Hookworm platelet inhibitor belongs to the cysteine-rich/antigen-5/pathogenesis-related 1 (CAP) protein family. It has been linked to integrin-antagonist, antiplatelet activity found in extracts of A. caninum hookworms.
Keywords: antigen V, hemostasis, parasitism
Abstract
Secreted protein components of hookworm species include a number of representatives of the cysteine-rich/antigen 5/pathogenesis-related 1 (CAP) protein family known as Ancylostoma-secreted proteins (ASPs). Some of these have been considered as candidate antigens for the development of vaccines against hookworms. The functions of most CAP superfamily members are poorly understood, but one form, the hookworm platelet inhibitor (HPI), has been isolated as a putative antagonist of the platelet integrins αIIbβ3 and α2β1. Here, the crystal structure of HPI is described and its structural features are examined in relation to its possible function. The HPI structure is similar to those of other ASPs and shows incomplete conservation of the sequence motifs CAP1 and CAP2 that are considered to be diagnostic of CAP superfamily members. The asymmetric unit of the HPI crystal contains a dimer with an extensive interaction interface, but chromatographic measurements indicate that it is primarily monomeric in solution. In the dimeric structure, the putative active-site cleft areas from both monomers are united into a single negatively charged depression. A potential Lys-Gly-Asp disintegrin-like motif was identified in the sequence of HPI, but is not positioned at the apex of a tight turn, making it unlikely that it interacts with the integrin. Recombinant HPI produced in Escherichia coli was found not to inhibit the adhesion of human platelets to collagen or fibrinogen, despite having a native structure as shown by X-ray diffraction. This result corroborates previous analyses of recombinant HPI and suggests that it might require post-translational modification or have a different biological function.
1. Introduction
Helminth species secrete a large number of proteins that play a role in invasion, feeding and modulation of the host immune system. Prominent among these are members of the cysteine-rich secretory/antigen 5/pathogenesis-related 1 (CAP) protein family, which in hookworms are known as Ancylostoma-secreted proteins (ASPs; Asojo, 2011 ▶; Asojo et al., 2005 ▶; Borloo et al., 2013 ▶; Gibbs et al., 2008 ▶; Mason et al., 2014 ▶; Osman et al., 2012 ▶). In the medically important hookworms A. caninum and Necator americanus, many of the most highly abundant, transcriptionally upregulated proteins secreted by the invasive L3 stage are ASP proteins, suggesting that these play a role in parasite establishment in the host (Datu et al., 2008 ▶). Two particularly prominent forms, ASP-1 and ASP-2, have been considered as potential hookworm vaccine antigens and other forms have been studied from additional helminth species for similar reasons (Bethony et al., 2005 ▶; McSorley & Loukas, 2010 ▶; Pearson et al., 2012 ▶). A positive correlation between the presence of anti-ASP-2 antibodies in immunized animals and a lack of heavy infestation has been observed, and in the same study immunization with ASP-2 from N. americanus was shown to be protective in laboratory models (Bethony et al., 2005 ▶).
CAP superfamily proteins are broadly distributed in eukaryotes, but the vast majority of them have not been functionally characterized. Neutrophil inhibitory factor (NIF) and hookworm platelet inhibitor (HPI) are secreted CAP superfamily members from A. caninum that have been associated with specific functions. NIF has been shown to block neutrophil adhesion to endothelial cells by binding to the I domain of the integrin receptor CD11a/CD18 expressed on the neutrophil surface (Moyle et al., 1994 ▶; Muchowski et al., 1994 ▶). Hookworm-derived HPI was identified as an inhibitor of platelet activation acting through a blockade of the fibrinogen receptor integrin αIIbβ3 and the collagen receptor integrin α2β1 (Chadderdon & Cappello, 1999 ▶; Del Valle et al., 2003 ▶). However, recombinant protein failed to inhibit platelet function. Tablysin-15, a CAP-domain protein from the saliva of the blood-feeding horse-fly Tabanus yao, has also been shown to modulate platelet and endothelial cell function by binding to integrins αIIbβ3 and αVβ3 via an Arg-Gly-Asp (RGD) sequence (Ma et al., 2011 ▶; Xu et al., 2012 ▶). Additionally, this protein contains a hydrophobic channel as part of its structure that is selective for binding of cysteinyl leukotrienes and may serve to regulate the inflammatory state and vascular tone of the host (Xu et al., 2012 ▶).
