A Cmr1-deficient functional Cmr complex composed of P. furiosus Cmr2–Cmr3, A. fulgidus Cmr4–Cmr5–Cmr6 and the 39-mer P. furiosus 7.01-crRNA was cocrystallized with single-stranded DNA complementary to the crRNA guide. X-ray diffraction data for the crystals were collected to 2.1 Å resolution using a synchrotron-radiation source.
Keywords: Cmr complex, CRISPR-Cas, crRNA
Abstract
Clustered regularly interspaced short palindromic repeat (CRISPR)-derived RNA (crRNA) and CRISPR-associated (Cas) proteins constitute a prokaryotic adaptive immune system (CRISPR–Cas system) that targets and degrades invading genetic elements. The type III-B CRISPR–Cas Cmr complex, composed of the six Cas proteins (Cmr1–Cmr6) and a crRNA, captures and cleaves RNA complementary to the crRNA guide sequence. Here, a Cmr1-deficient functional Cmr (CmrΔ1) complex composed of Pyrococcus furiosus Cmr2–Cmr3, Archaeoglobus fulgidus Cmr4–Cmr5–Cmr6 and the 39-mer P. furiosus 7.01-crRNA was prepared. The CmrΔ1 complex was cocrystallized with single-stranded DNA (ssDNA) complementary to the crRNA guide by the vapour-diffusion method. The crystals diffracted to 2.1 Å resolution using synchrotron radiation at the Photon Factory. The crystals belonged to the triclinic space group P1, with unit-cell parameters a = 75.5, b = 76.2, c = 139.2 Å, α = 90.3, β = 104.8, γ = 118.6°. The asymmetric unit of the crystals is expected to contain one CmrΔ1–ssDNA complex, with a Matthews coefficient of 2.03 Å3 Da−1 and a solvent content of 39.5%.
1. Introduction
The CRISPR (clustered regularly interspaced short palindromic repeats)–Cas (CRISPR-associated) system, an adaptive immune system in prokaryotes, targets invading genetic elements, such as phages and plasmids, for degradation (van der Oost et al., 2009 ▶; Karginov & Hannon, 2010 ▶; Marraffini & Sontheimer, 2010 ▶; Terns & Terns, 2011 ▶; Wiedenheft et al., 2012 ▶). CRISPR loci are composed of conserved repeated sequences separated by variable spacer sequences derived from previously encountered invading genetic materials (Bolotin et al., 2005 ▶; Mojica et al., 2005 ▶; Pourcel et al., 2005 ▶). After CRISPR loci are transcribed, the primary transcripts are processed within each repeat sequence to generate crRNAs (CRISPR RNAs) in a process catalyzed by the Cas6 endoribonuclease (Brouns et al., 2008 ▶; Carte et al., 2008 ▶; Hale et al., 2008 ▶). Subsequently, the crRNA and Cas protein(s) form an interference complex to degrade the invading nucleic acid complementary to the spacer region of the crRNA (Brouns et al., 2008 ▶; Hale et al., 2009 ▶; Jinek et al., 2012 ▶). Based on the signature Cas proteins Cas3, Cas9 and Cas10, the interference complexes are classified into the three major types I, II and III, respectively, which are further divided into several subtypes (Makarova et al., 2011 ▶).
