Abstract
Human immunodeficiency virus type 1 (HIV-1) Vpr induces cell cycle arrest at the G2/M transition and subsequently apoptosis. Here we examined the potential involvement of Wee-1 in Vpr-induced G2 arrest. Wee-1 is a cellular protein kinase that inhibits Cdc2 activity, thereby preventing cells from proceeding through mitosis. We previously showed that the levels of Wee-1 correlate with Vpr-mediated apoptosis. Here, we demonstrate that Vpr-induced G2 arrest correlated with delayed degradation of Wee-1 at G2/M. Experimental depletion of Wee-1 by a small interfering RNA directed to wee-1 mRNA alleviated Vpr-induced G2 arrest and allowed apparently normal progression through M into G1. Similar results were observed when cells were arrested at G2 following gamma irradiation. Thus, Wee-1 is integrally involved as a key cellular regulatory protein in the signal transduction pathway for HIV-1 Vpr-induced cell cycle arrest.
Human immunodeficiency virus type 1 (HIV-1) Vpr is a highly conserved 96-amino-acid virion-associated protein found among HIV-1, HIV-2, and simian immunodeficiency virus type 1 (13, 32). Vpr plays an important role in viral replication by facilitating infection of nondividing cells such as macrophages (2, 7, 31). Vpr has a weak trans-activating activity and functions to trans-activate the long terminal repeat prior to integration (19). We and others have shown that Vpr induces cell cycle arrest at the G2 cell cycle checkpoint and subsequently induces apoptosis in cells (3, 10, 14, 21, 25). These pathogenic properties of Vpr likely contribute to the immune deregulation and CD4+-T-cell depletion seen in HIV-1-infected individuals.
The phenotypic features of Vpr-induced cell cycle arrest are similar to those seen following DNA damage induced by ionizing radiation and alkylating agents (20). A recent study demonstrated that Vpr activates the DNA damage-signaling protein ATR (22). Together these results suggest that Vpr induces cell cycle arrest by using highly conserved cellular pathways for cell cycle regulation.
Although the detailed mechanism of Vpr-induced cell cycle arrest is as yet unknown, Vpr-induced G2 arrest is associated with inactivation of Cdc2, the key regulatory kinase element at the G2/M checkpoint (10, 14, 21). Entry into mitosis requires activation of Cdc2 following removal of inhibitory phosphates by Cdc25. Cdc2 kinase activity is regulated in an opposing manner by the kinase Wee-1. Thus, Cdc25 and Wee-1 compose a phosphatase-kinase switch by which Cdc2 is activated. Various pathways converge at this point, shifting the balance toward entry into mitosis or arrest at G2/M (5, 18).
Wee-1 is a nuclear protein that is subject to multiple levels of regulation including reversible phosphorylation, proteolysis, and protein-protein interactions (1, 15, 16, 29, 30). Wee-1 encodes a tyrosine-specific protein kinase that inactivates Cdc2 kinase activity by phosphorylation on Tyr15. The Wee-1 kinase is negatively regulated by phosphorylation in a cell cycle-specific manner. In Xenopus laevis oocytes N-terminal phosphorylation of serine 38 in Wee-1 correlates with reduced activity and stability of Wee-1 during mitosis and is important for conferring substrate specificity (6, 28). The C terminus of Wee-1 contains a 14-3-3 binding site thought to function as a positive regulator of Wee-1 (23, 29). In addition to phosphorylation, human Wee-1 is also regulated at the level of protein synthesis and stability. Wee-1 levels rise during the S and G2 phases of the cell cycle because of increased synthesis and then fall during M phase due to decreased synthesis and proteolytic degradation (16, 30). In Xenopus, Tome-1 regulates Cdc2 activity by targeting Wee-1 for degradation, thus shifting the balance toward Cdc25 phosphatase and activation of Cdc2 (1).
In this study we sought to further explore the mechanism of Vpr-induced G2 arrest and specifically the relation to Wee-1 kinase. We demonstrate that Vpr-induced cell cycle arrest results in increased levels of Wee-1 kinase in synchronized HeLa cells. We observed that Vpr-induced G2 arrest correlated with delayed degradation of Wee-1 and that Cdc2 activity is reduced at G2/M in the presence of Vpr. Similar results were observed when cells were arrested at G2 following gamma irradiation. Thus, Wee-1 likely serves as a key regulatory element in HIV-1 Vpr-induced cell cycle arrest.
MATERIALS AND METHODS
Cell culture, synchronization, and gamma irradiation.