Structurally, the CAP domain is characterized by an α/β core that is quite highly conserved despite a large degree of sequence variation within the family. This is followed by a hinge region, with a second cysteine-rich C-terminal domain known as the CRISP domain also being present in vertebrate forms (Gibbs et al., 2008 ▶). Previously characterized helminth ASP structures only possess the CAP domain and hinge region (Asojo, 2011 ▶; Asojo et al., 2005 ▶; Borloo et al., 2013 ▶; Mason et al., 2014 ▶). In this study, we describe the three-dimensional structure of HPI from A. caninum, which can be used to help to clarify the role of this protein in the biology of the organism and to understand any structural features that may underlie the reported activity of the protein as an integrin-targeting inhibitor of platelet activation.
2. Materials and methods
2.1. Macromolecule production
A cDNA encoding HPI with a six-histidine tag at the C-terminus was prepared synthetically (BioBasic Inc.), cloned into the expression vector pET-17b and then transformed into Escherichia coli strain BL21(DE3)pLysS. For the production of inclusion bodies, LB medium was inoculated with a 10% volume of a saturated culture of the expression strain. After growth at 37°C to an optical density of 0.8, IPTG was added to a concentration of 1 mM and the cultures were grown for an additional 3 h at 37°C. After pelleting, washing and freezing, the cells were lysed by sonication of the suspended pellet in 20 mM Tris–HCl pH 7.5, 150 mM NaCl and the resulting inclusion-body pellet was washed with 1% Triton X-100 in the same buffer, dissolved in 6 M guanidine hydrochloride pH 8.0 and reduced by adding DTT to a concentration of 10 mM. This solution was then refolded by adding it dropwise with stirring to an excess volume of 20 mM Tris–HCl pH 8.0, 0.3 M arginine, 1 mM EDTA, 0.2 mM GSSG, 1 mM GSH. After 48 h at 4°C, the protein was concentrated by ultrafiltration. The protein showed relatively good solubility in the refolding buffer, indicating a high yield of native material. Therefore, we skipped the metal-affinity purification step and applied the concentrated material directly onto a Sephacryl S-100 (16/60) column in order to remove aggregated material. A second step of ion-exchange chromatography on Mono Q yielded highly purified, apparently monomeric protein. After purification, the protein was dialyzed against 20 mM Tris–HCl pH 7.4, 150 mM NaCl. The oligomeric state of the recombinant HPI was determined by gel-filtration chromatography on Superdex 75 using an elution buffer consisting of 20 mM Tris–HCl pH 7.5, 0.15 M NaCl.
2.2. Crystallization
The purified HPI was crystallized using the hanging-drop vapour-diffusion method with 30% PEG 6000, 0.1 M HEPES pH 7.0. The crystals grew as clusters; the clusters were broken apart with a probe and single-crystal fragments were then flash-cooled in precipitant solution containing 10% glycerol (Table 1 ▶).
Table 1. Crystallization.
| Method | Hanging-drop vapour diffusion |
| Plate type | Linbro 24-well |
| Temperature (K) | 273 |
| Protein concentration (mM) | 1 |
| Buffer composition of protein solution | 10mM TrisHCl pH 8.0 |
| Composition of reservoir solution | 30% PEG 6000, 0.1M HEPES pH 7.0 |
| Volume and ratio of drop | 2l, 1:1 ratio |
| Volume of reservoir (ml) | 0.8 |
2.3. Data collection and processing
Diffraction data were collected on beamline 22-ID of the Southeast Regional Collaborative Access Team (SER-CAT) facility at the Advanced Photon Source (APS), Argonne National Laboratory. Data obtained at 100 K were integrated, merged and scaled using HKL-2000 (Otwinowski & Minor, 1997 ▶). Data-collection and processing statistics are given in Table 2 ▶.
Table 2. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | Beamline 22-ID, APS |
| Wavelength () | 0.97931 |
| Temperature (K) | 100 |
| Detector | MAR CCD 300mm |
| Crystal-to-detector distance (mm) | 175.0 |
| Rotation range per image () | 1.0 |
| Total rotation range () | 220 |
| Exposure time per image (s) | 1.0 |
| Space group | P21 |
| a, b, c () | 39.16, 51.98, 73.94 |
| , , () | 90, 90.1, 90 |
| Mosaicity () | 0.522 |
| Resolution range () | 301.6 |
| Total No. of reflections | 174768 |
| No. of unique reflections | 39309 |
| Completeness (%) | 99.2 (92.1) |
| Multiplicity | 4.4 (3.6) |
| I/(I) | 11.1 (3.4) |
| R r.i.m. † (%) | 8.6 (37.2) |
| Overall B factor from Wilson plot (2) | 12.4 |
R r.i.m. was estimated by multiplying the R merge value by the factor [N/(N 1)]1/2, where N is the data multiplicity.
