ficoll and a 52% Percoll gradient and subsequently an adherence step. Adherent monocytes were differentiated into immature DCs in the presence of interleukin-4 and granulocyte-macrophage colony-stimulating factor (500 and 800 U/ml, respectively; Schering-Plough, Brussels, Belgium). At day 6, the phenotype of the cultured DCs was confirmed by flow cytometric analysis. The DCs expressed high levels of major histocompatibility complex class I and II, CD11b, CD11c, and ICAM-1 and low levels of CD80 and CD86. DCs were matured by adding lipopolysaccharide (LPS, 2 μg/ml, Salmonella typhosa; Sigma Aldrich, St. Louis, Mo.) for 2 days. Maturation was verified by analysis of the expression of CD80, CD83, and CD86.
Stable THP-1 and K562 transfectants expressing wild-type L-SIGN, DC-SIGN, or DC-SIGN mutants (17) were generated as previously described (15).
Soluble DC-SIGN-Fc binding ELISA.
The DC-SIGN-Fc chimera (17) contained the extracellular portion of DC-SIGN (amino acid residues 64 to 404) fused at the C terminus to a human immunoglobulin G1-Fc fragment. The soluble DC-SIGN-Fc binding assay was performed as previously described (17). Soluble ligands were coated on enzyme-linked immunosorbent assay (ELISA) plates (Maxisorb plate; Nunc; 0.25 μg/well unless indicated) overnight at 4°C, followed by blocking with 1% bovine serum albumin for 30 min at 37°C. Soluble DC-SIGN-Fc (5 μg/ml) was added for 2 h at 37°C, and binding was determined by an anti-immunoglobulin G1 ELISA. Specificity was determined (unless indicated) in the presence of 50 μg of blocking antibodies or 100 μg of mannan per ml or 10 mM EGTA.
Fluorescent bead adhesion assay.
Binding of ligand-coated beads to cells was done as described by Geijtenbeek et al. (16). In short, streptavidin was covalently coupled to carboxylate-modified TransFluorSpheres (488/645 nm, 1.0 μm; Molecular Probes). The streptavidin-coated beads were incubated with biotinylated F(ab′)2 fragment goat anti-mouse immunoglobulin G (6 μg/ml; Jackson Immunoresearch), followed by overnight incubation with mouse anti-HCV E1 or E2 antibody (23C12 or 4H6B2) at 4°C. The beads were washed and incubated with 250 ng of purified HCV envelope glycoprotein E1 or E2 per ml overnight at 4°C. The fluorescent bead adhesion assay was performed as described before (18). In short, 50,000 cells were incubated with beads for 45 min at 37°C. Mannan, EGTA, and blocking antibodies against DC-SIGN and L-SIGN were used to determine the specificity of adhesion. Binding was measured by fluorescence-activated cell sorting analysis.
DC activation.
Immature DCs (0.12 × 106 cells/ml) were cultured in the presence of interleukin-4 (500 U/ml; Schering-Plough) and granulocyte-macrophage colony-stimulating factor (800 U/ml; Schering-Plough). The effect of HCV on activation was determined by incubating immature DCs (day 6) with HCV VLPs (12.5 μg/ml) in the absence or presence of LPS (10 ng/ml) for 48 h at 37°C. Activation was determined by cell surface expression of the costimulatory molecules CD83 and CD86 with phycoerythrin-conjugated antibodies.
Immunofluorescence microscopy.
Cells were incubated for 4 h or overnight at 37°C with HCV E1/E2 (30 μg/ml) or biotinylated Lex antigen (10 μg/ml). The transferrin receptor was detected by incubating the cells with Alexa Fluor 594-conjugated transferrin (10 μg/ml; Molecular Probes) for 15 min at 37°C prior to fixation. Labeled cells were fixed in 3% paraformaldehyde in phosphate-buffered saline for 15 min and permeabilized in phosphate-buffered saline-0.1% saponin prior to staining. Cells were stained in phosphate-buffered saline-0.5% bovine serum albumin with antibodies against HCV envelope glycoprotein E1 or E2 and against LAMP-1, EEA-1, or DC-SIGN and subsequently with FITC- or Alexa Fluor 488/594-conjugated secondary antibodies or with streptavidin-Alexa Fluor 488. Next, cells were allowed to adhere to poly-L-lysine-coated glass slides and mounted in antibleach reagent. Fixed slides were either imaged with a Zeiss Axiovert 200 Marianas inverted microscope (Intelligent Imaging Innovations, Denver, Colo.) equipped with a motorized stage (stepper-motor z axis increments, 0.1 μm), multiple fluorescence as well as bright-field channels, and a Cooke Sensicam cooled charge-coupled device camera (Cooke, Tonawanda, N.Y.; 1,280 by 1,024 pixels) with true 16-bit capability at 40× objective or examined with a Nikon Eclipse E800 fluorescence microscope, and pictures were captured with a digital Nikon DXM1200 camera at 40× objective. In both cases images were acquired in three independent series or sessions. Pictures were analyzed with SlideBook 4 digital microscopy software (Intelligent Imaging Innovations) or Jasc Paint Shop Pro software.
Liver section staining.