HeLa cells and 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% bovine calf serum and penicillin-streptomycin. Aliquots of 1.5 × 105 HeLa cells per well in a six-well plate or 1.0 × 106 cells per 60-mm-diameter plate (Becton Dickinson, Mountain View, Calif.) were seeded 1 day before synchronization. HeLa cells were synchronized at G1/S by culturing them in the presence of 2 mM thymidine (Sigma-Aldrich, St. Louis, Mo.) in DMEM-10% calf serum for 18 h. They were then allowed to recover in complete growth medium that lacked thymidine for 8 h and then propagated again in 2 mM thymidine for an additional 18 h. For gamma irradiation, the synchronized cells were exposed to gamma rays at a dose of 4,000 rads upon release from G1/S and medium was changed 3 h later.
Preparation of viral stocks.
HR′Thy, HR′EGFP, and HR′Vpr viruses were produced in 293T cells by cotransfection of 293T cells with 12.5 μg of pHR′ vector, 12.5 μg of pCMVΔR8.2ΔVpr, and 5 μg of pCMV VSV-G and collected as described previously (26). Virus stock was titrated to ensure that more than 95% of HeLa cells were arrested in G2 phase at 24 h postinfection. The equivalent of 2.0 μg of viral p24 was used to infect 1.0 × 106 HeLa cells. Equivalent amounts of HR′Thy and HR′EGFP virus were used as negative controls.
Infections and flow cytometry.
Cells were infected and analyzed for cell cycle status as previously described (27). Cell cycles were measured by DNA staining with propidium iodide (Sigma-Aldrich). All stained cells were acquired on a FACScan II cell sorter (Becton Dickinson) and analyzed with the Cell Quest software package. A total of 10,000 events were collected and analyzed in each sample.
Western blot and Cdc2 kinase assay.
The Western blot and Cdc2 kinase assay was performed as described previously (33). Wee-1, Cdc2, and β-actin antibodies were purchased from Santa Cruz Biotech, Santa Cruz, Calif. Anti-phospho-cdc2 (Tyr15) was from Cell Signaling, Beverly, Mass., and antihemagglutinin (anti-HA) was from Covance Inc., Princeton, N.J.
Real-time quantitative RT-PCR assay.
Real-time quantitative reverse transcription-PCR (RT-PCR) was performed to measure the level of wee-1 mRNA. Total RNA was prepared using the total RNA isolation kit (RNeasy; Qiagen, Valencia, Calif.) and treated with DNase I (RNase-free DNase set; Qiagen) according to the manufacturer's instructions. Quantification of the mRNA with wee-1 and glyceraldehyde-3-phosphate dehydrogenase (gapdh) was performed using ABI PRISM7700 (Applied Biosystems, Foster City, Calif.) with the QuantiTect SYBR Green RT-PCR kit (Qiagen). The reaction mixture contained QuantiTect SYBR Green RT-PCR Master Mix, QuantiTect RT Mix, each primer at 100 nM, and 25 ng (for wee-1) or 5 ng (for gapdh) of total RNA. After incubation for 30 min at 50°C (for the RT reaction) and then for 15 min at 95°C (for activation of Hot Start Taq polymerase), we carried out 45 cycles, with each cycle consisting of denaturation for 15 s at 95°C, annealing for 30 s at 59°C, and extension for 30 s at 72°C. The following primers were used in RT-PCR for these specific mRNAs: (i) human wee-1, 5′ primer, 5′-ATTTCTCTGCGTGGGCAGAAG-3′, and 3′ primer, 5′-CAAAAGGAGATCCTTCAACTCTGC-3′; (ii) human gapdh, 5′ primer, 5′-TGCACCACCAACTGCTTAGC-3′, and 3′ primer, 5′-GGCATGGACTGTGGTCATGAG-3′.
Pulse-chase analysis of endogenous Wee-1.