The subtype III-B interference complex (Cmr complex) consists of six Cmr proteins, Cmr1, Cmr2 (also known as Cas10), Cmr3, Cmr4, Cmr5 and Cmr6, as well as a crRNA, and captures RNA complementary to the spacer region of the crRNA for degradation (Hale et al., 2009 ▶; Staals et al., 2013 ▶). The crRNAs isolated from the Pyrococcus furiosus Cmr complex have lengths of 39 or 45 nucleotides (nt), with an invariant 8 nt tag at the 5′-end which is derived from the repeat sequence and is essential for CRISPR-mediated RNA interference (Hale et al., 2009 ▶, 2012 ▶). Variation in the lengths of the crRNAs, which differ in length by 6 nt, has also been reported in the crRNAs isolated from the Thermus thermophilus Cmr complex (Staals et al., 2013 ▶). The Cmr complex degrades the target RNA at multiple positions with 6 nt intervals via a 5′-ruler mechanism (Staals et al., 2013 ▶; Hale et al., 2014 ▶). The individual crystal structures of Cmr1, Cmr2dHD (a Cmr2 derivative lacking the N-terminal HD nuclease domain), Cmr3 and Cmr5, as well as cryoelectron microscopy (cryo-EM) structures of the P. furiosus and T. thermophilus Cmr complexes, have been determined (Cocozaki et al., 2012 ▶; Park et al., 2013 ▶; Shao et al., 2013 ▶; Osawa et al., 2013b ▶; Staals et al., 2013 ▶; Spilman et al., 2013 ▶; Sun et al., 2014 ▶). The cryo-EM reconstructions of the Cmr complexes revealed the presence of a helical filament in the complex which is composed of several Cmr4 subunits (Spilman et al., 2013 ▶; Staals et al., 2013 ▶). However, the mechanisms of crRNA recognition and the periodic target cleavages from the 5′-end of the crRNA by the Cmr complex have remained elusive because of a lack of structural information for the fully assembled functional Cmr complex at atomic resolution. Intriguingly, the subtype III-A interference complex (Csm complex), which resembles the structure of the Cmr complex as demonstrated by cryo-EM analyses (Rouillon et al., 2013 ▶; Staals et al., 2014 ▶), degraded the target RNA in a similar manner to that of the Cmr complex (Staals et al., 2014 ▶; Tamulaitis et al., 2014 ▶). Therefore, structural determination of the Cmr complex will also pave the way towards elucidating the RNA-silencing mechanism by the subtype III-A Csm complex. We now report the crystallization and preliminary X-ray diffraction analysis of the functional Cmr complex in order to clarify the crRNA-guided RNA-cleavage reaction.
2. Materials and methods
2.1. Protein preparation
The Cmr2dHD–Cmr3 complex from P. furiosus (PfCmr2dHD–Cmr3) was produced and purified as described previously (Osawa et al., 2013a ▶,b ▶). The operon gene encoding Cmr4–Cmr5–Cmr6 from Archaeoglobus fulgidus (AfCmr4–Cmr5–Cmr6) was PCR-amplified from genomic DNA and cloned into the NcoI–BamHI sites of pET-28b (Novagen). The AfCmr6 subunit in the AfCmr4–Cmr5–Cmr6 complex bears a C-terminal His6 tag, which is derived from the PCR primer. Expression plasmids were transformed into Escherichia coli strain BL21-CodonPlus(DE3)-RIL and protein expression was induced with 0.1 mM IPTG when the culture reached an optical cell density (OD600) of ∼0.8, followed by shaking at 293 K for 18 h. The cells were harvested and lysed by sonication in 20 mM Tris–HCl pH 8.0, 300 mM NaCl, 10 mM imidazole, 1 mM PMSF. The cleared lysate was subjected to heat treatment at 338 K for 20 min. The heat-denatured proteins were removed by centrifugation and the supernatant was applied onto an Ni–NTA (Qiagen) column equilibrated with 20 mM Tris–HCl pH 8.0, 300 mM NaCl, 10 mM imidazole. The AfCmr4–Cmr5–Cmr6 complex was eluted with the same buffer containing 250 mM imidazole. The eluate from the Ni–NTA column was dialyzed against 20 mM Tris–HCl pH 7.0, 200 mM NaCl. The dialyzed solution was loaded onto a HiTrap Heparin column (GE Healthcare) and the flowthrough fraction containing the complex was collected and dialyzed against 20 mM Tris–HCl pH 7.0, 400 mM NaCl. The dialysate was then loaded onto a RESOURCE Q column (GE Healthcare) and the flowthrough fraction containing the AfCmr4–Cmr5–Cmr6 complex was collected and dialyzed against 20 mM Tris–HCl pH 8.0, 200 mM NaCl, 1 mM DTT. The purified AfCmr4–Cmr5–Cmr6 complex was concentrated to approximately 10 mg ml−1 using Amicon Ultra-15 centrifugal filters (30K molecular-weight cutoff; Merck Millipore). The protein concentration of the AfCmr4–Cmr5–Cmr6 complex was determined using a molar extinction coefficient of 158 600 M −1 cm−1 at 280 nm.