2.4. Structure solution and refinement
The structure of HPI was determined by molecular replacement with Phaser (McCoy et al., 2007 ▶) using the structure of Na-ASP-2 (PDB entry 1u53; Asojo et al., 2005 ▶) from N. americanus as a search model. The molecular-replacement solution contained two molecules in the asymmetric unit and was refined by rigid-body refinement in REFMAC5 (Murshudov et al., 2011 ▶) to give an R cryst of 43% and an R free of 47%. The HPI model was completed by numerous rebuilding and refinement cycles using phenix.refine (Afonine et al., 2012 ▶) and Coot (Emsley et al., 2010 ▶). The quality of the model was evaluated using MolProbity (Chen et al., 2010 ▶). Statistics of data collection and refinement are given in Table 3 ▶.
Table 3. Structure solution and refinement.
| Resolution range () | 28.811.60 |
| Completeness (%) | 99.2 |
| No. of reflections, working set | 37332 |
| No. of reflections, test set | 1980 |
| Final R cryst (%) | 15.7 |
| Final R free (%) | 19.7 |
| No. of non-H atoms | |
| Protein | 2852 |
| Water | 370 |
| Total | 3222 |
| R.m.s. deviations | |
| Bonds () | 0.008 |
| Angles () | 1.08 |
| Average B factors (2) | |
| Protein | 15.6 |
| Water | 28.1 |
| Ramachandran plot | |
| Most favored (%) | 97.4 |
| Allowed (%) | 100 |
| PDB code | 4tpv |
2.5. Platelet-adhesion assays
Platelet-rich plasma was obtained under informed consent from medication-free platelet donors participating in a National Institutes of Health Institutional Review Board-approved protocol of the Department of Transfusion Medicine (DTM/NIH, Blood Bank). For platelet-adhesion assays, platelet-rich plasma was incubated with calcein-AM (2 µM, Calbiochem) for 30 min at room temperature and centrifuged in the presence of EDTA (5 mM) and apyrase (0.2 units ml−1). Platelets were resuspended (2 × 105 µl−1) in Tyrode’s buffer (no additions; Francischetti et al., 1997 ▶). Inhibition of platelet adhesion to immobilized collagen was examined by fluorometry. Microfluor Black microtiter 96-well plates (Thermo Labsystems, Franklin, Massachusetts, USA) were coated with 50 µl (1 µg per well in phosphate-buffered saline; PBS) of fibrillar (Horm) or soluble collagen I (BD Biosciences) or fibrinogen (50 µg per well) overnight at 4°C in PBS pH 7.4. Wells were washed with PBS and blocked with 2%(w/v) BSA (in PBS). Calcein-labeled platelets (2 × 105 µl−1) were incubated with HPI or EDTA for 20 min, and 50 µl of platelets was added to each well. After 1 h, the wells were washed three or four times with Tyrode-BSA and adhesion was estimated by fluorescence (excitation at 485 nm and emission at 530 nm).