Human liver tissue was obtained from biopsies of therapeutic surgeon and cryofrozen; 8-μm sections were placed on gelatin-coated slides and stored at −80°C. The sections were incubated with HCV VLPs (10 μg/ml) for 2 h and stained with the mouse anti-HCV antibody 23C12 and an Alexa Fluor 594-conjugated secondary antibody or with the human anti-HCV antibody 1C4 and a FITC-conjugated secondary antibody. Binding of HCV envelope glycoprotein was blocked by incubation in the presence of 50 μg of antibody AZN-D2 per ml. L-SIGN was detected by staining the liver sections with an anti-L-SIGN antibody and Alexa Fluor 594-conjugated secondary antibody.
Sections were analyzed with a Nikon Eclipse E800 fluorescence microscope, and pictures were captured with a digital Nikon DXM1200 camera at 20× objective.
RESULTS
DC-SIGN interacts with HCV envelope glycoproteins E1 and E2.
The interaction of DC-SIGN with HCV envelope glycoproteins was investigated with the DC-SIGN-Fc binding assay (17). Purified HCV envelope glycoproteins E1 and E2 produced in either mammalian cells or the yeast H. polymorpha are reconstituted into VLPs. Recombinant DC-SIGN-Fc specifically interacted with both mammalian and yeast HCV envelope glycoproteins E1, E2, and mixed E1/E2 VLPs. This interaction could be blocked by the DC-SIGN-specific antibody AZN-D1, the calcium chelator EGTA, and the polycarbohydrate mannan (Fig. 1A). No difference in binding was observed between yeast and mammalian envelope glycoproteins (Fig. 1A).
FIG. 1.
DC-SIGN specifically binds HCV envelope glycoproteins E1 and E2. (A) DC-SIGN binds both H. polymorpha and mammalian cell-produced HCV envelope glycoproteins. DC-SIGN interaction with HCV envelope glycoproteins E1 and E2 (0.25 μg/well) produced by either the yeast H. polymorpha or mammalian cells with a recombinant vaccinia virus, was determined in an Fc-based ELISA. The specificity of the binding was confirmed with the DC-SIGN-specific blocking antibody AZN-D1 (50 μg/ml), mannan (100 μg/ml), and the calcium chelator EGTA (5 mM). (B) DC-SIGN binds more strongly to HCV envelope glycoprotein E2 than to E1. HCV glycoproteins E1 and E2 were titrated (0 to 16 and 0 to 14 μM, respectively), and DC-SIGN-Fc binding was determined as described above. DC-SIGN-specific antibody AZN-D1 (50 μg/ml) was used to specifically block the interaction. (C) DC-SIGN has a higher affinity for envelope glycoprotein E2 than for envelope glycoproteins E1 and gp120. Mammalian cell-produced glycoproteins E1 and E2 (10 nM) and gp120 (2 nM) were coated, and DC-SIGN-Fc binding was determined as described above. Mannan was titrated (0 to 1,000 μg/ml) to block the interaction. Binding is represented as a percentage of maximal binding. (D) DC-SIGN binding to HCV envelope glycoproteins E1 and E2 and HIV-1 envelope gp120 is equally dependent on calcium. Viral envelope glycoproteins E1 and E2 (10 nM) and gp120 (2 nM) were coated, and DC-SIGN-Fc binding was measured as described above in the presence of calcium (0 to 5 mM). Binding is represented as a percentage of maximal binding at 5 mM calcium. Standard deviations were <2%.
Further experiments were performed with HCV envelope glycoproteins produced in mammalian cells. DC-SIGN bound more strongly to glycoprotein E2 than to E1 (Fig. 1B), and the binding could be specifically blocked by anti-DC-SIGN antibodies (Fig. 1B). The interaction of DC-SIGN with HCV envelope glycoproteins E1 and E2 was further investigated by titration of mannan to block the interaction. Mannan could completely block the interaction of DC-SIGN with both E1 and E2 (Fig. 1C), although higher concentrations of mannan were necessary to block HCV E2 binding than to block HCV E1 and HIV-1 gp120 binding to DC-SIGN (Fig. 1C). This indicates that DC-SIGN has the same affinity for HCV glycoprotein E1 and HIV-1 gp120 and a higher affinity for HCV glycoprotein E2.
The calcium dependency of the DC-SIGN interaction with the HCV envelope glycoproteins was investigated. The interaction of DC-SIGN with HCV envelope glycoproteins E1 and E2 and HIV-1 gp120 was equally dependent on calcium (Fig. 1D) with the calcium concentration at which binding is half of the maximum binding (Ki50) between 0.15 and 0.25 mM. At calcium concentrations lower than 0.1 mM, the binding of these three ligands was almost completely abolished. These data demonstrate that DC-SIGN binds both HCV glycoprotein E1 and E2 as well as HIV-1 gp120.
Cellular DC-SIGN and L-SIGN bind to HCV envelope glycoproteins.
K562 cells stably expressing DC-SIGN and L-SIGN were used to investigate the binding of cellular DC-SIGN and L-SIGN to HCV envelope glycoproteins. The transfectants expressed similar high levels of DC-SIGN and L-SIGN (Fig. 2A), as shown with the DC-SIGN- and L-SIGN-specific antibody AZN-D2. K562 transfectants expressing either DC-SIGN or L-SIGN strongly bound to both HCV envelope glycoproteins E1 and E2 (Fig. 2B), whereas mock-transfected K562 cells did not bind the HCV glycoproteins (data not shown). The observed binding of both DC-SIGN and L-SIGN to the HCV glycoproteins was comparable and could be completely blocked by antibodies against DC-SIGN and L-SIGN (Fig. 2B).
FIG. 2.