2.2. Complex formation
The complex composed of PfCmr2dHD–Cmr3 and AfCmr4–Cmr5–Cmr6, which is referred to as chimeric Cmr23456 (ChiCmr23456), was formed by mixing the purified PfCmr2dHD–Cmr3 and AfCmr4–Cmr5–Cmr6 complexes in a 1.3:1 molar ratio. The mixture was incubated at 343 K for 10 min and on ice for 5 min. After centrifugation, the supernatant containing ChiCmr23456 was purified by gel-filtration chromatography on a HiLoad 16/60 Superdex 200 column (GE Healthcare) previously equilibrated with 20 mM Tris–HCl pH 8.0, 200 mM NaCl, 1 mM DTT. Fractions that were rich in ChiCmr23456 were combined and concentrated to approximately 10 mg ml−1. The protein concentration of ChiCmr23456 was determined using a molar extinction coefficient of 306 660 M −1 cm−1 at 280 nm.
The Cmr1-deficient chimeric Cmr complex (ChiCmrΔ1) was reconstituted by mixing the purified ChiCmr23456 complex and the 39-mer P. furiosus 7.01-crRNA in a molar ratio of 1.3:1 in the presence of 9.7 mM MgCl2. The mixture was incubated at 343 K for 10 min and then on ice for 5 min. To this mixture, an equimolar amount of the 31-mer single-stranded DNA (ssDNA) complementary to the Pf7.01-crRNA guide was added. The final protein concentration of the mixture was adjusted to 5.1 mg ml−1 with buffer consisting of 20 mM Tris–HCl pH 8.0, 200 mM NaCl, 5 mM MgCl2, 1 mM DTT. The solution was then incubated at 343 K for 10 min, followed by gradual cooling to 298 K to form the ChiCmrΔ1–ssDNA complex. After an incubation on ice for 5 min, the supernatant was used for crystallization. The nucleotide sequences of the Pf7.01-crRNA and the ssDNA used in the reconstitution experiment are AUUGAAAGUUGUAGUAUGCGGUCCUUGCGGCUGAGAGCA and TGCTCTCAGCCGCAAGGACCGCATACTACAA, respectively.
2.3. Crystallization
Crystallization conditions were screened by the sitting-drop vapour-diffusion method at 293 K. We tested several screening kits, Crystal Screen, Crystal Screen 2, Crystal Screen Lite, Crystal Screen Cryo, Natrix, PEG/Ion, Index and SaltRx (Hampton Research), for the initial crystallization experiment. Sitting drops were prepared by mixing 1 µl reservoir solution with 1 µl sample solution and were equilibrated against 100 µl reservoir solution. Crystals of the ChiCmrΔ1–ssDNA complex grew in condition No. 89 [15%(w/v) PEG 3350, 100 mM succinic acid pH 7.0] from the Index screen. The crystallization conditions were then optimized by changing the concentration of the precipitant using the hanging-drop vapour-diffusion method. Hanging drops were prepared by combining 1 µl reservoir solution with 1 µl sample solution and were equilibrated against 500 µl reservoir solution. Finally, crystals suitable for X-ray diffraction analysis were obtained under conditions consisting of 13–15%(w/v) PEG 3350, 100 mM succinic acid pH 7.0. The crystals of the complex grew within one week to maximum dimensions of 200 × 200 × 20 µm.
2.4. X-ray data collection
For data collection under cryogenic conditions, the crystals of the ChiCmrΔ1–ssDNA complex were transferred into a cryoprotectant solution consisting of 20%(v/v) glycerol in the reservoir solution (cryo-condition I). The crystals were immediately mounted in a cryoloop and flash-cooled in a nitrogen stream at 95 K. X-ray diffraction data were collected on beamline BL-17A at the Photon Factory, Ibaraki, Japan using an ADSC Q270 CCD detector. We only obtained ∼3.1 Å resolution diffraction data using the crystals cryoprotected using cryo-condition I. To improve the diffraction quality of the crystals, we optimized the cryo-conditions by changing the cryoprotection method and the cryoprotectant solution. To gradually increase the concentration of the cryoprotectant, we added 1 µl of the cryoprotectant solution to the crystallization droplet and then removed 1 µl of solution from the resultant droplet. We repeated this procedure several times over 2–4 min. Finally, we obtained higher resolution (∼2.1 Å) diffraction data by using a cryoprotectant solution consisting of 30%(v/v) PEG 400 and 2%(w/v) trehalose in the reservoir solution (cryo-condition II). Diffraction data were processed with XDS (Kabsch, 2010 ▶). The data-processing statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics.