3. Results and discussion
3.1. The structure of HPI
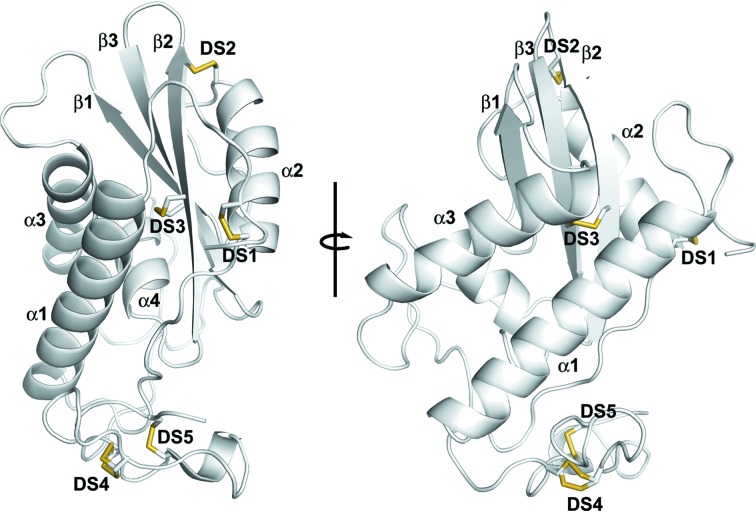
Recombinant HPI (3–187) containing a C-terminal histidine tag was crystallized in space group P21 with two monomers in the asymmetric unit (Fig. 1 ▶). Each monomer consists of an α/β/α structure typical of CAP-domain proteins, as well as a shorter C-terminal hinge region (Fig. 1 ▶). The secondary-structural elements of the protein are arranged similarly to other ASPs with the pattern α1–α2–β1–α3–α4–β2–β3 (Fig. 1 ▶). The protein is stabilized by five disulfide bonds that are equivalent to the conserved disulfides contained in other members of the CAP superfamily, as shown in the structure-based alignment in Fig. 2 ▶. Three of these disulfides lie in the CAP domain itself and link Cys7 to Cys48 of α2 (disulfide bond 1), Cys61 of α2 to Cys129 of β2 (disulfide bond 2) and Cys124 of β2 to Cys137 of β3 (disulfide bond 3) (Figs. 1 ▶ and 2 ▶). The C-terminal hinge region of HPI then begins at the conserved residue Gly154 [part of the conserved GX(PV) motif] and is similar in conformation to other ASP proteins (Gibbs et al., 2008 ▶). It is stabilized by two conserved disulfide bonds linking Cys157 to Cys169 (disulfide bond 4) and Cys160 to Cys178 (disulfide bond 5) (Figs. 1 ▶ and 2 ▶). Based on the ASP-categorization system of Osman et al. (2012 ▶), HPI belongs to the group 3 ASPs, since it is missing two conserved histidine residues characteristic of group 1 ASPs that lie in the loop between α2 and β1 and on helix α4 (Osman et al., 2012 ▶; Asojo et al., 2005 ▶, 2011 ▶; Assumpção et al., 2013 ▶; Mason et al., 2014 ▶). These two residues have been suggested to form a metal-binding site lying at the base of a cleft that could be involved in the hypothesized proteolytic activities of CAP-domain proteins or in the copper-dependent superoxide dismutase activity reported for a CAP-domain protein from the saliva of the blood-feeding insect Dipetalogaster maxima (Asojo et al., 2005 ▶, 2011 ▶; Assumpção et al., 2013 ▶; Mason et al., 2014 ▶). In HPI, the two histidine residues are replaced by Pro65 and Asn110 (Fig. 2 ▶). Group 2 ASPs differ from group 1 and 3 proteins in that they lack disulfide bond 2 shown in the amino-acid sequence alignment in Fig. 2 ▶.
Figure 1.
Ribbon diagram of the HPI model. The left and right panels are related by a rotation of approximately 90° around the axis shown. α-Helices are labeled α1–α4 and β-strands are labeled β1–β3. The disulfide bonds discussed in the text are labeled DS1–DS5.
Figure 2.
Structural relationships among ASPs. Structure-based alignment of HPI with Na-ASP-2 from N. americanus (PDB entry 1u53; Asojo et al., 2005 ▶), Ac-ASP-7 from A. caninum (second domain, PDB entry 3nt8; Asojo, 2011 ▶) and Oo-ASP-1 from Ostertagia ostertagi (PDB entry 4g2u; Borloo et al., 2013 ▶). The CAP1–CAP4 motifs are highlighted in green. Conserved disulfide bonds 1–5 are highlighted in black. The two histidine positions found in the ‘active-site’ cleft are marked by hash marks and the candidate disintegrin motifs are marked by asterisks. The consensus sequences for CAP1–CAP4 in PROSITE nomenclature are as follows: CAP1, [GDER][HR][FYWH][TVS][QA][LIVM][LIVMA]Wxx[STN]; CAP2, [LIVMFYH][LIVMFY]XC[NQRHS]Yx[PARH]x[GL]N[LIVMFYWDN]; CAP3, HNxxR; CAP4 G[EQ]N[ILV] (Gibbs et al., 2008 ▶).