HCV envelope glycoproteins are bound by cellular DC-SIGN and L-SIGN. (A) K562 transfectants express similar levels of DC-SIGN and L-SIGN. K562 cells were transfected with DC-SIGN or L-SIGN as described in Materials and Methods. Expression was measured by fluorescence-activated cell sorting staining with the DC-SIGN- and L-SIGN-specific antibody AZN-D2. Dotted lines represent the isotype control, and black lines represent AZN-D2 staining. Mean fluorescence indices were >700. (B) Cellular DC-SIGN and L-SIGN bind to both envelope glycoproteins E1 and E2. HCV envelope glycoprotein binding by K562 cells expressing DC-SIGN or L-SIGN was measured with a fluorescently coated bead adhesion assay. The L-SIGN- and DC-SIGN-specific blocking antibody AZN-D2 (50 μg/ml) was used to determine the specificity of the interaction. (C) The DC-SIGN Val351 mutant binds both HCV envelope glycoproteins E1 and E2. Binding of K562 cells transfected with DC-SIGN V351G to glycoprotein E1- and E2-coated beads was investigated in the presence and absence of the DC-SIGN-specific antibody AZN-D2 (50 μg/ml) and EGTA (10 μM). Standard deviations were <5%.
We used DC-SIGN mutants containing specific mutations in their ligand-binding site in order to investigate the binding site of DC-SIGN for HCV envelope glycoprotein E1 and E2 in more detail. The C-type lectin domain of DC-SIGN contains two calcium binding sites (16): site 1, positioned at the auxiliary site of DC-SIGN, and site 2, located near the primary ligand binding site. The Ca2+ ion at site 1 coordinates the correct positioning of the loops forming the primary binding site (13, 17). To investigate the role of this calcium ion, four mutations were generated. Mutating Asp320 (D320A), Glu324 (E324A), Asn350 (N350A), or Asp355 (D355A) to alanine resulted in the loss of calcium binding at site 1 and subsequent loss of binding to HCV envelope glycoproteins (Table 1), as was also previously shown for both ICAM-3 and HIV-1 gp120 (17). Changing Asp366, which coordinates calcium binding at site 2, to an alanine residue (D366A) resulted in complete loss of binding to both HCV and HIV-1 glycoproteins (Table 1). The amino acid residues that are in close contact with the Ca2+ at site 2 (Glu347, Asn349, Glu354, and Asn365) form the core of the ligand-binding site (17). Changing Glu347 (E347Q) to Gln or Asn349 (N349D) and Asn365 (N365D) to Asp also resulted in complete loss of binding to HCV envelope glycoproteins E1 and E2 and HIV-1 gp120 (Table 1).
TABLE 1.
Identification of ligand binding site of DC-SIGN for HCV and HIV-1 envelope glycoproteins
| DC-SIGN expressed by K562 transfectant | Fluorescent bead adhesion
|
||
|---|---|---|---|
| gp120 | E1 | E2 | |
| Wild type | 45 | 50 | 45 |
| E347Q | 4 | 3 | 7 |
| N349D | 0 | 5 | 9 |
| N356D | 0 | 5 | 9 |
| D366A | 3 | 4 | 5 |
| D320A | 7 | 4 | 6 |
| E324A | 0 | 4 | 7 |
| N350A | 1 | 4 | 8 |
| D355A | 2 | 4 | 8 |
Recently, we demonstrated that the binding site of DC-SIGN for its cellular ligand ICAM-3 is distinct from that of its pathogen ligands HIV-1 gp120 (17) and mycobacterial ManLAM (19), since a specific mutation in DC-SIGN (V351G) abolished ICAM-3 but not HIV-1 gp120 and ManLAM binding. Interestingly, the DC-SIGN V351G mutant also interacted with HCV E1 as well as E2 (Fig. 2C), demonstrating that both HIV-1 and HCV bind similarly to DC-SIGN at a site distinct from that of the cellular ligand ICAM-3.
HCV VLPs are internalized by DC-SIGN and L-SIGN transfectants.
DC-SIGN can function as an antigen receptor, internalizing antigens (12). With a DC-SIGN-specific antibody as an antigen, it was shown that DC-SIGN rapidly targets the antigen to lysosomal compartments for degradation and subsequent presentation on major histocompatibility complex class II (12). In order to investigate the internalization and intracellular targeting of HCV VLPs after capture by both DC-SIGN and L-SIGN, we used stable transfectants of the erythroleukemic cell line K562 and the monocytic cell line THP-1. Both DC-SIGN- and L-SIGN-transfected K562 and THP-1 cells efficiently bound HCV E1/E2 VLPs (Fig. 3A).
FIG. 3.
Internalization pathway of DC-SIGN and L-SIGN is dependent on cellular background. (A) DC-SIGN and L-SIGN expressed by K562 and THP-1 transfectants bind HCV VLPs similarly. Binding of HCV E1/E2 VLP to K562 and THP-1 transfectants with both DC-SIGN and L-SIGN was measured. Interaction was blocked by the DC-SIGN- and L-SIGN-specific antibody AZN-D2 (50 μg/ml). Standard deviations were <5%. (B to E) DC-SIGN- or L-SIGN-bound HCV is targeted to the early endosomes (transferrin positive) in THP-1 transfectants, in contrast to the lysosomal (LAMP-1 positive) targeting in K562 transfectants. K562 (B and C) and THP-1 (D and E) cells expressing DC-SIGN or L-SIGN were incubated overnight with HCV VLPs. HCV was detected with a human anti-HCV antibody and a FITC-labeled secondary antibody. Intracellular targeting was determined by staining the endosomal compartments with a mouse antibody against the lysosomal and late endosomal LAMP-1-specific and Alexa Fluor 594-labeled secondary antibody (B and D) or by coincubating the cells for 15 min with Alexa Fluor 594-labeled transferrin, which is specifically transported to the early endosomes (C and E). Cells were analyzed by fluorescence microscopy.