Synchronized HeLa cells were cultured for 7 h after infection with HR′Vpr or HR′EGFP virus and were washed once with methionine- and cysteine-free DMEM, followed by starvation in the same medium supplemented with 10% dialyzed fetal calf serum (FCS; Gibco BRL, Grand Island, N.Y.) for 30 min. The cells were pulse-labeled at 37°C by adding 100 μCi of 35S-labeled l-methionine and l-cysteine (Redivue Pro-Mix l-[35S] in vitro cell labeling mix; Amersham Biosciences, Uppsala, Sweden) per 106 cells in methionine- and cysteine-free medium supplemented with 10% dialyzed FCS and glutamine for 10 min. It has been reported that [35S]methionine inhibits cell growth (11, 12). We titrated the levels and labeling time of [35S]methionine in order to avoid this effect (data not shown). The pulse-labeling was stopped by washing the cells twice with DMEM, and DMEM-10% FCS containing unlabeled methionine and cysteine at a concentration of 200 μM was added to cultures for the chase periods indicated. After a chase, the cells were washed with ice-cold phosphate-buffered saline and were lysed in 1 ml of ice-cold radioimmunoprecipitation assay buffer (1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.2% NP-40, 150 mM NaCl, 5 mM EDTA, 10 mM Tris-HCl [pH 7.4], 10 mM NaF, 10 mM β-glycerophosphate, 1 mM Na3VO4) containing protease inhibitor cocktail (P1860; Sigma-Aldrich) and phosphatase inhibitor cocktails (P5726 and P2850; Sigma-Aldrich). The lysate was clarified by centrifugation at 12,000 rpm in an Eppendorf microcentrifuge at 4°C for 10 min, and the supernatants were used for immunoprecipitation.
For immunoprecipitation, the supernatants were divided into two parts: one part was immunoprecipitated with anti-Wee-1 polyclonal antibody (SC-325; Santa Cruz Biotech) and the other part was immunoprecipitated with normal rabbit immunoglobulin G (I5006; Sigma-Aldrich) as a negative control. The immunoprecipitated complex was washed with radioimmunoprecipitation assay buffer and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE; 4 to 12% polyacrylamide gel). The gels were dried and exposed to a phosphor screen. The positions of the radiolabeled proteins were determined by using a PhosphorImager with ImageQuant software (Molecular Dynamics Inc.).
siRNA.
A 21-nucleotide (nt) RNA duplex with symmetric 2-nt 3′ (2′-deoxy) thymidine overhangs corresponding to human Wee-1, encoding nt 954 to 972, was synthesized and purified (Dharmacon Research, Inc.). RNA sequences were as follows: sense, 5′-GAGGCUGGAUGGAUGCAUUdTdT-3′, and antisense, 5′-AAUGCAUCCAUCCAGCCUCdTdT-3′. A luciferase small interfering RNA (siRNA) was synthesized as described above. RNA sequences were as follows: sense, 5′-CUUACGCUGAGUACUUCGAdTdT-3′, and antisense, 5′-UCGAAGUACUCAGCGUAAGdTdT-3′. HeLa cells (1.5 × 105 cells per well of a six-well plate) were transfected with Oligofectamine (Invitrogen, Carlsbad, Calif.) as described elsewhere (9, 33).
RESULTS
Expression of Vpr correlates with an increase in the level of Wee-1 at G2/M.
A recombinant lentivirus vector encoding HIV-1 Vpr was used to infect a population of HeLa cells that was synchronized at the G1/S border by a double thymidine block and release protocol (23). Flow cytometry was used to monitor the ability of cells to traverse the cell cycle after release from the block (Fig. 1A). Cells infected with control virus HR′EGFP proceeded normally through the S, G2, and M phases of the cell cycle. Mitosis occurred 11 h after release at G1/S. In agreement with our previous observations the majority of Vpr-infected cells (>95%) were arrested in G2 (Fig. 1A).
FIG. 1.
Vpr stabilizes Wee-1 in Vpr-infected HeLa cells. HeLa cells (1.0 × 106) were synchronized at G1/S by a double thymidine block and then infected with equivalent amounts (2.0 μg of viral p24) of HR′Vpr or HR′EGFP. Infected cells were cultured for various times as indicated prior to analysis. (A) Cells were stained with propidium iodide and analyzed with a FACScan cell sorter. A total of 10,000 events were collected and analyzed in each sample. (B) Whole-cell lysate (10 μg) was separated by SDS-7.5% (Wee-1) or 15% (others) PAGE and probed with antibodies to Wee-1, phosphorylated Cdc2, total Cdc2, HA-tagged Vpr, and actin (as a loading control). Data shown are representative of Western blotting results obtained in three independent experiments.
Western blot analysis was used to monitor the levels of Wee-1 and Cdc2 after G1/S release (Fig. 1B). As expected, the level of Wee-1 in cells infected with control virus decreased at 11 and 12 h after release as a result of protein degradation during M phase. We observed an elevated level of Wee-1 in Vpr-infected cells at the same time points.