Based on previous comparative studies, two amino-acid sequence motifs, designated CAP1 and CAP2, have been catalogued in the PROSITE database (Sigrist et al., 2012 ▶) and shown to be diagnostic for members of the CAP superfamily (Fig. 2 ▶). These conserved motifs lie in the region of the cleft containing the two ‘active-site’ histidine residues of group 1 ASPs (Gibbs et al., 2008 ▶). Recently, the CAP1 consensus sequence has been expanded to include additional conserved residues, most notably a highly conserved cysteine lying C-terminal to the originally described motif (Borloo et al., 2013 ▶). HPI shows only partial conservation of the expanded CAP1, with 11 of 15 residues matching the consensus, while CAP2 adheres to the consensus at six of nine positions. Two additional motifs, CAP3 and CAP4, have more recently been described by Gibbs et al. (2008 ▶). HPI matches the consensus for CAP3 at all five positions and that for CAP4 at three of four positions.
3.2. Assessment of integrin-binding function
Extracts of A. caninum have been shown to prevent the aggregation and adhesion of platelets in response to a variety of agonists, including collagen, ADP, thrombin and epinephrine, suggesting the presence of an inhibitor that blocks integrin interactions with activating ligands (Chadderdon & Cappello, 1999 ▶; Del Valle et al., 2003 ▶). HPI was originally isolated from extract preparations based on its ability to inhibit the binding of fibrinogen and collagen to their respective platelet integrins αIIbβ3 and α2β1 (Del Valle et al., 2003 ▶). Inhibitory integrin-binding proteins known as disintegrins derived from snake venoms and other sources contain an Arg-Gly-Asp (RGD) sequence motif or a variant of this sequence that has been proven to be the primary integrin–inhibitor interaction site (Assumpcao et al., 2012 ▶; Wermelinger et al., 2009 ▶). The structures of integrins αIIbβ3 and αvβ3 in complex with Arg-Gly-Asp peptides reveal that the residues of the motif interact with the head structure of the integrin in a groove located between the α and β subunits (Xiao et al., 2004 ▶; Xiong et al., 2002 ▶). Inhibitors containing the motif normally present a projecting loop containing a tight turn with the RGD sequence at its apex. The only CAP-domain protein with a confirmed integrin-inhibitory function is the horse-fly salivary protein tablysin-15, which possesses an RGD sequence at the apex of a tight turn lying in an N-terminal extension of the CAP domain (Xu et al., 2012 ▶). Two possible disintegrin motifs related to RGD are present in the HPI sequence (Fig. 2 ▶), with the most likely being Lys144-Gly145-Asp146 (KGD; Assumpcao et al., 2012 ▶). The KGD motif is a known active variant of RGD and occurs in the sequence of HPI at a location that is C-terminal to β3 at the beginning of the hinge region. It does not, however, lie at the apex of a tight turn as in tablysin-15. Rather, the lysine side chain is buried and the aspartate, while on the surface, does not appear to project sufficiently to interact with the integrin. The second possible motif, Lys37-Thr38-Ser39 (KTS; Marcinkiewicz et al., 2003 ▶), has been shown to interact with integrin α1β1 in other proteins and lies near the beginning of α1. This sequence is also not completely exposed and does not appear to be oriented in a manner consistent with integrin interaction.
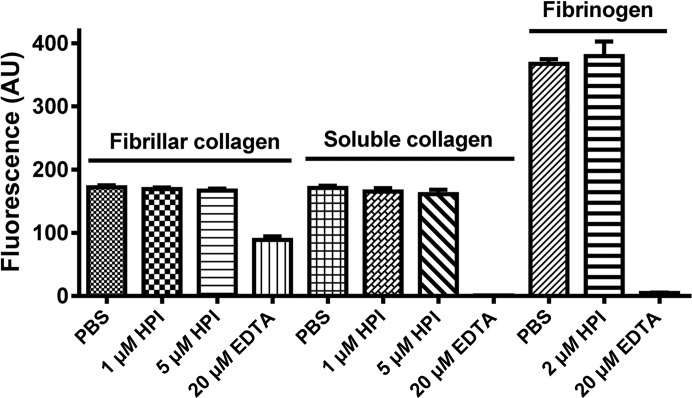
Recombinant HPI produced after isolation and cloning has been reported by Del Valle et al. (2003 ▶) to be unable to block the adhesion of platelets to either collagen or fibrinogen, suggesting a possible folding problem with the recombinant material produced in E. coli. We have tested the refolded recombinant protein used for this study for its ability to block platelet adhesion to collagen and fibrinogen. While the crystal structure shows that the protein is properly folded, no inhibition of the adhesion of calcein AM-loaded platelets to soluble collagen, fibrillar collagen or fibrinogen was observed (Fig. 3 ▶), indicating that the E. coli-produced protein is inactive. This suggests that HPI may require removal of the His-tag modification, may require additional post-translational modification for activation or may have a different biological function than suggested to this point.