Transfectants were incubated overnight with E1 AND E2 VLPs. Intracellular compartments were subsequently stained with an antibody against LAMP-1, a marker for lysosomal and late endosomal vesicles, and with transferrin, which accumulates in early endosomes through transferrin receptor uptake (9). Cells were analyzed by immunofluorescence microscopy. In all transfectants, HCV VLPs were rapidly internalized by DC-SIGN and L-SIGN and targeted to the endosomal pathway (Fig. 3B). Remarkably, our results demonstrate that the internalization pathway of both DC-SIGN and L-SIGN is dependent on the cell type. Both DC-SIGN and L-SIGN expressed by K562 cells targeted HCV E1/E2 VLPs to the LAMP-1-positive compartments (Fig. 3B), not to the transferrin-positive early endosomes (Fig. 3C). In contrast, HCV VLPs internalized by DC-SIGN and L-SIGN expressed by THP-1 cells were not targeted to the lysosomes. In these cells, HCV VLPs resided not within LAMP-1-positive compartments (Fig. 3D) but in transferrin-positive vesicles (Fig. 3E).
The internalization was specific for both DC-SIGN and L-SIGN, since both mock-transfected K562 and THP-1 cells did not internalize HCV VLPs (data not shown). Thus, both DC-SIGN and L-SIGN efficiently internalize HCV VLPs, but the internalization pathway is dependent on the cell type, suggesting that cellular origin is important. Therefore, we investigated antigen uptake with DC-SIGN on primary cells.
DC-SIGN on DCs targets HCV to early endosomes.
DCs express high levels of various pathogen receptors such as DC-SIGN (Fig. 4A) and the mannose receptor (12). We investigated the interaction of immature DCs with the different HCV envelope glycoproteins. The contribution of DC-SIGN on immature DCs in the binding to HCV envelope glycoproteins was investigated with specific blocking antibodies. Immature DCs showed strong binding to HCV E1, E2, and E1/E2 VLPs. This binding could be blocked by a specific anti-DC-SIGN antibody, mannan, and EGTA, but not by an antibody against mannose receptor (Fig. 4B), demonstrating that DC-SIGN is the major receptor for HCV envelope glycoproteins on immature DCs.
FIG. 4.
DCs strongly bind to HCV glycoprotein E1 and E2 through DC-SIGN. (A) Immature DCs express high levels of DC-SIGN. LPS-matured DCs express lower levels of DC-SIGN. Monocyte-derived DCs were isolated as described in Materials and Methods. Expression of DC-SIGN was measured by fluorescence-activated cell sorting staining with the DC-SIGN-specific antibody AZN-D2. Open histograms, isotype control; solid histograms, AZN-D2 staining. (B and C) Immature DCs and mature DCs bind strongly to HCV envelope glycoproteins E1 and E2 and mixed HCV E1/E2 VLPs via DC-SIGN. Immature (B) and LPS-matured (C) DC binding to HCV envelope glycoproteins was determined by a fluorescent bead adhesion assay. Specificity was determined by anti-DC-SIGN antibody AZN-D2 (50 μg/ml), mannan (100 μg/ml), EGTA (10 μM), and anti-mannose re- ceptor antibody (clone 19) (50 μg/ml). Standard deviations were <5%. (D) HCV VLPs do not affect DC activation or maturation. Immature DCs were incubated with HCV (12.5 μg/ml) alone, LPS (10 μg/ml) alone, or HCV and LPS together for 48 h, and activation was determined by measuring the expression of CD83 and CD86. Dotted lines, isotype controls; solid histograms with thin lines, incubations without HCV; open histograms with thick lines, incubations without or with LPS in combination with HCV VLPs. Upper panel, incubations without LPS; lower panel, incubations with LPS. One representative experiment out of three is shown.
Moreover, mature DCs also captured the different HCV envelope glycoproteins, and this could be blocked by mannan, EGTA, and an antibody against DC-SIGN but not by a mannose receptor-specific antibody (Fig. 4C). This indicates that on mature DCs, DC-SIGN is also the major receptor for HCV envelope glycoproteins. However, this binding was less strong than the binding by immature DCs due to downregulation of DC-SIGN expression (Fig. 4A).
Next, we investigated the effect of HCV on DC activation and maturation. Activation was determined by measuring the expression levels of the maturation markers CD83 and CD86. Immature DCs were incubated with HCV VLPs for 48 h. CD83 and CD86 expression did not change compared to that in DCs incubated without HCV (Fig. 4D), demonstrating that HCV VLPs do not induce maturation. We have demonstrated that mycobacterial lipoarabinomannan binding to DC-SIGN prevents DC activation (19). In contrast, HCV had no effect on LPS-induced DC activation (Fig. 4D). This supports recent data showing that DCs function normally in chronic HCV-infected patients (30), suggesting that HCV evades DC immunity in a different way. Therefore, the fate of HCV VLPs upon binding by immature DCs was investigated.