The electrophoretic mobility of Cdc2 can be used as an indicator of cell cycle position and to assess the phosphorylation status of Cdc2. The higher-molecular-weight species represents the phosphorylated form of Cdc2 (Tyr15) that is inactive. The smaller species is the active unphosphorylated form of the kinase. We monitored the mobility of Cdc2 using antibodies that allowed us to distinguish the phosphorylation status of Cdc2 as cells cycled from G1/S through M. As previously reported, in the control cells we observed the disappearance of the upper band (phosphorylated form of Cdc2) at 10 h and then a decrease in the overall level of Cdc2 at 12 h as cells went through mitosis (Fig. 1B). In contrast equivalent amounts of the phosphorylated and unphosphorylated forms of Cdc2 were observed in the Vpr-infected cells throughout the time course. The transient increase in the level of Wee-1 and the presence of the phosphorylated form of Cdc2 in Vpr-arrested cells are consistent with the cell cycle-inhibitory role of Wee-1 at the G2/M checkpoint.
Vpr causes a delay in reduction of the levels of Wee-1 at G2/M.
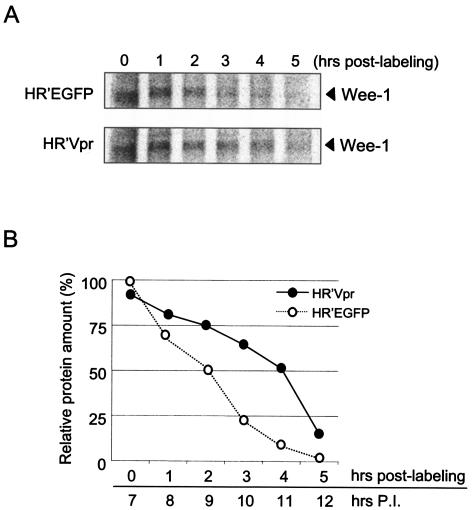
The results presented above demonstrate that an increase in the levels of Wee-1 following infection with HR′Vpr is paralleled by increased levels of the phosphorylated (inactive) form of Cdc2. The relationship between induction of G2 arrest and increased levels of Wee-1 at the G2/M transition over time suggested the hypothesis that a delay in reduction of Wee-1 may be responsible for the failure of Vpr-arrested cells to progress through mitosis. To further test this hypothesis, we determined the level of 35S-labeled Wee-1 in Vpr-infected cells by pulse-chase analysis (Fig. 2A). Cells were released from G1/S at the time of infection and 35S-pulse-labeled 7 h after infection/release. In HR′EGFP-infected cells the level of labeled Wee-1 decreased following release at G1/S. Wee-1 had a half-life of approximately 2.5 h. In contrast a sustained level of labeled Wee-1 is observed in the presence of Vpr. Over the 5-h time course there is a delay in degradation of Wee-1 relative to cells infected with HR′EGFP. In this case the half-life of Wee-1 is about twice as long as it is in the absence of Vpr (Fig. 2B).
FIG. 2.
The level of Wee-1 remains stable following Vpr-induced arrest. (A) HeLa cells (2.0 × 106) were synchronized at G1/S by a double thymidine block. They were then infected with equivalent amounts of HR′Vpr or HR′EGFP (4.0 μg of viral p24). Five hours after infection cells were subjected to a 30-min period of serum starvation and pulse-labeled for 10 min with 35S-labeled methionine-cysteine. This was followed by a chase period for 5 h. Cells were analyzed during the chase period at the time points indicated. Equivalent amounts of cell lysates were immunoprecipitated with antibody specific for Wee-1. Antibody-bound immune complexes were separated by SDS-10% PAGE. 35S-labeled Wee-1 was detected by autoradiography. Data shown are representative of results from three independent experiments. (B) Quantitation of Wee-1 levels by densitometry. The signal obtained at the starting point (0 h) for HR′EGFP virus-infected HeLa cells was arbitrarily set to 100%. P.I., postinfection.
Quantitative RT-PCR analysis of wee-1 mRNA.
Vpr has been shown to weakly transactivate transcription from heterologous promoters (4). In order to determine if the increased level of Wee-1 was due to an increase in the level of wee-1 mRNA, we used quantitative real-time RT-PCR to monitor levels of wee-1 mRNA as cells transitioned from G1/S to M (Fig. 3). Total RNA was isolated from cells infected with HR′EGFP or HR′Vpr. The level of mRNA was determined relative to gapdh mRNA. Our results indicate that the level of wee-1 mRNA in Vpr-infected cells over time was not significantly different from that in control cells. Thus, the delayed loss of Wee-1 protein appears to be the major cause of increased levels of Wee-1 in the presence of Vpr.
FIG. 3.