Values in parentheses are for the last shell.
| Cryo-condition I | Cryo-condition II | |
|---|---|---|
| Space group | P1 | P1 |
| Unit-cell parameters (, ) | a = 79.0, b = 79.6, c = 142.9, = 90.6, = 103.4, = 115.4 | a = 75.5, b = 76.2, c = 139.2, = 90.3, = 104.8, = 118.6 |
| Wavelength () | 0.98 | 0.98 |
| Resolution () | 503.1 (3.283.10) | 502.1 (2.222.10) |
| Measured reflections | 214115 | 1125455 |
| Unique reflections | 54626 | 148845 |
| R merge † | 0.077 (0.491) | 0.075 (0.505) |
| I/(I) | 14.3 (2.7) | 18.3 (4.4) |
| Completeness (%) | 98.3 (95.7) | 97.4 (94.4) |
| Multiplicity | 3.9 (3.9) | 7.6 (7.5) |
| Wilson B factor (2) | 70.1 | 39.7 |
| Mosaicity () | 0.162 | 0.216 |
R
merge = 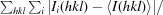
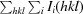 , where I
i(hkl) is the observed intensity and I(hkl) is the average intensity over symmetry-equivalent measurements.
, where I
i(hkl) is the observed intensity and I(hkl) is the average intensity over symmetry-equivalent measurements.
3. Results and discussion
Since we found that the Cmr1-deficient P. furiosus Cmr complex (PfCmrΔ1) exhibits crRNA-dependent RNA cleavage activity (Osawa et al., unpublished work), we tried to crystallize PfCmrΔ1 and the PfCmrΔ1–ssDNA complex. However, we were unable to obtain any crystals of these complexes. We then tried to reconstitute the A. fulgidus Cmr complex, but we could not prepare the AfCmr2–Cmr3 complex because of its low expression level in E. coli cells. Therefore, we sought to reconstitute the chimeric Cmr1-deficient Cmr complex (ChiCmrΔ1) composed of PfCmr2dHD–Cmr3, AfCmr4–Cmr5–Cmr6 and Pf7.01-crRNA. Since the Cmr proteins from P. furiosus and A. fulgidus exhibit sequence identities of 27.1–42.2% (Table 2 ▶) and the 5′-tag sequence of the crRNA is the same in these two archaeal species, we expected the hybrid complex to adopt the physiologically relevant architecture. The PfCmr2dHD–Cmr3 and AfCmr4–Cmr5–Cmr6 complexes were individually overexpressed in E. coli cells and were purified using several chromatographic steps. The purified PfCmr2dHD–Cmr3 and AfCmr4–Cmr5–Cmr6 complexes were mixed in a 1.3:1 molar ratio, and the resulting ChiCmr23456 complex was purified by gel-filtration chromatography, confirming the interaction between these two subcomplexes. The purified ChiCmr23456 complex was mixed with Pf7.01-crRNA to reconstitute ChiCmrΔ1 (Fig. 1 ▶). We confirmed that ChiCmrΔ1 degraded the target RNA of the crRNA guide region in a sequence-specific manner and that ChiCmrΔ1 produced the same cleavage product as those generated by PfCmrΔ1 (Osawa et al., unpublished work). These findings clearly showed that the hybrid complex is functional and that the Cmr proteins from P. furiosus and A. fulgidus are interchangeable. We will report these results in more detail in the near future.
Table 2. Sequence identities of the Cmr proteins from P. furiosus and A. fulgidus .
| Sequence identity (%) | |
|---|---|
| Cmr1 | 27.1 |
| Cmr2 | 29.6 |
| Cmr3 | 33.3 |
| Cmr4 | 42.2 |
| Cmr5 | 27.5 |
| Cmr6 | 37.5 |
Figure 1.