Immature DCs were incubated with HCV VLPs for 4 h or overnight, and colocalization with the lysosomal marker LAMP-1 or early endosomal markers EEA-1 (35) and transferrin was analyzed. After 4 h, colocalization of the HCV VLPs was observed with transferrin-positive but not with LAMP-1-positive vesicles. Even after 24 h, HCV was still present primarily in EEA-1-positive vesicles (Fig. 5A), as was observed for THP-1 transfectants (Fig. 3D and E). HCV VLP internalization was DC-SIGN dependent, since blocking of DC-SIGN with a DC-SIGN antibody completely abolished internalization of HCV VLPs (data not shown). Subsequently, DC-SIGN colocalization with HCV VLPs after incubation was investigated. After incubation at 4°C, both HCV VLPs and DC-SIGN were localized at the surface of the DCs (Fig. 5B). When incubated at 37°C for 4 h to allow internalization, internalized HCV VLPs colocalized with DC-SIGN (Fig. 5B). Even after prolonged incubation (24 h), DC-SIGN colocalized with HCV VLPs, suggesting that, besides a role in internalization, DC-SIGN has a role in intracellular routing of HCV VLPs.
FIG. 5.
DC-SIGN on immature DCs targets HCV VLPs to early endosomes but Lewis X antigen to lysosomes. (A) Immature DCs were incubated with HCV VLPs (30 μg/ml) for 4 h or overnight. HCV was detected with a human anti-HCV antibody and a FITC-labeled secondary antibody. Intracellular targeting was determined by staining the endosomal compartments with a mouse antibody against the lysosome- and late endosome-specific marker LAMP-1 or the early endosome-specific marker EEA-1 and an Alexa Fluor 594-labeled secondary antibody or by coincubating the cells for 15 min with Alexa Fluor 594-labeled transferrin, which is specifically transported to early endosomes. (B) Immature DCs were incubated with HCV VLPs (30 μg/ml) for 4 h or overnight at 37 or 4°C. HCV was detected as described for panel A. Localization of DC-SIGN was determined with the DC-SIGN-specific antibody AZN-D2 and an Alexa Fluor 594-labeled sec- secondary antibody. Cells were analyzed on a 3i Marianas digital imaging microscopy workstation with SlideBook software. (C) DC-SIGN on immature DCs targets its ligand Lewis X antigen to late endosomes and lysosomes. Immature DCs were incubated with Lewis X (10 μg/ml) for 4 h. Intracellular targeting was determined by staining the endosomal compartments with a mouse antibody against the lysosome- and late endosome-specific marker LAMP-1 or by coincubating the cells for 15 min with Alexa Fluor 594-labeled transferrin, which is specifically transported to early endosomes. Cells were analyzed by fluorescence microscopy.
Strikingly, the internalization pathway of DC-SIGN on immature DCs is dependent on the ligand, since the carbohydrate blood group antigen Lewis X and mycobacterial ManLAM were efficiently captured and internalized by immature DCs through DC-SIGN and targeted to transferrin negative, LAMP-1-positive lysosomes (Fig. 5C) (19) but not on the ligand-binding site, as ManLAM binds the same site as HCV E1 and E2 and HIV gp120. Together, these data suggest that HCV targets DC-SIGN to enter DCs and escape degradation by preventing targeting to the lysosomes.
HCV interact with LSECs in situ.
In the liver, hepatocytes are the main target of HCV, and although putative HCV receptors have been identified, it is unclear how HCV infects hepatocytes. We have shown that cell lines transfected with L-SIGN can interact with HCV envelope glycoproteins (Fig. 2). L-SIGN is expressed on LSECs (5, 37), the liver sinusoidal endothelial cells that are in close contact with the blood and could capture HCV from the blood and transmit it to the hepatocytes. However, these cells are difficult to isolate. Therefore, we used an in situ binding assay to investigate the major HCV binding cells in the liver.
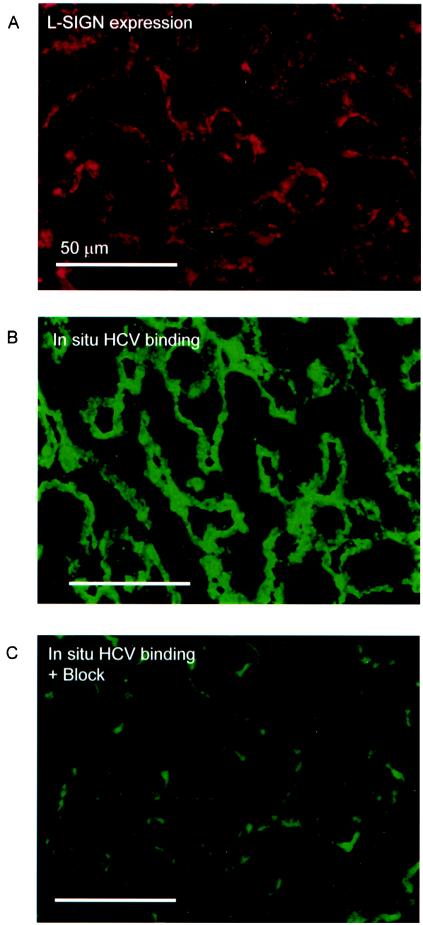
L-SIGN was stained in liver tissue sections with an anti-L-SIGN antibody and analyzed by immunofluorescence microscopy (Fig. 6A). HCV envelope glycoprotein binding in situ was investigated by incubating the liver sections with HCV VLPs. VLPs were detected with anti-HCV E1 antibody and fluorescently labeled secondary antibody (Fig. 6B). The staining pattern of HCV VLPs was similar to that of L-SIGN staining in the sections, suggesting that the HCV binds to L-SIGN-expressing LSECs. Moreover, HCV binding to LSEC was specifically inhibited by a blocking antibody against L-SIGN (Fig. 6C), demonstrating that this binding was mediated by the C-type lectin L-SIGN. Strikingly, HCV VLPs interacted only with the LSECs and not with hepatocytes that express the putative HCV receptor asialoglycoprotein receptor and are the main target cells for HCV. These data suggest that HCV is captured by L-SIGN that mediates internalization of HCV, and thus LSECs act as an HCV reservoir and may transmit the virus to hepatocytes.