Increased level of Wee-1 does not correlate with increased mRNA level. The levels of wee-1 mRNA were quantitated with a one-step real-time quantitative PCR assay. Data are shown relative to signal intensities against gapdh mRNA. The bars represent the standard deviations of three experiments. The signal obtained from HR′EGFP virus-infected HeLa cells at the zero time point was arbitrarily set to 1.
Cdc2 activity is reduced at G2/M in HR′Vpr-infected cells.
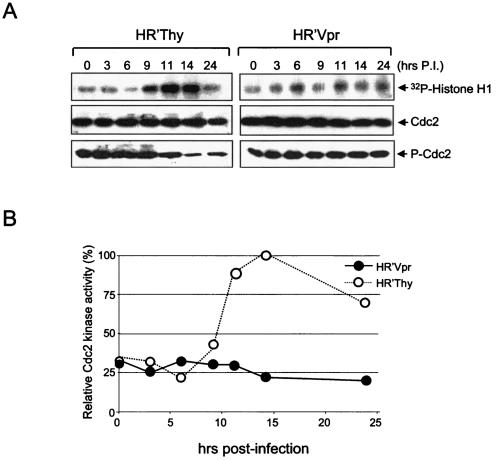
We investigated whether Vpr had an effect on Cdc2 kinase activity. Wee-1 regulates Cdc2 kinase activity through phosphorylation of Tyr15, resulting in inactivation of Cdc2 and cell cycle arrest at the G2/M checkpoint. Our previous finding that the level of Wee-1 was elevated in Vpr-infected cells lent support to the idea that we should expect to see a decrease in Cdc2 kinase activity in the presence of Vpr. We immunoprecipitated Cdc2 from HeLa cells infected with HR′Thy or HR′Vpr at various time points after release at G1/S. Utilizing a histone H1 phosphorylation assay, we observed three- to fourfold-less Cdc2 kinase activity specifically in Vpr-infected cells 14 h after release (Fig. 4). This result is consistent with the elevated level of Tyr15 phosphorylation of Cdc2 that we observed in Vpr-infected cells.
FIG. 4.
Cdc2 kinase activity is suppressed during G2/M transition in HR′Vpr-infected cells. (A) HeLa cells (1.5 × 105) were synchronized at G1/S by a double thymidine block. They were then infected with equivalent amounts of HR′Vpr or HR′Thy (0.3 μg of viral p24). Whole-cell lysates were prepared at different time points after infection. Cdc2 was isolated from lysates, and the kinase assay was performed in the presence of histone H1 substrate. Proteins were resolved by SDS-12% PAGE and detected by Western blotting with anti-Cdc2 and anti-phosphorylated Cdc2 antibodies. P.I., postinfection. (B) Cdc2 kinase activity after normalization by the amount of Cdc2. The activity obtained 14 h after infection with HR′Thy was arbitrarily set to 100%.
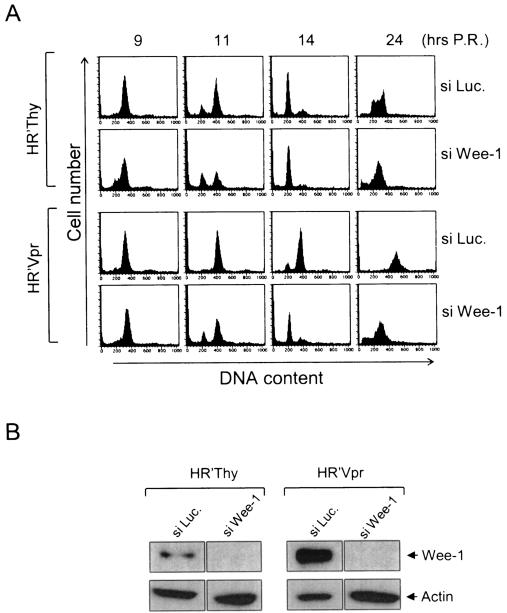
Depletion of Wee-1 by siRNA Wee-1 alleviates Vpr-induced cell cycle arrest.