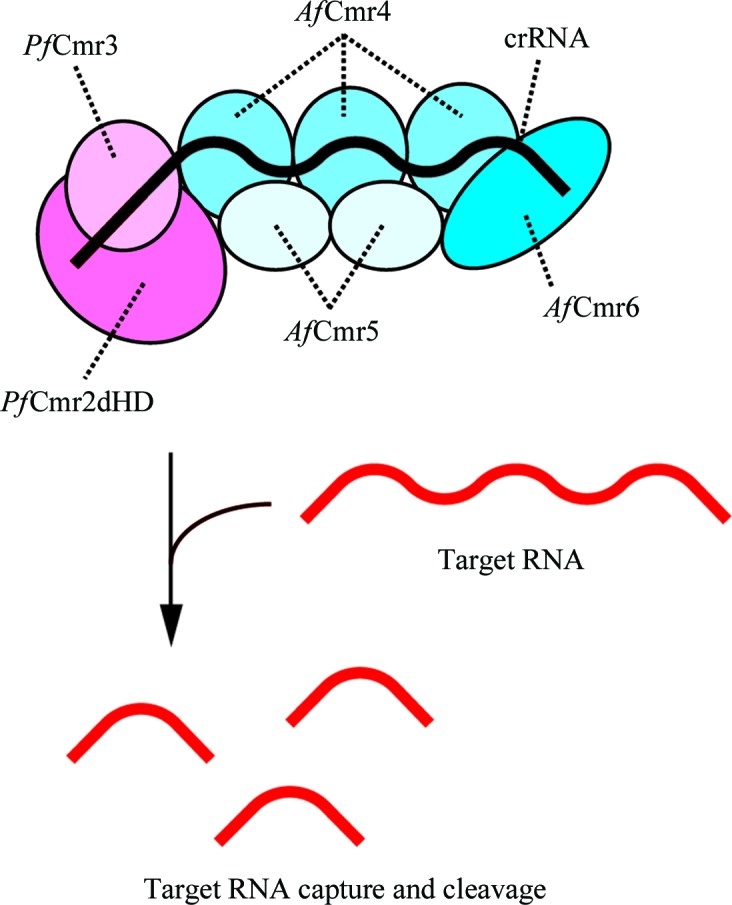
Schematic diagram of the reconstituted ChiCmrΔ1 complex and its functional activity.
For crystallization, ChiCmrΔ1 was mixed with ssDNA complementary to the guide region of the Pf7.01-crRNA to form the ChiCmrΔ1–ssDNA complex. Crystallization screening was performed by the sitting-drop vapour-diffusion method at 293 K. The crystals were obtained under conditions consisting of 15%(w/v) PEG 3350, 100 mM succinic acid pH 7.0. We subsequently optimized the crystallization conditions by the hanging-drop vapour-diffusion method and obtained complex crystals suitable for X-ray diffraction experiments using the reservoir conditions 13–15%(w/v) PEG 3350, 100 mM succinic acid pH 7.0. The crystals of the ChiCmrΔ1–ssDNA complex grew to maximum dimensions of 200 × 200 × 20 µm within a week (Fig. 2 ▶ a). Electrophoretic analysis of the crystals by SDS–PAGE revealed that the crystals contained all of the Cmr protein components (Fig. 2 ▶ b). Although we did not confirm the presence of the crRNA and the ssDNA at this stage, these nucleic acids were also present in the crystals, as described below.
Figure 2.
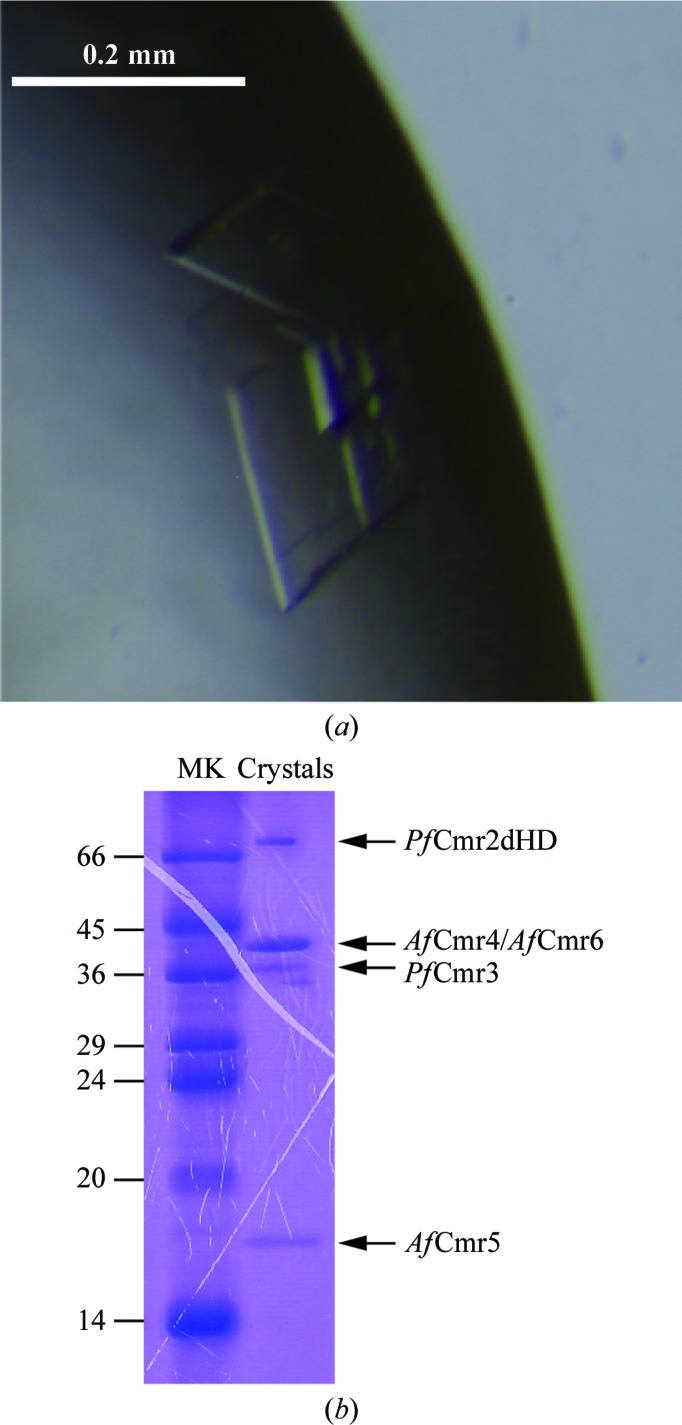
(a) Crystals of the ChiCmrΔ1–ssDNA complex. (b) Electrophoretic analysis of the crystals by SDS–PAGE. Since the molecular mass of AfCmr4 (39.8 kDa) is almost the same as that of AfCmr6 (40.2 kDa), it is difficult to distinguish these protein bands by SDS–PAGE analysis. Lane MK contains molecular-mass markers (labelled in kDa).