FIG. 6.
HCV interacts with L-SIGN-expressing LSECs in situ. (A) L-SIGN is expressed by human LSECs, as was determined by staining of liver tissue with an L-SIGN-specific antibody. (B) Binding of HCV VLPs by liver tissue was determined by incubating liver sections with HCV VLPs (10 μg/ml) for 2 h at 37°C. HCV binding was detected with a human anti-HCV antibody and FITC-labeled secondary antibody. (C) HCV VLP binding to LSECs is specifically blocked by the L-SIGN-specific antibody AZN-D2. Sections were incubated with AZN-D2 (50 μg/ml) for 30 min at room temperature before HCV VLPs were added as described for panel A. Sections were analyzed by fluorescence microscopy with a 20× objective. Bars, 50 μm.
DISCUSSION
Our results demonstrate that HCV is efficiently captured by DCs through DC-SIGN and by LSECs through L-SIGN. The C-type lectins interacted with both envelope glycoproteins E1 and E2. Strikingly, internalized HCV VLPs were targeted by DC-SIGN on immature DCs to early endosomal compartments, where HCV is protected from degradation. Similarly, HCV captured by L-SIGN expressed on THP-1 was targeted to early endosomal compartments. In contrast, another ligand of DC-SIGN, Lewis X blood group antigen, was targeted to the lysosomes. Thus, HCV may target DC-SIGN and L-SIGN to use DCs and LSECs, respectively, as reservoirs to facilitate viral dissemination.
Because of the lack of a suitable cell culture system for in vitro propagation of HCV and the unavailability of virions in sufficient quantities, truncated and secreted versions of the HCV envelope glycoproteins have been used as soluble surrogates for native virus particles. Here, we used VLPs of HCV envelope glycoproteins E1 and E2. The envelope glycoproteins E1 and E2 were produced in either the yeast H. polymorpha or mammalian cells with a recombinant vaccinia virus and assembled into a VLP of noncovalently linked E1 or E2 homomers or E1/E2 heteromers. We used these purified HCV VLPs to investigate their interaction with both DC-SIGN and L-SIGN.
Both cellular DC-SIGN and L-SIGN bound strongly to envelope glycoproteins E1 and E2 alone as well as to the E1/E2 heterodimer particles (Fig. 2). The interactions were specifically inhibited by blocking antibodies against DC-SIGN and L-SIGN. Recently, it was shown that both DC-SIGN and L-SIGN interact with HCV envelope glycoprotein E2 (14, 31, 38) and envelope glycoprotein E1 (38). Here we demonstrate that DC-SIGN bound more strongly to envelope glycoprotein E2 than to glycoprotein E1. Lozach et al. (31) demonstrated that the interaction of DC-SIGN with glycoprotein E2 is dependent on N-linked glycosylations. HCV envelope glycoprotein E2 has 11 potential N-linked glycosylation sites, whereas envelope glycoprotein E1 has only six sites (34), which can explain the stronger binding of DC-SIGN to HCV envelope glycoprotein E2. Furthermore, we demonstrate that the interactions of DC-SIGN with HCV envelope glycoproteins E1 and E2 and with HIV-1 envelope protein gp120 were equally dependent on calcium (Fig. 1E), suggesting that these viral envelope glycoproteins interact at the same binding site in DC-SIGN.
Indeed, site-directed mutagenesis demonstrated that both HCV glycoproteins E1 and E2 bound the primary ligand-binding site in DC-SIGN through coordination with the primary Ca2+ ion at site 2 (Table 1). Moreover, HCV envelope glycoproteins E1 and E2, like the pathogenic ligands HIV-1 gp120 and mycobacterial ManLAM, bound to the V351G DC-SIGN mutant, whereas the cellular ligand ICAM-3 does not bind this mutant (17, 19). These observations further suggest that DC-SIGN may distinguish between different types of ligand and may tailor its responses specifically to the ligand that it recognizes.
DC-SIGN functions as a more universal pathogen receptor (46), and the pathogens HIV-1 and M. tuberculosis target DC-SIGN to escape immune surveillance (15, 19, 27). The interaction of HCV envelope glycoproteins E1 and E2 with both DC-SIGN and L-SIGN suggests that DC-SIGN and L-SIGN may be involved in the pathogenesis of HCV. Little is known about the dissemination of HCV, and the role of the immune system in the pathogenesis of HCV is complex and largely unknown. It has been shown that HCV may affect DCs; during chronic HCV infection, DCs are impaired in their immune function (3, 4). In contrast, a recent study demonstrated that monocyte-derived DCs from patients suffering from chronic HCV infection are functionally normal (30). Here we demonstrate that immature DCs bind strongly to HCV envelope glycoprotein E1, E2, and E1/E2 VLPs through DC-SIGN, since the interactions were completely blocked by antibodies against DC-SIGN (Fig. 4B). Mature DCs also captured HCV E1, E2, and E1/E2 VLPs through DC-SIGN, although less efficiently than immature DCs (Fig. 4C). This may be due to the lower expression of DC-SIGN on mature DCs than on immature DCs (Fig. 4A) (38). The complete inhibition of the interaction of both immature and mature DCs with HCV particles by antibodies against DC-SIGN demonstrates that DC-SIGN is the primary receptor for HCV on both immature and mature DCs, not the HCV E2 receptor CD81, which is also expressed by DCs (38).