Our results indicate that increased levels of Wee-1 prior to M at the G2/M transition correlate with cell cycle arrest induced by Vpr, leading to the hypothesis that the higher levels of Wee-1 are directly responsible for the failure of Vpr-infected cells to progress into mitosis. We tested this hypothesis further by using an siRNA directed to wee-1 mRNA that specifically eliminates Wee-1 expression. Since the timing of Wee-1 depletion within the cell cycle is critical for this experiment, we utilized cells synchronized at G1/S by a double thymidine block. Wee-1 siRNA was transfected into the cells immediately following release from the double thymidine block. Four hours later, cells were infected with HR′Vpr and the effect of Wee-1 depletion upon Vpr-mediated cell cycle arrest was monitored over time as the cells progressed through the G2/M transition. Control cells were transfected with an irrelevant siRNA directed against luciferase and infected with control virus HR′Thy. We confirmed that the siRNA knocks down Wee-1 levels by analysis of Wee-1 levels at 14 h postrelease from the double thymidine block by Western blot analysis (Fig. 5B). Cell cycle progression was analyzed about the G2/M transition (9 to 14 h following release). As expected, in control cells with siRNA to luciferase mRNA (siLuc.), the virion-associated Vpr in HR′Vpr induces cell cycle arrest within 11 to 14 h, whereas normal cells (siLuc. and HR′Thy) progressed through mitosis into G1. In contrast, transfection of siRNA to wee-1 mRNA (siWee-1) prior to introduction of Vpr resulted in a nearly normal cell cycle progression through the G2/M checkpoint (compare HR′Thy/siLuc. with HR′Vpr/siWee-1). We also counted the viable cell number by trypan blue dye exclusion and confirmed that the number of cells with HR′Vpr/siWee-1 was similar to that of control cells (HR′Thy/siLuc.) or cells with siWee-1 alone (HR′Thy/siWee-1) at 14 h after infection, indicating progression through mitosis and cell division (data not shown). Cells depleted of Wee-1 without Vpr showed a slightly accelerated progression into G1 (Fig. 5A, compare lanes 1 and 2 at 14 h), consistent with the depletion of Wee-1. Thus, the presence of Wee-1 during G2 is essential for the induction of cell cycle arrest by Vpr. As previously reported, at later time points (24 to 48 h) in the presence of Vpr, siWee-1, or both, cell death via apoptosis is evident (data not shown) (25, 33).
FIG. 5.
Wee-1 depletion by siRNA Wee-1 alleviates Vpr-induced arrest. HeLa cells (1.5 × 105) were synchronized at G1/S by double thymidine block and transfected with siWee-1 or small interfering luciferase (siLuc.). Cells were then infected with HR′Vpr or HR′Thy virus (0.3 μg of viral p24). (A) Cell cycle profiles were determined at various time points following infection. P.R., postrelease. (B) Fourteen hours after infection whole-cell lysates were prepared and analyzed by Western blotting.
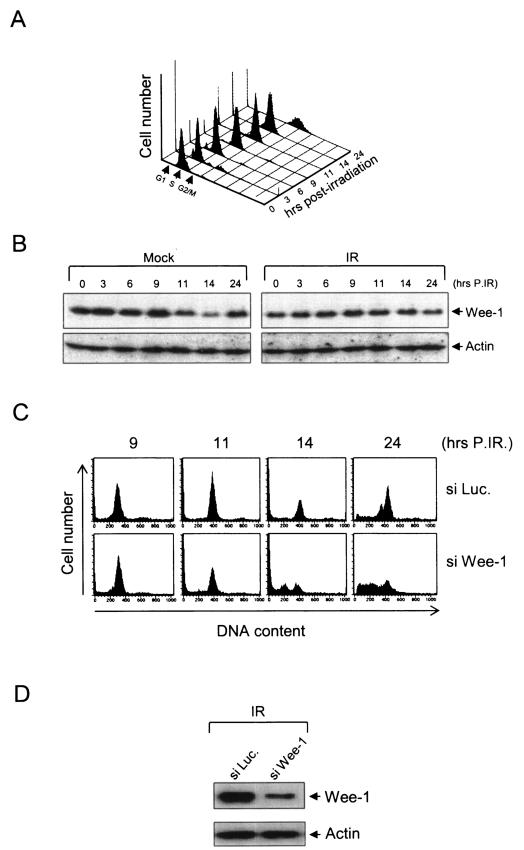
Depletion of Wee-1 by siWee-1 partially alleviates gamma irradiation-induced G2/M arrest.