Figure 3.
Adhesion of platelets to collagen and fibrinogen in the presence and absence of HPI. Microtiter plates coated with fibrillar collagen, soluble collagen or fibrinogen were blocked with BSA and exposed to calcein-labeled platelets that had been pre-incubated with HPI for 20 min. After 1 h, the plate was washed and adhesion was measured by fluorescence.
3.3. Oligomeric state of HPI
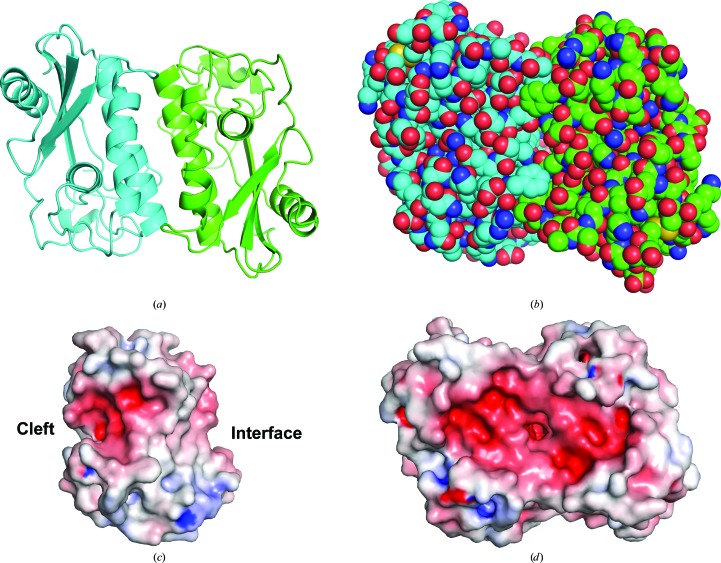
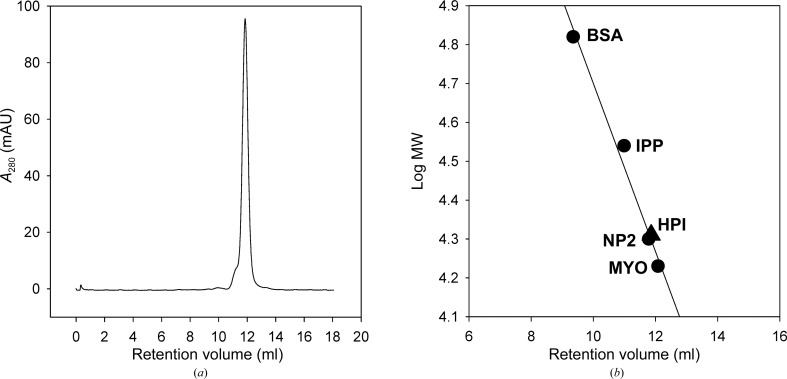
The two HPI monomers contained in the asymmetric unit are related by a rotation around a twofold axis that runs approximately perpendicular to the longitudinal axes of α1 and α3 in both molecules (Figs. 4 ▶ a and 4 ▶ b). The contact interface consists of a four-helix bundle made up of α1 and α3 from each monomer. The interface, as determined by analysis with PISA (Krissinel & Henrick, 2007 ▶), is relatively large, with 1117 Å2 of surface (per monomer) being buried, and has a calculated interaction energy (ΔG) of −10.1 kcal mol−1. A notable surface feature of the HPI monomer is a large negatively charged patch that lies directly adjacent to the interaction interface and includes the cleft corresponding to that containing the two conserved histidine residues found in group 1 ASPs as well as other CAP-domain proteins (Fig. 4 ▶ c). In the dimeric model, the negatively charged areas belonging to each of the two monomers align almost perfectly to form a negatively charged surface depression that measures approximately 38 Å across in its longest dimension (Fig. 4 ▶ d). The dimer interface contains a number of hydrogen-bond and electrostatic interactions between the two chains, which are listed in Table 4 ▶. Although PISA calculations suggest that the dimer would be stable in solution, gel-filtration chromatography on Superdex 75 indicated that recombinant HPI remains largely monomeric in an elution-buffer system containing 150 mM sodium chloride, making the relevance of the dimeric form uncertain (Fig. 5 ▶).