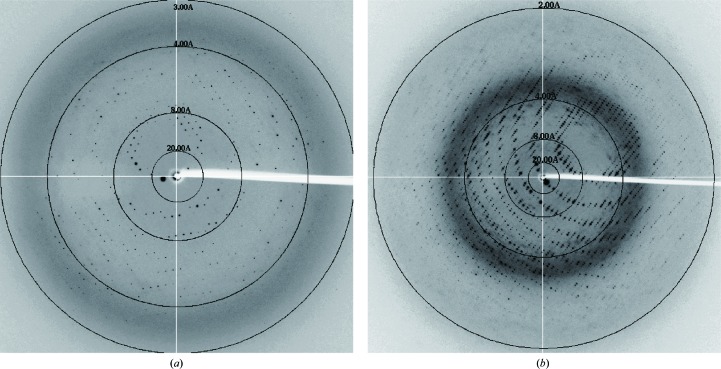
The crystals were initially cryoprotected under cryo-condition I, but the resulting crystals only diffracted to a maximum resolution of 3.1 Å (Fig. 3 ▶ a). Furthermore, these crystals did not always diffract to ∼3.1 Å resolution. Therefore, it was difficult to prepare consistently well diffracting crystals under cryo-condition I. To improve the quality and resolution of the diffraction data, we screened and optimized the cryo-conditions. Finally, we found that the crystals cryoprotected using PEG 400 and trehalose as cryoprotectants (cryo-condition II) consistently diffracted to ∼2.1 Å resolution (Fig. 3 ▶ b), a much higher resolution than that obtained with cryo-condition I. These findings demonstrated that exploration of the cryo-conditions is crucial to obtain high-quality diffraction data. The crystals of the ChiCmrΔ1–ssDNA complex belonged to the triclinic space group P1 (Table 1 ▶). The unit-cell parameters of the crystals cryoprotected under cryo-condition I were a = 79.0, b = 79.6, c = 142.9 Å, α = 90.6, β = 103.4, γ = 115.4° (Table 1 ▶). In contrast, those of the crystals cryoprotected under cryo-condition II were a = 75.5, b = 76.2, c = 139.2 Å, α = 90.3, β = 104.8, γ = 118.6° (Table 1 ▶). Therefore, soaking the crystals in the cryo-condition II solution caused lattice shrinking, which led to the significant improvement of the resolution limit during the diffraction experiments.