Gamma irradiation induces cell cycle arrest at different stages of the cell cycle. The mechanisms of arrest are complex and involve multiple pathways (28). In some cells, including HeLa cells, DNA damage induced by gamma irradiation induces a cell cycle arrest at G2/M that phenotypically resembles Vpr-induced arrest (Fig. 6A). We observed that the level of Wee-1 is increased in gamma-irradiated cells (Fig. 6B). As expected, the level of Wee-1 increases and remains constant throughout the 24-h time point. In control cells a decrease in Wee-1 is seen at 11 h postinfection (at the G2/M transition) followed by an increased level of Wee-1 at 24 h when the majority of cells proceeded to S phase. Thus, Vpr and gamma irradiation-mediated cell cycle arrest give a similar phenotype in regard to Wee-1 levels. These results indicate that, like Vpr, increased levels of Wee-1 prior to M at the G2/M transition correlate with cell cycle arrest induced by gamma irradiation. We tested the role of Wee-1 directly in a fashion similar to that above by depleting Wee-1 with an siRNA to wee-1 mRNA. Cells were synchronized at G1/S by a double thymidine block and exposed to gamma irradiation following release. Cells were immediately transfected with siLuc or siWee-1. The effect of Wee-1 depletion upon gamma irradiation-mediated cell cycle arrest was monitored as the cells progressed through the G2/M transition. Western blotting analysis of Wee-1 levels at 14 h post-release from the double thymidine block confirmed the effect of siWee-1 (Fig. 6D). Cell cycle progression was analyzed about the G2/M transition (9 to 14 h following release). As expected, in control cells with siRNA Luc, the gamma irradiation induced cell cycle arrest within 11 to 14 h (Fig. 6C). In contrast, transfection of siRNA to wee-1 mRNA following gamma irradiation significantly alleviated the radiation-induced G2 arrest.
FIG. 6.
Gamma irradiation-induced G2 arrest results in stabilization of Wee-1. HeLa cells (1.5 × 105) were synchronized by double thymidine block at G1/S and gamma irradiated with 4,000 rads following release. (A) Cells were collected at various times after gamma irradiation, stained with propidium iodide, and analyzed with a FACScan cell sorter. (B) Cell lysates were analyzed at the indicated time points with an antibody specific for Wee-1. (C) HeLa cells were transfected with siRNA to wee-1 (siWee-1) or luciferase (siLuc.) for 4 h following release and irradiation. Cells were then collected for cell cycle analysis at the indicated time points. (D) Fourteen hours after release siWee-1-transfected cell lysates were analyzed for Wee-1 expression. IR, irradiation; P.IR, postirradiation.
DISCUSSION
Previous studies established the role of Wee-1 kinase in negative regulation of Cdc2 activity. Our results demonstrate that, in cells arrested in G2 by Vpr, Wee-1 levels are elevated. Most importantly, depletion of Wee-1 by an siRNA prior to infection with Vpr virus demonstrated that elevated levels of Wee-1 are essential for the induction of cell cycle arrest by Vpr. These results support our hypothesis that a delay in reducing the levels of Wee-1 may be one determinant responsible for the failure of Vpr-arrested cells to progress through mitosis.
The greater levels of Wee-1 in the presence of Vpr correlated with an increase in the level of the phosphorylated form of Cdc2 and a decrease in Cdc2 kinase activity. The observed reduction in Cdc2 kinase activity is consistent with the inhibitory role of Wee-1 via Tyr15 phosphorylation of Cdc2 (24). Ultimately, insufficient levels of Cdc2 kinase activity are responsible for the failure of cells to progress into mitosis.
The regulation of Wee-1 activity is complex. Here, we find that at least one reason for increased levels of Wee-1 is due to enhanced protein stability. Wee-1 is a target for ubiquitination in Xenopus oocytes (17) and caspase-3 cleavage in human Jurkat cells (34). Vpr expression has also been reported to result in increased nuclear herniations resulting in transient cytoplasmic release of Wee-1 green fluorescent protein fusion proteins (8). It is possible that a change in subcellular compartmentalization reduces the stability and or activity of Wee-1. It has also been reported that Wee-1 kinase activity is regulated by changes in phosphorylation; however, we did not examine that directly here.
We previously demonstrated that a decline in Wee-1 levels subsequent to Vpr-mediated cell cycle arrest correlated with the apoptosis that occurs 1 to 3 days following prolonged cell cycle arrest by Vpr. Ectopic overexpression of Wee-1 during the Vpr-mediated apoptotic phase results in an attenuation of the apoptosis (33). Taking that together with the results presented here, we propose that Wee-1 has two roles in the deregulation of cell cycle progression by Vpr. First, elevated levels of Wee-1, mediated principally through enhanced protein stability, are critical for the initiation of G2/M cell cycle arrest induced by Vpr. Second, once cell cycle arrest is initiated by Vpr, subsequent decreases in Wee-1 levels appear to be important for the induction of Vpr-mediated apoptosis. During normal cell cycle progression, Wee-1 is established as a negative regulator of Cdc2 kinase. This role of Wee-1 is likely to be the reason why elevated levels of Wee-1 in the presence of Vpr lead to cell cycle arrest at G2/M. The role of Wee-1 in apoptosis is less well understood. We previously reported an enhanced Cdc2 kinase activity concomitant with the declines in Wee-1 levels and proposed that this inappropriate activation of Cdc2 may lead to cell death via an abnormal mitotic process known as mitotic catastrophe (33). Thus, the dual and opposing effect of Vpr on Wee-1 at different points in the cell cycle may indicate the role of Wee-1 in linking cell cycle arrest to induction of apoptosis.