Figure 4.
Oligomeric state and electrostatic surface properties of HPI. (a) Ribbon diagram of the dimeric structure of the asymmetric unit with chain A colored green and chain B colored cyan. The two molecules are related by a twofold rotation axis perpendicular to the plane of the page. The dimer interface is made up primarily of elements of α1 and α3. (b) Space-filling diagram of the model from (a) showing packing of the interface. (c) Electrostatic map of the surface of the HPI monomer. Negatively charged surface areas are colored red and positively charged areas are colored blue. The dimer interface and negatively charged cleft are labeled. (d) Electrostatic map of the HPI dimer showing the joining of the anionic surfaces of HPI monomers into a large, negatively charged depression.
Table 4. Hydrogen-bonding and salt-bridge interactions at the dimer interface in HPI.
| Chain B residue | Distance () | Chain A residue |
|---|---|---|
| Arg34NH1 | 3.17 | Glu19OE1 |
| Lys37NZ | 3.13 | Asp16OD1 |
| Asn81ND2 | 2.93 | Asn35OD1 |
| Trp91NE1 | 2.95 | Asn88OD1 |
| Glu23OE1 | 2.68 | Arg34NE |
| Glu23OE2 | 2.69 | Arg34NH2 |
| Asn35OD1 | 3.02 | Asn81ND2 |
| Asn88OD1 | 3.06 | Trp91NE1 |
| Asp172O | 2.89 | Gln174NE2 |
Figure 5.
Solution oligomeric state of HPI. (a) Recombinant HPI was applied onto a Superdex 75 (10/300) column and eluted with 20 mM Tris–HCl pH 7.5, 150 mM NaCl. (b) A series of standard proteins were chromatographed on the same column in the same buffer system: bovine serum albumin (BSA; 66.1 kDa), Rhodnius prolixus inositol polyphosphate phosphatase (IPP; 34.7 kDa), nitrophorin 2 (NP2; 19.9 kDa) and horse heart myoglobin (MYO; 17 kDa). The elution volume and calculated mass of the HPI monomer are also indicated with a triangle. The results indicate that HPI is monomeric under these conditions.
Supplementary Material
PDB reference: hookworm platelet inhibitor, 4tpv
Acknowledgments
The authors thank D. Garboczi and A. Gittis for discussions and Van Pham for technical assistance. We also thank the staff of the Southeast Regional Collaborative Access Team, Advanced Photon Source, Argonne National Laboratory for assistance with X-ray data collection. Use of the Advanced Photon Source beamlines was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract No. W-31-109-Eng-38. This work was supported by the intramural research program of the NIAID, National Institutes of Health.
References
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Asojo, O. A. (2011). Acta Cryst. D67, 455–462. [DOI] [PMC free article] [PubMed]
- Asojo, O. A., Goud, G., Dhar, K., Loukas, A., Zhan, B., Deumic, V., Liu, S., Borgstahl, G. E. & Hotez, P. J. (2005). J. Mol. Biol. 346, 801–814. [DOI] [PubMed]
- Asojo, O. A., Koski, R. A. & Bonafé, N. (2011). Acta Cryst. D67, 847–855. [DOI] [PMC free article] [PubMed]
- Assumpção, T. C. F., Ma, D., Schwarz, A., Reiter, K., Santana, J. M., Andersen, J. F., Ribeiro, J. M. C., Nardone, G., Yu, L. L. & Francischetti, I. M. B. (2013). J. Biol. Chem. 288, 14341–14361. [DOI] [PMC free article] [PubMed]
- Assumpcao, T. C. F., Ribeiro, J. M. C. & Francischetti, I. M. B. (2012). Toxins, 4, 296–322. [DOI] [PMC free article] [PubMed]
- Bethony, J., Loukas, A., Smout, M., Brooker, S., Mendez, S., Plieskatt, J., Goud, G., Bottazzi, M. E., Zhan, B., Wang, Y., Williamson, A., Lustigman, S., Correa-Oliveira, R., Xiao, S. & Hotez, P. J. (2005). FASEB J. 19, 1743–1745. [DOI] [PubMed]