Figure 3.
Diffraction patterns of the ChiCmrΔ1–ssDNA complex cryoprotected under cryo-condition I (a) and cryo-condition II (b).
crRNAs of two different lengths (a 39-mer and a 45-mer) have been observed in the P. furiosus Cmr complex (Hale et al., 2009 ▶, 2012 ▶). A recent study suggested that the 39-mer and 45-mer crRNAs are components of complexes with protein stoichiometries Cmr11–Cmr21–Cmr31–Cmr43–Cmr52–Cmr61 and Cmr11–Cmr21–Cmr31–Cmr44–Cmr53–Cmr61, respectively (Benda et al., 2014 ▶). Therefore, we assumed that the stoichiometry of the ChiCmrΔ1–ssDNA complex is 1:1:3:2:1:1:1 Cmr2dHD:Cmr3:Cmr4:Cmr5:Cmr6:Pf7.01-crRNA:ssDNA, with a molecular mass of 331 kDa, on the basis of the molecular masses of Cmr2dHD (78.0 kDa), Cmr3 (36.3 kDa), Cmr4 (39.8 kDa), Cmr5 (17.4 kDa), Cmr6 (40.2 kDa), Pf7.01-crRNA (12.6 kDa) and ssDNA (9.4 kDa). Therefore, the asymmetric unit of the crystal is expected to contain one ChiCmrΔ1–ssDNA complex, with a Matthews coefficient of 2.03 Å3 Da−1 and a solvent content of 39.5%. Since the crystal structures of PfCmr2dHD, PfCmr3 and AfCmr5 have been determined (Cocozaki et al., 2012 ▶; Park et al., 2013 ▶; Shao et al., 2013 ▶; Osawa et al., 2013b ▶), the ChiCmrΔ1–ssDNA complex structure is expected to be solved by a combination of the molecular-replacement and single-wavelength anomalous dispersion methods, with selenium as the anomalous scattering atom. Quite recently, we have succeeded in determining the complex structure by this method. We confirmed that the crystals contained the crRNA and the ssDNA, as well as all of the Cmr protein subunits in the stoichiometry described above. Intriguingly, the subunit interfaces of the P. furiosus and A. fulgidus proteins contain many residues that are conserved between these two archaeal species, thus supporting their interchangeability in the formation of the active complex. These results will be reported in detail in the near future.
Acknowledgments
We thank the beamline staff at BL-17A of the Photon Factory, Ibaraki, Japan for technical assistance during data collection. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (JSPS) and grants from the Kato Memorial Bioscience Foundation, the Kurata Memorial Hitachi Science and Technology Foundation and the Institute for Fermentation, Osaka (IFO) to TN.
References
- Benda, C., Ebert, J., Scheltema, R. A., Schiller, H. B., Baumgärtner, M., Bonneau, F., Mann, M. & Conti, E. (2014). Mol. Cell, 56, 43–54. [DOI] [PubMed]
- Bolotin, A., Quinquis, B., Sorokin, A. & Ehrlich, S. D. (2005). Microbiology, 151, 2551–2561. [DOI] [PubMed]
- Brouns, S. J., Jore, M. M., Lundgren, M., Westra, E. R., Slijkhuis, R. J., Snijders, A. P., Dickman, M. J., Makarova, K. S., Koonin, E. V. & van der Oost, J. (2008). Science, 321, 960–964. [DOI] [PMC free article] [PubMed]
- Carte, J., Wang, R., Li, H., Terns, R. M. & Terns, M. P. (2008). Genes Dev. 22, 3489–3496. [DOI] [PMC free article] [PubMed]
- Cocozaki, A. I., Ramia, N. F., Shao, Y., Hale, C. R., Terns, R. M., Terns, M. P. & Li, H. (2012). Structure, 20, 545–553. [DOI] [PMC free article] [PubMed]