REFERENCES
- 1.Ayad, N. G., S. Rankin, M. Murakami, J. Jebanathirajah, S. Gygi, and M. W. Kirschner. 2003. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell 113:101-113. [DOI] [PubMed] [Google Scholar]
- 2.Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623-631. [DOI] [PubMed] [Google Scholar]
- 3.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky, M., and A. Adzhubei. 1999. Viral protein R of HIV-1. Rev. Med. Virol. 9:39-49. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, T. R., and W. G. Dunphy. 1994. Cdc2 regulatory factors. Curr. Opin. Cell Biol. 6:877-882. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, T. R., Z. Tang, and W. G. Dunphy. 1993. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell 72:919-929. [DOI] [PubMed] [Google Scholar]
- 7.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 8.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 9.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 10.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, V. W., and D. S. Heikka. 2000. Radiolabeling revisited: metabolic labeling with 35S-methionine inhibits cell cycle progression, proliferation, and survival. FASEB J. 14:448-454. [DOI] [PubMed] [Google Scholar]
- 12.Hu, V. W., D. S. Heikka, P. B. Dieffenbach, and L. Ha. 2001. Metabolic radiolabeling: experimental tool or Trojan horse? 35S-Methionine induces DNA fragmentation and p53-dependent ROS production. FASEB J. 15:1562-1568. [DOI] [PubMed] [Google Scholar]
- 13.Huang, L.-M., and K.-T. Jeang. 1995. HIV Vpr: roles in viral replication and cellular metabolism, p. III-3-III-9. In G. Myers, B. Hahn, J. Mellors, and L. Henderson (ed.), Human retroviruses and AIDS. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 14.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Y. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, J., A. Kumagai, and W. G. Dunphy. 2001. Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell 12:551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan, C. H., and P. Russell. 1995. Cell cycle regulation of human Wee1. EMBO J. 14:2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael, W. M., and J. Newport. 1998. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science 282:1886-1889. [DOI] [PubMed] [Google Scholar]
- 18.Nurse, P. 1997. Checkpoint pathways come of age. Cell 91:865-867. [DOI] [PubMed] [Google Scholar]
- 19.Poon, B., and I. S. Chen. 2003. Human immunodeficiency virus type 1 (HIV-1) Vpr enhances expression from unintegrated HIV-1 DNA. J. Virol. 77:3962-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon, B., J. B. Jowett, S. A. Stewart, R. W. Armstrong, G. M. Rishton, and I. S. Y. Chen. 1997. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J. Virol. 71:3961-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roshal, M., B. Kim, Y. Zhu, P. Nghiem, and V. Planelles. 2003. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J. Biol. Chem. 278:25879-25886. [DOI] [PubMed] [Google Scholar]
- 23.Rothblum-Oviatt, C. J., C. E. Ryan, and H. Piwnica-Worms. 2001. 14-3-3 binding regulates catalytic activity of human Wee1 kinase. Cell Growth Differ. 12:581-589. [PubMed] [Google Scholar]
- 24.Russell, P., and P. Nurse. 1987. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49:559-567. [DOI] [PubMed] [Google Scholar]
- 25.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Y. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Y. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang, Z., T. R. Coleman, and W. G. Dunphy. 1993. Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 12:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Y., C. Jacobs, K. E. Hook, H. Duan, R. N. Booher, and Y. Sun. 2000. Binding of 14-3-3beta to the carboxyl terminus of Wee1 increases Wee1 stability, kinase activity, and G2-M cell population. Cell Growth Differ. 11:211-219. [PubMed] [Google Scholar]
- 30.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westervelt, P., T. Henkel, D. B. Trowbridge, J. Orenstein, J. Heuser, H. E. Gendelman, and L. Ratner. 1992. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J. Virol. 66:3925-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, X. F., M. Matsuda, M. Essex, and T. H. Lee. 1990. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J. Virol. 64:5688-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan, H., Y. M. Xie, and I. S. Y. Chen. 2003. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J. Virol. 77:2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, B. B., H. Li, J. Yuan, and M. W. Kirschner. 1998. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA 95:6785-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]