- Borloo, J., Geldhof, P., Peelaers, I., Van Meulder, F., Ameloot, P., Callewaert, N., Vercruysse, J., Claerebout, E., Strelkov, S. V. & Weeks, S. D. (2013). Acta Cryst. D69, 493–503. [DOI] [PubMed]
- Chadderdon, R. C. & Cappello, M. (1999). J. Infect. Dis. 179, 1235–1241. [DOI] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Datu, B. J. D., Gasser, R. B., Nagaraj, S. H., Ong, E. K., O’Donoghue, P., McInnes, R., Ranganathan, S. & Loukas, A. (2008). PLoS Negl. Trop. Dis. 2, e130. [DOI] [PMC free article] [PubMed]
- Del Valle, A., Jones, B. F., Harrison, L. M., Chadderdon, R. C. & Cappello, M. (2003). Mol. Biochem. Parasitol. 129, 167–177. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Francischetti, I. M. B., Saliou, B., Leduc, M., Carlini, C. R., Hatmi, M., Randon, J., Faili, A. & Bon, C. (1997). Toxicon, 35, 1217–1228. [DOI] [PubMed]
- Gibbs, G. M., Roelants, K. & O’Bryan, M. K. (2008). Endocr. Rev. 29, 865–897. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Ma, D., Xu, X., An, S., Liu, H., Yang, X., Andersen, J. F., Wang, Y., Tokumasu, F., Ribeiro, J. M. C., Francischetti, I. M. B. & Lai, R. (2011). Thromb. Haemost. 105, 1032–1045. [DOI] [PMC free article] [PubMed]
- Marcinkiewicz, C., Weinreb, P. H., Calvete, J. J., Kisiel, D. G., Mousa, S. A., Tuszynski, G. P. & Lobb, R. R. (2003). Cancer Res. 63, 2020–2023. [PubMed]
- Mason, L., Tribolet, L., Simon, A., von Gnielinski, N., Nienaber, L., Taylor, P., Willis, C., Jones, M. K., Sternberg, P. W., Gasser, R. B., Loukas, A. & Hofmann, A. (2014). Int. J. Biochem. Cell Biol. 50, 146–155. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- McSorley, H. J. & Loukas, A. (2010). Parasite Immunol. 32, 549–559. [DOI] [PubMed]
- Moyle, M., Foster, D. L., McGrath, D. E., Brown, S. M., Laroche, Y., De Meutter, J., Stanssens, P., Bogowitz, C. A., Fried, V. A., Ely, J. A., Soule, H. R. & Vlasuk, G. P. (1994). J. Biol. Chem. 269, 10008–10015. [PubMed]
- Muchowski, P. J., Zhang, L., Chang, E. R., Soule, H. R., Plow, E. F. & Moyle, M. (1994). J. Biol. Chem. 269, 26419–26423. [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Osman, A., Wang, C. K., Winter, A., Loukas, A., Tribolet, L., Gasser, R. B. & Hofmann, A. (2012). Biotechnol. Adv. 30, 652–657. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Pearson, M. S., Tribolet, L., Cantacessi, C., Periago, M. V., Valerio, M. A., Jariwala, A. R., Hotez, P., Diemert, D., Loukas, A. & Bethony, J. (2012). J. Allergy Clin. Immunol. 130, 13–21. [DOI] [PubMed]
- Sigrist, C. J. A., de Castro, E., Cerutti, L., Cuche, B. A., Hulo, N., Bridge, A., Bougueleret, L. & Xenarios, I. (2012). Nucleic Acids Res. 41, D344–D347. [DOI] [PMC free article] [PubMed]
- Wermelinger, L. S., Geraldo, R. B., Frattani, F. S., Rodrigues, C. R., Juliano, M. A., Castro, H. C. & Zingali, R. B. (2009). Arch. Biochem. Biophys. 482, 25–32. [DOI] [PubMed]
- Xiao, T., Takagi, J., Coller, B. S., Wang, J.-H. & Springer, T. A. (2004). Nature (London), 432, 59–67. [DOI] [PMC free article] [PubMed]
- Xiong, J.-P., Stehle, T., Zhang, R., Joachimiak, A., Frech, M., Goodman, S. L. & Arnaout, M. A. (2002). Science, 296, 151–155. [DOI] [PubMed]
- Xu, X., Francischetti, I. M. B., Lai, R., Ribeiro, J. M. C. & Andersen, J. F. (2012). J. Biol. Chem. 287, 10967–10976. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: hookworm platelet inhibitor, 4tpv