HCV VLPs were rapidly internalized upon capture by both DC-SIGN and L-SIGN (Fig. 3). Depending on the cell type, the captured HCV VLPs were targeted differently to intracellular compartments by these C-type lectins. In the cell line THP-1, captured HCV VLPs were targeted to transferrin-positive nonlysosomal compartments, whereas VLPs captured by the erythroleukemic cell line K562, expressing either DC-SIGN or L-SIGN, were targeted to LAMP-1-positive lysosomal compartments (Fig. 3). These results demonstrate that there is a difference in internalization pathway between these cell lines. HCV VLPs captured by primary immature DCs through DC-SIGN were targeted to early endosomal vesicles, where they resided for over 24 h, similar to the THP-1 transfectants. This indicates that the internalization pathway observed in THP-1 transfectants may represent a more native situation, as found in DCs. In addition, it was recently demonstrated that transmission of HIV-1 is also cell type dependent (45). DC-SIGN does not facilitate HIV-1 infection of DCs but protects the virus from degradation by internalizing the virus in nonlysosomal compartments (15, 27). The DC-SIGN-bound HIV-1 is efficiently transmitted to recipient CD4+ T cells upon coculture of DCs with CD4+ T cells, which results in a productive infection of T cells (15).
Like DCs, THP-1 cells expressing DC-SIGN continue to infect T cells with HIV-1 for several days, whereas DC-SIGN-expressing 293 cells are infective only for a short period. However, HIV-1 is not retained in early endosomal compartments like HCV or in lysosomes but in other, undefined vesicles (27, 45), which may explain why transmission in these cells is possible over a prolonged period. The internalization pathway of DC-SIGN-bound ligands is dependent not only on the cell type but also on the ligand, as another carbohydrate-containing ligand of DC-SIGN, Lewis X antigen, was rapidly internalized and targeted to the lysosomal compartment in both immature DCs and THP-1 transfectants (Fig. 5C and data not shown).
Altogether, these results support a role for DC-SIGN in the capture of HCV virions by immature DCs, similar to HIV-1, and show that THP-1 transfectants are a suitable model for pathogen internalization. A recent study suggests that the THP-1 cells used to study DC-SIGN and L-SIGN function have B-cell-like characteristics (48). Indeed, the THP-1 cells used in this study express CD19 (data not shown) and therefore may be of B-cell origin. Interestingly, B cells have an antigen-processing and -presenting machinery similar to that of DCs, and our results demonstrate that DC-SIGN expressed by these THP-1 cells behaves as it does on immature DCs. Therefore, THP-1 cells are a suitable model for studying pathogen internalization.
Hepatocytes are productively infected by HCV, and the hepatocyte-specific asialoglycoprotein receptor has been proposed as an HCV receptor (41). However, we demonstrate here that not hepatocytes but LSECs bind HCV VLPs in primary liver tissue (Fig. 6), suggesting that LSECs capture HCV from the blood and transfer HCV to hepatocytes. Moreover, L-SIGN, like DC-SIGN, has been shown to function in HIV-1 transmission (5), and L-SIGN expressed by LSECs, could capture HCV from the blood and transfer it to hepatocytes. A similar mechanism was demonstrated for another hepatic virus, hepatitis B virus (6). This virus enters LSECs, and Breiner et al. (6) proposed that the virus travels via endosomal compartments to the hepatocytes that are subsequently infected. Our data further indicate that both LSECs and DCs may efficiently capture and protect the virus and function as HCV reservoirs.
Our recent results suggest that mycobacteria target DC-SIGN by secreting ManLAM to downregulate DC-mediated immune responses and thus promote pathogen survival (19). It is not clear whether viruses are able to suppress DC functions by binding DC-SIGN through a similar mechanism. The immunosuppressive setting that characterizes infections with cytomegalovirus and HIV-1, two viruses that interact with DC-SIGN, implies that such a mechanism of immunomodulation may exist (reviewed in reference 46). It was suggested that DCs isolated from patients suffering from chronic HCV infections have an impaired maturation and immune-stimulatory function (3, 4). However, our data indicate that immature DCs are not affected by HCV VLPs, and furthermore, Longman et al. recently showed that DCs from chronically infected patients are not impaired in their immune function (30). This suggests that HCV may target DC-SIGN to escape lysosomal degradation in DCs and thereby evade immunity. Although further investigations into the function of DC-SIGN and L-SIGN will be necessary to determine their importance in HCV infections, our results suggest that both DC-SIGN and L-SIGN may be potential targets for designing strategies to combat HCV infections.
Acknowledgments
I.S.L. was supported by the Dutch Digestive Diseases Foundation (MLDS; grant no. WS 01-36) and the AIDS Fund (grant no. 5008). A.N.L. was supported by the Dutch Scientific Research Organization (NWO; grant no. 015.000.023).