- Hale, C. R., Cocozaki, A., Li, H., Terns, R. M. & Terns, M. P. (2014). Genes Dev. 28, 2432–2443. [DOI] [PMC free article] [PubMed]
- Hale, C., Kleppe, K., Terns, R. M. & Terns, M. P. (2008). RNA, 14, 2572–2579. [DOI] [PMC free article] [PubMed]
- Hale, C. R., Zhao, P., Olson, S., Duff, M. O., Graveley, B. R., Wells, L., Terns, R. M. & Terns, M. P. (2009). Cell, 139, 945–956. [DOI] [PMC free article] [PubMed]
- Hale, C. R., Majumdar, S., Elmore, J., Pfister, N., Compton, M., Olson, S., Resch, A. M., Glover, C. V. III, Graveley, B. R., Terns, R. M. & Terns, M. P. (2012). Mol. Cell, 45, 292–302. [DOI] [PMC free article] [PubMed]
- Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A. & Charpentier, E. (2012). Science, 337, 816–821. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Karginov, F. V. & Hannon, G. J. (2010). Mol. Cell, 37, 7–19. [DOI] [PMC free article] [PubMed]
- Makarova, K. S., Haft, D. H., Barrangou, R., Brouns, S. J., Charpentier, E., Horvath, P., Moineau, S., Mojica, F. J., Wolf, Y. I., Yakunin, A. F., van der Oost, J. & Koonin, E. V. (2011). Nature Rev. Microbiol. 9, 467–477. [DOI] [PMC free article] [PubMed]
- Marraffini, L. A. & Sontheimer, E. J. (2010). Nature Rev. Genet. 11, 181–190. [DOI] [PMC free article] [PubMed]
- Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J. & Soria, E. (2005). J. Mol. Evol. 60, 174–182. [DOI] [PubMed]
- Oost, J. van der, Jore, M. M., Westra, E. R., Lundgren, M. & Brouns, S. J. (2009). Trends Biochem. Sci. 34, 401–407. [DOI] [PubMed]
- Osawa, T., Inanaga, H. & Numata, T. (2013a). Acta Cryst. F69, 585–587. [DOI] [PMC free article] [PubMed]
- Osawa, T., Inanaga, H. & Numata, T. (2013b). J. Mol. Biol. 425, 3811–3823. [DOI] [PubMed]
- Park, J.-H., Sun, J., Park, S.-Y., Hwang, H.-J., Park, M.-Y., Shin, M. & Kim, J.-S. (2013). FEBS Lett. 587, 562–568. [DOI] [PubMed]
- Pourcel, C., Salvignol, G. & Vergnaud, G. (2005). Microbiology, 151, 653–663. [DOI] [PubMed]
- Rouillon, C., Zhou, M., Zhang, J., Politis, A., Beilsten-Edmands, V., Cannone, G., Graham, S., Robinson, C. V., Spagnolo, L. & White, M. F. (2013). Mol. Cell, 52, 124–134. [DOI] [PMC free article] [PubMed]
- Shao, Y., Cocozaki, A. I., Ramia, N. F., Terns, R. M., Terns, M. P. & Li, H. (2013). Structure, 21, 376–384. [DOI] [PMC free article] [PubMed]
- Spilman, M., Cocozaki, A., Hale, C., Shao, Y., Ramia, N., Terns, R., Terns, M., Li, H. & Stagg, S. (2013). Mol. Cell, 52, 146–152. [DOI] [PMC free article] [PubMed]
- Staals, R. H. et al. (2013). Mol. Cell, 52, 135–145. [DOI] [PMC free article] [PubMed]
- Staals, R. H. et al. (2014). Mol. Cell, 56, 518–530. [DOI] [PMC free article] [PubMed]
- Sun, J., Jeon, J.-H., Shin, M., Shin, H.-C., Oh, B.-H. & Kim, J.-S. (2014). Acta Cryst. D70, 535–543. [DOI] [PubMed]
- Tamulaitis, G., Kazlauskiene, M., Manakova, E., Venclovas, Č., Nwokeoji, A., Dickman, M., Horvath, P. & Siksnys, V. (2014). Mol. Cell, 56, 506–517. [DOI] [PubMed]
- Terns, M. P. & Terns, R. M. (2011). Curr. Opin. Microbiol. 14, 321–327. [DOI] [PMC free article] [PubMed]
- Wiedenheft, B., Sternberg, S. H. & Doudna, J. A. (2012). Nature (London), 482, 331–338. [DOI] [PubMed]