Liver sections were a kind gift of the Department of Pathology, Vrije Universiteit Medical Center, Amsterdam, The Netherlands.
REFERENCES
- 1.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 4.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 5.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. Van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breiner, K., H. Schaller, and P. Knolle. 2001. Endothelial cell-mediated uptake of a hepatitis B virus: A new concept of liver targeting of hepatotropic microorganisms. Hepatology 34:803-808. [DOI] [PubMed] [Google Scholar]
- 7.Cerny, A., J. G. McHutchison, C. Pasquinelli, M. E. Brown, M. A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F. V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Investig. 95:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover, A., A. L. Schwartz, and H. F. Lodish. 1983. Sorting and recycling of cell surface receptors and endocytosed ligands: the asialoglycoprotein and transferrin receptors. J. Cell Biochem. 23:107-130. [DOI] [PubMed] [Google Scholar]
- 10.Di Bisceglie, A. M. 1995. Hepatitis C and hepatocellular carcinoma. Semin. Liver Dis. 15:64-69. [DOI] [PubMed] [Google Scholar]
- 11.Dittmann, S., M. Roggendorf, J. Durkop, M. Wiese, B. Lorbeer, and F. Deinhardt. 1991. Long-term persistence of hepatitis C virus antibodies in a single source outbreak. J. Hepatol. 13:323-327. [DOI] [PubMed] [Google Scholar]
- 12.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. Van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, J. P., R. J. Durso, R. R. Arrigale, G. P. Donovan, P. J. Maddon, T. Dragic, and W. C. Olson. 2003. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA 100:4498-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. Van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 16.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. Van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., G. C. van Duijnhoven, S. J. van Vliet, E. Krieger, G. Vriend, C. G. Figdor, and Y. Van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314-11320. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., Y. Van Kooyk, S. J. van Vliet, M. H. Renes, R. A. Raymakers, and C. G. Figdor. 1999. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood 94:754-764. [PubMed] [Google Scholar]
- 19.Geijtenbeek, T. B., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gish, R. G., K. P. Qian, S. Quan, Y. L. Xu, I. Pike, A. Polito, R. DiNello, and J. Y. Lau. 1997. Concordance between hepatitis C virus serotyping assays. J. Viral Hepat. 4:421-422. [DOI] [PubMed] [Google Scholar]
- 21.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 23.Hijikata, M., N. Kato, Y. Ootsuyama, M. Nakagawa, and K. Shimotohno. 1991. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc. Natl. Acad. Sci. USA 88:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houghton, M., A. Weiner, J. Han, G. Kuo, and Q. L. Choo. 1991. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology 14:381-388. [PubMed] [Google Scholar]
- 25.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R. W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knolle, P. A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 174:21-34. [DOI] [PubMed] [Google Scholar]
- 27.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 28.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 72:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, C., B. D. Lindenbach, B. M. Pragai, D. W. McCourt, and C. M. Rice. 1994. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J. Virol. 68:5063-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longman, R. S., A. H. Talal, I. M. Jacobson, M. L. Albert, and C. M. Rice. 2004. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood 103:1026-1029. [DOI] [PubMed] [Google Scholar]
- 31.Lozach, P. Y., H. Lortat-Jacob, d. L. de Lacroix, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278:20358-20366. [DOI] [PubMed] [Google Scholar]
- 32.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamura, T., and Y. Matsuura. 1993. Structural proteins of hepatitis C virus. Trends Microbiol. 1:229-231. [DOI] [PubMed] [Google Scholar]
- 34.Op De Beeck, L. Cocquerel, and J. Dubuisson. 2001. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 82:2589-2595. [DOI] [PubMed] [Google Scholar]
- 35.Patki, V., J. Virbasius, W. S. Lane, B. H. Toh, H. S. Shpetner, and S. Corvera. 1997. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 94:7326-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 37.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehermann, B., K. M. Chang, J. G. McHutchison, R. Kokka, M. Houghton, and F. V. Chisari. 1996. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Investig. 98:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunier, B., M. Triyatni, L. Ulianich, P. Maruvada, P. Yen, and L. D. Kohn. 2003. Role of the asialoglycoprotein receptor in binding and entry of hepatitis C virus structural proteins in cultured human hepatocytes. J. Virol. 77:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarselli, E., H. Ansuini, R. Cerino, R. M. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21:5017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeff, L. B., Z. Buskell-Bales, E. C. Wright, S. J. Durako, H. J. Alter, F. L. Iber, F. B. Hollinger, G. Gitnick, R. G. Knodell, and R. P. Perrillo. 1992. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N. Engl. J. Med. 327:1906-1911. [DOI] [PubMed] [Google Scholar]
- 44.Takamizawa, A., C. Mori, I. Fuke, S. Manabe, S. Murakami, J. Fujita, E. Onishi, T. Andoh, I. Yoshida, and H. Okayama. 1991. Structure and organization of the hepatitis C virus genome isolated from human carriers. J. Virol. 65:1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trumpfheller, C., C. G. Park, J. Finke, R. M. Steinman, and A. Granelli-Piperno. 2003. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 15:289-298. [DOI] [PubMed] [Google Scholar]
- 46.Van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 47.Wellnitz, S., B. Klumpp, H. Barth, S. Ito, E. Depla, J. Dubuisson, H. E. Blum, and T. F. Baumert. 2002. Binding of hepatitis C virus-like particles derived from infectious clone H77C to defined human cell lines. J. Virol. 76:1181-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]